Human Testicular Tissue Digestion, Testicular Cell Selection, and Downstream Characterization for Reproductive Purposes: A Scoping Review
Abstract
1. Introduction
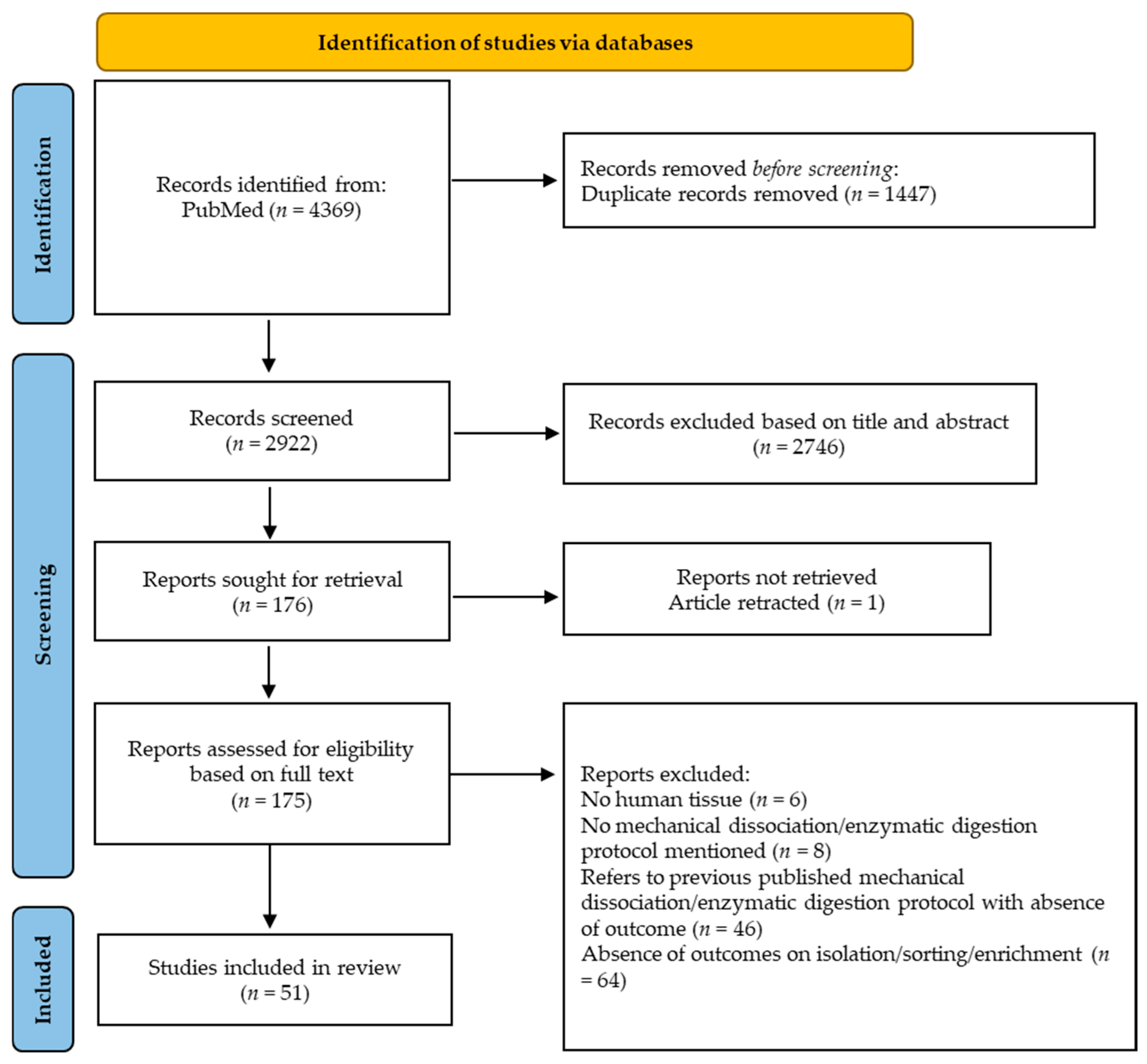
2. Materials and Methods
2.1. Study Design
2.2. Literature Search
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction and Interpretation
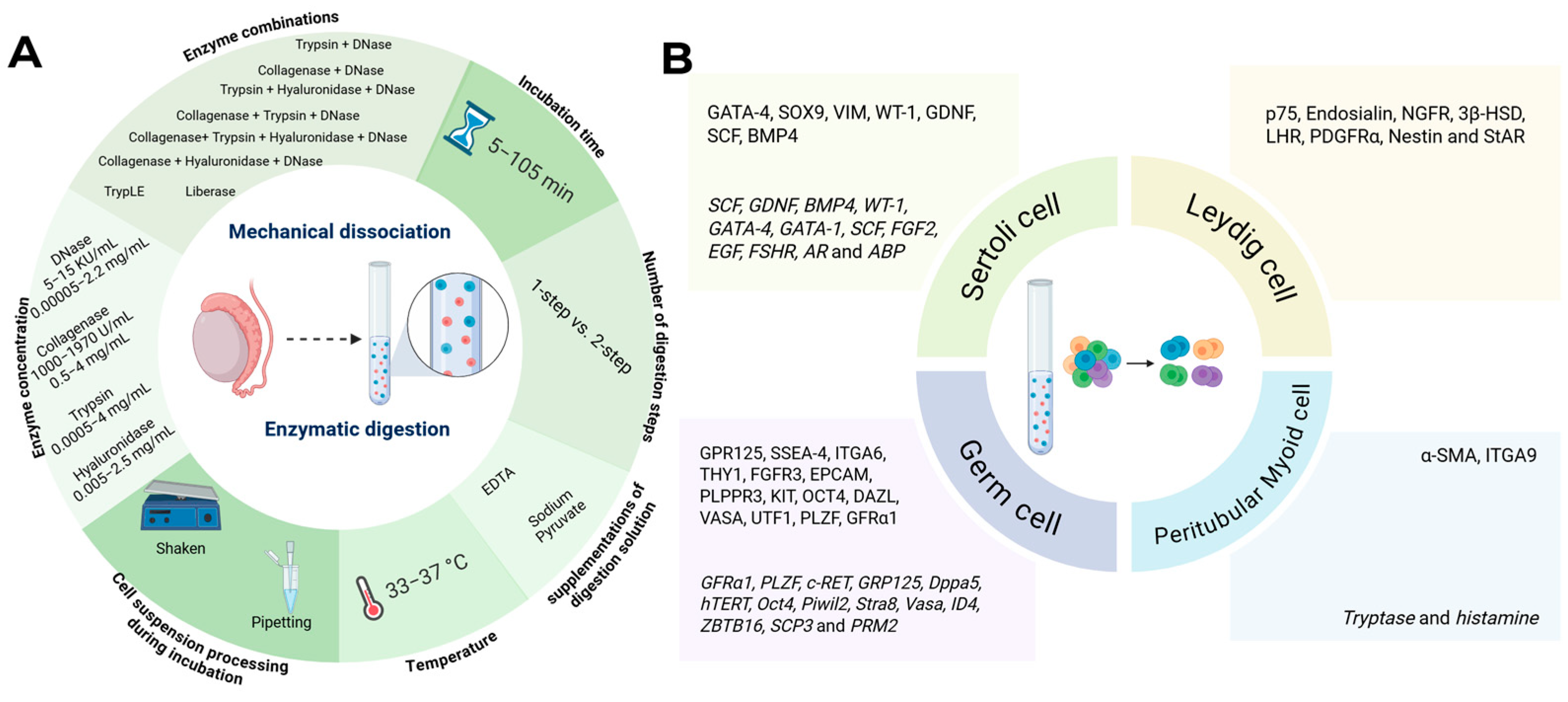
3. Results and Discussion
3.1. Testicular Tissue Dissociation/Digestion to Obtain Single-Cell Suspensions
3.1.1. Testicular Tissue Characteristics Influencing Testicular Dissociation/Digestion Outcomes
3.1.2. Protocol-Related Factors Influencing Testicular Dissociation/Digestion Outcomes
| Mechanical Dissociation Applied to Human Testicular Tissue | |||||
| Reference | Age of Donor | Medical Diagnosis/Tissue Source/Anatomopathology Number of Donors (n) Tissue Weight/Size | Fresh/Frozen | Dissociation Protocol | Main Outcomes |
| Kurpisz et al., 1988 [35] | 32–38 years | Azoospermia n = NA | NA | Battery of chilled razor blades (automated) | Single-cell suspension containing spermatocytes, spermatids and 20–25% extensively damaged Sertoli cells |
| Schneider et al., 2015 [33] | NA | Sex reassignment surgery n = 76 (500 mg) | Fresh n = 6 Frozen n = 7 | Medimachine dissociation (automated) for 15 min | Mechanical dissociation of cryopreserved tissue resulted in significantly higher total cell yield compared to enzymatic digestion 38.04% vs. 15.72% viability after mechanical dissociation using fresh or cryopreserved tissue, respectively 75.93% vs. 73.99% viability after enzymatic digestion using fresh or cryopreserved tissue, respectively 2-fold lower spermatogonia (UTF1), 1.5-fold lower germ cell (MAGEA4), 2-fold lower Sertoli cell (VIM), and 5-fold lower Peritubular Myoid cell (ACTA2) expression when Medimachine dissociation was applied on fresh tissue compared to enzymatic digestion 7.95 ± 4.38% and 26.92 ± 6.52% SALL4+ cells when cryopreserved tissue was enzymatically digested compared to mechanical dissociation 19.58 ± 7.88% and 27.33 ± 9.49% SALL4+ cells when fresh tissue was enzymatically digested compared to mechanical dissociation |
| Enzymatic Digestion Applied to Human Testicular Tissue | |||||
| Reference | Age of Donor | Medical Diagnosis/Tissue Source/Anatomopathology Number of Donors (n) Tissue Weight/Size | Fresh/Frozen | Digestion Protocol | Main Outcomes |
| Berensztein et al., 1992 [48] | Prepubertal | Cadaver testis n = 7 | Fresh | Step 1: 5 KU/mL DNase + 1.182 × 103 U/mL collagenase at 37 °C for 20 min Sedimentation for 5 min Centrifugation Step 2: 15 KU/mL DNase + 1.970 × 103 U/mL collagenase at 37 °C for 20 min Filter 0.4 mm | 1.13 ± 0.40 × 108 cell yield/g after 1st step of digestion 2.45 ± 0.32 × 108 cell yield/g at the end of digestion |
| Brook et al., 2001 [49] | 22–35 years | Biopsy with normal spermatogenesis n = 11 Orchiectomy whole testes n = 8 | Fresh | Mincing (3 × 3 × 3 mm3) Step 1: 1 mg/mL collagenase I at 37 °C for 12–20 min Filter 120 µM Step 2: 6 µg/mL bovine pancreatic trypsin + 2 mM EDTA + 16 µg/mL ovine hyaluronidase + 0.4 µg/mL DNase I + 0.2 mM sodium pyruvate at 37 °C for 12–15 min 500 µg/mL Soybean trypsin inhibitor Centrifugation 500× g at 4 °C for 10 min | 66% viability after digestion |
| Liu et al., 2011 [47] | Fetal 6–7 months | Miscarriage n = Not available (NA) | Fresh | Mincing Step 1: 1 mg/mL collagenase I at 37 °C for 10 min Centrifugation Supernatant removed Step 2: 0.25% Trypsin at 37 °C for 10–15 min Centrifugation Filter 200 mesh | 4.86 ± 1.19 × 106 cell yield after digestion 91.07 ± 2.16% viability after digestion |
| Nowroozi et al., 2011 [50] | 25–52 years | Non-obstructive azoospermia (NOA) n = 47 (100–200 mg/fragment) | Fresh | 0.5 mg/L collagenase + 0.5 mg/L Trypsin + 0.5 mg/L hyaluronidase + 0.05 mg/L DNase for 20 min at 37 °C with agitation | 93.40 ± 5.04% viability after digestion |
| Izadyar et al., 2011 [51] | NA | Obstructive azoospermia (OA) testicular sperm extraction (TESE) n = 29 Orchiectomy with normal spermatogenesis n = 2 | Fresh | Step 1: 1 mg/mL collagenase A + 10 U/mL DNase at 37 °C for 15 min Gravity sedimentation and discard of supernatant Step 2: 1.5 mg/mL collagenase A + 1.5 mg/mL hyaluronidase V + 0.5 mg/mL Trypsin + 10 U/mL DNase at 37 °C for 20 min Filter Centrifuge 400× g for 10 min | 0.5 × 106 cell yield after digestion 87% viability after digestion |
| Mirzapour et al., 2011 [52] | 28–50 years | Azoospermia (maturation arrest) n = 20 (100–200 mg/patient) | Fresh | Step 1: 0.5 mg/mL Trypsin + 0.5 mg/mL Hyaluronidase + 0.05 mg/mL DNase at 37 °C for 20 min Centrifugation 112 Relative centrifugal force (RCF) for 4 min Wash Dulbecco’s modified eagle medium (DMEM) Step 2: 0.5 mg/mL Trypsin + 0.5 mg/mL Hyaluronidase + 0.05 mg/mL DNase at 37 °C for 5 min Centrifugation 542 RCF for 4 min at 37 °C Filter 70 µM nylon | 93.40 ± 5.04% viability after digestion |
| Koruji et al., 2012 [53] | 32–50 years | NOA TESE n = 20 | Fresh | Mincing Step 1: 1 mg/mL collagenase I + 1 mg/mL Hyaluronidase + 1 mg/mL Trypsin + 0.05 mg/mL DNase at 37 °C for 30 min with shaking and pipetting Centrifugation 2 min 1100 revolutions per minute (rpm) 3 × wash in DMEM Step 2: repetition step 1 for 30–45 min Filter 40 µM | ≥92% viability after 24 h differential plating |
| Riboldi et al., 2012 [54] | NA | OA n = 9 NOA TESE n = 11 | Fresh | Mincing (1 mm3) Step 1: 1000 IU/mL collagenase IA for 20 min at 37 °C on shaker Step 2: TrypLE select for 10 min at 37 °C on shaker Filter 50 µM Centrifugation 1000 rpm for 5 min | 75% viability after digestion OA: 4.16 ± 4.90 × 106 cell yield after digestion NOA: 1.99 ± 1.97 × 106 cell yield after digestion |
| Zohni et al., 2012 [55] | 39–50 years | OA n = 18 (mean biopsy weight 67.1 ± 8.3 mg/patient) | Fresh | Step 1: 1 mg/mL collagenase I + 1 mg/mL collagenase IV + 1 mg/mL Hyaluronidase + 1 mg/mL DNase I at 33 °C for 15 min with periodic shaking Centrifugation 500× g for 5 min Step 2: 0.5 mg/mL Trypsin + 1 mg/mL DNase I at 33 °C for 5 min | 8.6 ± 0.4 × 104 cell yield/mg after digestion 5.8 × 106 cell yield/patient 95.5 ± 1.7% viability after digestion |
| Pacchiarotti et al., 2013 [46] | 25–40 years | Sex reassignment surgery n = 5 | Fresh Frozen | Mincing Liberase (0.3 U/mL collagenase I and II + 1000 U/mL Thermolysin) at 37 °C shaking 110 RPM for 1.75 h Filter 100 µm centrifugation 400 × g for 5 min at 4 °C | Fresh: 42.5 ± 9.3 × 106 cell yield/g with 90.1 ± 1.3% viability after digestion Fresh: 0.6 ± 0.1 × 106 spermatogonia stem cell (SSEA-4+), 1.6 ± 0.5 × 106 Leydig cell (LHR+) and 16.6 ± 4.1 × 106 germ cell (VASA+) cell yield/g after digestion Frozen 15.9 ± 4.4 × 106 cell yield/g with 74.0 ± 2.2% viability after digestion Frozen: 0.3 ± 0.1 × 106 Spermatogonial stem cell (SSEA-4+), 22 ± 0.9 × 106 Leydig cell (LHR+) and 10.9 ± 3.3 × 106 germ cell (VASA+) cell yield/g after digestion |
| Kossack et al., 2013 [56] | NA | Biopsy with normal spermatogenesis n = 4 Klinefelter patients n = 3 | Fresh | Step 1: 1 mg/mL collagenase IA at 37 °C for 30 min Centrifugation 438 × g for 5 min Remove supernatant Step 2: 4 mg/mL Trypsin + 2.2 mg/mL DNase I for 10 min at 37 °C | 2.91 ± 1.21 × 106 cell yield after digestion of normal spermatogenesis samples 2.87 ± 2.03 × 106 cell yield after digestion of Klinefelter patient samples |
| Zheng et al., 2014 [45] | 13–40 years | Cadaver testis n = NA (0.5–2 g/experiment) | Fresh Frozen | Mincing Step 1: 1 mg/mL collagenase IV + 0.7 mg/mL DNase in HBSS at 37 °C for 15 min Step 2: 0.25% Trypsin/EDTA + 0.7 mg/mL DNase in HBSS at 37 °C with periodic rocking for 5 min Filter 40 µM | Fresh: 29 ± 16 × 106 cell yield/g after digestion Frozen: significant lower yield after digestion, exact cellular yield not reported |
| Guo et al., 2015 [57] | 22–35 years | OA n = 50 | Fresh | Step 1: 2 mg/mL collagenase IV + 1 µg/mL DNase I at 34 °C for 15 min Step 2: 4 mg/mL collagenase IV + 2.5 mg/mL hyaluronidase + 2 mg/mL trypsin + 1 µg/mL DNase I | ≥98% viability after overnight differential plating |
| Jabari et al., 2023 [58] | 15, 21, and 26 years old | Cadaver testis n = 3 | Fresh | Mincing Step 1: 1 mg/mL collagenase I + 1 mg/mL hyaluronidase + 1 mg/mL Trypsin + 0.05 mg/mL DNase at 37 °C with 150 cycles/min shaker for 30 min Centrifugation 1100 rpm for 4 min Wash in DMEM Step 2: Repetition step 1 for 25 min Filter 40 µm | >91% viability after digestion |
| Nikmahzar et al., 2023 [59] | 28, 32, and 44 years | Cadaver testis n = 3 | Fresh | Step 1: 1 mg/mL collagenase IV + 1 mg/mL hyaluronidase at 37 °C for 10 min at 150 cycles/min shaken Centrifugation at 1100 rpm for 10 min Step 2: 1 mg/mL collagenase + 0.5 mg/mL DNase I + 1 mg/mL hyaluronidase at 37 °C for 10 min Filter 100 µM and 40 µM | 70% viability after digestion |
3.2. Testicular Cell Selection/Enrichment, and Characterization
3.2.1. Sertoli Cells
Selection/Enrichment of SCs by Density Gradient Separation
Selection/Enrichment of SCs by Differential Plating
Selection/Enrichment of SCs by Combinatory Strategies
3.2.2. Leydig Cells
Selection/Enrichment of LCs by Density Gradient Separation
Selection/Enrichment of LCs by Flow Cytometry
3.2.3. Peritubular Myoid Cells
Selection/Enrichment of PTMCs by Explant Growth Culture
Selection/Enrichment of PTMCs by Flow Cytometry
3.2.4. Germ Cells
Selection/Enrichment of SSCs by MACS
Selection/Enrichment of SSCs by Flow Cytometry
Selection/Enrichment of GCs by Differential Plating
Selection/Enrichment of GCs by Flow Cytometry
Selection/Enrichment of GCs by MACS
Selection/Enrichment of GCs by Combinatory Approaches
| Sertoli Cell Selection/Enrichment Strategies Applied on Human Testicular Tissue | |||||
| Reference | Age of Donor | Medical Diagnosis/Tissue Source/Anatomopathology Number of Donors (n) Tissue Weight/Size | Fresh/Frozen | Selection/Enrichment Strategy After Enzymatic Digestion + Verification Technique | Main Outcomes |
| Lipshultz et al., 1982 [69] | Adult | Sex reassignment surgery n = NA | Fresh | Differential plating and culture for 45 days Morphological identification | >95% pure Sertoli cell culture after 45 days 75–85% cell viability after 45 days |
| Teng et al., 2005 [67] | 28–42 years | Cadaver testis n = NA | Fresh | Differential plating and culture for 28 days Morphological identification | >90% pure Sertoli cell culture after 28 days >95% cell viability after 28 days |
| Chui et al., 2011 [68] | 12–36 years | Cadaver testis n = 7 | Fresh | Differential plating and culture for 20 days Morphological identification + Flow cytometry (GATA-4/SOX9) + RT-PCR (SCF, GDNF and BMP4) | ≥95% pure Sertoli cells expressing Sertoli cell (SCF, GDNF, and BMP4) markers with 90% viability after 20 days culture |
| Mirzapour et al., 2011 [52] | 28–50 years | Azoospermia (maturation arrest) n = 20 (100–200 mg/sample) | Fresh | Differential plating to (un)-DSA coated dishes and culture for 72 h ICC (VIM) | >95% pure Sertoli cells after 72 h culture No statistically significant difference in Sertoli cell purity when using DSA-coated dishes compared to uncoated dishes |
| Riboldi et al., 2012 [54] | NA | Obstructive azoospermia (OA) n = 9 Non-obstructive azoospermia (NOA) TESE n = 11 | Fresh | Differential plating and culture | 95% pure Sertoli cell after differential plating |
| Guo et al., 2015 [57] | 22–35 years | OA n = 50 | Fresh | Differential plating and overnight culture RT-PCR (WT-1, GATA-4, GATA-1, GDNF, BMP4, SCF, FGF2, EGF, FSHR, AR and ABP) + ICC (WT-1, GDNF, SCF, BMP4, VIM, PCNA, and GATA-4) | 98% Sertoli cell viability after overnight culture 95% pure Sertoli cell culture <5% of the enriched cells expressed Peritubular Myoid cells (α-SMA) or Leydig cell (CYP11A1) markers |
| Gaur et al., 2018 [70] | 26–56 years | Cadaver testis n = 5 | Fresh | Multiple gravity sedimentation steps ICC (GATA-4) | >95% pure Sertoli cell culture |
| Leydig Cell Selection/Enrichment Strategies Applied on Human Testicular Tissue | |||||
| Reference | Age of Donor | Medical Diagnosis/Tissue Source/Anatomopathology Number of Donors (n) Tissue Weight/Size | Fresh/Frozen | Selection/Enrichment Strategy after Enzymatic Digestion + Verification Technique | Main Outcomes |
| Simpson et al., 1987 [74] | 57–85 years | Orchiectomy for prostatic carcinoma n = 10 (Testis fragment weight used 5–8 g/patient) | NA | Discontinuous Percoll gradient Immunohistochemistry (3β-HSD) | After DPG 3 bands were obtained Band 1: 95–97% of all testicular cells, 12–28% pure Leydig cells accounting for 77–95% of all Leydig cells, 10.6–16.6 × 106 Leydig cell yield Band 2: 2–4% of all testicular cells, 48–70% pure Leydig cells accounting for 4–18% of all Leydig cells, 0.7–5.7 × 106 Leydig cell yield Band 3: 0.7–1.5% of all testicular cells, 30–56% pure Leydig cell yield accounting for 1–6% of all Leydig cells, 0.3–1.9 × 106 Leydig cell yield Overall, 11.6–24.2 × 106 Leydig cell yield |
| Qureshi et al., 1993 [72] | 54–89 years | Orchiectomy for prostatic carcinoma n = 27 (Testes paired weight 6.6–59.48 g) | Fresh | Discontinuous Percoll Gradient Immunohistochemistry (3β-HSD) | 60–77% pure Leydig cells obtained after DPG 139.12 ± 81.89 × 106 Leydig cell yield/testis |
| Sivakumar et al., 2006 [75] | 60–70 years | Orchiectomy for prostatic carcinoma n = NA | Fresh | Discontinuous Percoll Gradient Immunohistochemistry (3β-HSD) | 95% cell viability after DPG 0.5 × 106 Leydig cell yield/mL after DPG |
| Bilinska et al., 2009 [76] | 60–67 years | Orchiectomy for prostatic carcinoma n = 4 | Fresh | Discontinuous Percoll Gradient Morphological identification ICC (3β-HSD/LHR) | DPG 34–60%: 80–83% pure Leydig cells 94% Leydig cell viability |
| Zhang et al., 2017 [73] | 18 and 19 years 23, 25, 28, 32 years | Cadaver testis n = 2 OA n = 4 | NA | FACS (p75) | p75 sorted stem Leydig cells account for 1.79% of the total cell population. |
| Xia et al., 2020 [77] | 56–60 years 57–67 years | Cadaver testis n = 2 Orchiectomy for prostatic carcinoma n = 2 | NA | FACS (Endosialin) ICC (PDGFRα, NGFR, and Nestin) | Endosialin+ sorted stem Leydig cells accounted for 0.31 ± 0.03% of the entire cell population with >98% expressing Leydig cell (PDGFRα, NGFR, and Nestin) markers |
| Han et al., 2025 [79] | NA | OA TESE n = 3 | Fresh | FACS (ITGA9/NGFR) ICC (3β-HSD, α-SMA, StAR) | ITGA9+/NGFR−sorted cells account for 0.2% of the total cell population, ITGA9+/NGFR+ sorted cells account for 0.68% of the total cell population and 95% expressed Peritubular Myoid cell (α-SMA) marker ITGA9−/NGFR + sorted cells were Leydig cell (3β-HSD, StAR) marker positive |
| Peritubular Myoid Cell Selection/Enrichment Strategies Applied on Human Testicular Tissue | |||||
| Reference | Age of Donor | Medical Diagnosis/Tissue Source/Anatomopathology Number of Donors (n) Tissue Weight/Size | Fresh/Frozen | Selection/Enrichment Strategy + Verification Technique | Main Outcomes |
| Albrecht et al., 2006 [81] | 29, 32, 32, 34, 35, 36, 40, 41, 46, 47 years | OA with normal spermatogenesis n = 8 Varicocele with slightly reduced spermatogenesis n = 2 | Fresh | Explant growth culture Morphological identification + immunohistochemistry (FSH, LH-r, α-SMA, THY1) + RT-PCR (Tryptase, Histamine) | Peritubular Myoid cells become visible after 1–2 weeks and expressed Peritubular Myoid cell (α-SMA, Tryptase and Histamine) and germ cell (THY1) markers while not expressing Sertoli cell (FSH) and Leydig cell (LH-r) markers |
| Landreh et al., 2014 [82] | 31–52 years | OA NOA n = NA | NA | Explant growth culture Immunohistochemistry (PDGFR-α, α-SMA, StAR) | Almost all outgrowth cells expressed Peritubular Myoid marker (α-SMA) and Leydig cell (StAR) marker and 79–90% of them were Leydig cell (PDGFR-α) marker positive |
| Rolland et al., 2019 [84] | Average 51 years Average 80 years | Cadaver testis n = 8 Orchiectomy n = 9 | NA | Explant growth culture | Peritubular Myoid cells grow out after 2–3 weeks |
| Han et al., 2025 [79] | NA | OA TESE normal spermatogenesis n = 3 | Fresh | FACS (ITGA9/NGFR) ICC (3β-HSD, α-SMA, StAR) | ITGA9+/NGFR−sorted cells account for 0.2% of the total cell population, ITGA9+/NGFR+ sorted cells account for 0.68% of the total cell population and 95% expressed Peritubular Myoid cell (α-SMA) marker ITGA9−/NGFR+ sorted cells were Leydig cell (3β-HSD, StAR) marker positive |
| Germ Cell Selection/Enrichment Strategies Applied on Human Testicular Tissue | |||||
| Reference | Age of Donor | Medical Diagnosis/Tissue Source/Anatomopathology Number of Donors (n) Tissue Weight/Size | Fresh/Frozen | Selection/Enrichment Strategy after Enzymatic Digestion + Verification Technique | Main Outcomes |
| He et al., 2010 [92] | 16–58 years | Cadaver testis n = 5 | Fresh | Differential plating + MACS (GPR125) ICC (GPR125) | 6 × 106 germ cell yield/g after differential plating 3 × 104 germ cell yield after MACS >95% pure germ cell after MACS cells could be proliferated 5-fold during 14-day culture while retaining phenotypical characteristics of SSCs |
| Liu et al., 2011 [47] | Fetal 6–7 months | Miscarriage n = NA | Fresh | Discontinuous Percoll gradient followed by 3 h-differential plating ICC (SSEA-4, OCT4) | 86.7% pure OCT4+ germ cell after DPG and differential plating of which the majority expressed germ cell marker SSEA-4 |
| Izadyar et al., 2011 [51] | NA | OA TESE n = 29 Orchiectomy with normal spermatogenesis n = 2 | Fresh | MACS (SSEA-4) RT-PCR (c-KIT, GFRα1, PLZF, c-RET, GPR125, Dppa5, and hTERT) + Xenotransplantation | SSEA-4+ sorted germ cells account for 13.3 ± 1.4% of the entire single-cell suspension and had significantly higher expression of SSC-specific genes compared to SSEA-4- sorted cells SSEA-4+ sorted cells showed 40–50-fold HNP+ cells after xenotransplantation compared to unsorted single-cell suspension xenotransplantation |
| Mirzapour et al., 2011 [52] | 28–50 years | Azoospermia (maturation arrest) n = 20 (100–200 mg/patient) | Fresh | Differential plating to DSA-coated dishes for 2–3 h and culture ICC (OCT4) + RT-PCR (Oct4, Nanog, Piwil2, Stra8, Vasa, Bax, and DMC1) + Xenotransplantation | 95% pure germ cell after differential plating and SSC colonies expressed key germ cell markers after 2 weeks of culture |
| Nickkholgh et al., 2014 [88] | NA | Orchiectomy (prostate cancer) n = 2 | Frozen | Culture for 50 days followed by MACS (GPR125 or ITGA6 in combination with HLA) qRT-PCR (ID4) + Xenotransplantation | 5.3 ± 3.8% ITGA6+, 1.99 ± 1.5% HLA−/ITGA6+, 1.89 ± 0.9% GPR125+, and 2.33 ± 0.7% HLA−/GPR125+ after MACS higher expression of undifferentiated germ cells (ID4) in ITGA6+ and HLA−/GPR125+ sorted fraction Xenotransplantation: No significant difference among GPR125+/HLA− or ITGA6+/HLA− or GPR125+ or ITGA6+ MACS sorted fractions regarding SSC colony formation |
| Smith et al., 2014 [95] | Adult | Normal spermatogenesis n = 13 | NA | FACS (SSEA-4, THY1) RT-PCR (ZBTB16, GFRa1), ICC (DAZL, VASA) | SSEA-4+ sorted germ cells expressed undifferentiated germ cell (DAZL, VASA) markers and had significantly higher undifferentiated germ cell (1.9-fold ZBTB16, 10-fold GFRa1, and 3-fold GPR125) marker expression compared to THY1+ sorted germ cell fractions |
| Von Kopylow et al., 2016 [93] | Adult | OA with normal spermatogenesis n = 37 Meiotic arrest n = 3 (30 mg/testis) | Fresh | MACS (FGFR3) + Micromanipulation cell-picking Morphological identification + ICC (UTF1) | 54–138 and 220–280 UTF1+ undifferentiated germ cell yield after micromanipulation in normal spermatogenesis and meiotic arrest patients, respectively 100% pure UTF1+ undifferentiated germ cells compared to 1–2% UTF1+ cells in unsorted control with viability 95% |
| Medrano et al., 2016 [65] | Adult | Bilateral orchiectomy (prostate cancer, normal spermatogenesis) n = 3 | Frozen | Differential plating 24 h or FACS (HLA−/EPCAM+) ICC (VASA/UTF1) | 27% pure VASA+/UTF1+ undifferentiated germ cells in sorted compared to 13% unsorted fraction 112 undifferentiated germ cell/cm2 yield compared to unsorted cells and differentially plated cells 61 and 49 undifferentiated germ cells/cm2, respectively 50 and 8 VASA+/UTF1+ undifferentiated germ cells/cm2, in floating and adherent fraction after 24 h differential plating, respectively |
| Tan et al., 2020 [89] | 30–50 years | vasectomy reversal n = 29 | Frozen | FACS (PLPPR3 or KIT) Xenotransplantation | Xenotransplantation: 38-fold SSC-activity enrichment of PLPPR3+ SSCs compared to unsorted cells |
| Salem et al., 2023 [91] | 22, 25, and 28 years | Cadaver testis n = 3 | Fresh | Differential plating Morphological identification + ICC (PLZF, GFRA1) + RT-PCR (GFRA1, PLZF, SCP3, and PRM2) + xenotransplantation | GFRA1+ and PLZF+ SSC colonies were visible after 2 weeks of culture Xenotransplantation: SSC colonies were positive for undifferentiated germ cell markers (PLZF and GFRA1), and differentiated germ cell markers (SCP3 and PRM2) after 8 weeks |
3.3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3β-HSD | 3β-Hydroxysteroid Dehydrogenase |
| α-SMA | Alpha Smooth Muscle Actin |
| ABP | Androgen binding protein |
| AR | Androgen receptor |
| ACTA2 | Actin alpha 2 |
| BAX | BCL2 associated X |
| BMP4 | Bone morphogenetic protein 4 |
| CYP11A1 | Cytochrome P450 family 11 subfamily A member 1 |
| DAZL | Deleted in Azoospermia-like |
| DMC1 | DNA meiotic recombinase 1 |
| DMEM | Dulbecco’s modified eagle medium |
| DPG | Discontinuous Percoll gradient |
| DPPA5 | Developmental pluripotency associated 5 |
| DSA | Datura Stramonium Agglutinin |
| ECM | Extracellular Matrix |
| EDTA | Ethylenediaminetetraacetic acid |
| EGF | Epidermal growth factor |
| EPCAM | Epithelial Cell Adhesion Molecule |
| FACS | Fluorescent-Activated Cell Sorting |
| FGF2 | Fibroblast growth factor 2 |
| FGFR3 | Fibroblast Growth Factor Receptor 3 |
| FSH | Follicle stimulating hormone |
| GATA-1 | GATA Binding Protein 1 |
| GATA-4 | GATA Binding Protein 4 |
| GC | Germ Cell |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFRA | Growth Factor Receptor A |
| GPR125 | G-Protein Coupled Receptor 125 |
| HBSS | Hanks’ balanced salt solution |
| HLA | Human Leukocyte Antigen |
| HNP | Human nuclear protein |
| hTERT | Human telomerase reverse transcriptase |
| ICC | Immunocytochemistry |
| ITGA6 | Integrin Subunit Alpha 6 |
| ITGA9 | Integrin Subunit Alpha 9 |
| ITT | Immature Testicular Tissue |
| IU | International unit |
| IVF-ICSI | In Vitro Fertilization–Intracytoplasmatic Sperm Injection |
| IVM | In Vitro Maturation |
| KU | Kunitz unit |
| LC | Leydig Cell |
| LHR | Luteinizing Hormone Receptor |
| MACS | Magnetic-Activated Cell Sorting |
| MAGEA4 | Melanoma-associated antigen 4 |
| MRD-PCR | Minimal Residual Disease Polymerase Chain Reaction |
| NA | Not available |
| NGFR | Nerve Growth Factor Receptor |
| NOA | Non-obstructive azoospermia |
| OA | Obstructive azoospermia |
| OCT4 | Octamer-Binding Transcription Factor 4 |
| PCNA | Proliferating cell nuclear antigen |
| PDGFR-α | Platelet-Derived Growth Factor Receptor Alpha |
| PIWIL2 | Piwi-like RNA-mediated gene silencing 2 |
| PLPPR3 | Phospholipid Phosphatase Related 3 |
| PLZF | Promyelocytic leukemia zinc finger |
| PRM2 | Protamine 2 |
| PTMC | Peritubular Myoid Cell |
| RCF | Relative centrifugal force |
| RPM | Revolutions per minute |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SALL4 | Sal-like protein 4 |
| SC | Sertoli Cell |
| SCF | Stem cell factor |
| SCP3 | Synaptonemal complex protein 3 |
| sLC | Stem Leydig Cell |
| SOX9 | SRY-box 9 |
| SSC | Spermatogonial Stem Cell |
| SSEA-4 | Stage-Specific Embryonic Antigen-4 |
| StAR | Steroidogenic Acute Regulatory Protein |
| STRA8 | Stimulated By Retinoic Acid 8 |
| TESE | Testicular sperm extraction |
| THY1 | Thymocyte Antigen 1 |
| UTF1 | Undifferentiated Embryonic Cell Transcription Factor 1 |
| VIM | Vimentin |
| WT-1 | Wilms’ tumor gene 1 |
| ZBTB16 | Zinc finger and BTB domain-containing protein 16 |
References
- Botta, L.; Gatta, G.; Capocaccia, R.; Stiller, C.; Cañete, A.; Maso, L.D.; Innos, K.; Mihor, A.; Erdmann, F.; Spix, C.; et al. Long-term survival and cure fraction estimates for childhood cancer in Europe (EUROCARE-6): Results from a population-based study. Lancet Oncol. 2022, 23, 1525–1536. [Google Scholar] [CrossRef]
- Silva, N.d.P.; Gini, A.; Dolya, A.; Colombet, M.; Soerjomataram, I.; Youlden, D.; Stiller, C.; Steliarova-Foucher, E.; Aitken, J.; Bray, F.; et al. Prevalence of childhood cancer survivors in Europe: A scoping review. EJC Paediatr. Oncol. 2024, 3, 100155. [Google Scholar] [CrossRef] [PubMed]
- Howell, S.J.; Shalet, S.M. Spermatogenesis After Cancer Treatment: Damage and Recovery. JNCI Monogr. 2005, 2005, 12–17. [Google Scholar] [CrossRef]
- Rivkees, S.A.; Crawford, J.D. The Relationship of Gonadal Activity and Chemotherapy-Induced Gonadal Damage. JAMA 1988, 259, 2123–2125. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Kawashima, T.; Stovall, M.; Leisenring, W.; Sklar, C.A.; Mertens, A.C.; Donaldson, S.S.; Byrne, J.; Robison, L.L. Fertility of Male Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2010, 28, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Green, D.M.; Liu, W.; Kutteh, W.H.; Ke, R.W.; Shelton, K.C.; Sklar, C.A.; Chemaitilly, W.; Pui, C.-H.; Klosky, J.L.; Spunt, S.L.; et al. Cumulative alkylating agent exposure and semen parameters in adult survivors of childhood cancer: A report from the St Jude Lifetime Cohort Study. Lancet Oncol. 2014, 15, 1215–1223. [Google Scholar] [CrossRef]
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E.; et al. Clinical Ascertainment of Health Outcomes Among Adults Treated for Childhood Cancer. JAMA 2013, 309, 2371–2381. [Google Scholar] [CrossRef]
- Brignardello, E.; Felicetti, F.; Castiglione, A.; Chiabotto, P.; Corrias, A.; Fagioli, F.; Ciccone, G.; Boccuzzi, G. Endocrine health conditions in adult survivors of childhood cancer: The need for specialized adult-focused follow-up clinics. Eur. J. Endocrinol. 2013, 168, 465–472. [Google Scholar] [CrossRef]
- Wyns, C.; Curaba, M.; Petit, S.; Vanabelle, B.; Laurent, P.; Wese, J.-F.; Donnez, J. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum. Reprod. 2011, 26, 737–747. [Google Scholar] [CrossRef]
- Wyns, C.; Kanbar, M.; Giudice, M.G.; Poels, J. Fertility preservation for prepubertal boys: Lessons learned from the past and update on remaining challenges towards clinical translation. Hum. Reprod. Updat. 2020, 27, 433–459. [Google Scholar] [CrossRef]
- Stukenborg, J.-B.; Wyns, C. Fertility sparing strategies for pre- and peripubertal male cancer patients. Ecancermedicalscience 2020, 14, 1016. [Google Scholar] [CrossRef]
- Jensen, C.F.S.; Dong, L.; Gul, M.; Fode, M.; Hildorf, S.; Thorup, J.; Hoffmann, E.; Cortes, D.; Fedder, J.; Andersen, C.Y.; et al. Fertility preservation in boys facing gonadotoxic cancer therapy. Nat. Rev. Urol. 2021, 19, 71–83. [Google Scholar] [CrossRef]
- Wyns, C.; Curaba, M.; Vanabelle, B.; Van Langendonckt, A.; Donnez, J. Options for fertility preservation in prepubertal boys. Hum. Reprod. Updat. 2010, 16, 312–328. [Google Scholar] [CrossRef]
- Duffin, K.; Neuhaus, N.; Andersen, C.Y.; Barraud-Lange, V.; Braye, A.; Eguizabal, C.; Feraille, A.; Ginsberg, J.P.; Gook, D.; Goossens, E.; et al. A 20-year overview of fertility preservation in boys: New insights gained through a comprehensive international survey. Hum. Reprod. Open 2024, 2024, hoae010. [Google Scholar] [CrossRef] [PubMed]
- Goossens, E.; Jahnukainen, K.; Mitchell, R.; van Pelt, A.; Pennings, G.; Rives, N.; Poels, J.; Wyns, C.; Lane, S.; Rodriguez-Wallberg, K.; et al. Fertility preservation in boys: Recent developments and new insights. Hum. Reprod. Open 2020, 2020, hoaa016. [Google Scholar] [CrossRef] [PubMed]
- Kanbar, M.; de Michele, F.; Giudice, M.G.; Desmet, L.; Poels, J.; Wyns, C. Long-term follow-up of boys who have undergone a testicular biopsy for fertility preservation. Hum. Reprod. 2020, 36, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Fayomi, A.P.; Peters, K.; Sukhwani, M.; Valli-Pulaski, H.; Shetty, G.; Meistrich, M.L.; Houser, L.; Robertson, N.; Roberts, V.; Ramsey, C.; et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019, 363, 1314–1319, Erratum in Science 2019, 364, eaax4999. [Google Scholar] [CrossRef]
- Kourta, D.; Kanbar, M.; Amorim, C.A.; Wyns, C. Cancer cell contamination and decontamination methods for ovaries and testes: Special focus on prepubertal gonads with a view to safe fertility restoration. Hum. Reprod. 2023, 38, 780–798. [Google Scholar] [CrossRef]
- Kourta, D.; Camboni, A.; Saussoy, P.; Kanbar, M.; Poels, J.; Wyns, C. Evaluating testicular tissue for future autotransplantation: Focus on cancer cell contamination and presence of spermatogonia in tissue cryobanked for boys diagnosed with a hematological malignancy. Hum. Reprod. 2024, 39, 486–495. [Google Scholar] [CrossRef]
- Hou, M.; Andersson, M.; Eksborg, S.; Soder, O.; Jahnukainen, K. Xenotransplantation of testicular tissue into nude mice can be used for detecting leukemic cell contamination. Hum. Reprod. 2007, 22, 1899–1906. [Google Scholar] [CrossRef]
- Jahnukainen, K.; Hou, M.; Petersen, C.; Setchell, B.; Söder, O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001, 61, 706–710. [Google Scholar]
- Patience, C.; YTakeuchi, R.A. Weiss, Zoonosis in xenotransplantation. Curr. Opin. Immunol. 1998, 10, 539–542. [Google Scholar] [CrossRef]
- de Michele, F.; Poels, J.; Vermeulen, M.; Ambroise, J.; Gruson, D.; Guiot, Y.; Wyns, C. Haploid Germ Cells Generated in Organotypic Culture of Testicular Tissue From Prepubertal Boys. Front. Physiol. 2018, 9, 1413. [Google Scholar] [CrossRef]
- Abofoul-Azab, M.; AbuMadighem, A.; Lunenfeld, E.; Kapelushnik, J.; Shi, Q.; Pinkas, H.; Huleihel, M. Development of Postmeiotic Cells In Vitro from Spermatogonial Cells of Prepubertal Cancer Patients. Stem Cells Dev. 2018, 15, 1007–1020. [Google Scholar] [CrossRef]
- Hermann, B.P.; Sukhwani, M.; Winkler, F.; Pascarella, J.N.; Peters, K.A.; Sheng, Y.; Valli, H.; Rodriguez, M.; Ezzelarab, M.; Dargo, G.; et al. Spermatogonial Stem Cell Transplantation into Rhesus Testes Regenerates Spermatogenesis Producing Functional Sperm. Cell Stem Cell 2012, 11, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Zielen, A.C.; Peters, K.A.; Shetty, G.; Gross, D.A.; Hanna, C.B.; Dovey, S.L.; Wecht, A.; Cannon, G.M.; Meistrich, M.L.; Hsieh, M.; et al. Ultrasound-Guided Rete Testis Approach to Sperm Aspiration and Spermatogonial Stem Cell Transplantation in Patients with Azoospermia. medRxiv 2025. [Google Scholar] [CrossRef] [PubMed]
- Kanbar, M.; Vermeulen, M.; Wyns, C. Organoids as tools to investigate the molecular mechanisms of male infertility and its treatments. Reproduction 2021, 161, R103–R112. [Google Scholar] [CrossRef] [PubMed]
- Baert, Y.; Rombaut, C.; Goossens, E. Scaffold-Based and Scaffold-Free Testicular Organoids from Primary Human Testicular Cells. Methods Mol Biol 2019, 1576, 283–290. [Google Scholar]
- Dovey, S.L.; Valli, H.; Hermann, B.P.; Sukhwani, M.; Donohue, J.; Castro, C.A.; Chu, T.; Sanfilippo, J.S.; Orwig, K.E. Eliminating malignant contamination from therapeutic human spermatogonial stem cells. J. Clin. Investig. 2013, 4, 1833–1843. [Google Scholar] [CrossRef]
- Geens, M.; Goossens, E.; Tournay, H. Cell selection by selective matrix adhesion is not sufficiently efficient for complete malignant cell depletion from contaminated human testicular cell suspensions. Fertil. Steril. 2011, 95, 787–791. [Google Scholar] [CrossRef]
- Human Testicular Tissue Digestion, Testicular Cells Selection and Downstream Characterization for Reproductive Purposes: A Scoping Review. Available online: https://osf.io/82mp4/ (accessed on 9 September 2025).
- Verheyen, G.; Popovic-Todorovic, B.; Tournaye, H. Processing and selection of surgically-retrieved sperm for ICSI: A review. Basic Clin. Androl. 2017, 27, 6. [Google Scholar] [CrossRef]
- Schneider, F.; Redmann, K.; Wistuba, J.; Schlatt, S.; Kliesch, S.; Neuhaus, N. Comparison of enzymatic digestion and mechanical dissociation of human testicular tissues. Fertil. Steril. 2015, 104, 302–311.e3. [Google Scholar] [CrossRef]
- Harichandan, A.; Sivasubramaniyan, K.; Hennenlotter, J.; Poths, S.; Bedke, J.; Kruck, S.; Stenzl, A.; Bühring, H.-J. Molecular Signatures of Primary Human Spermatogonial Progenitors and Its Neighboring Peritubular Stromal Compartment. Stem Cells Dev. 2017, 26, 263–273. [Google Scholar] [CrossRef]
- Kurpisz, M.; Mapp, P.; Łukaszyk, A.; Ogilvie, J.; Festenstein, H.; Sachs, J. Characterization of two monoclonal antibodies raised against human testicular cells. Andrologia 1988, 20, 304–310. [Google Scholar] [CrossRef]
- Reichard, A.; Asosingh, K. Best Practices for Preparing a Single Cell Suspension from Solid Tissues for Flow Cytometry. Cytom. Part A 2018, 95, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, M.; Rong, M.; Zhang, X.; Wang, G.; Liu, Y.; Li, H.; Duan, S. The pros and cons of mechanical dissociation and enzymatic digestion in patient-derived organoid cultures for solid tumor. Cell Organoid 2024, 1, 9410009. [Google Scholar] [CrossRef]
- Lam, D.M.K.; Furrer, R.; Bruce, W.R. The Separation, Physical Characterization, and Differentiation Kinetics of Spermatogonial Cells of the Mouse. Proc. Natl. Acad. Sci. USA 1970, 65, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. Protein secretions of Sertoli cells. Int. Rev. Cytol. 1988, 110, 133–156. [Google Scholar]
- Berardo, C.; Ferrigno, A.; Siciliano, V.; Richelmi, P.; Vairetti, M.; Di Pasqua, L.G. Isolation of rat hepatocytes for pharmacological studies on metabotropic glutamate receptor (mGluR) subtype 5: A comparison between collagenase I versus collagenase IV. Eur. J. Histochem. 2020, 64, 3123. [Google Scholar] [CrossRef]
- Crabbé, E.; Verheyen, G.; Tournaye, H.; Van Steirteghem, A. The use of enzymatic procedures to recover testicular germ cells. Hum. Reprod. 1997, 12, 1682–1687. [Google Scholar] [CrossRef][Green Version]
- Dong, L.; Gul, M.; Hildorf, S.; Pors, S.E.; Kristensen, S.G.; Hoffmann, E.R.; Cortes, D.; Thorup, J.; Andersen, C.Y. Xeno-Free Propagation of Spermatogonial Stem Cells from Infant Boys. Int. J. Mol. Sci. 2019, 20, 5390. [Google Scholar] [CrossRef]
- Pakhomov, O.; Posokhov, Y. Detecting changes of testicular interstitial cell membranes with a fluorescent probe after incubation and cryopreservation with cryoprotective agents. Cryobiology 2025, 118, 105194. [Google Scholar] [CrossRef] [PubMed]
- Unni, S.; Kasiviswanathan, S.; D’sOuza, S.; Khavale, S.; Mukherjee, S.; Patwardhan, S.; Bhartiya, D. Efficient cryopreservation of testicular tissue: Effect of age, sample state, and concentration of cryoprotectant. Fertil. Steril. 2012, 97, 200–208.e1. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Thomas, A.; Schmidt, C.; Dann, C. Quantitative detection of human spermatogonia for optimization of spermatogonial stem cell culture. Hum. Reprod. 2014, 29, 2497–2511. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarotti, J.; Ramos, T.; Howerton, K.; Greilach, S.; Zaragoza, K.; Olmstead, M.; Izadyar, F. Developing a Clinical-Grade Cryopreservation Protocol for Human Testicular Tissue and Cells. BioMed Res. Int. 2013, 2013, 930962. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Z.; Xiong, T.; Tang, W. Isolation and characterization of human spermatogonial stem cells. Reprod. Biol. Endocrinol. 2011, 9, 141. [Google Scholar] [CrossRef]
- Berensztein, E.B.; Belgorosky, A.; Rivarola, M.A. Primary culture of prepubertal human testicular cells isolated from testes collected at necropsy. Eur. J. Endocrinol. 1992, 127, 66–71. [Google Scholar] [CrossRef]
- Brook, P.F.; Radford, J.A.; Shalet, S.M.; Joyce, A.D.; Gosden, R.G. Isolation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertil. Steril. 2001, 75, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Nowroozi, M.R.; Ahmadi, H.; Rafiian, S.; Mirzapour, T.; Movahedin, M. In Vitro Colonization of Human Spermatogonia Stem Cells: Effect of Patient’s Clinical Characteristics and Testicular Histologic Findings. Urology 2011, 78, 1075–1081. [Google Scholar] [CrossRef]
- Izadyar, F.; Wong, J.; Maki, C.; Pacchiarotti, J.; Ramos, T.; Howerton, K.; Yuen, C.; Greilach, S.; Zhao, H.H.; Chow, M.; et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum. Reprod. 2011, 26, 1296–1306. [Google Scholar] [CrossRef]
- Mirzapour, T.; Movahedin, M.; Ibrahim, T.A.T.; Koruji, M.; Haron, A.W.; Nowroozi, M.R.; Rafieian, S.H. Effects of basic fibroblast growth factor and leukaemia inhibitory factor on proliferation and short-term culture of human spermatogonial stem cells. Andrologia 2011, 44, 41–55. [Google Scholar] [CrossRef]
- Koruji, M.; Shahverdi, A.; Janan, A.; Piryaei, A.; Lakpour, M.R.; Sedighi, M.A.G. Proliferation of small number of human spermatogonial stem cells obtained from azoospermic patients. J. Assist. Reprod. Genet. 2012, 29, 957–967. [Google Scholar] [CrossRef]
- Riboldi, M.; Rubio, C.; Pellicer, A.; Gil-Salom, M.; Simón, C. In vitro production of haploid cells after coculture of CD49f+ with Sertoli cells from testicular sperm extraction in nonobstructive azoospermic patients. Fertil. Steril. 2012, 98, 580–590.e4. [Google Scholar] [CrossRef] [PubMed]
- Zohni, K.; Zhang, X.; Tan, S.L.; Chan, P.; Nagano, M. CD9 Is Expressed on Human Male Germ Cells That Have a Long-Term Repopulation Potential after Transplantation into Mouse Testes1. Biol. Reprod. 2012, 87, 27. [Google Scholar] [CrossRef] [PubMed]
- Kossack, N.; Terwort, N.; Wistuba, J.; Ehmcke, J.; Schlatt, S.; Schöler, H.; Kliesch, S.; Gromoll, J. A combined approach facilitates the reliable detection of human spermatogonia in vitro. Hum. Reprod. 2013, 28, 3012–3025. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Hai, Y.; Yao, C.; Chen, Z.; Hou, J.; Li, Z.; He, Z. Long-term culture and significant expansion of human Sertoli cells whilst maintaining stable global phenotype and AKT and SMAD1/5 activation. Cell Commun. Signal. 2015, 13, 20. [Google Scholar] [CrossRef]
- Jabari, A.; Gholami, K.; Khadivi, F.; Koruji, M.; Amidi, F.; Gilani, M.A.S.; Mahabadi, V.P.; Nikmahzar, A.; Salem, M.; Movassagh, S.A.; et al. In vitro complete differentiation of human spermatogonial stem cells to morphologic spermatozoa using a hybrid hydrogel of agarose and laminin. Int. J. Biol. Macromol. 2023, 235, 123801. [Google Scholar] [CrossRef]
- Nikmahzar, A.; Khadivi, F.; Koruji, M.; Jahanshahi, M.; Tarazjani, M.D.; Shabani, M.; Abbasi, Y.; Abbasi, M. Evaluation of Apoptosis-related Genes and Hormone Secretion Profiles Using Three Dimensional Culture System of Human Testicular Organoids. Galen. Med. J. 2023, 12, e2805. [Google Scholar]
- Sohni, A.; Tan, K.; Song, H.-W.; Burow, D.; de Rooij, D.G.; Laurent, L.; Hsieh, T.-C.; Rabah, R.; Hammoud, S.S.; Vicini, E.; et al. The Neonatal and Adult Human Testis Defined at the Single-Cell Level. Cell Rep. 2019, 26, 1501–1517.e4. [Google Scholar] [CrossRef]
- Guo, J.; Nie, X.; Giebler, M.; Mlcochova, H.; Wang, Y.; Grow, E.J.; DonorConnect; Kim, R.; Tharmalingam, M.; Matilionyte, G.; et al. The Dynamic Transcriptional Cell Atlas of Testis Development during Human Puberty. Cell Stem Cell 2020, 26, 262–276.e4. [Google Scholar] [CrossRef]
- Kaur, G.; Thompson, L.A.; Dufour, J.M. Sertoli cells–Immunological sentinels of spermatogenesis. Semin. Cell Dev. Biol. 2014, 30, 36–44. [Google Scholar] [CrossRef]
- David, S.; Orwig, K.E. Spermatogonial Stem Cell Culture in Oncofertility. Urol. Clin. N. Am. 2020, 47, 227–244. [Google Scholar] [CrossRef]
- Kato, Y.; Nagao, Y. Effect of polyvinylpyrrolidone on sperm function and early embryonic development following intracytoplasmic sperm injection in human assisted reproduction. Reprod. Med. Biol. 2012, 11, 165–176. [Google Scholar] [CrossRef]
- Medrano, J.V.; Rombaut, C.; Simon, C.; Pellicer, A.; Goossens, E. Human spermatogonial stem cells display limited proliferation in vitro under mouse spermatogonial stem cell culture conditions. Fertil. Steril. 2016, 106, 1539–1549.e8. [Google Scholar] [CrossRef]
- Gat, I.; Maghen, L.; Filice, M.; Wyse, B.; Zohni, K.; Jarvi, K.; Lo, K.C.; Fisher, A.G.; Librach, C. Optimal culture conditions are critical for efficient expansion of human testicular somatic and germ cells in vitro. Fertil. Steril. 2017, 107, 595–605.e7. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Xue, W.-J.; Ding, X.-M.; Feng, X.-S.; Xiang, H.-L.; Jiang, Y.-Z.; Tian, P.-X. Isolation and culture of adult Sertoli cells and their effects on the function of co-cultured allogeneic islets in vitro. Chin. Med. J. 2005, 118, 1857–1862. [Google Scholar] [PubMed]
- Chui, K.; Trivedi, A.; Cheng, C.Y.; Cherbavaz, D.B.; Dazin, P.F.; Huynh, A.L.T.; Mitchell, J.B.; Rabinovich, G.A.; Noble-Haeusslein, L.J.; John, C.M. Characterization and Functionality of Proliferative Human Sertoli Cells. Cell Transplant. 2011, 20, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Lipshultz, L.I.; Murthy, L.; Tindall, D.J. Characterization of Human Sertoli Cells in Vitro*. J. Clin. Endocrinol. Metab. 1982, 55, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Ramathal, C.; Pera, R.A.R.; Turek, P.J.; John, C.M. Isolation of human testicular cells and co-culture with embryonic stem cells. Reproduction 2018, 155, 151–164. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- Qureshi, S.J.; Sharpe, R.M. Evaluation of possible determinants and consequences of Leydig cell heterogeneity in man. Int. J. Androl. 1993, 16, 293–305. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Deng, C.; Jiang, M.H.; Feng, X.; Xia, K.; Li, W.; Lai, X.; Xiao, H.; Ge, R.-S.; et al. Transplanted human p75-positive stem Leydig cells replace disrupted Leydig cells for testosterone production. Cell Death Dis. 2017, 8, e3123. [Google Scholar] [CrossRef]
- Simpson, B.J.B.; Wu, F.C.W.; Sharpe, R.M. Isolation of Human Leydig Cells Which Are Highly Responsive to Human Chorionic Gonadotropin. J. Clin. Endocrinol. Metab. 1987, 65, 415–422. [Google Scholar] [CrossRef]
- Sivakumar, R.; Sivaraman, P.B.; Mohan-Babu, N.; Jainul-Abideen, I.M.; Kalliyappan, P.; Balasubramanian, K. Radiation Exposure Impairs Luteinizing Hormone Signal Transduction and Steroidogenesis in Cultured Human Leydig Cells. Toxicol. Sci. 2006, 91, 550–556. [Google Scholar] [CrossRef]
- Bilinska, B.; Kotula-Balak, M.; Sadowska, J. Morphology and function of human Leydig cells in vitro. Immunocytochemical and radioimmunological analyses. Eur. J. Histochem. 2009, 53, 35–42. [Google Scholar] [CrossRef]
- Xia, K.; Ma, Y.; Feng, X.; Deng, R.; Ke, Q.; Xiang, A.P.; Deng, C. Endosialin defines human stem Leydig cells with regenerative potential. Hum. Reprod. 2020, 35, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; Walker, W.H. The regulation of spermatogenesis by androgens. Semin. Cell Dev. Biol. 2014, 30, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhao, L.; Luo, J.; Shi, C.; Yao, C.; Ji, Z.; Xu, J.; Xu, S.; Tian, R.; Zhi, E.; et al. Identification and isolation of human testicular peritubular myoid cells and Leydig cells by a combination of ITGA9 and NGFR. Reprod. Biol. Endocrinol. 2025, 23, 82. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, A. Human testicular peritubular cells: More than meets the eye. Reproduction 2013, 145, R107–R116. [Google Scholar] [CrossRef]
- Albrecht, M.; RämSch, R.; KöhN, F.M.; Schwarzer, J.U.; Mayerhofer, A. Isolation and Cultivation of Human Testicular Peritubular Cells: A New Model for the Investigation of Fibrotic Processes in the Human Testis and Male Infertility. J. Clin. Endocrinol. Metab. 2006, 91, 1956–1960. [Google Scholar] [CrossRef]
- Landreh, L.; Spinnler, K.; Schubert, K.; Häkkinen, M.R.; Auriola, S.; Poutanen, M.; Söder, O.; Svechnikov, K.; Mayerhofer, A. Human Testicular Peritubular Cells Host Putative Stem Leydig Cells With Steroidogenic Capacity. J. Clin. Endocrinol. Metab. 2014, 99, E1227–E1235. [Google Scholar] [CrossRef] [PubMed]
- Liebich, A.; Schmid, N.; Koupourtidou, C.; Herrmann, C.; Dietrich, K.-G.; Welter, H.; Ninkovic, J.; Mayerhofer, A. The Molecular Signature of Human Testicular Peritubular Cells Revealed by Single-Cell Analysis. Cells 2022, 11, 3685. [Google Scholar] [CrossRef]
- Rolland, A.D.; Evrard, B.; Darde, T.A.; Le Béguec, C.; Le Bras, Y.; Bensalah, K.; Lavoué, S.; Jost, B.; Primig, M.; Dejucq-Rainsford, N.; et al. RNA profiling of human testicular cells identifies syntenic lncRNAs associated with spermatogenesis. Hum. Reprod. 2019, 34, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Gille, A.-S.; Lapoujade, C.; Wolf, J.-P.; Fouchet, P.; Barraud-Lange, V. Contribution of Single-Cell Transcriptomics to the Characterization of Human Spermatogonial Stem Cells: Toward an Application in Male Fertility Regenerative Medicine? Int. J. Mol. Sci. 2019, 20, 5773. [Google Scholar] [CrossRef]
- Givelet, M.; Firlej, V.; Lassalle, B.; Gille, A.S.; Lapoujade, C.; Holtzman, I.; Jarysta, A.; Haghighirad, F.; Dumont, F.; Jacques, S.; et al. Transcriptional profiling of β-2M(-)SPα-6(+)THY1(+) spermatogonial stem cells in human spermatogenesis. Stem Cell Rep. 2022, 17, 936–952. [Google Scholar] [CrossRef] [PubMed]
- Lapoujade, C.; Blanco, M.; Givelet, M.; Gille, A.S.; Allemand, I.; Lenez, L.; Thiounn, N.; Roux, S.; Wolf, J.P.; Patrat, C.; et al. Characterisation and hierarchy of the spermatogonial stem cell compartment in human spermatogenesis by spectral cytometry using a 16-colors panel. Cell. Mol. Life Sci. 2024, 82, 15. [Google Scholar] [CrossRef]
- Nickkholgh, B.; Mizrak, S.C.; Korver, C.M.; van Daalen, S.K.; Meissner, A.; Repping, S.; van Pelt, A.M. Enrichment of spermatogonial stem cells from long-term cultured human testicular cells. Fertil. Steril. 2014, 102, 558–565.e5. [Google Scholar] [CrossRef]
- Tan, K.; Song, H.-W.; Thompson, M.; Munyoki, S.; Sukhwani, M.; Hsieh, T.-C.; Orwig, K.E.; Wilkinson, M.F. Transcriptome profiling reveals signaling conditions dictating human spermatogonia fate in vitro. Proc. Natl. Acad. Sci. USA 2020, 117, 17832–17841. [Google Scholar] [CrossRef]
- Movassagh, S.A.; Dehkordi, M.B.; Pourmand, G.; Gholami, K.; Talebi, A.; Esfandyari, S.; Jabari, A.; Samadian, A.; Abbasi, M. Isolation, identification and differentiation of human spermatogonial cells on three-dimensional decellularized sheep testis. Acta Histochem. 2020, 122, 151623. [Google Scholar] [CrossRef]
- Salem, M.; Khadivi, F.; Feizollahi, N.; Khodarahmian, M.; Marghmaleki, M.S.; Ayub, S.; Chegini, R.; Bashiri, Z.; Abbasi, Y.; Naji, M.; et al. Melatonin Promotes Differentiation of Human Spermatogonial Stem Cells Cultured on Three-Dimensional Decellularized Human Testis Matrix. Urol. J. 2023, 21, 250–264. [Google Scholar] [CrossRef]
- He, Z.; Kokkinaki, M.; Jiang, J.; Dobrinski, I.; Dym, M. Isolation, Characterization, and Culture of Human Spermatogonia1. Biol. Reprod. 2010, 82, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Von Kopylow, K.; Schulze, W.; Salzbrunn, A.; Spiess, A.-N. Isolation and gene expression analysis of single potential human spermatogonial stem cells. Mol. Hum. Reprod. 2016, 22, 229–239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, Y.; Niu, M.; Yao, C.; Hai, Y.; Yuan, Q.; Liu, Y.; Guo, Y.; Li, Z. Fractionation of human spermatogenic cells using STA-PUT gravity sedimentation and their miRNA profiling. Sci. Rep. 2015, 5, 8084. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.F.; Yango, P.; Altman, E.; Choudhry, S.; Poelzl, A.; Zamah, A.M.; Rosen, M.; Klatsky, P.C.; Tran, N.D. Testicular Niche Required for Human Spermatogonial Stem Cell Expansion. STEM CELLS Transl. Med. 2014, 3, 1043–1054. [Google Scholar] [CrossRef]
- Davidoff, M.S.; Breucker, H.; Holstein, A.F.; Seidl, K. Cellular architecture of the lamina propria of human seminiferous tubules. Cell Tissue Res. 1990, 262, 253–261. [Google Scholar] [CrossRef]
- Frungieri, M.B.; Calandra, R.S.; Lustig, L.; Meineke, V.; Köhn, F.M.; Vogt, H.-J.; Mayerhofer, A. Number, distribution pattern, and identification of macrophages in the testes of infertile men. Fertil. Steril. 2002, 78, 298–306. [Google Scholar] [CrossRef]
- Meineke, V.; Frungieri, M.B.; Jessberger, B.; Vogt, H.-J.; Mayerhofer, A. Human testicular mast cells contain tryptase: Increased mast cell number and altered distribution in the testes of infertile men. Fertil. Steril. 2000, 74, 239–244. [Google Scholar] [CrossRef]
- Hussein, M.R.; Abou-Deif, E.S.; Bedaiwy, M.A.; Said, T.M.; Mustafa, M.G.; Nada, E.; Ezat, A.; Agarwal, A. Phenotypic characterization of the immune and mast cell infiltrates in the human testis shows normal and abnormal spermatogenesis. Fertil. Steril. 2005, 83, 1447–1453. [Google Scholar] [CrossRef]
- Hentrich, A.; Wolter, M.; Szardening-Kirchner, C.; Lüers, G.H.; Bergmann, M.; Kliesch, S.; Konrad, L. Reduced numbers of Sertoli, germ, and spermatogonial stem cells in impaired spermatogenesis. Mod. Pathol. 2011, 24, 1380–1389, Erratum in Mod. Pathol. 2017, 30, 311. [Google Scholar] [CrossRef]
- Dobrinski, I.; Ogawa, T.; Avarbock, M.R.; Brinster, R.L. Computer assisted image analysis to assess colonization of recipient seminiferous tubules by spermatogonial stem cells from transgenic donor mice. Mol. Reprod Dev. 1999, 53, 142–148. [Google Scholar] [CrossRef]
- Fujita, K.; Ohta, H.; Tsujimura, A.; Takao, T.; Miyagawa, Y.; Takada, S.; Matsumiya, K.; Wakayama, T.; Okuyama, A. Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. J. Clin. Investig. 2005, 115, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Kwaspen, L.; Kanbar, M.; Wyns, C. Mapping the Development of Human Spermatogenesis Using Transcriptomics-Based Data: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 6925. [Google Scholar] [CrossRef] [PubMed]

| Testicular Cell Selection Strategy | Main Achievements | Research Gaps for Clinical Applications |
|---|---|---|
| Density gradient separation | 95% Leydig cell (LC) purity, 95% viability | Not tested for cancer decontamination purposes Toxicity of silica-based gradients Low cell recovery Limited standardization |
| Differential plating | >95% Sertoli cell (SC) purity, 98% viability 95% germ cell (GC) purity | Not tested for cancer decontamination purposes Limited standardization Need for identification of optimal protein-coating of culture plates Impossible to isolate specific GC subtypes Limited capacity to achieve cell-type specific separation |
| Flow cytometry | 98% LC purity 95% Peritubular Myoid cell purity 14% GC enrichment 38-fold Spermatogonial stem cell (SSC)-activity No tumor formation after Fluorescent-activated cell sorting (FACS)-sorted xenotransplantation of putative SSCs [29] | Questionable invasive capacities of the cancer cell line used in the decontamination protocol Lack of optimal markers for 100% pure testicular cell selection Additional fluorescent dye removal steps required |
| Explant growth culture | Peritubular Myoid cells (PTMCs) outgrowth after 2–3 weeks | Not tested for cancer decontamination purposes Only applicable for PTMCs |
| Magnetic-activated cell sorting (MACS) | 95% GC purity 40-fold SSC-activity 0.9–4.6% leukemic cells remaining in the sorted fraction [30] | Inefficient cancer cell decontamination Lack of optimal markers for 100% testicular cell selection Risk of magnetic-bead contamination requiring magnetic-bead removal steps |
| Queries |
|---|
| Leydig cell AND isolation |
| Leydig cell AND marker |
| Macrophage AND isolation AND testis |
| Macrophage AND marker AND testis |
| Peritubular cell AND isolation |
| Peritubular cell AND marker |
| Sertoli cell AND isolation |
| Sertoli cell AND marker |
| Spermatogonia AND isolation |
| Spermatogonia AND marker |
| Spermatogonial stem cell AND isolation |
| Spermatogonial stem cell AND marker |
| Testicular cell AND isolation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Windt, S.; Nekounazar Azad, N.; Wyns, C. Human Testicular Tissue Digestion, Testicular Cell Selection, and Downstream Characterization for Reproductive Purposes: A Scoping Review. Int. J. Mol. Sci. 2025, 26, 10150. https://doi.org/10.3390/ijms262010150
De Windt S, Nekounazar Azad N, Wyns C. Human Testicular Tissue Digestion, Testicular Cell Selection, and Downstream Characterization for Reproductive Purposes: A Scoping Review. International Journal of Molecular Sciences. 2025; 26(20):10150. https://doi.org/10.3390/ijms262010150
Chicago/Turabian StyleDe Windt, Sven, Neguine Nekounazar Azad, and Christine Wyns. 2025. "Human Testicular Tissue Digestion, Testicular Cell Selection, and Downstream Characterization for Reproductive Purposes: A Scoping Review" International Journal of Molecular Sciences 26, no. 20: 10150. https://doi.org/10.3390/ijms262010150
APA StyleDe Windt, S., Nekounazar Azad, N., & Wyns, C. (2025). Human Testicular Tissue Digestion, Testicular Cell Selection, and Downstream Characterization for Reproductive Purposes: A Scoping Review. International Journal of Molecular Sciences, 26(20), 10150. https://doi.org/10.3390/ijms262010150






