Facile Synthesis of N-vinylindoles via Knoevenagel Condensation: Molecular Features and Biological Activities
Abstract
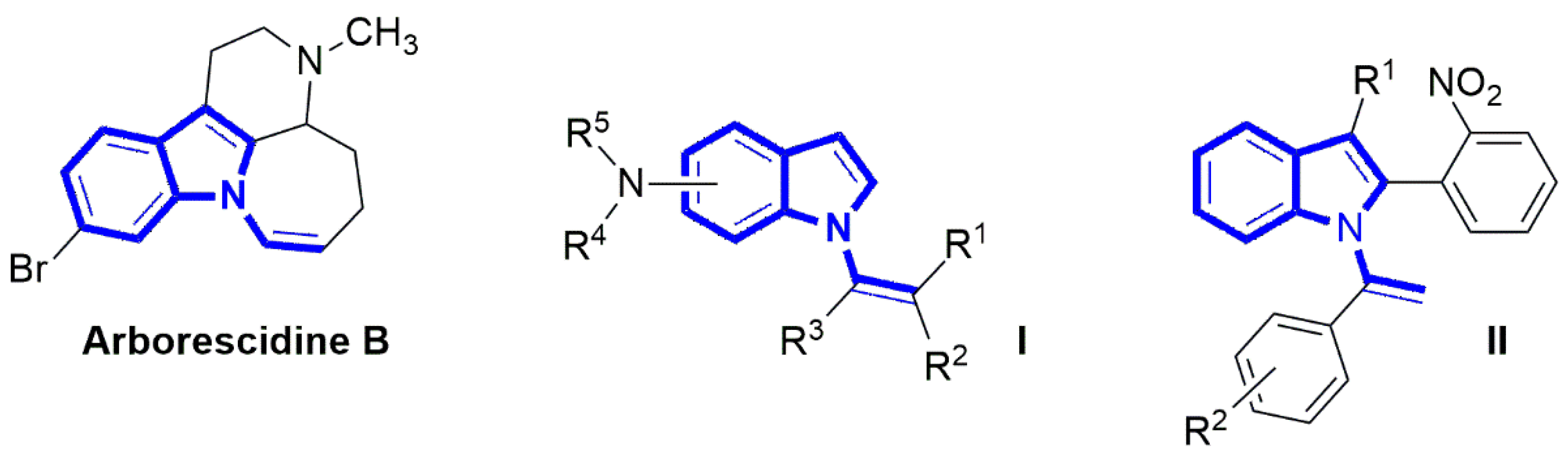
1. Introduction
2. Results and Discussion
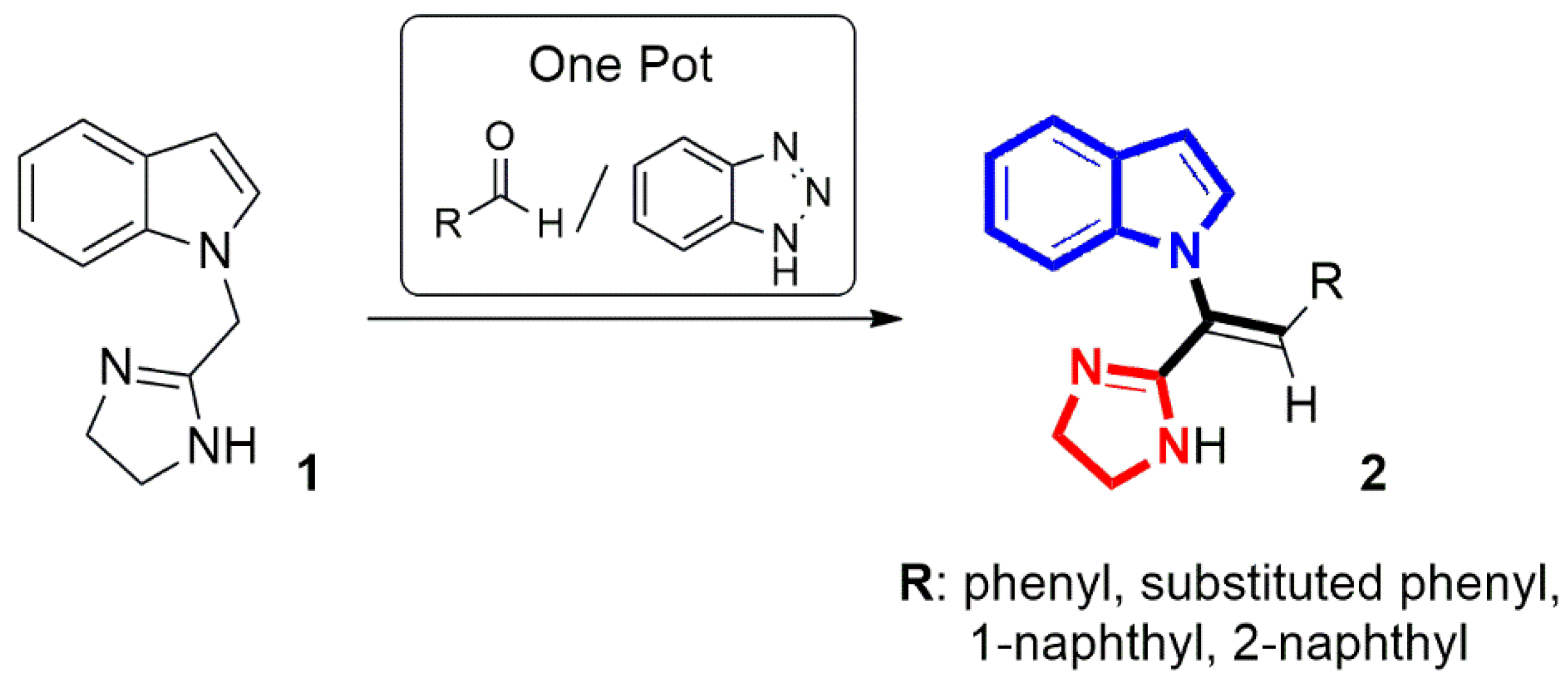
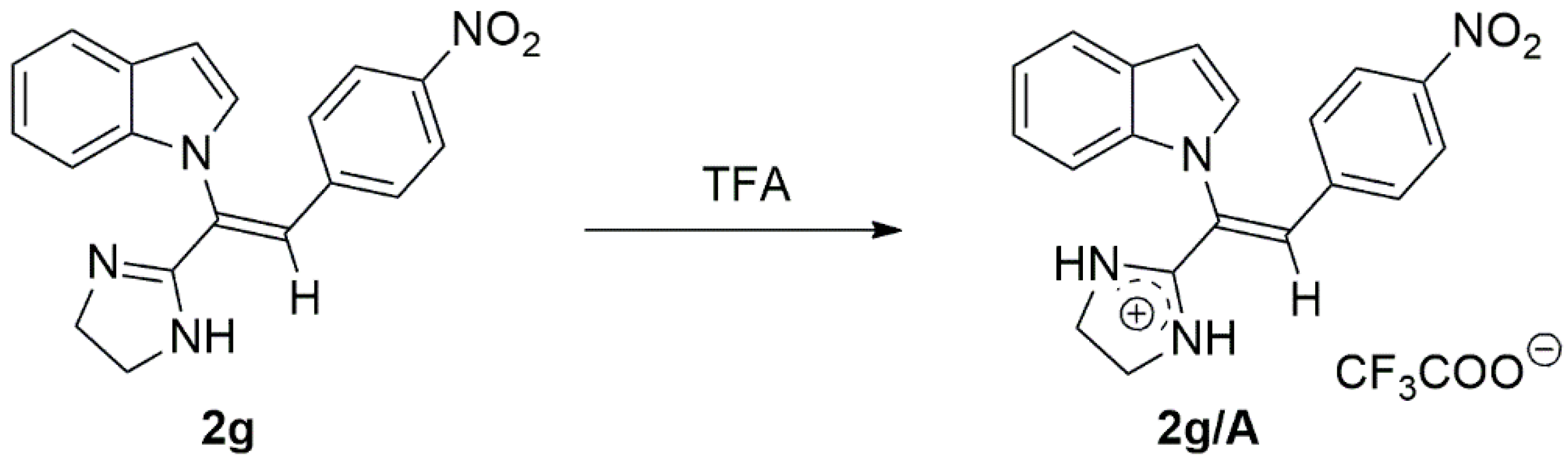
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. In Vitro Anticancer Activity
Cytotoxic Activity of N-vinylindole Derivatives on Cancer Cell Lines
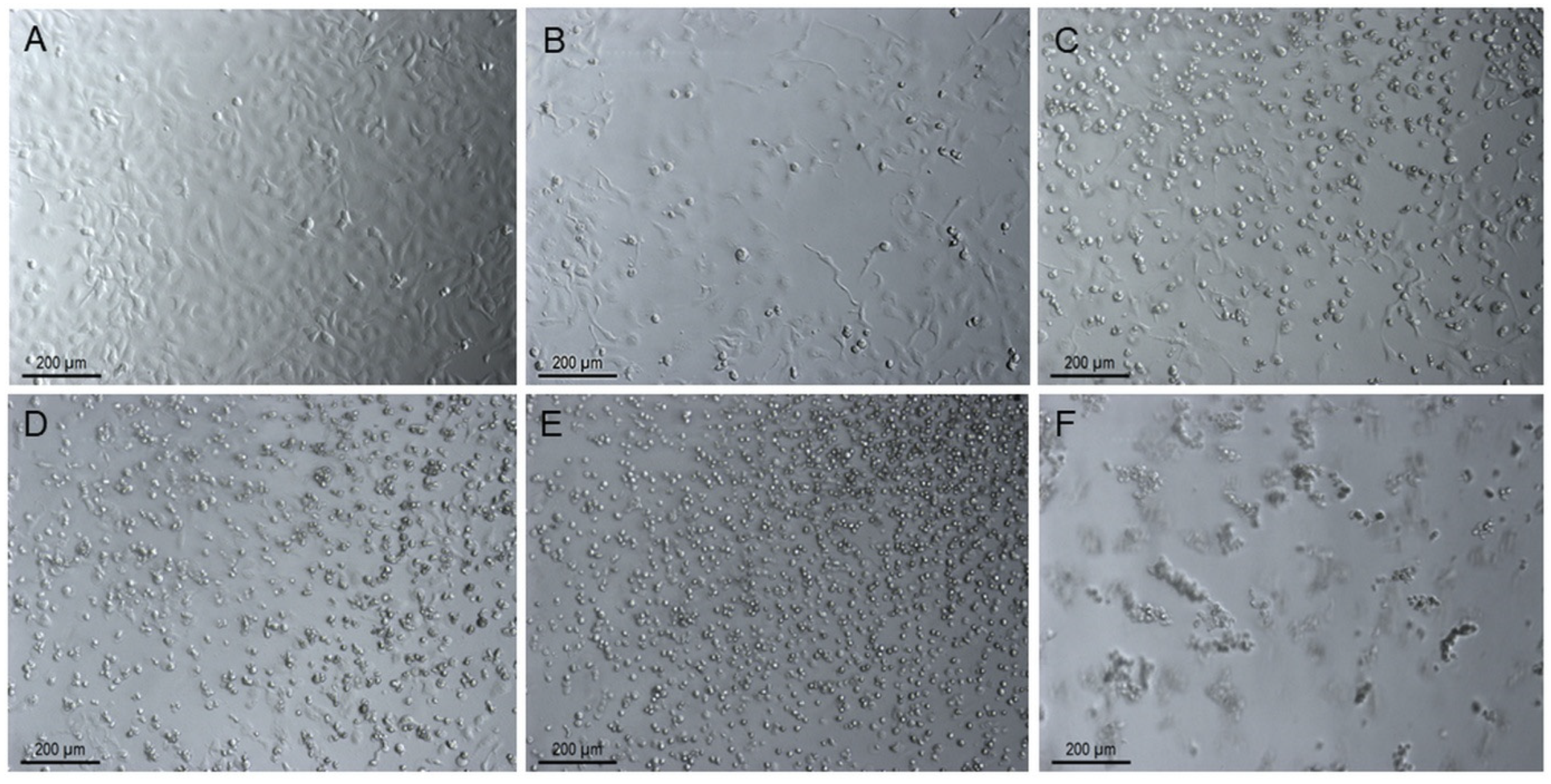
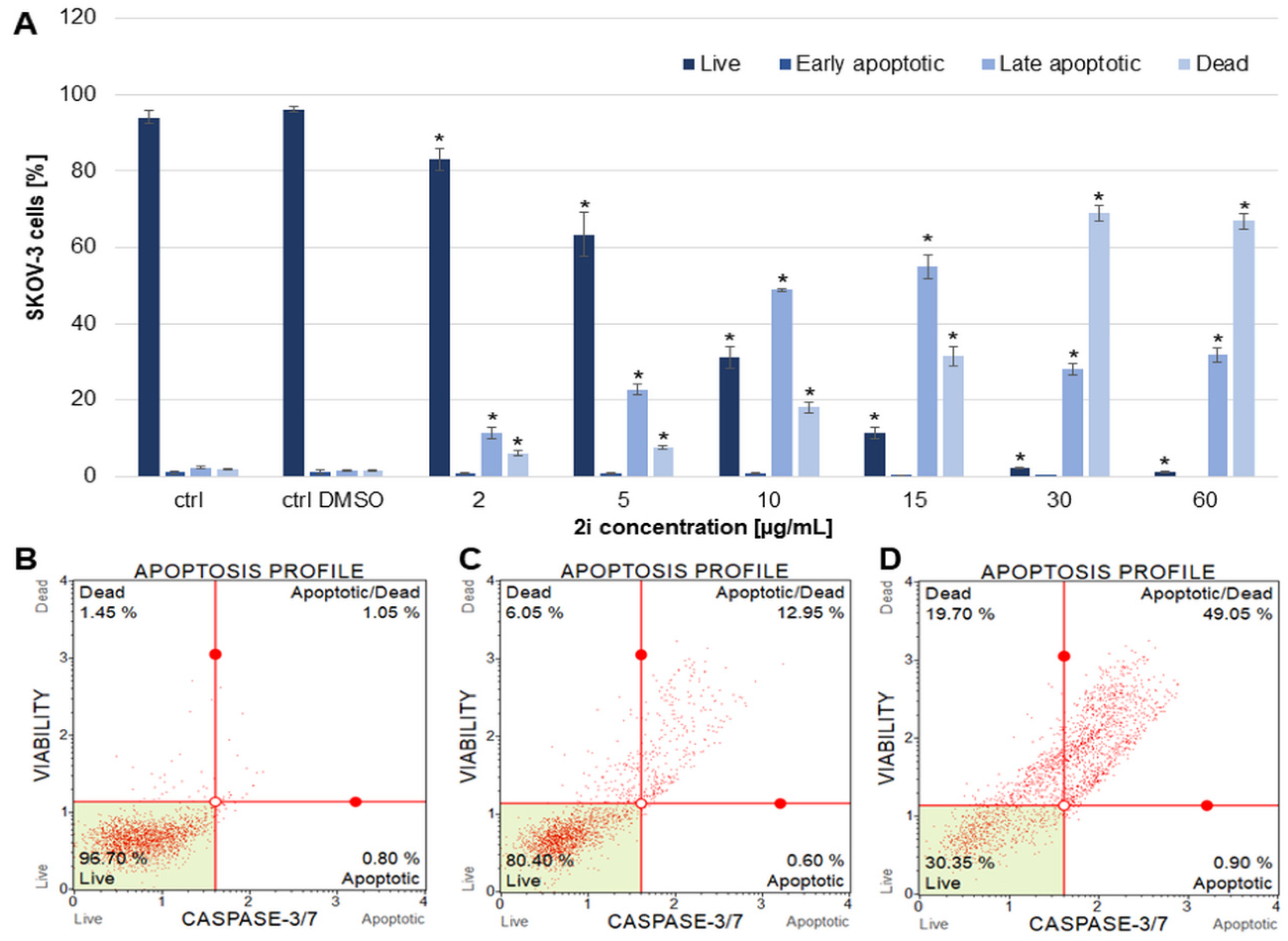
Estimation of the Apoptotic Effect of 2i in SKOV-3 Cells
Cell Cycle Arrest Analysis of SKOV-3 Cells Treated with 2i
2.2.2. In Vitro Antimicrobial Activity
2.2.3. Effect on the AGE2-BSA/sRAGE Interaction
3. Materials and Methods
3.1. Experimental Section
3.1.1. General Information
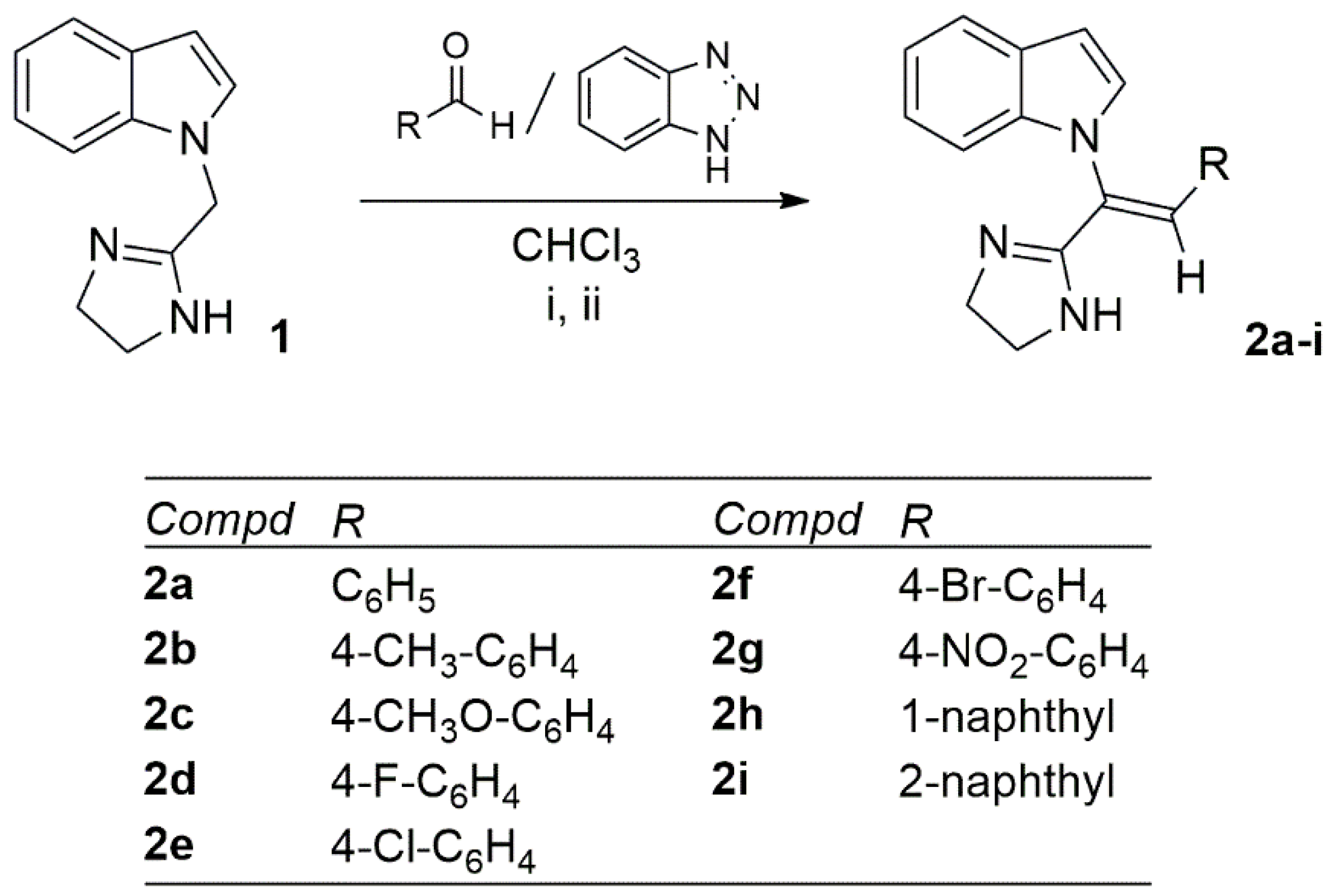
3.1.2. General Procedure for the Synthesis of (Z)-1-[2-aryl-1-(4,5-dihydro-1H-imidazol-2-yl)vinyl]-1H-indoles 2a-f
- (Z)-1-[1-(4,5-dihydro-1H-imidazol-2-yl)-2-(phenyl)vinyl]-1H-indole (2a)
- (Z)-1-[1-(4,5-dihydro-1H-imidazol-2-yl)-2-(p-tolyl)vinyl]-1H-indole (2b)
- (Z)-1-[1-(4,5-dihydro-1H-imidazol-2-yl)-2-(4-methoxyphenyl)vinyl]-1H-indole (2c)
- (Z)-1-[1-(4,5-dihydro-1H-imidazol-2-yl)-2-(4-fluorophenyl)vinyl]-1H-indole (2d)
- (Z)-1-[1-(4,5-dihydro-1H-imidazol-2-yl)-2-(4-nitrophenyl)vinyl]-1H-indole (2g)
- (Z)-1-[1-(4,5-dihydro-1H-imidazol-2-yl)-2-(naphthalen-1-yl)vinyl]-1H-indole (2h)
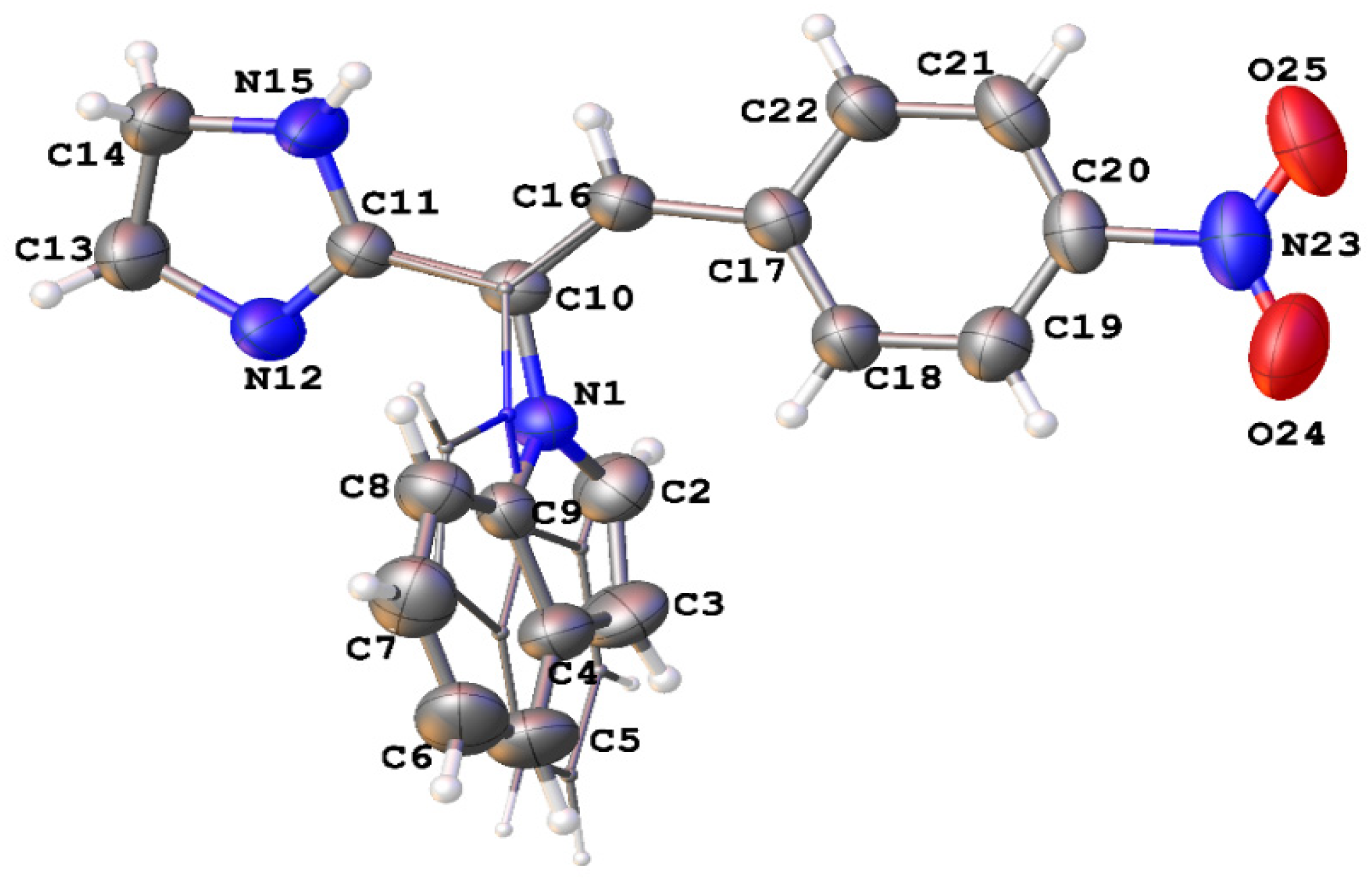
3.1.3. Single-Crystal X-Ray Diffraction Experiments
3.2. Biological Studies
3.2.1. Anticancer Studies
Cell Lines
MTT Assay
Caspase-3/7 Assay
Cell Cycle Assay
Statistical Analysis
3.2.2. Microbroth Dilution Assay
3.2.3. ELISA AGE2-BSA/sRAGE Inhibitory Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, H.-L.; Liu, J.; Fang, W.-Y.; Ravindar, L.; Rakesh, K.P. Indole-based derivatives as potential antibacterial activity against methicillin-resistance Staphylococcus aureus (MRSA). Eur. J. Med. Chem. 2020, 194, 112245. [Google Scholar] [CrossRef]
- Chandal, N.; Kalia, R.; Dey, A.; Tambat, R.; Mahey, N.; Nandanwar, H. Synthetic indole derivatives as an antibacterial agent inhibiting respiratory metabolism of multidrug-resistant gram-positive bacteria. Commun. Biol. 2024, 7, 1489. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bhutani, C.; Khanna, P.; Talwar, S.; Singh, S.K.; Khanna, L. Recent report on indoles as a privileged anti-viral scaffold in drug discovery. Eur. J. Med. Chem. 2025, 281, 117017. [Google Scholar] [CrossRef] [PubMed]
- Barresi, E.; Baglini, E.; Poggetti, V.; Castagnoli, J.; Giorgini, D.; Salerno, S.; Taliani, S.; Da Settimo, F. Indole-based compounds in the development of anti-neurodegenerative agents. Molecules 2024, 29, 2127. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Celik, I.; Chinnam, S.; Çevik, U.A.; Tallei, T.E.; Nizam, A.; Walode, S.G. Analgesic and anti-inflammatory potential of indole derivatives. Polycycl. Aromat. Compd. 2022, 43, 7732–7753. [Google Scholar] [CrossRef]
- Baramaki, I.; Altıntop, M.D.; Arslan, R.; Altınok, F.A.; Özdemir, A.; Dallali, I.; Hasan, A.; Türkmen, N.B. Design, synthesis, and in vivo evaluation of a new series of indole-chalcone hybrids as analgesic and anti-Inflammatory agents. ACS Omega 2024, 9, 12175–12183. [Google Scholar] [CrossRef]
- Siddiqui, N.; Andalip; Bawa, S.; Ali, R.; Afzal, O.; Akhtar, M.J.; Azad, B.; Kumar, R. Antidepressant potential of nitrogen-containing heterocyclic moieties: An updated review. J. Pharm. Bioallied. Sci. 2011, 3, 194–212. [Google Scholar]
- Hashemi, S.M.; Emami, S.; Masihi, P.H.; Shakiba, A.; Dehestani, L.; Ahangar, N. Synthesis of 2-aryl-3-triazolyl-indoles from phenacyltriazole-derived hydrazones: Exploring new scaffolds for anticonvulsant activity. J. Mol. Struct. 2023, 1276, 134704. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, J.; Luo, L.; Gao, Y.; Bao, H.; Li, P.; Zhang, H. Research progress of indole compounds with potential antidiabetic activity. Eur. J. Med. Chem. 2021, 223, 113665. [Google Scholar] [CrossRef]
- Peroutka, S.J.; McCarthy, B.G. Sumatriptan (GR 43175) interacts selectively with 5-HT1B and 5-HT1D binding sites. Eur. J. Pharmacol. 1989, 163, 133–136. [Google Scholar] [CrossRef]
- Poeggeler, B.; Singh, S.K.; Pappolla, M.A. Tryptophan in nutrition and health. Int. J. Mol. Sci. 2022, 23, 5455. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.M.; Farid, A.; Panda, S.S.; Bekheit, M.S.; Dinkins, H.; Fayad, W.; Girgis, A.S. Indole compounds in oncology: Therapeutic potential and mechanistic insights. Pharmaceuticals 2024, 17, 922. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Sakr, W.A.; Rahman, K.W. Mechanisms and therapeutic implications of cell death induction by indole compounds. Cancers 2011, 3, 2955–2974. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Mandi, P.; Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Synthesis, molecular docking, and QSAR study of sulfonamide-based indoles as aromatase inhibitors. Eur. J. Med. Chem. 2017, 143, 1604–1615. [Google Scholar] [CrossRef]
- Singla, R.; Prakash, K.; Gupta, K.B.; Upadhyay, S.; Dhiman, M.; Jaitak, V. Identification of novel indole based heterocycles as selective estrogen receptor modulator. Bioorg. Chem. 2018, 79, 72–88. [Google Scholar] [CrossRef]
- Mondal, D.; Amin, S.A.; Moinul, M.; Das, K.; Jha, T.; Gayen, S. How the structural properties of the indole derivatives are important in kinase targeted drug design? A case study on tyrosine kinase inhibitors. Bioorganic Med. Chem. 2022, 53, 116534. [Google Scholar] [CrossRef]
- Hong, Y.; Zhu, Y.; He, Q.; Gu, S. Indole derivatives as tubulin polymerization inhibitors for the development of promising anticancer agents. Bioorganic Med. Chem. 2022, 55, 116597. [Google Scholar] [CrossRef]
- Ahmad, A.; Biersack, B.; Li, Y.; Kong, D.; Bao, B.; Schobert, R.; Padhye, S.B.; Sarkar, F.H. Targeted regulation of PI3K/Akt/mTOR/NF-κB signaling by indole compounds and their derivatives: Mechanistic details and biological implications for cancer therapy. Anticancer Agents Med. Chem. 2013, 13, 1002–1013. [Google Scholar] [CrossRef]
- Dadashpour, S.; Emami, S. Indole in the target-based design of anticancer agents: A versatile scaffold with diverse mechanisms. Eur. J. Med. Chem. 2018, 150, 9–29. [Google Scholar] [CrossRef]
- Tasic, G.; Simic, M.; Popovic, S.; Husinec, S.; Maslak, V.; Savic, V. Indirect N-vinylation of indoles via isomerization of N-allyl derivatives: Synthesis of (±)-debromoarborescidine B. Tetrahedron Lett. 2013, 54, 4536–4539. [Google Scholar] [CrossRef]
- Ghosh, A.; Bainbridge, D.T.; Stanley, L.M. Enantioselective model synthesis and progress toward the putative structure of Yuremamine. J. Org. Chem. 2016, 81, 7945–7951. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Boonnak, N.; Padwa, A. N-alkenyl indoles as useful intermediates for alkaloid synthesis. J. Org. Chem. 2011, 76, 9488–9496. [Google Scholar] [CrossRef]
- Schultz, A.G.; Malachowski, W.P.; Pan, Y. Asymmetric total synthesis of (+)-apovincamine and a formal synthesis of (+)-vincamine. Demonstration of a practical “Asymmetric Linkage” between aromatic carboxylic acids and chiral acyclic substrates. J. Org. Chem. 1997, 62, 1223–1229. [Google Scholar] [CrossRef]
- Din Belle, D.; Tolvanen, A.; Lounasmaa, M. Total syntheses of tacamine-type indole alkaloids of Tabernaemontana eglandulosa. Tetrahedron 1996, 52, 11361–11378. [Google Scholar] [CrossRef]
- England, D.B.; Padwa, A. General access to the Vinca and Tacaman alkaloids using a Rh(II)-catalyzed cyclization/cycloaddition cascade. J. Org. Chem. 2008, 73, 2792–2802. [Google Scholar] [CrossRef]
- Roche, M.; Bignon, J.; Brion, J.-D.; Hamze, A.; Alami, M. Tandem one-pot palladium-catalyzed coupling of hydrozones, haloindoles, and amines: Synthesis of amino-N-vinylindoles and their effect on human colon carcinoma cells. J. Org. Chem. 2014, 79, 7583–7592. [Google Scholar] [CrossRef]
- Pecnard, S.; Liu, X.; Provot, O.; Retailleau, P.; Tran, C.; Hamze, A. Mo-catalyzed cyclization of N-vinylindoles and skatoles: Synthesis of dihydroindolo[1,2-c]quinazolines and dihydroindolo[3,2-b]-indoles, and evaluation of their anticancer activities. Org. Chem. Front. 2024, 11, 1668–1677. [Google Scholar] [CrossRef]
- Maki, Y.; Mori, H.; Endo, T. Xanthate-mediated controlled radical polymerization of N-vinylindole derivatives. Macromolecules 2007, 40, 6119–6130. [Google Scholar] [CrossRef]
- Brustolin, F.; Castelvetro, V.; Ciardelli, F.; Ruggeri, G.; Colligiani, A. Synthesis and characterization of different poly(1-vinylindole)s for photorefractive materials. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 253–262. [Google Scholar] [CrossRef]
- Lebedev, A.Y.; Izmer, V.V.; Kazyul’kin, D.N.; Beletskaya, I.P.; Voskoboynikov, A.Z. Palladium-catalyzed stereocontrolled vinylation of azoles and phenothiazine. Org. Lett. 2002, 4, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Fridkin, G.; Boutard, N.; Lubell, W.D. β,β-disubstituted C- and N-vinylindoles from one-step condensations of aldehydes and indole derivatives. J. Org. Chem. 2009, 74, 5603–5606. [Google Scholar] [CrossRef]
- Kennedy, D.F.; Nova, A.; Willis, A.C.; Eisenstein, O.; Messerle, B.A. The mechanism of N-vinylindole formation via tandem imine formation and cycloisomerisation of o-ethynylanilines. Dalton Trans. 2009, 10296–10304. [Google Scholar] [CrossRef]
- Li, H.; Boonnak, N.; Padwa, A. Synthesis of N-vinyl substituted indoles and their acid-catalyzed behaviour. Tetrahedron Lett. 2011, 52, 2062–2064. [Google Scholar] [CrossRef]
- Roche, M.; Frison, G.; Brion, J.-D.; Provot, O.; Hamze, A. Csp2-N bond formation via ligand-free Pd-catalyzed oxidative coupling reaction of N-tosylhydrazones and indole derivatives. J. Org. Chem. 2013, 78, 8485–8495. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Cheng, G.; Shen, J.; Cui, X. Palladium-catalyzed oxidative coupling of N-tosylhydrazones with indoles: Synthesis of N-vinylindoles. Org. Lett. 2013, 15, 3022–3025. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Zhang, X.; Yu, L.-T.; Mao, W.-T.; Chen, C.-Z.; Xu, K. Synthesis of N-vinylindoles through copper catalyzed cyclization reaction of N-(2-alkynylphenyl)imine. Org. Biomol. Chem. 2015, 13, 6931–6934. [Google Scholar] [CrossRef]
- Nie, Z.; Lv, H.; Li, H.; Su, M.; Yang, T.; Luo, W.; Liu, Q.; Guo, C. Synthesis of terminal N-vinylazoles from aromatic aldehydes, DMSO, and azoles based DMSO as terminal carbon synthon. Adv. Synth. Catal. 2021, 363, 4621–4626. [Google Scholar] [CrossRef]
- Xu, P.-P.; Liao, J.-Y.; Zhang, J.-J.; Shi, W.-M.; Liang, C.; Su, G.-F.; Mo, D.-L. Nickel(II)-catalyzed [3+2] cycloaddition of nitrones and allenoates to access N-vinylindoles and N-vinylpyrroles. Org. Lett. 2021, 23, 7482–7486. [Google Scholar] [CrossRef]
- Palomba, M.; Dias, I.F.C.; Cocchioni, M.; Marini, F.; Santi, C.; Bagnoli, L. Vinylation of N-heteroarenes through addition/elimination reactions of vinyl selenones. Molecules 2023, 28, 6026. [Google Scholar] [CrossRef]
- Balestri, L.J.I.; Beveridge, J.; Gising, J.; Odell, L.R. Synthesis of N-alkenylated heterocycles via T3P-promoted condensation with ketones. J. Org. Chem. 2024, 89, 11203–11214. [Google Scholar] [CrossRef]
- Saczewski, J.; Hudson, A.; Scheinin, M.; Wasilewska, A.; Saczewski, F.; Rybczynska, A.; Fredousi, M.; Laurila, J.M.; Boblewski, K.; Lehmann, A.; et al. Transfer of SAR information from hypotensive indazole to indole derivatives acting at α-adrenergic receptors: In vitro and in vivo studies. Eur. J. Med. Chem. 2016, 115, 406–415. [Google Scholar] [CrossRef]
- Krasavin, M. Biologically active compounds based on the privileged 2-imidazoline scaffold: The world beyond adrenergic/imidazoline receptor modulators. Eur. J. Med. Chem. 2015, 97, 525–537. [Google Scholar] [CrossRef]
- Fritz, G. RAGE: A single receptors fits for multiple ligands. Trends Biochem. Sci. 2011, 36, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, Y.; Huang, Y.; Deng, H. Pathophysiology of RAGE in inflammatory diseases. Front. Immunol. 2022, 13, 931473. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Fukami, K. RAGE signaling regulates the progression of diabetic complications. Front. Pharmacol. 2023, 14, 1128872. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Shekhtman, A.; Schmidt, A.M. RAGE/DIAPH1 and atherosclerosis through an evolving lens: Viewing the cell from “inside–out”. Atherosclerosis 2024, 394, 117304. [Google Scholar] [CrossRef]
- Kinscherf, N.A.; Pehar, M. Role and therapeutic potential of RAGE signaling in neurodegeneration. Curr. Drug Targets 2022, 23, 1191–1209. [Google Scholar] [CrossRef]
- Vianello, E.; Beltrami, A.P.; Aleksova, A.; Janjusevic, M.; Fluca, A.L.; Corsi Romanelli, M.M.; La Sala, L.; Dozio, E. The advanced glycation end-products (AGE)–receptor for AGE system (RAGE): An inflammatory pathway linking obesity and cardiovascular diseases. Int. J. Mol. Sci. 2025, 26, 3707. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeong, M.S.; Jang, S.B. Molecular characteristics of RAGE and advances in small-molecule inhibitors. Int. J. Mol. Sci. 2021, 22, 6904. [Google Scholar] [CrossRef]
- Korcz, M.; Sączewski, F.; Bednarski, P.J.; Kornicka, A. Synthesis, structure, chemical stability, and in vitro cytotoxic properties of novel quinoline-3-carbaldehyde hydrazones bearing a 1,2,4-triazole or benzotriazole moiety. Molecules 2018, 23, 1497. [Google Scholar] [CrossRef]
- Lopez-Sanchez, A.; Bertnard, H.C. Pt(iv) anticancer prodrugs bearing an oxaliplatin scaffold: What do we know about their bioactivity? Inorg. Chem. Front. 2024, 11, 1639–1667. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Tran, D.N.; Hoang, T.T.H.; Nandanwar, S.; Ho, V.T.T.X.; Pham, V.T.; Vu, H.D.; Nguyen, X.H.; Nguyen, H.T.; Nguyen, T.V.; Nguyen, T.K.V.; et al. Dual anticancer and antibacterial activity of fluorescent naphthoimidazolium salts. RSC Adv. 2023, 13, 36430–36438. [Google Scholar] [CrossRef]
- Kapron, B.; Czarnomysy, R.; Paneth, A.; Wujec, M.; Bielawski, K.; Bielawska, A.; Swiatek, L.; Rajtar, B.; Polz-Dacewicz, M.; Plech, T. Dual antibacterial and anticancer activity of 4−benzoyl−1-dichlorobenzoylthiosemicarbazide derivatives. Anti-Cancer Agents Med. Chem. 2018, 18, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Kisiel-Nawrot, E.; Pindjakova, D.; Latocha, M.; Bak, A.; Kozik, V.; Suwinska, K.; Cizek, A.; Jampilek, J.; Zięba, A. Towards anticancer and antibacterial agents: Design and synthesis of 1,2,3-triazol-quinobenzothiazine derivatives. Int. J. Mol. Sci. 2023, 24, 13250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, Y.; Shi, L.; Li, L.; Zhang, D.; Gong, Z.; Wu, Q. Activation and modulation of the AGEs-RAGE axis: Implications for inflammatory pathologies and therapeutic interventions—A review. Pharmacol. Res. 2024, 206, 107282. [Google Scholar] [CrossRef] [PubMed]
- Dobrucki, I.T.; Miskalis, A.; Nelappana, M.; Applegate, C.; Wozniak, M.; Czerwinski, A.; Kalinowski, L.; Dobrucki, L.W. Receptor for advanced glycation end-products: Biological significance and imaging applications. WIREs Nanomed. Nanobiotech. 2024, 16, e1935. [Google Scholar] [CrossRef]
- Faruqui, T.; Khan, M.S.; Akhter, Y.; Khan, S.; Rafi, Z.; Saeed, M.; Han, I.; Choi, E.-H.; Yadav, D.K. RAGE inhibitors for targeted therapy of cancer: A comprehensive review. Int. J. Mol. Sci. 2023, 24, 266. [Google Scholar] [CrossRef]
- El-Far, A.H.; Sroga, G.; Al Jaouni, S.K.; Mousa, S.A. Role and mechanisms of RAGE-ligand complexes and RAGE-inhibitors in cancer progression. Int. J. Mol. Sci. 2020, 21, 3613. [Google Scholar] [CrossRef]
- Ko, S.Y.; Ko, H.A.; Shieh, T.M.; Chang, W.C.; Chen, H.I.; Chang, S.S.; Lin, I.H. Cell migration is regulated by AGE-RAGE interaction in human oral cancer cells in vitro. PLoS ONE 2014, 16, e110542. [Google Scholar] [CrossRef]
- Mollace, A.; Coluccio, M.L.; Donato, G.; Mollace, V.; Malara, N. Cross-talks in colon cancer between RAGE/AGEs axis and inflammation/immunotherapy. Oncotarget 2021, 12, 1281–1295. [Google Scholar] [CrossRef]
- Deng, R.; Mo, F.; Chang, B.; Zhang, Q.; Ran, H.; Yang, S.; Zhu, Z.; Hu, L.; Su, Q. Glucose-derived AGEs enhance human gastric cancer metastasis through RAGE/ERK/Sp1/MMP2 cascade. Oncotarget 2017, 8, 104216–104226. [Google Scholar] [CrossRef]
- Coser, M.; Neamtu, B.M.; Pop, B.; Cipaian, C.R.; Crisan, M. RAGE and its ligands in breast cancer progression and metastasis. Oncol Rev. 2025, 18, 1507942. [Google Scholar] [CrossRef] [PubMed]
- Applegate, C.C.; Nelappana, M.B.; Nielsen, E.A.; Kalinowski, L.; Dobrucki, I.T.; Dobrucki, L.W. RAGE as a novel biomarker for prostate cancer: A systematic review and meta-analysis. Cancers 2023, 15, 4889. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, F.; Karimi, J.; Goodarzi, M.T.; Saidijam, M.; Khodadadi, I.; Razavi, A.N.; Nankali, M. Overexpression of receptor for advanced glycation end products (RAGE) in ovarian cancer. Cancer Biomark. 2017, 18, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Song, Y.; Zhou, L.; Li, W.; Zhu, X. Downregulation of RAGE inhibits cell proliferation and induces apoptosis via regulation of PI3K/AKT pathway in cervical squamous cell carcinoma. Onco Targets Ther. 2020, 13, 2385–2397. [Google Scholar] [CrossRef]
- Singh, H.; Agrawal, D.K. Therapeutic potential of targeting the receptor for advanced glycation end products (RAGE) by small molecule inhibitors. Drug Dev. Res. 2022, 83, 1257–1269. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction. CrysAlisPro Software System, Version 1.171.38.46; Rigaku Corporation: Oxford, UK, 2015.
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0, 2024. Available online: http://www.eucast.org (accessed on 1 September 2005).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs for Antifungal Agents, Version 10.0, 2020. Available online: http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/ (accessed on 1 September 2025).
- Hałasa, R.; Turecka, K.; Orlewska, C.; Werel, W. Comparison of fluorescence optical respirometry and microbroth dilution methods for testing antimicrobial compounds. J. Microbiol. Methods 2014, 107, 98–105. [Google Scholar] [CrossRef]
- Turecka, K.; Chylewska, A.; Kawiak, A.; Waleron, K. Antifungal activity and mechanism of action of the Co(III) coordination complexes with diamine chelate ligands against reference and clinical strains of Candida spp. Front. Microbiol. 2018, 9, 1594. [Google Scholar] [CrossRef]



| Compound | IC50 ± SD (μg/mL) a | |||
|---|---|---|---|---|
| Cell Line | ||||
| HeLa | SKOV-3 | AGS | HaCaT | |
| 2a | 8.96 ± 0.33 | 9.81 ± 0.45 | 17.25 ± 0.68 | 11.70 ± 1.31 |
| 2b | 8.19 ± 0.40 | 9.69 ± 0.62 | 16.23 ± 1.20 | 12.09 ± 0.55 |
| 2c | 7.86 ± 0.59 | 8.80 ± 0.46 | 10.29 ± 0.08 | 9.11 ± 1.58 |
| 2d | 8.65 ± 0.68 | 10.25 ± 0.45 | 15.48 ± 0.72 | 7.37 ± 0.28 |
| 2e | 3.13 ± 0.30 | 4.40 ± 0.16 | 11.37 ± 0.74 | 3.43 ± 0.05 |
| 2f | 2.37 ± 0.15 | 2.76 ± 0.18 | 11.12 ± 1.24 | 3.13 ± 0.09 |
| 2g | 5.60 ± 0.57 | 7.06 ± 0.62 | 10.14 ± 0.08 | 9.15 ± 0.74 |
| 2h | 10.09 ± 1.12 | 10.39 ± 0.10 | 18.90 ± 1.81 | 17.95 ± 1.93 |
| 2i | 2.03 ± 0.05 | 2.45 ± 0.13 | 5.68 ± 0.41 | 3.02 ± 0.18 |
| Oxaliplatin b | 35.76 ± 1.72 | 102.16 ± 9.26 | 17.90 ± 1.20 | NT c |
| Vinblastine sulfate b | 0.005 ± 0.0004 | 0.008 ± 0.0003 | NT c | NT c |
| Compound b | Microorganism | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus ATCC 6535 | S. aureus MRSA 12673 | E. coli ATCC 8739 | C. albicans ATCC 10231 | C. glabrata ATCC 2001 | ||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MFC | MIC | MFC | |
| μg/mL | ||||||||||
| 2a | 110 | 220 | 220 | >220 | 220 | 220 | >220 | >220 | >220 | >220 |
| 2b | 55 | 110 | 220 | >220 | 220 | >220 | 220 | >220 | 220 | >220 |
| 2c | 110 | 110 | >220 | >220 | 220 | >220 | >220 | >220 | >220 | >220 |
| 2d | 120 | 240 | 120 | >240 | 240 | >240 | >240 | >240 | >240 | >240 |
| 2e | 30 | 60 | 60 | 240 | 120 | 240 | 120 | 240 | 240 | 240 |
| 2f | 27.5 | 55 | 55 | 110 | 110 | 110 | 110 | 110 | 220 | 220 |
| 2g | 55 | 110 | 110 | >220 | 220 | >220 | >220 | >220 | >220 | >220 |
| 2h | 120 | 240 | 120 | 240 | >240 | >240 | >240 | >240 | 240 | 240 |
| 2i | 15 | 30 | 30 | 30 | >240 | >240 | 120 | 120 | 60 | 60 |
| Ampicillin | 8 | 8 | 512 | 512 | 128 | 128 | - | - | - | - |
| Ketoconazole | - | - | - | - | - | - | >128 | >128 | 64 | 128 |
| Compound | R | Inhibition (%) |
|---|---|---|
| 2b | 4-CH3-C6H4 | NA b |
| 2d | 4-F-C6H4 | 12 |
| 2e | 4-Cl-C6H4 | 34.7 |
| 2f | 4-Br-C6H4 | 46.6 |
| 2h | 1-naphthyl | 36.3 |
| 2i | 2-naphthyl | 49.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornicka, A.; Stefanowicz-Hajduk, J.; Turecka, K.; Furman, C.; Gdaniec, M.; Balewski, Ł. Facile Synthesis of N-vinylindoles via Knoevenagel Condensation: Molecular Features and Biological Activities. Int. J. Mol. Sci. 2025, 26, 10149. https://doi.org/10.3390/ijms262010149
Kornicka A, Stefanowicz-Hajduk J, Turecka K, Furman C, Gdaniec M, Balewski Ł. Facile Synthesis of N-vinylindoles via Knoevenagel Condensation: Molecular Features and Biological Activities. International Journal of Molecular Sciences. 2025; 26(20):10149. https://doi.org/10.3390/ijms262010149
Chicago/Turabian StyleKornicka, Anita, Justyna Stefanowicz-Hajduk, Katarzyna Turecka, Christophe Furman, Maria Gdaniec, and Łukasz Balewski. 2025. "Facile Synthesis of N-vinylindoles via Knoevenagel Condensation: Molecular Features and Biological Activities" International Journal of Molecular Sciences 26, no. 20: 10149. https://doi.org/10.3390/ijms262010149
APA StyleKornicka, A., Stefanowicz-Hajduk, J., Turecka, K., Furman, C., Gdaniec, M., & Balewski, Ł. (2025). Facile Synthesis of N-vinylindoles via Knoevenagel Condensation: Molecular Features and Biological Activities. International Journal of Molecular Sciences, 26(20), 10149. https://doi.org/10.3390/ijms262010149








