Structure–Function Insights into Frog Skin Peptides Reveal Potent Inhibition of West Nile Virus Entry
Abstract
1. Introduction
2. Results
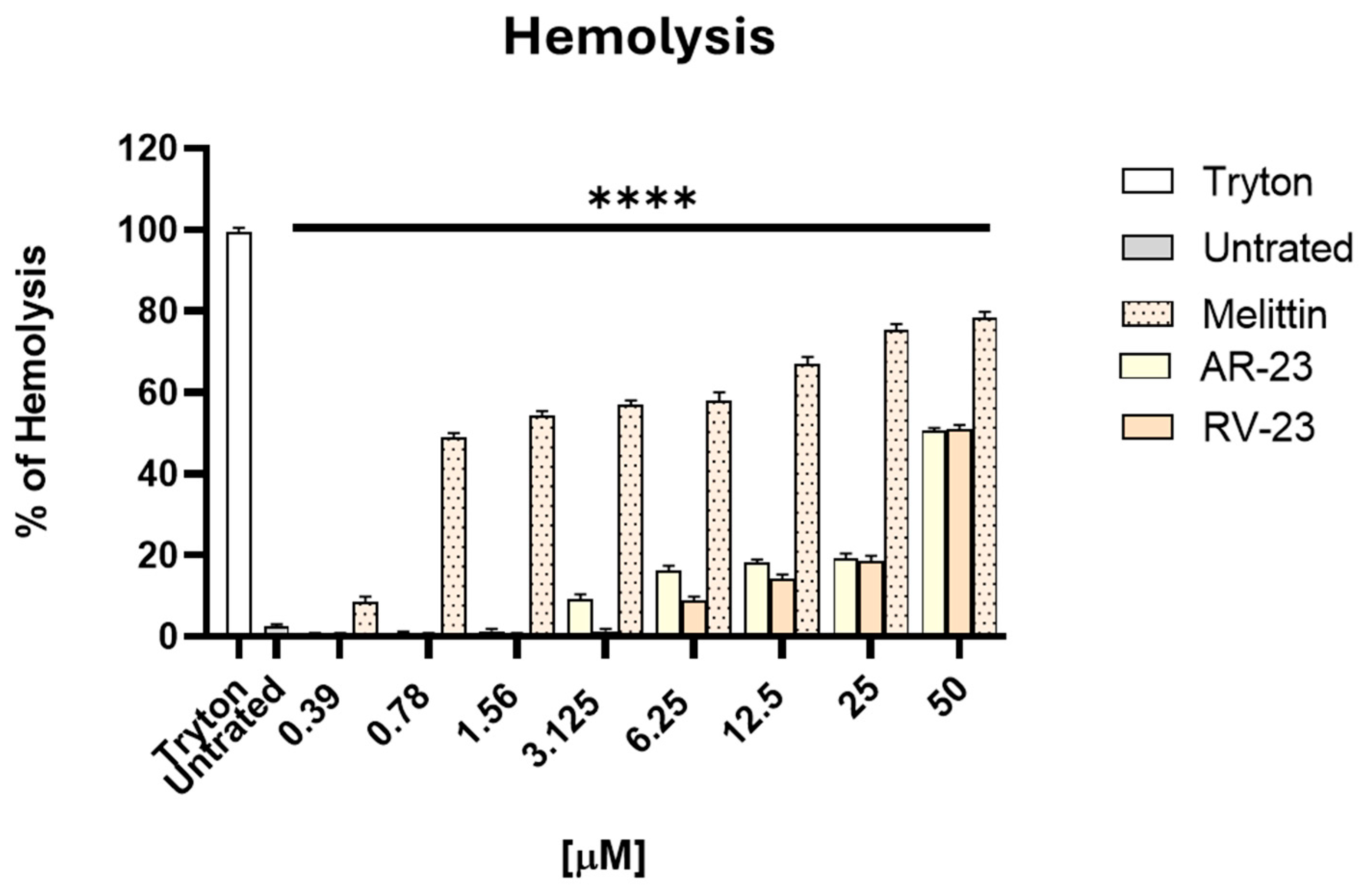
2.1. Evaluation of Peptides’ Cytotoxicity
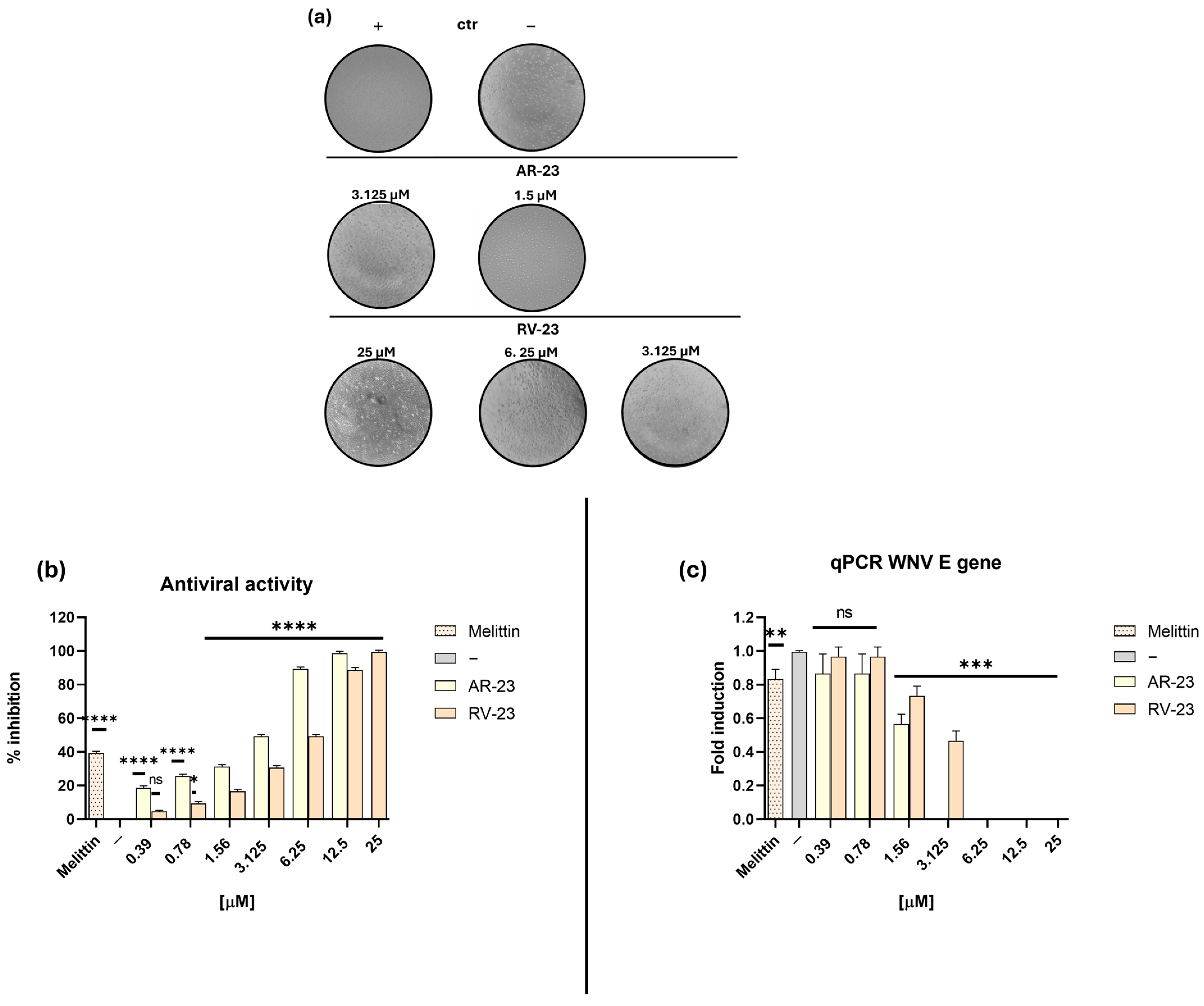
2.2. Antiviral Properties of Peptides
2.3. Molecular Docking Analysis
3. Discussion
4. Materials and Methods
4.1. Preparation and Characterization of AVPs
4.2. Hemolytic Assay
4.3. Cells and Viruses
4.4. Antiviral Activity by 50% Tissue Culture Infectious Dose (TCID50)
4.5. Molecular Analysis: Real-Time PCR
4.6. Molecular Docking Studies
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Laureti, M.; Narayanan, D.; Rodriguez-Andres, J.; Fazakerley, J.K.; Kedzierski, L. Flavivirus Receptors: Diversity, Identity, and Cell Entry. Front. Immunol. 2018, 9, 2180. [Google Scholar] [CrossRef] [PubMed]
- van Leur, S.W.; Heunis, T.; Munnur, D.; Sanyal, S. Pathogenesis and virulence of flavivirus infections. Virulence 2021, 12, 2814–2838. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.N.; Ploss, A. Emerging mosquito-borne flaviviruses. mBio 2024, 15, e0294624. [Google Scholar] [CrossRef]
- Paull, S.H.; Horton, D.E.; Ashfaq, M.; Rastogi, D.; Kramer, L.D.; Diffenbaugh, N.S.; Kilpatrick, A.M. Drought and immunity determine the intensity of West Nile virus epidemics and climate change impacts. Proc. Biol. Sci. 2017, 284, 20162078. [Google Scholar] [CrossRef]
- Singh, P.; Khatib, M.N.; Ballal, S.; Kaur, M.; Nathiya, D.; Sharma, S.; Prasad, G.V.S.; Sinha, A.; Gaidhane, A.M.; Mohapatra, P.; et al. West Nile Virus in a changing climate: Epidemiology, pathology, advances in diagnosis and treatment, vaccine designing and control strategies, emerging public health challenges–A comprehensive review. Emerg. Microbes Infect. 2025, 14, 2437244. [Google Scholar] [CrossRef]
- Farooq, Z.; Sjodin, H.; Semenza, J.C.; Tozan, Y.; Sewe, M.O.; Wallin, J.; Rocklov, J. European projections of West Nile virus transmission under climate change scenarios. One Health 2023, 16, 100509. [Google Scholar] [CrossRef]
- Carrasco, L.; Utrilla, M.J.; Fuentes-Romero, B.; Fernandez-Novo, A.; Martin-Maldonado, B. West Nile Virus: An Update Focusing on Southern Europe. Microorganisms 2024, 12, 2623. [Google Scholar] [CrossRef]
- Ricco, M.; Peruzzi, S.; Balzarini, F. Epidemiology of West Nile Virus Infections in Humans, Italy, 2012-2020: A Summary of Available Evidences. Trop. Med. Infect. Dis. 2021, 6, 61. [Google Scholar] [CrossRef]
- Moirano, G.; Richiardi, L.; Calzolari, M.; Merletti, F.; Maule, M. Recent rapid changes in the spatio-temporal distribution of West Nile Neuro-invasive Disease in Italy. Zoonoses Public Health 2020, 67, 54–61. [Google Scholar] [CrossRef]
- Roberts, J.A.; Kim, C.Y.; Hwang, S.A.; Hassan, A.; Covington, E.; Heydari, K.; Lyerly, M.; Sejvar, J.J.; Hasbun, R.; Prasad, M.; et al. Clinical, Prognostic, and Longitudinal Functional and Neuropsychological Features of West Nile Virus Neuroinvasive Disease in the United States: A Systematic Review and Meta-Analysis. Ann. Neurol. 2025, 98, 93–106. [Google Scholar] [CrossRef]
- Habarugira, G.; Suen, W.W.; Hobson-Peters, J.; Hall, R.A.; Bielefeldt-Ohmann, H. West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and “One Health” Implications. Pathogens 2020, 9, 589. [Google Scholar] [CrossRef]
- Varga, J.F.A.; Bui-Marinos, M.P.; Katzenback, B.A. Frog Skin Innate Immune Defences: Sensing and Surviving Pathogens. Front. Immunol. 2018, 9, 3128. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yu, H.; Lv, T.; Chen, Q.; Luo, H.; Zhang, H. Progress in the classification, optimization, activity, and application of antimicrobial peptides. Front. Microbiol. 2025, 16, 1582863. [Google Scholar] [CrossRef] [PubMed]
- Chianese, A.; Ambrosino, A.; Giugliano, R.; Palma, F.; Parimal, P.; Acunzo, M.; Monti, A.; Doti, N.; Zannella, C.; Galdiero, M.; et al. Frog Skin Peptides Hylin-a1, AR-23, and RV-23: Promising Tools Against Carbapenem-Resistant Escherichia coli and Klebsiella pneumoniae Infections. Antibiotics 2025, 14, 374. [Google Scholar] [CrossRef] [PubMed]
- Zannella, C.; Chianese, A.; Palomba, L.; Marcocci, M.E.; Bellavita, R.; Merlino, F.; Grieco, P.; Folliero, V.; De Filippis, A.; Mangoni, M.; et al. Broad-Spectrum Antiviral Activity of the Amphibian Antimicrobial Peptide Temporin L and Its Analogs. Int. J. Mol. Sci. 2022, 23, 2060. [Google Scholar] [CrossRef]
- Bartels, E.J.H.; Dekker, D.; Amiche, M. Dermaseptins, Multifunctional Antimicrobial Peptides: A Review of Their Pharmacology, Effectivity, Mechanism of Action, and Possible Future Directions. Front. Pharmacol. 2019, 10, 1421. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Smith, P.B.; Ledeczi, A.M.; Rowe, J.M.; Reinert, L.K. Caerin 1 Antimicrobial Peptides That Inhibit HIV and Neisseria May Spare Protective Lactobacilli. Antibiotics 2020, 9, 661. [Google Scholar] [CrossRef]
- VanCompernolle, S.E.; Taylor, R.J.; Oswald-Richter, K.; Jiang, J.; Youree, B.E.; Bowie, J.H.; Tyler, M.J.; Conlon, J.M.; Wade, D.; Aiken, C.; et al. Antimicrobial peptides from amphibian skin potently inhibit human immunodeficiency virus infection and transfer of virus from dendritic cells to T cells. J. Virol. 2005, 79, 11598–11606. [Google Scholar] [CrossRef]
- Zhang, S.K.; Ma, Q.; Li, S.B.; Gao, H.W.; Tan, Y.X.; Gong, F.; Ji, S.P. RV-23, a Melittin-Related Peptide with Cell-Selective Antibacterial Activity and High Hemocompatibility. J. Microbiol. Biotechnol. 2016, 26, 1046–1056. [Google Scholar] [CrossRef]
- Urban, E.; Nagy, E.; Pal, T.; Sonnevend, A.; Conlon, J.M. Activities of four frog skin-derived antimicrobial peptides (temporin-1DRa, temporin-1Va and the melittin-related peptides AR-23 and RV-23) against anaerobic bacteria. Int. J. Antimicrob. Agents 2007, 29, 317–321. [Google Scholar] [CrossRef]
- Chianese, A.; Iovane, V.; Zannella, C.; Capasso, C.; Nastri, B.M.; Monti, A.; Doti, N.; Montagnaro, S.; Pagnini, U.; Iovane, G.; et al. Synthetic Frog-Derived-like Peptides: A New Weapon against Emerging and Potential Zoonotic Viruses. Viruses 2023, 15, 1804. [Google Scholar] [CrossRef]
- Chianese, A.; Zannella, C.; Palma, F.; Di Clemente, L.; Monti, A.; Doti, N.; De Filippis, A.; Galdiero, M. Melittin-Related Peptides Interfere with Sandfly Fever Naples Virus Infection by Interacting with Heparan Sulphate. Microorganisms 2023, 11, 2446. [Google Scholar] [CrossRef] [PubMed]
- Memariani, H.; Memariani, M.; Moravvej, H.; Shahidi-Dadras, M. Melittin: A venom-derived peptide with promising anti-viral properties. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Sun, C.; Xu, N.; Liu, W. The current landscape of the antimicrobial peptide melittin and its therapeutic potential. Front. Immunol. 2024, 15, 1326033. [Google Scholar] [CrossRef] [PubMed]
- Enayathullah, M.G.; Parekh, Y.; Banu, S.; Ram, S.; Nagaraj, R.; Kumar, B.K.; Idris, M.M. Gramicidin S and melittin: Potential anti-viral therapeutic peptides to treat SARS-CoV-2 infection. Sci. Rep. 2022, 12, 3446. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Kuhn, R.J.; Rossmann, M.G. A structural perspective of the flavivirus life cycle. Nat. Rev. Microbiol. 2005, 3, 13–22. [Google Scholar] [CrossRef]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. Structure of the dengue virus envelope protein after membrane fusion. Nature 2004, 427, 313–319. [Google Scholar] [CrossRef]
- Goswami, S.; Chowdhury, J.P. Antiviral attributes of bee venom as a possible therapeutic approach against SARS-CoV-2 infection. Future Virol. 2023, 18, 1021–1030. [Google Scholar] [CrossRef]
- Praphawilai, P.; Kaewkod, T.; Suriyaprom, S.; Panya, A.; Disayathanoowat, T.; Tragoolpua, Y. Anti-Herpes Simplex Virus and Anti-Inflammatory Activities of the Melittin Peptides Derived from Apis mellifera and Apis florea Venom. Insects 2024, 15, 109. [Google Scholar] [CrossRef]
- Hur, J.; Kim, K.; Lee, S.; Park, H.; Park, Y. Melittin-induced alterations in morphology and deformability of human red blood cells using quantitative phase imaging techniques. Sci. Rep. 2017, 7, 9306. [Google Scholar] [CrossRef]
- Askari, P.; Namaei, M.H.; Ghazvini, K.; Hosseini, M. In vitro and in vivo toxicity and antibacterial efficacy of melittin against clinical extensively drug-resistant bacteria. BMC Pharmacol. Toxicol. 2021, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Chianese, A.; Zannella, C.; Monti, A.; De Filippis, A.; Doti, N.; Franci, G.; Galdiero, M. The Broad-Spectrum Antiviral Potential of the Amphibian Peptide AR-23. Int. J. Mol. Sci. 2022, 23, 883. [Google Scholar] [CrossRef] [PubMed]
- Modis, Y.; Ogata, S.; Clements, D.; Harrison, S.C. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 2003, 100, 6986–6991. [Google Scholar] [CrossRef]
- Guha, S.; Ferrie, R.P.; Ghimire, J.; Ventura, C.R.; Wu, E.; Sun, L.; Kim, S.Y.; Wiedman, G.R.; Hristova, K.; Wimley, W.C. Applications and evolution of melittin, the quintessential membrane active peptide. Biochem. Pharmacol. 2021, 193, 114769. [Google Scholar] [CrossRef]
- Gordon-Grossman, M.; Zimmermann, H.; Wolf, S.G.; Shai, Y.; Goldfarb, D. Investigation of model membrane disruption mechanism by melittin using pulse electron paramagnetic resonance spectroscopy and cryogenic transmission electron microscopy. J. Phys. Chem. B 2012, 116, 179–188. [Google Scholar] [CrossRef]
- Kashiwada, A.; Mizuno, M.; Hashimoto, J. pH-Dependent membrane lysis by using melittin-inspired designed peptides. Org. Biomol. Chem. 2016, 14, 6281–6288. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, S.; Li, J.; Yuan, B.; Yang, K.; Ma, Y. Molecular details on the intermediate states of melittin action on a cell membrane. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2234–2241. [Google Scholar] [CrossRef]
- Chen, H.; Dong, Y.; Shi, F.; Li, F. Modulation of Membrane-Disruptive Activity of Melittin via N- and C-Terminal PEGylation Strategies. Bioconjug Chem. 2025, 36, 1438–1447. [Google Scholar] [CrossRef]
- Buonocore, C.; Giugliano, R.; Della Sala, G.; Palma Esposito, F.; Tedesco, P.; Folliero, V.; Galdiero, M.; Franci, G.; de Pascale, D. Evaluation of Antimicrobial Properties and Potential Applications of Pseudomonas gessardii M15 Rhamnolipids towards Multiresistant Staphylococcus aureus. Pharmaceutics 2023, 15, 700. [Google Scholar] [CrossRef]



| AVPs | Characteristics | |||
|---|---|---|---|---|
| Binding ΔG (kcal/mol) | Ki (hypothetical) (pM) | Domains involved | Residues | |
| AR-23 | –14.3 | 32 | DI, DII | 45 |
| RV-23 | –13.9 | 65 | DII | 43 |
| GENE | SEQUENCE (5′→3′) |
|---|---|
| E | F: GCAACATGGGTGGATTTGGT |
| R: GGGTCAGCACGTTTGTCATT | |
| GAPDH | F: CCTTTCATTGAGCTCCAT |
| R: CGTACATGGGAGCGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannella, C.; Chianese, A.; Giugliano, R.; Stefanizzi, V.; Monti, A.; Doti, N.; Palazzotto, E.; Bonura, F.; Giammanco, G.M.; Mastino, A.; et al. Structure–Function Insights into Frog Skin Peptides Reveal Potent Inhibition of West Nile Virus Entry. Int. J. Mol. Sci. 2025, 26, 10148. https://doi.org/10.3390/ijms262010148
Zannella C, Chianese A, Giugliano R, Stefanizzi V, Monti A, Doti N, Palazzotto E, Bonura F, Giammanco GM, Mastino A, et al. Structure–Function Insights into Frog Skin Peptides Reveal Potent Inhibition of West Nile Virus Entry. International Journal of Molecular Sciences. 2025; 26(20):10148. https://doi.org/10.3390/ijms262010148
Chicago/Turabian StyleZannella, Carla, Annalisa Chianese, Rosa Giugliano, Valeria Stefanizzi, Alessandra Monti, Nunzianna Doti, Emilia Palazzotto, Floriana Bonura, Giovanni M. Giammanco, Antonio Mastino, and et al. 2025. "Structure–Function Insights into Frog Skin Peptides Reveal Potent Inhibition of West Nile Virus Entry" International Journal of Molecular Sciences 26, no. 20: 10148. https://doi.org/10.3390/ijms262010148
APA StyleZannella, C., Chianese, A., Giugliano, R., Stefanizzi, V., Monti, A., Doti, N., Palazzotto, E., Bonura, F., Giammanco, G. M., Mastino, A., De Grazia, S., Marino-Merlo, F., Galdiero, M., & De Filippis, A. (2025). Structure–Function Insights into Frog Skin Peptides Reveal Potent Inhibition of West Nile Virus Entry. International Journal of Molecular Sciences, 26(20), 10148. https://doi.org/10.3390/ijms262010148














