Berberine Alleviates Intestinal Inflammation by Disrupting Pathological Macrophage–Epithelial Crosstalk in Macrophage–Organoid Co-Culture Model
Abstract
1. Introduction
2. Results
2.1. BBR Antagonised LPS-Induced Inflammation on Murine Intestinal Organoids
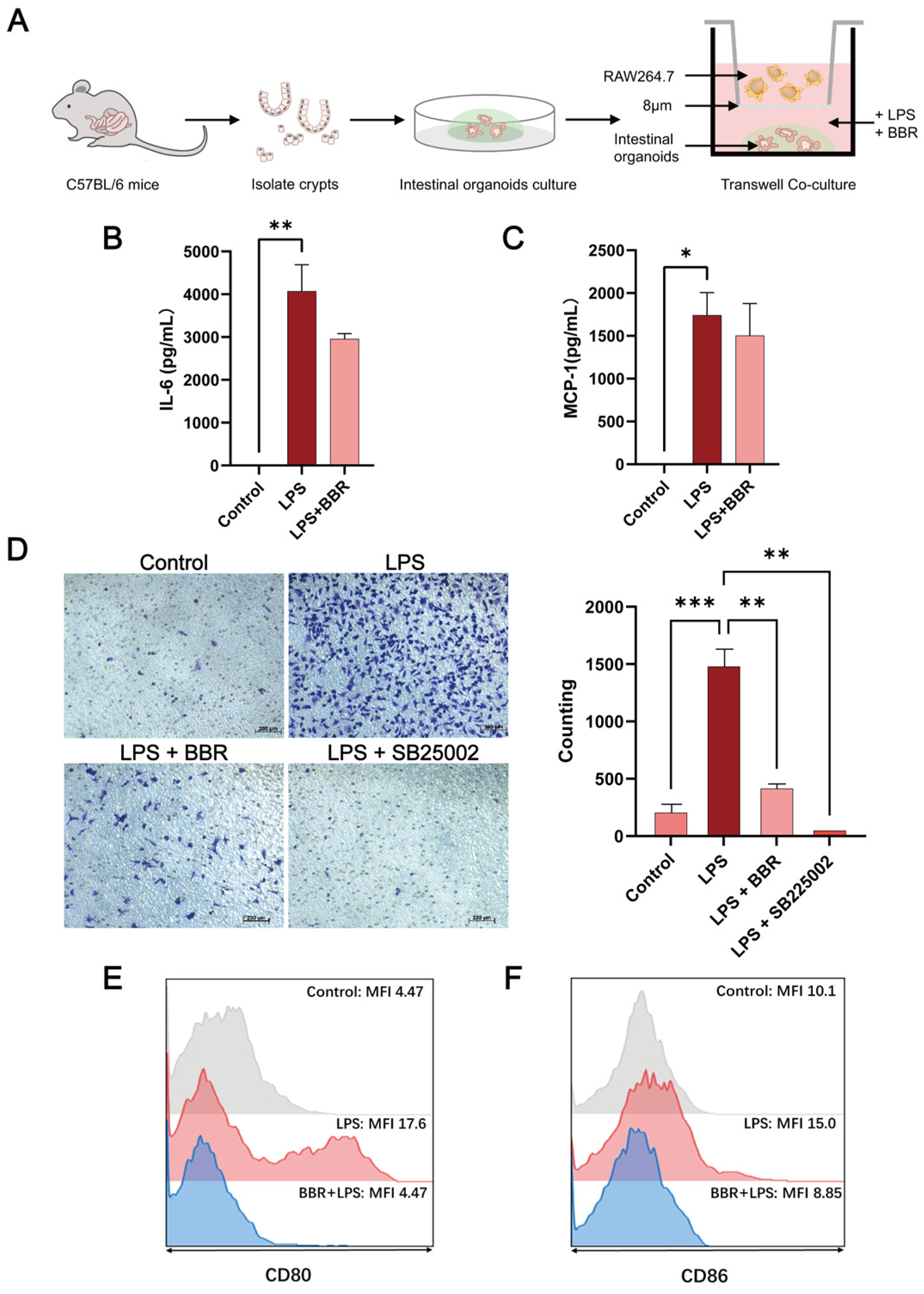
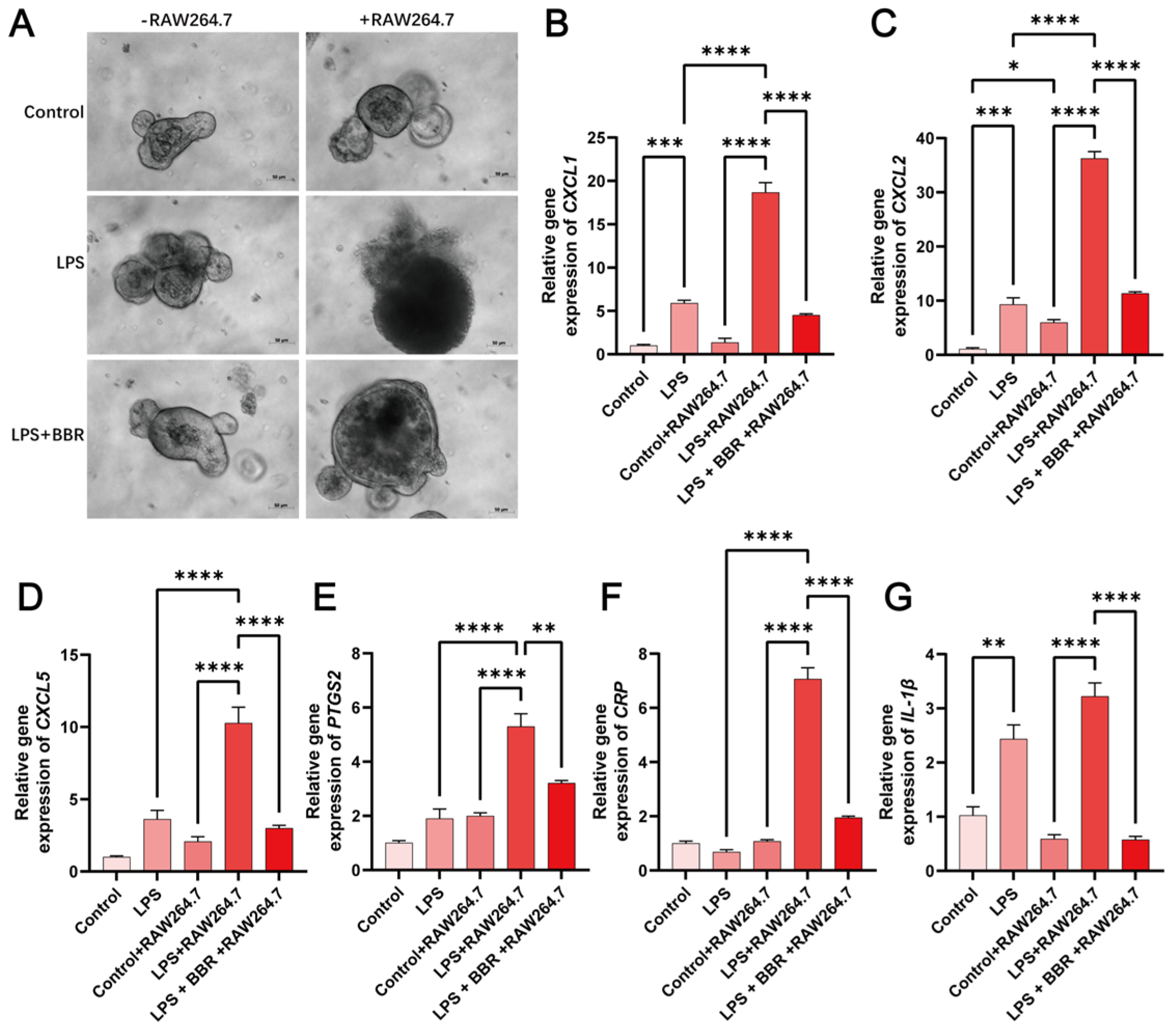
2.2. BBR Has Anti-Inflammatory and Barrier-Protective Effects in a Macrophage–Intestinal Organoids Co-Culture Model
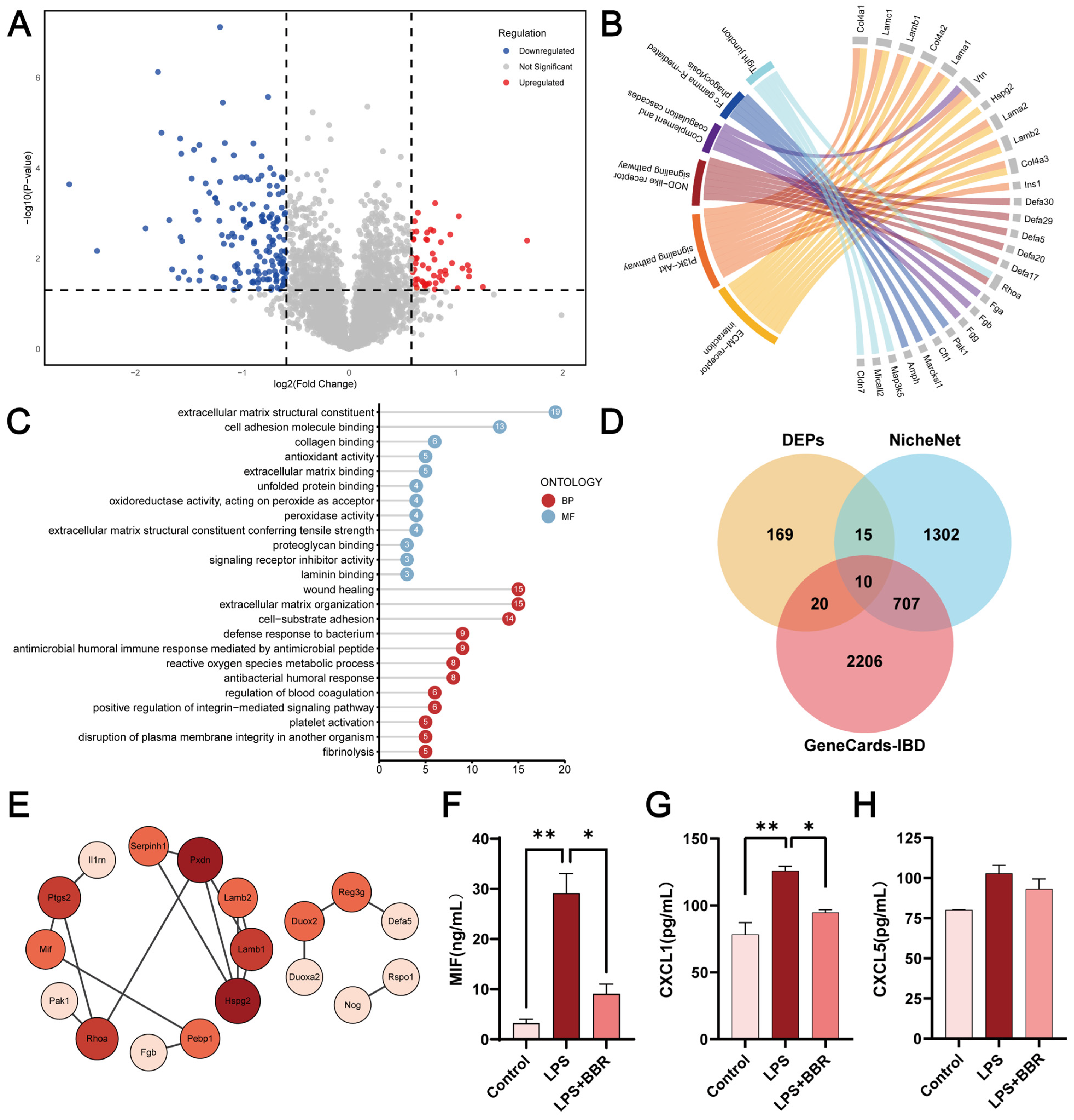
2.3. BBR Regulated Epithelial–Macrophage Interaction
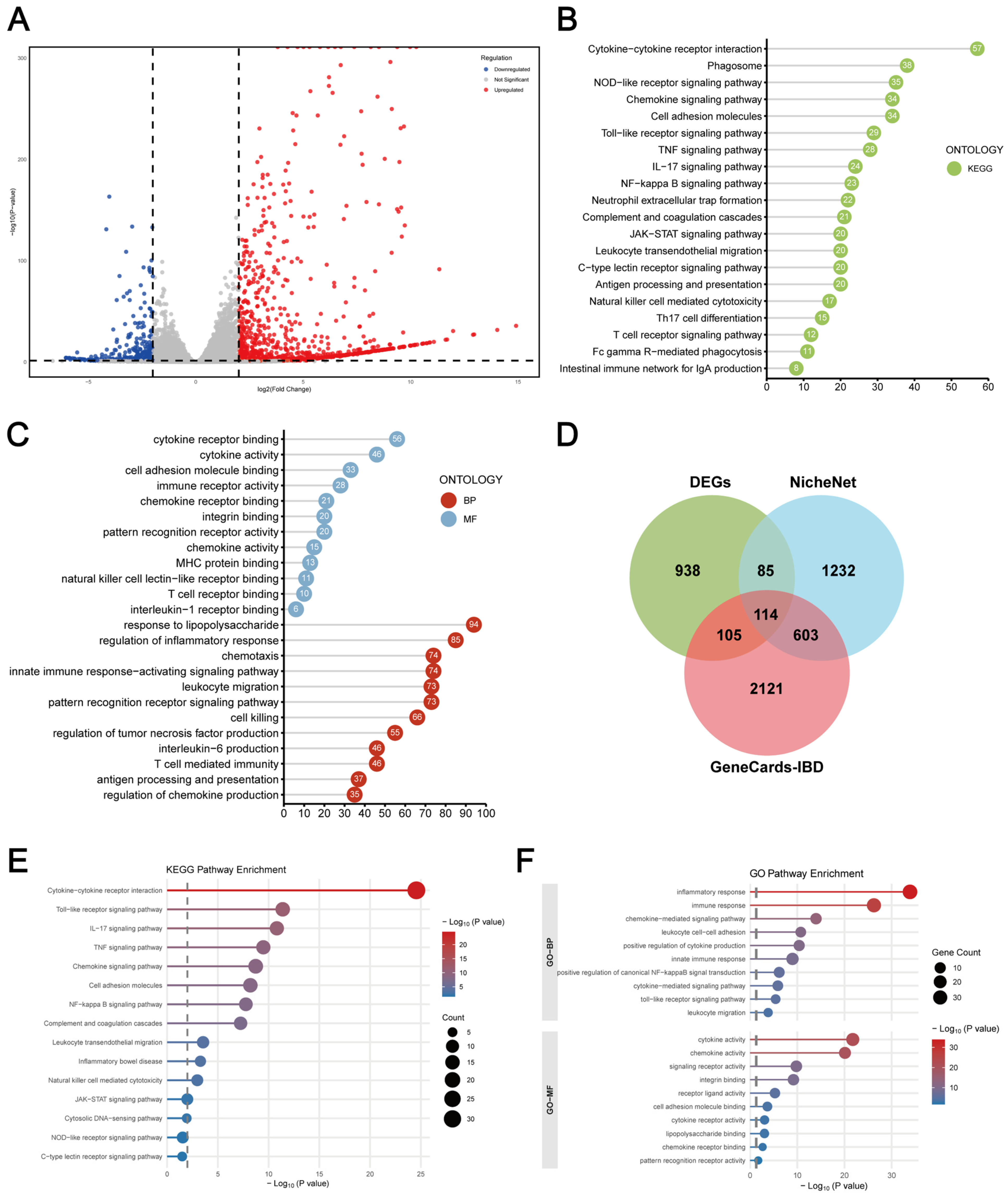
2.4. Macrophage–Intestinal Organoids Co-Culture Exhibits a More Robust Inflammatory Microenvironment
3. Discussion
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Mice
4.3. Cell Culture
4.4. Crypt Isolation and Organoid Culture
4.5. Cell Viability Assay
4.6. EdU Cell Proliferation Assay
4.7. FITC-Dextran Permeability Assay
4.8. Transwell Co-Culture of Murine Intestinal Organoid and RAW264.7 Cells
4.9. Transwell Migration Assay
4.10. Flow Cytometry Analysis
4.11. Immunofluorescence
4.12. Western Blot Assay
4.13. Enzyme-Linked Immunosorbent Assay (ELISA)
4.14. Real-Time Quantitative PCR Analysis
4.15. Proteomic Analysis
4.16. RNA Sequencing (RNA-Seq) Analysis
4.17. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imenshahidi, M.; Hosseinzadeh, H. Berberine and Barberry (Berberis vulgaris): A Clinical Review. Phytother. Res. PTR 2019, 33, 504–523. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, C.; Zou, J.; Zhou, Z.; Zhang, J.; Yan, Y.; Liang, Y.; Tang, G.; Chen, G.; Xu, X.; et al. Coptidis Rhizoma and Berberine as Anti-Cancer Drugs: A 10-Year Updates and Future Perspectives. Pharmacol. Res. 2025, 216, 107742. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Ma, J.; Ma, Y.; Cheng, Z.; Gan, S.; Yuan, G.; Liu, D.; Li, S.; Liu, Y.; Xue, X.; et al. Berberine Ursodeoxycholate for the Treatment of Type 2 Diabetes: A Randomized Clinical Trial. JAMA Netw. Open 2025, 8, e2462185. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Yang, H.; Li, T.; Luo, J.; Zhou, L.; Chen, R.; Han, L.; Lin, Y. Molecular Mechanisms, Targets and Clinical Potential of Berberine in Regulating Metabolism: A Review Focussing on Databases and Molecular Docking Studies. Front. Pharmacol. 2024, 15, 1368950. [Google Scholar] [CrossRef]
- Li, H.; Feng, C.; Fan, C.; Yang, Y.; Yang, X.; Lu, H.; Lu, Q.; Zhu, F.; Xiang, C.; Zhang, Z.; et al. Intervention of Oncostatin M-Driven Mucosal Inflammation by Berberine Exerts Therapeutic Property in Chronic Ulcerative Colitis. Cell Death Dis. 2020, 11, 271. [Google Scholar] [CrossRef]
- Li, C.; Xi, Y.; Li, S.; Zhao, Q.; Cheng, W.; Wang, Z.; Zhong, J.; Niu, X.; Chen, G. Berberine Ameliorates TNBS Induced Colitis by Inhibiting Inflammatory Responses and Th1/Th17 Differentiation. Mol. Immunol. 2015, 67, 444–454. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Jiang, T.; Gong, J.; Niu, L.; Li, N. Berberine Prevents Damage to the Intestinal Mucosal Barrier during Early Phase of Sepsis in Rat through Mechanisms Independent of the NOD-like Receptors Signaling Pathway. Eur. J. Pharmacol. 2014, 730, 1–7. [Google Scholar] [CrossRef]
- Zhu, C.; Li, K.; Peng, X.-X.; Yao, T.-J.; Wang, Z.-Y.; Hu, P.; Cai, D.; Liu, H.-Y. Berberine a Traditional Chinese Drug Repurposing: Its Actions in Inflammation-Associated Ulcerative Colitis and Cancer Therapy. Front. Immunol. 2022, 13, 1083788. [Google Scholar] [CrossRef]
- Dong, Y.; Fan, H.; Zhang, Z.; Jiang, F.; Li, M.; Zhou, H.; Guo, W.; Zhang, Z.; Kang, Z.; Gui, Y.; et al. Berberine Ameliorates DSS-Induced Intestinal Mucosal Barrier Dysfunction through Microbiota-Dependence and Wnt/β-Catenin Pathway. Int. J. Biol. Sci. 2022, 18, 1381–1397. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, P.; Zhu, Z.; Zhou, L.; Li, J.; Zhou, R.; Kan, Y.; Li, Y.; Yu, X.; Zhao, J.; et al. Acetylation of p65Lys310 by P300 in Macrophages Mediates Anti-Inflammatory Property of Berberine. Redox Biol. 2023, 62, 102704. [Google Scholar] [CrossRef]
- Li, Y.-H.; Xiao, H.-T.; Hu, D.-D.; Fatima, S.; Lin, C.-Y.; Mu, H.-X.; Lee, N.P.; Bian, Z.-X. Berberine Ameliorates Chronic Relapsing Dextran Sulfate Sodium-Induced Colitis in C57BL/6 Mice by Suppressing Th17 Responses. Pharmacol. Res. 2016, 110, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Deng, H.; Li, Y.; Fan, T.; Liu, Y.; Tang, S.; Wei, W.; Liu, X.; Guo, X.; Jiang, J.; et al. Berberine Directly Targets the NEK7 Protein to Block the NEK7–NLRP3 Interaction and Exert Anti-Inflammatory Activity. J. Med. Chem. 2021, 64, 768–781. [Google Scholar] [CrossRef]
- Xiao, Y.; Cui, Y.; Zhang, Y.; Fu, W.; Liu, Y.; Liu, F. Berberine Hydrochloride Enhances Innate Immunity to Protect against Pathogen Infection via P38 MAPK Pathway. Front. Immunol. 2025, 16, 1536143. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Hua, W.; Wei, Q.; Fang, X.; Zhao, Z.; Ge, C.; Liu, C.; Chen, C.; Tao, Y.; et al. Berberine Inhibits Macrophage M1 Polarization via AKT1/SOCS1/NF-κB Signaling Pathway to Protect against DSS-Induced Colitis. Int. Immunopharmacol. 2018, 57, 121–131. [Google Scholar] [CrossRef]
- Haftcheshmeh, S.M.; Abedi, M.; Mashayekhi, K.; Mousavi, M.J.; Navashenaq, J.G.; Mohammadi, A.; Momtazi-Borojeni, A.A. Berberine as a Natural Modulator of Inflammatory Signaling Pathways in the Immune System: Focus on NF-κB, JAK/STAT, and MAPK Signaling Pathways. Phytother. Res. PTR 2022, 36, 1216–1230. [Google Scholar] [CrossRef]
- Jeong, H.W.; Hsu, K.C.; Lee, J.-W.; Ham, M.; Huh, J.Y.; Shin, H.J.; Kim, W.S.; Kim, J.B. Berberine Suppresses Proinflammatory Responses through AMPK Activation in Macrophages. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E955–E964. [Google Scholar] [CrossRef]
- Yan, F.; Wang, L.; Shi, Y.; Cao, H.; Liu, L.; Washington, M.K.; Chaturvedi, R.; Israel, D.A.; Cao, H.; Wang, B.; et al. Berberine Promotes Recovery of Colitis and Inhibits Inflammatory Responses in Colonic Macrophages and Epithelial Cells in DSS-Treated Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G504–G514. [Google Scholar] [CrossRef]
- Dinesh, P.; Rasool, M. Berberine, an Isoquinoline Alkaloid Suppresses TXNIP Mediated NLRP3 Inflammasome Activation in MSU Crystal Stimulated RAW 264.7 Macrophages through the Upregulation of Nrf2 Transcription Factor and Alleviates MSU Crystal Induced Inflammation in Rats. Int. Immunopharmacol. 2017, 44, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Cheng, Z.; Wu, F.; Hu, M.; Liu, Z.; Dong, R.; Chen, G. Berberine in the Treatment of Ulcerative Colitis: A Possible Pathway through Tuft Cells. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 134, 111129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cui, D.; Yuan, W.; Chen, C.; Liu, Q. Berberine Represses Wnt/β-Catenin Pathway Activation via Modulating the microRNA-103a-3p/Bromodomain-Containing Protein 4 Axis, Thereby Refraining Pyroptosis and Reducing the Intestinal Mucosal Barrier Defect Induced via Colitis. Bioengineered 2022, 13, 7392–7409. [Google Scholar] [CrossRef]
- Sato, T.; Vries, R.G.; Snippert, H.J.; van de Wetering, M.; Barker, N.; Stange, D.E.; van Es, J.H.; Abo, A.; Kujala, P.; Peters, P.J.; et al. Single Lgr5 Stem Cells Build Crypt-Villus Structures In Vitro without a Mesenchymal Niche. Nature 2009, 459, 262–265. [Google Scholar] [CrossRef]
- Lechuga, S.; Braga-Neto, M.B.; Naydenov, N.G.; Rieder, F.; Ivanov, A.I. Understanding Disruption of the Gut Barrier during Inflammation: Should We Abandon Traditional Epithelial Cell Lines and Switch to Intestinal Organoids? Front. Immunol. 2023, 14, 1108289. [Google Scholar] [CrossRef]
- Macedo, M.H.; Dias Neto, M.; Pastrana, L.; Gonçalves, C.; Xavier, M. Recent Advances in Cell-Based In Vitro Models to Recreate Human Intestinal Inflammation. Adv. Sci. Weinh. Baden-Wurtt. Ger. 2023, 10, e2301391. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.-F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1α-Responsive Genes Involved in Oxidative Phosphorylation Are Coordinately Downregulated in Human Diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Hibiya, S.; Tsuchiya, K.; Hayashi, R.; Fukushima, K.; Horita, N.; Watanabe, S.; Shirasaki, T.; Nishimura, R.; Kimura, N.; Nishimura, T.; et al. Long-Term Inflammation Transforms Intestinal Epithelial Cells of Colonic Organoids. J. Crohns Colitis 2017, 11, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, S.; Chen, L.; Pan, X.; Wen, Z.; Chen, Y.; Zhang, L.; Liu, J.; Chen, D. Lipopolysaccharide Induced Intestinal Epithelial Injury: A Novel Organoids-Based Model for Sepsis In Vitro. Chin. Med. J. 2022, 135, 2232–2239. [Google Scholar] [CrossRef]
- Hentschel, V.; Seufferlein, T.; Armacki, M. Intestinal Organoids in Coculture: Redefining the Boundaries of Gut Mucosa Ex Vivo Modeling. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G693–G704. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.J.; Barningham, L.; Campbell, E.L.; Cerovic, V.; Duckworth, C.A.; Luu, L.; Wastling, J.; Derricott, H.; Coombes, J.L. A Novel In Vitro Model of the Small Intestinal Epithelium in Co-Culture with “gut-like” Dendritic Cells. Discov. Immunol. 2023, 2, kyad018. [Google Scholar] [CrossRef]
- Moraitis, I.; Taelman, J.; Arozamena, B.; Mularoni, L.; Wienskowska, O.; Sanjuan Garriga, X.; Arregui, L.; Stefanovic, M.; Modolell Farré, I.; Guedea, F.; et al. Mucosal Macrophages Govern Intestinal Regeneration in Response to Injury. Gastroenterology 2025, 169, 119–135.e26. [Google Scholar] [CrossRef]
- Schreurs, R.R.C.E.; Baumdick, M.E.; Drewniak, A.; Bunders, M.J. In Vitro Co-Culture of Human Intestinal Organoids and Lamina Propria-Derived CD4+ T Cells. STAR Protoc. 2021, 2, 100519. [Google Scholar] [CrossRef]
- Hentschel, V.; Govindarajan, D.; Seufferlein, T.; Armacki, M. An Adaptable Protocol to Generate a Murine Enteroid-Macrophage Co-Culture System. Int. J. Mol. Sci. 2024, 25, 7944. [Google Scholar] [CrossRef] [PubMed]
- Staab, J.F.; Lemme-Dumit, J.M.; Latanich, R.; Pasetti, M.F.; Zachos, N.C. Co-Culture System of Human Enteroids/Colonoids with Innate Immune Cells. Curr. Protoc. Immunol. 2020, 131, e113. [Google Scholar] [CrossRef]
- Kakni, P.; Truckenmüller, R.; Habibović, P.; van Griensven, M.; Giselbrecht, S. A Microwell-Based Intestinal Organoid-Macrophage Co-Culture System to Study Intestinal Inflammation. Int. J. Mol. Sci. 2022, 23, 15364. [Google Scholar] [CrossRef]
- Li, N.; Gu, L.; Qu, L.; Gong, J.; Li, Q.; Zhu, W.; Li, J. Berberine Attenuates Pro-Inflammatory Cytokine-Induced Tight Junction Disruption in an In Vitro Model of Intestinal Epithelial Cells. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2010, 40, 1–8. [Google Scholar] [CrossRef]
- Meng, G.; Li, P.; Du, X.; Feng, X.; Qiu, F. Berberine Alleviates Ulcerative Colitis by Inhibiting Inflammation through Targeting IRGM1. Phytomedicine Int. J. Phytother. Phytopharm. 2024, 133, 155909. [Google Scholar] [CrossRef]
- Sun, J.; Zeng, Q.; Wu, Z.; Huang, L.; Sun, T.; Ling, C.; Zhang, B.; Chen, C.; Wang, H. Berberine Inhibits NLRP3 Inflammasome Activation and Proinflammatory Macrophage M1 Polarization to Accelerate Peripheral Nerve Regeneration. Neurother. J. Am. Soc. Exp. Neurother. 2024, 21, e00347. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, C.; Lu, H.; Feng, C.; He, P.; Yang, X.; Xiang, C.; Zuo, J.; Tang, W. Protective Role of Berberine on Ulcerative Colitis through Modulating Enteric Glial Cells–Intestinal Epithelial Cells–Immune Cells Interactions. Acta Pharm. Sin. B 2020, 10, 447–461. [Google Scholar] [CrossRef]
- Yang, L.; Akanyibah, F.A.; Yao, D.; Jin, T.; Mao, F. The Role of COX-2 and Its Use as a Therapeutic Target in IBD and Related Colorectal Cancer. Arch. Biochem. Biophys. 2025, 771, 110516. [Google Scholar] [CrossRef] [PubMed]
- Izadparast, F.; Riahi-Zajani, B.; Yarmohammadi, F.; Hayes, A.W.; Karimi, G. Protective Effect of Berberine against LPS-Induced Injury in the Intestine: A Review. Cell Cycle Georget. Tex. 2022, 21, 2365–2378. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, N.P.; Murphy, E.A.; Price, R.L.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.S. Chemokine and Cytokine Levels in Inflammatory Bowel Disease Patients. Cytokine 2016, 77, 44–49. [Google Scholar] [CrossRef]
- Song, Z.; Li, X.; Xie, J.; Han, F.; Wang, N.; Hou, Y.; Yao, J. Associations of Inflammatory Cytokines with Inflammatory Bowel Disease: A Mendelian Randomization Study. Front. Immunol. 2023, 14, 1327879. [Google Scholar] [CrossRef] [PubMed]
- Jurickova, I.; Dreskin, B.W.; Angerman, E.; Bonkowski, E.; Nguyen, J.; Villarreal, R.; Tominaga, K.; Iwasawa, K.; Braun, T.; Takebe, T.; et al. Eicosatetraynoic Acid Regulates Profibrotic Pathways in an Induced Pluripotent Stem Cell-Derived Macrophage-Human Intestinal Organoid Model of Crohn’s Disease. J. Crohns Colitis 2025, 19, jjae139. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, T.; Wu, S.; Yang, C.; Bai, M.; Shu, K.; Li, K.; Zhang, G.; Jin, Z.; He, F.; et al. iProX: An Integrated Proteome Resource. Nucleic Acids Res. 2019, 47, D1211–D1217. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ma, J.; Liu, Y.; Chen, Z.; Xiao, N.; Lu, Y.; Fu, Y.; Yang, C.; Li, M.; Wu, S.; et al. iProX in 2021: Connecting Proteomics Data Sharing with Big Data. Nucleic Acids Res. 2022, 50, D1522–D1527. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Zhang, S.; Zhu, J.; Tang, B.; Wang, A.; Dong, L.; Zhang, Z.; Yu, C.; Sun, Y.; et al. The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Genom. Proteom. Bioinform. 2021, 19, 578–583. [Google Scholar] [CrossRef]
- CNCB-NGDC Members and Partners. Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2025. Nucleic Acids Res. 2025, 53, D30–D44. [Google Scholar] [CrossRef]



| Name | Sequence |
|---|---|
| cxcl1 F | GCACCCAAACCGAAGTCATAG |
| cxcl1 R | CGTTACTTGGGGACACCTTTTAG |
| cxcl2 F | CAGACAGAAGTCATAGCCACTC |
| cxcl2 R | CTCTTTGGTTCTTCCGTTGAGG |
| cxcl5 F | TGCCCTACGGTGGAAGTCATA |
| cxcl5 R | TGCATTCCGCTTAGCTTTCTTT |
| cox2 F | TGCACTATGGTTACAAAAGCTGG |
| cox2 R | TCAGGAAGCTCCTTATTTCCCTT |
| crp F | TTCCCAAGGAGTCAGATACTTCC |
| crp R | TCAGAGCAGTGTAGAAATGGAGA |
| il1b F | GAAATGCCACCTTTTGACAGTG |
| il1b R | TGGATGCTCTCATCAGGACAG |
| gapdh F | AGGTCGGTGTGAACGGATTTG |
| gapdh R | TGTAGACCATGTAGTTGAGGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Li, M.; Chen, T.; Wang, C.; Zhou, H.; Zhou, T.; Jia, R.; Chen, Y.; Hu, Q. Berberine Alleviates Intestinal Inflammation by Disrupting Pathological Macrophage–Epithelial Crosstalk in Macrophage–Organoid Co-Culture Model. Int. J. Mol. Sci. 2025, 26, 10161. https://doi.org/10.3390/ijms262010161
Han Y, Li M, Chen T, Wang C, Zhou H, Zhou T, Jia R, Chen Y, Hu Q. Berberine Alleviates Intestinal Inflammation by Disrupting Pathological Macrophage–Epithelial Crosstalk in Macrophage–Organoid Co-Culture Model. International Journal of Molecular Sciences. 2025; 26(20):10161. https://doi.org/10.3390/ijms262010161
Chicago/Turabian StyleHan, Yuncong, Mengting Li, Tian Chen, Chen Wang, Hong Zhou, Tunan Zhou, Runqing Jia, Ying Chen, and Qin Hu. 2025. "Berberine Alleviates Intestinal Inflammation by Disrupting Pathological Macrophage–Epithelial Crosstalk in Macrophage–Organoid Co-Culture Model" International Journal of Molecular Sciences 26, no. 20: 10161. https://doi.org/10.3390/ijms262010161
APA StyleHan, Y., Li, M., Chen, T., Wang, C., Zhou, H., Zhou, T., Jia, R., Chen, Y., & Hu, Q. (2025). Berberine Alleviates Intestinal Inflammation by Disrupting Pathological Macrophage–Epithelial Crosstalk in Macrophage–Organoid Co-Culture Model. International Journal of Molecular Sciences, 26(20), 10161. https://doi.org/10.3390/ijms262010161








