The Microbiome as a Protagonist of Xylophagous Insects in Adaptation to Environmental Conditions and Climate Change
Abstract
1. Introduction
2. Diversity and Ecological–Physiological Characteristics of Xylophagous Insects
2.1. Overview of Major Groups of Xylophagous Insects and Their Ecological Roles
2.2. The Structure and Functions of the Digestive System of Xylophagous Insects: Adaptations to Wood Feeding
3. Microorganisms and Their Role in Symbiosis with Xylophagous Insects
3.1. Role of Microbiota in the Digestion of Xylophagous Insects
3.2. Role of Gut Microbiota in Immune System Maintenance and Protection Against Pathogens
3.3. The Role of the Microbiota in the Detoxification of Harmful Wood-Derived Compounds
| Function of Microbiota | Molecular Mechanisms | Example Genera of Microorganisms | Key Enzymes/Molecules | Notes/Role in Adaptation | Sources |
|---|---|---|---|---|---|
| Cellulose degradation | Hydrolysis of cellulose to glucose | Bacteroidetes, Firmicutes, and Proteobacteria (bacteria), Trichonympha and Pyrsonympha (protists) | Cellulases (endoglucanases, exoglucanases, β-glucosidases) | Provides a basic energy source for the host and microbiota | [43,44,48] |
| Hemicellulose degradation | Hydrolysis of hemicellulose to sugars | Bacteroides (bacteria) | Hemicellulases | Allows use of additional carbohydrate sources | [50] |
| Lignin degradation | Oxidation of lignin polymers | Streptomyces (bacteria), Phanerochaete (fungi) | Lignin peroxidases, manganese peroxidases, laccases (primarily fungal, often acting in wood) | Reduces wood toxicity, improves access to cellulose and hemicellulose | [54] |
| Redox balance in the gut | Utilization of hydrogen and CO2 produced during cellulose fermentation | Methanobrevibacter and Methanosphaera (archaea) | Methanogenesis (e.g., methyl-CoM reductase) | Maintains optimal fermentation conditions, prevents accumulation of toxic products | [48,58] |
| Atmospheric nitrogen fixation | Conversion of N2 into ammonia | Klebsiella, Pantoea, (bacteria) | Nitrogenase (e.g., protein NifH) | Provides nitrogen for synthesis of amino acids and proteins | [52,53] |
| Synthesis of vitamins and amino acids | Biosynthesis of B-group vitamins and essential amino acids | Enterobacter sp. and Pantoea sp. (bacteria) | Vitamin and amino acid-synthesizing enzymes | Compensates for nutrient deficiency in wood, promotes insect growth and development | [57] |
| Stimulation of immune response | Induction of antimicrobial peptide expression | Resident microbiota | Antimicrobial peptides | Maintains immune homeostasis, prevents pathogenic inflammation | [68] |
| Competitive exclusion of pathogens | Competition for nutrients and binding sites | Resident microbiota | - | Prevents colonization by pathogens, limits growth of pathogenic microorganisms | [72,73] |
| Production of antimicrobial substances | Synthesis of bacteriocins and organic acids | Bacillus, Pseudomonas (bacteria) | Bacteriocins and organic acids | Actively suppresses growth and colonization of pathogens | [74] |
| Immunomodulation and maintenance of gut homeostasis | Support of gut homeostasis, production of mucin and short-chain fatty acids | Resident microbiota | Mucin, short-chain fatty acids | Reduces inflammation, immunomodulation | [69,75,76] |
| Resin and terpene metabolism | Oxidation and modification of toxic compounds | Pseudomonas, Sphingomonas (bacteria), Trametes (fungi) | Oxidoreductases, laccases | Reduces toxicity of wood resins and terpenes, facilitates wood digestion | [75,79,80,81,82] |
| Phenol degradation | Oxidation of phenolic compounds | Phanerochaete, Pleurotus (fungi) | Lignin peroxidase, manganese peroxidase | Eliminates toxic phenols, improves habitat and digestion | [75,84] |
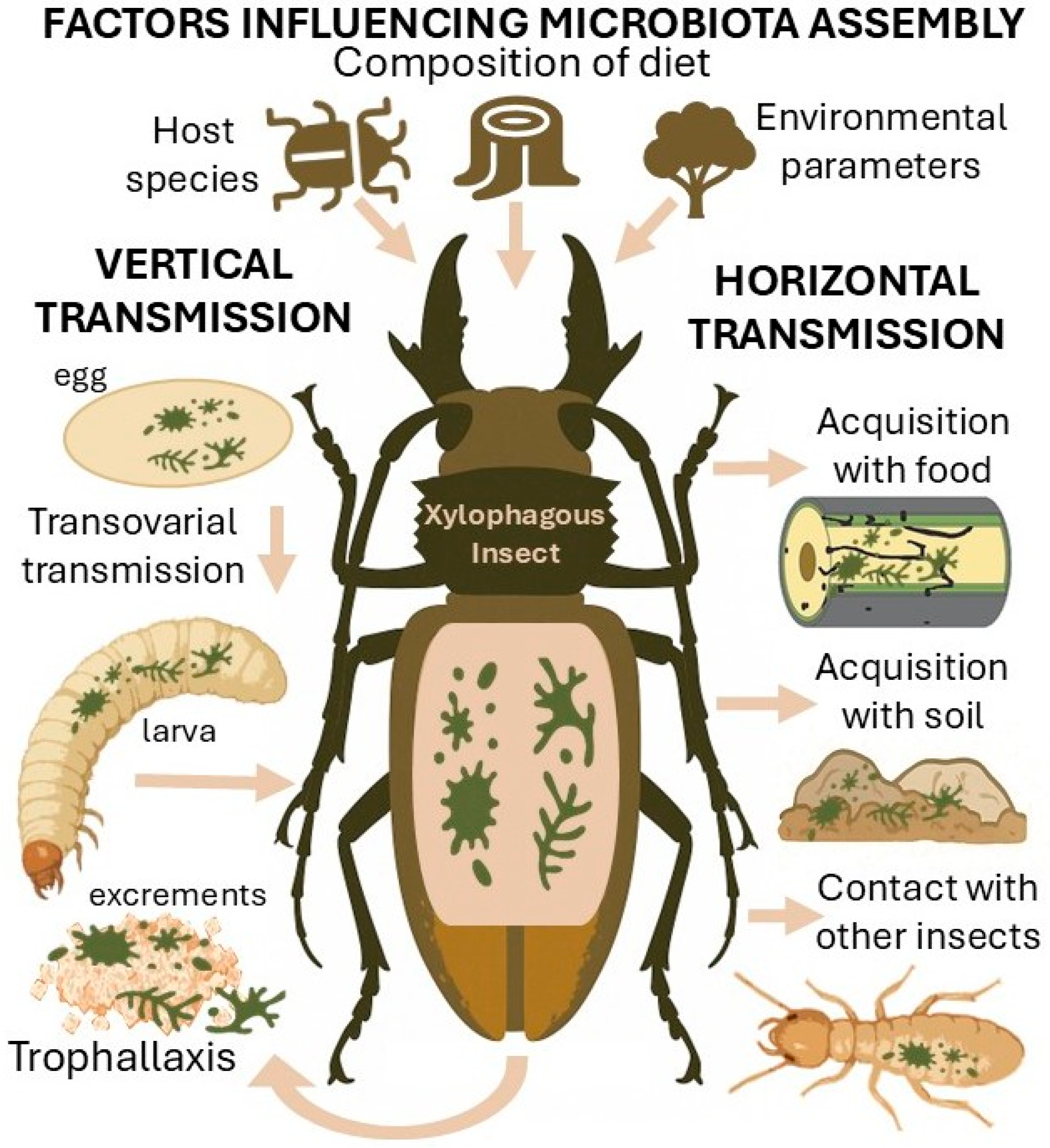
3.4. Transmission Pathways of the Microbiota and Factors Influencing Its Assembly
4. Impact of Environmental and Climate Changes on Microbiota and Adaptation of Xylophagous Insects
4.1. Impact of Rising Temperatures on the Microbiota and Adaptation of Xylophagous Insects
4.2. Impact of Humidity Changes (Droughts, Floods) on Microbiota and Adaptation of Xylophagous Insects
4.3. Impact of Environmental Pollution (Heavy Metals, Pesticides) on Microbiota and Adaptation of Xylophagous Insects
4.4. Adaptive Potential and Limitations of Xylophagous Insect Microbiome in Supporting Forest Ecosystem Resilience Against Climate Change
5. Ecological Consequences of Changes in Xylophagous Insect Microbiota Under Climate Change
5.1. Disruption of Wood Decomposition and Nutrient Cycling
5.2. Spread of Invasive Species
5.3. Alteration of Key Biotic Interactions
6. Future Research Directions and Practical Perspectives
6.1. Methodological Considerations and Integrative Approaches
6.2. Hypothesis-Driven Mechanistic Research
6.3. Practical Applications and Associated Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seibold, S.; Rammer, W.; Hothorn, T.; Seidl, R.; Ulyshen, M.D.; Lorz, J.; Cadotte, M.W.; Lindenmayer, D.B.; Adhikari, Y.P.; Aragón, R.; et al. The Contribution of Insects to Global Forest Deadwood Decomposition. Nature 2021, 597, 77–81. [Google Scholar] [CrossRef]
- Zou, J.-Y.; Cadotte, M.W.; Bässler, C.; Brandl, R.; Baldrian, P.; Borken, W.; Stengel, E.; Luo, Y.-H.; Müller, J.; Seibold, S. Wood Decomposition Is Increased by Insect Diversity, Selection Effects, and Interactions between Insects and Microbes. Ecology 2023, 12, e4184. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Wood Decomposition as Influenced by Invertebrates. Biol. Rev. 2016, 91, 70–85. [Google Scholar] [CrossRef]
- Harmon, M.E. The Role of Woody Detritus in Biogeochemical Cycles: Past, Present, and Future. Biogeochemistry 2021, 154, 349–369. [Google Scholar] [CrossRef]
- Pastorelli, R.; De Meo, I.; Lagomarsino, A. The Necrobiome of Deadwood: The Life after Death. Ecologies 2023, 4, 20–38. [Google Scholar] [CrossRef]
- Grove, S.J. Saproxylic Insect Ecology and the Sustainable Management of Forests. Annu. Rev. Ecol. Syst. 2002, 3, 1–23. [Google Scholar] [CrossRef]
- Gimmel, M.L.; Ferro, M.L. General Overview of Saproxylic Coleoptera. In Saproxylic Insects: Diversity, Ecology and Conservation; Ulyshen, M.D., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 51–128. ISBN 978-3-319-75937-1. [Google Scholar]
- Kajtoch, Ł.; Gronowska, M.; Plewa, R.; Kadej, M.; Smolis, A.; Jaworski, T.; Gutowski, J.M. A Review of Saproxylic Beetle Intra- and Interspecific Genetics: Current State of the Knowledge and Perspectives. Eur. Zool. J. 2022, 89, 481–501. [Google Scholar] [CrossRef]
- Jouquet, P.; Traoré, S.; Choosai, C.; Hartmann, C.; Bignell, D. Influence of Termites on Ecosystem Functioning. Ecosystem Services Provided by Termites. Eur. J. Soil Biol. 2011, 47, 215–222. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Saproxylic Diptera. In Saproxylic Insects: Diversity, Ecology and Conservation; Ulyshen, M.D., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 167–192. ISBN 978-3-319-75937-1. [Google Scholar]
- Mlynarek, J.J.; Taillefer, A.G.; Wheeler, T.A. Saproxylic Diptera Assemblages in a Temperate Deciduous Forest: Implications for Community Assembly. PeerJ 2018, 6, e6027. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, T.; Plewa, R.; Hilszczański, J.; Szczepkowski, A.; Horak, J. Saproxylic Moths Reveal Complex Within-Group and Group-Environment Patterns. J. Insect Conserv. 2016, 20, 677–690. [Google Scholar] [CrossRef]
- Beza-Beza, C.F.; Wiegmann, B.M.; Ware, J.A.; Petersen, M.; Gunter, N.; Cole, M.E.; Schwarz, M.; Bertone, M.A.; Young, D.; Mikaelyan, A. Chewing through Challenges: Exploring the Evolutionary Pathways to Wood-feeding in Insects. BioEssays 2024, 46, 2300241. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The Gut Microbiota of Insects–Diversity in Structure and Function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C.; Silva, C.P. Role of Microorganisms in Digestion and Nutrition. In Molecular Physiology and Evolution of Insect Digestive Systems; Terra, W.R., Ferreira, C., Silva, C.P., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 185–191. ISBN 978-3-031-39233-7. [Google Scholar]
- Douglas, A.E. Multiorganismal Insects: Diversity and Function of Resident Microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Mondal, S.; Somani, J.; Roy, S.; Babu, A.; Pandey, A.K. Insect Microbial Symbionts: Ecology, Interactions, and Biological Significance. Microorganisms 2023, 11, 2665. [Google Scholar] [CrossRef] [PubMed]
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest Disturbances under Climate Change. Nat. Clim. Change 2017, 7, 395–402. [Google Scholar] [CrossRef]
- Arias, P.; Bellouin, N.; Coppola, E.; Jones, R.; Krinner, G.; Marotzke, J.; Naik, V.; Palmer, M.; Plattner, G.-K.; Rogelj, J. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Technical Summary; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Hartmann, H.; Bastos, A.; Das, A.J.; Esquivel-Muelbert, A.; Hammond, W.M.; Martínez-Vilalta, J.; McDowell, N.G.; Powers, J.S.; Pugh, T.A.M.; Ruthrof, K.X.; et al. Climate Change Risks to Global Forest Health: Emergence of Unexpected Events of Elevated Tree Mortality Worldwide. Annu. Rev. Plant Biol. 2022, 73, 673–702. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Kane, J.M.; Anderegg, L.D.L. Consequences of Widespread Tree Mortality Triggered by Drought and Temperature Stress. Nat. Clim. Change 2013, 3, 30–36. [Google Scholar] [CrossRef]
- Jang, S.; Kikuchi, Y. Impact of the Insect Gut Microbiota on Ecology, Evolution, and Industry. Curr. Opin. Insect Sci. 2020, 41, 33–39. [Google Scholar] [CrossRef]
- Toriti, M.; Durand, A.; Fohrer, F. Atlas of the Most Common Xylophagous Insects. In Traces of Common Xylophagous Insects in Wood: Atlas of Identification-Western Europe; Toriti, M., Durand, A., Fohrer, F., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 25–208. ISBN 978-3-030-66391-9. [Google Scholar]
- Akbulut, S.; Stamps, W.T. Insect Vectors of the Pinewood Nematode: A Review of the Biology and Ecology of Monochamus Species. For. Pathol. 2011, 42, 89–99. [Google Scholar] [CrossRef]
- Six, D.L. Ecological and Evolutionary Determinants of Bark Beetle—Fungus Symbioses. Insects 2012, 3, 339–366. [Google Scholar] [CrossRef]
- Six, D.L. A Major Symbiont Shift Supports a Major Niche Shift in a Clade of Tree-killing Bark Beetles. Ecol. Entomol. 2020, 45, 190–201. [Google Scholar] [CrossRef]
- Demidko, D.A.; Demidko, N.N.; Mikhaylov, P.V.; Sultson, S.M. Biological Strategies of Invasive Bark Beetles and Borers Species. Insects 2021, 12, 367. [Google Scholar] [CrossRef]
- Shin, N.R.; Shin, S.; Okamura, Y.; Kirsch, R.; Lombard, V.; Svacha, P.; Denux, O.; Augustin, S.; Henrissat, B.; McKenna, D.D.; et al. Larvae of Longhorned Beetles (Coleoptera; Cerambycidae) Have Evolved a Diverse and Phylogenetically Conserved Array of Plant Cell Wall Degrading Enzymes. Syst. Entomol. 2021, 46, 784–797. [Google Scholar] [CrossRef]
- Rossa, R.; Goczał, J. Global Diversity and Distribution of Longhorn Beetles (Coleoptera: Cerambycidae). Eur. Zool. J. 2021, 88, 289–302. [Google Scholar] [CrossRef]
- Siegert, C.; Clay, N.; Pace, K.; Vissa, S.; Hofstetter, R.W.; Leverón, O.; Riggins, J.J. Bark Beetle-Driven Community and Biogeochemical Impacts in Forest Ecosystems: A Review. Ann. Entomol. Soc. Am. 2024, 117, 163–183. [Google Scholar] [CrossRef]
- Singh, V.V.; Naseer, A.; Mogilicherla, K.; Trubin, A.; Zabihi, K.; Roy, A.; Jakuš, R.; Erbilgin, N. Understanding Bark Beetle Outbreaks: Exploring the Impact of Changing Temperature Regimes, Droughts, Forest Structure, and Prospects for Future Forest Pest Management. Rev. Environ. Sci. Biotechnol. 2024, 23, 257–290. [Google Scholar] [CrossRef]
- Chouvenc, T.; Šobotník, J.; Engel, M.S.; Bourguignon, T. Termite Evolution: Mutualistic Associations, Key Innovations, and the Rise of Termitidae. Cell. Mol. Life Sci. 2021, 78, 2749–2769. [Google Scholar] [CrossRef]
- Hassan, B.; Morrell, J.J. Termite Testing Methods: A Global Review. J. Test. Eval. 2021, 49, 4607–4636. [Google Scholar] [CrossRef]
- Saputra, A.; Sari, V.; Ayu, F.; Bachry, S.; Susanti, A. Termites Attack on Residential Houses at Sialangmuggu, Tuah Madani, Pekanbaru. J. Eng. Sci. Technol. Manag. 2022, 2, 80–86. [Google Scholar] [CrossRef]
- Krivosheina, N.P. Macromycete Fruit Bodies as a Habitat for Dipterans (Insecta, Diptera). Entomol. Rev. 2008, 88, 778–792. [Google Scholar] [CrossRef]
- Terra, W.R.; Ferreira, C. Evolutionary Trends of Digestion and Absorption in the Major Insect Orders. Arthropod Struct. Dev. 2020, 56, 100931. [Google Scholar] [CrossRef]
- Krenn, H.W. Form and Function of Insect Mouthparts. In Insect Mouthparts: Form, Function, Development and Performance; Krenn, H.W., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 9–46. ISBN 978-3-030-29654-4. [Google Scholar]
- Watanabe, H.; Tokuda, G. Cellulolytic Systems in Insects. Annu. Rev. Entomol. 2010, 55, 609–632. [Google Scholar] [CrossRef] [PubMed]
- Terra, W.R.; Ferreira, C. Chapter 74-Digestive System. In Encyclopedia of Insects, 2nd ed.; Resh, V.H., Cardé, R.T., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 273–281. ISBN 978-0-12-374144-8. [Google Scholar]
- Serrão, J.E.; Santos, H.C.P. Chapter 4-Intestinal Tract. In Insect Anatomy; Moussian, B., Ed.; Academic Press: San Diego, CA, USA, 2025; pp. 145–180. ISBN 978-0-323-85619-5. [Google Scholar]
- Warnecke, F.; Luginbühl, P.; Ivanova, N.; Ghassemian, M.; Richardson, T.H.; Stege, J.T.; Cayouette, M.; McHardy, A.C.; Djordjevic, G.; Aboushadi, N.; et al. Metagenomic and Functional Analysis of Hindgut Microbiota of a Wood-Feeding Higher Termite. Nature 2007, 450, 560–565. [Google Scholar] [CrossRef]
- Liu, J.; Song, K.; Teng, H.; Zhang, B.; Li, W.; Xue, H.; Yang, X. Endogenous Cellulolytic Enzyme Systems in the Longhorn Beetle Mesosa Myops (Insecta: Coleoptera) Studied by Transcriptomic Analysis. Acta Biochim. Biophys. Sin. 2015, 47, 741–748. [Google Scholar] [CrossRef]
- Anand, A.A.P.; Vennison, S.J.; Sankar, S.G.; Prabhu, D.I.G.; Vasan, P.T.; Raghuraman, T.; Geoffrey, C.J.; Vendan, S.E. Isolation and Characterization of Bacteria from the Gut of Bombyx Mori That Degrade Cellulose, Xylan, Pectin and Starch and Their Impact on Digestion. J. Insect Sci. 2010, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, M.; Guerrero, R. The Holobiont Concept: The Case of Xylophagous Termites and Cockroaches. Symbiosis 2016, 68, 49–60. [Google Scholar] [CrossRef]
- Winder, R.S.; Macey, D.E.; Cortese, J. Dominant Bacteria Associated with Broods of Mountain Pine Beetle, Dendroctonus Ponderosae (Coleoptera: Curculionidae, Scolytinae). J. Entomol. Soc. Br. Columbia 2010, 107, 43–56. [Google Scholar]
- Grünwald, S.; Pilhofer, M.; Höll, W. Microbial Associations in Gut Systems of Wood- and Bark-Inhabiting Longhorned Beetles [Coleoptera: Cerambycidae]. Syst. Appl. Microbiol. 2010, 33, 25–34. [Google Scholar] [CrossRef]
- Chabanol, E.; Gendrin, M. Insects and Microbes: Best Friends from the Nursery. Curr. Opin. Insect Sci. 2024, 66, 101270. [Google Scholar] [CrossRef]
- Hongoh, Y. Diversity and Genomes of Uncultured Microbial Symbionts in the Termite Gut. Biosci. Biotechnol. Biochem. 2010, 74, 1145–1151. [Google Scholar] [CrossRef]
- Desai, M.S.; Brune, A. Bacteroidales Ectosymbionts of Gut Flagellates Shape the Nitrogen-Fixing Community in Dry-Wood Termites. ISME J. 2012, 6, 1302–1313. [Google Scholar] [CrossRef]
- Brune, A. Symbiotic Digestion of Lignocellulose in Termite Guts. Nat. Rev. Microbiol. 2014, 12, 168–180. [Google Scholar] [CrossRef]
- Scully, E.D.; Geib, S.M.; Carlson, J.E.; Tien, M.; McKenna, D.; Hoover, K. Functional Genomics and Microbiome Profiling of the Asian Longhorned Beetle (Anoplophora Glabripennis) Reveal Insights into the Digestive Physiology and Nutritional Ecology of Wood Feeding Beetles. BMC Genom. 2014, 15, 1096. [Google Scholar] [CrossRef]
- Ayayee, P.; Rosa, C.; Ferry, J.G.; Felton, G.; Saunders, M.; Hoover, K. Gut Microbes Contribute to Nitrogen Provisioning in a Wood-Feeding Cerambycid. Environ. Entomol. 2014, 43, 903–912. [Google Scholar] [CrossRef]
- Bar-Shmuel, N.; Behar, A.; Segoli, M. What Do We Know about Biological Nitrogen Fixation in Insects? Evidence and Implications for the Insect and the Ecosystem. Insect Sci. 2019, 27, 392–403. [Google Scholar] [CrossRef]
- Prewitt, L.; Kang, Y.; Kakumanu, M.L.; Williams, M. Fungal and Bacterial Community Succession Differs for Three Wood Types during Decay in a Forest Soil. Microb. Ecol. 2014, 68, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hong, K.; Ma, L.; Hao, X. Effect of Time Series on the Degradation of Lignin by Trametes Gibbosa: Products and Pathways. Int. J. Biol. Macromol. 2024, 281, 136236. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.; Bauer, E.; Strauss, A.S.; Vogel, H.; Marz, M.; Kaltenpoth, M. Vitamin Supplementation by Gut Symbionts Ensures Metabolic Homeostasis in an Insect Host. Proc. Biol. Sci. 2014, 281, 20141838. [Google Scholar] [CrossRef]
- Douglas, A.E. The B Vitamin Nutrition of Insects: The Contributions of Diet, Microbiome and Horizontally Acquired Genes. Curr. Opin. Insect Sci. 2017, 23, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Hackstein, J.H.P. (Endo)Symbiotic Methanogenic Archaea; Springe: Cham, Switzerland, 2018; Volume 19, ISBN 978-3-319-98836-8. [Google Scholar]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin Degradation: Microorganisms, Enzymes Involved, Genomes Analysis and Evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef]
- Liu, N.; Yan, X.; Zhang, M.; Xie, L.; Wang, Q.; Huang, Y.; Zhou, X.; Wang, S.; Zhou, Z. Microbiome of Fungus-Growing Termites: A New Reservoir for Lignocellulase Genes. Appl. Environ. Microbiol. 2011, 77, 48–56. [Google Scholar] [CrossRef]
- Moredo, N.; Lorenzo, M.; Domínguez, A.; Moldes, D.; Cameselle, C.; Sanroman, A. Enhanced Ligninolytic Enzyme Production and Degrading Capability of Phanerochaete Chrysosporium and Trametes Versicolor. World J. Microbiol. Biotechnol. 2003, 19, 665–669. [Google Scholar] [CrossRef]
- Konan, D.; Ndao, A.; Koffi, E.; Elkoun, S.; Robert, M.; Rodrigue, D.; Adjallé, K. Biodecomposition with Phanerochaete Chrysosporium: A Review. AIMS Microbiol. 2024, 10, 1068–1101. [Google Scholar] [CrossRef] [PubMed]
- Haridas, S.; Wang, Y.; Lim, L.; Massoumi Alamouti, S.; Jackman, S.; Docking, R.; Robertson, G.; Birol, I.; Bohlmann, J.; Breuil, C. The Genome and Transcriptome of the Pine Saprophyte Ophiostoma Piceae, and a Comparison with the Bark Beetle-Associated Pine Pathogen Grosmannia Clavigera. BMC Genom. 2013, 14, 373. [Google Scholar] [CrossRef] [PubMed]
- Trollip, C.; Carnegie, A.J.; Dinh, Q.; Kaur, J.; Smith, D.; Mann, R.; Rodoni, B.; Edwards, J. Ophiostomatoid Fungi Associated with Pine Bark Beetles and Infested Pines in South-Eastern Australia, Including Graphilbum Ipis-Grandicollis Sp. Nov. IMA Fungus 2021, 12, 24. [Google Scholar] [CrossRef]
- Yuki, M.; Kuwahara, H.; Shintani, M.; Izawa, K.; Sato, T.; Starns, D.; Hongoh, Y.; Ohkuma, M. Dominant Ectosymbiotic Bacteria of Cellulolytic Protists in the Termite Gut Also Have the Potential to Digest Lignocellulose. Environ. Microbiol. 2015, 17, 4942–4953. [Google Scholar] [CrossRef]
- Ohkuma, M. Symbioses of Flagellates and Prokaryotes in the Gut of Lower Termites. Trends Microbiol. 2008, 16, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Gile, G.H. Protist Symbionts of Termites: Diversity, Distribution, and Coevolution. Biol. Rev. 2024, 99, 622–652. [Google Scholar] [CrossRef]
- Zeng, T.; Jaffar, S.; Xu, Y.; Qi, Y. The Intestinal Immune Defense System in Insects. Int. J. Mol. Sci. 2022, 23, 15132. [Google Scholar] [CrossRef]
- Bai, S.; Yao, Z.; Raza, M.F.; Cai, Z.; Zhang, H. Regulatory Mechanisms of Microbial Homeostasis in Insect Gut. Insect Sci. 2021, 28, 286–301. [Google Scholar] [CrossRef]
- Khan, S.A.; Kojour, M.A.M.; Han, Y.S. Recent Trends in Insect Gut Immunity. Front. Immunol. 2023, 14, 1272143. [Google Scholar] [CrossRef]
- Franzenburg, S.; Walter, J.; Künzel, S.; Wang, J.; Baines, J.F.; Bosch, T.C.G.; Fraune, S. Distinct Antimicrobial Peptide Expression Determines Host Species-Specific Bacterial Associations. Proc. Natl. Acad. Sci. USA 2013, 110, E3730–E3738. [Google Scholar] [CrossRef] [PubMed]
- Stecher, B.; Hardt, W.-D. Mechanisms Controlling Pathogen Colonization of the Gut. Curr. Opin. Microbiol. 2011, 14, 82–91. [Google Scholar] [CrossRef]
- Schmidt, K.; Engel, P. Mechanisms Underlying Gut Microbiota–Host Interactions in Insects. J. Exp. Biol. 2021, 224, jeb207696. [Google Scholar] [CrossRef]
- Cotter, P.D.; Hill, C.; Ross, R.P. Bacteriocins: Developing Innate Immunity for Food. Nat. Rev. Microbiol. 2005, 3, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Shamjana, U.; Vasu, D.A.; Hembrom, P.S.; Nayak, K.; Grace, T. The Role of Insect Gut Microbiota in Host Fitness, Detoxification and Nutrient Supplementation. Antonie Leeuwenhoek 2024, 117, 71. [Google Scholar] [CrossRef]
- Mann, E.R.; Lam, Y.K.; Uhlig, H.H. Short-Chain Fatty Acids: Linking Diet, the Microbiome and Immunity. Nat. Rev. Immunol. 2024, 24, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Scharf, M.E. Challenges and Physiological Implications of Wood Feeding in Termites. Curr. Opin. Insect Sci. 2020, 41, 79–85. [Google Scholar] [CrossRef]
- Chamani, M.; Dadpour, M.; Dehghanian, Z.; Panahirad, S.; Chenari Bouket, A.; Oszako, T.; Kumar, S. From Digestion to Detoxification: Exploring Plant Metabolite Impacts on Insect Enzyme Systems for Enhanced Pest Control. Insects 2025, 16, 392. [Google Scholar] [CrossRef]
- Yu, S.J. Detoxification Mechanisms in Insects. In Encyclopedia of Entomology; Springer: Dordrecht, The Netherlands, 2008; pp. 1187–1201. ISBN 978-1-4020-6359-6. [Google Scholar]
- Boone, C.K.; Keefover-Ring, K.; Mapes, A.C.; Adams, A.S.; Bohlmann, J.; Raffa, K.F. Bacteria Associated with a Tree-Killing Insect Reduce Concentrations of Plant Defense Compounds. J. Chem. Ecol. 2013, 39, 1003–1006. [Google Scholar] [CrossRef]
- Adams, A.S.; Aylward, F.O.; Adams, S.M.; Erbilgin, N.; Aukema, B.H.; Currie, C.R.; Suen, G.; Raffa, K.F. Mountain Pine Beetles Colonizing Historical and Naïve Host Trees Are Associated with a Bacterial Community Highly Enriched in Genes Contributing to Terpene Metabolism. Appl. Environ. Microbiol. 2013, 79, 3468–3475. [Google Scholar] [CrossRef]
- Lu, K.; Song, Y.; Zeng, R. The Role of Cytochrome P450-Mediated Detoxification in Insect Adaptation to Xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef]
- Ge, S.-X.; Shi, F.-M.; Pei, J.-H.; Hou, Z.-H.; Zong, S.-X.; Ren, L.-L. Gut Bacteria Associated With Monochamus Saltuarius (Coleoptera: Cerambycidae) and Their Possible Roles in Host Plant Adaptations. Front. Microbiol. 2021, 12, 687211. [Google Scholar] [CrossRef]
- Szwajkowska-Michałek, L.; Stuper-Szablewska, K.; Krzyżaniak, M.; Łakomy, P. A Bioactive Compounds Profile Present in the Selected Wood Rot. Forests 2022, 13, 1242. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant Systems in Insects. Arch. Insect Biochem. Physiol. 1995, 29, 187–197. [Google Scholar] [CrossRef]
- Bright, M.; Bulgheresi, S. A Complex Journey: Transmission of Microbial Symbionts. Nat. Rev. Microbiol. 2010, 8, 218–230. [Google Scholar] [CrossRef]
- Geib, S.M.; Jimenez-Gasco, M.d.M.; Carlson, J.E.; Tien, M.; Jabbour, R.; Hoover, K. Microbial Community Profiling to Investigate Transmission of Bacteria Between Life Stages of the Wood-Boring Beetle, Anoplophora Glabripennis. Microb. Ecol. 2009, 58, 199–211. [Google Scholar] [CrossRef]
- Weiss, M.R. Defecation Behavior and Ecology of Insects. Annu. Rev. Entomol. 2006, 51, 635–661. [Google Scholar] [CrossRef] [PubMed]
- Brune, A.; Dietrich, C. The Gut Microbiota of Termites: Digesting the Diversity in the Light of Ecology and Evolution. Annu. Rev. Microbiol. 2015, 69, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Chen, S. Biological Pretreatment of Biomass in Wood-Feeding Termites. In Biological Conversion of Biomass for Fuels and Chemicals: Explorations from Natural Utilization Systems; Royal Society of Chemistry Publishing: Cambridge, UK, 2013; pp. 177–194. [Google Scholar]
- Biedermann, P.H.W.; Vega, F.E. Ecology and Evolution of Insect-Fungus Mutualisms. Annu. Rev. Entomol. 2020, 65, 431–455. [Google Scholar] [CrossRef]
- Rosenberg, E.; Zilber-Rosenberg, I. Reconstitution and Transmission of Gut Microbiomes and Their Genes between Generations. Microorganisms 2022, 10, 70. [Google Scholar] [CrossRef]
- Skelton, J.; Johnson, A.J.; Jusino, M.A.; Bateman, C.C.; Li, Y.; Hulcr, J. A Selective Fungal Transport Organ (Mycangium) Maintains Coarse Phylogenetic Congruence between Fungus-Farming Ambrosia Beetles and Their Symbionts. Proc. R. Soc. B 2019, 286, 20182127. [Google Scholar] [CrossRef]
- Shapira, M. Gut Microbiotas and Host Evolution: Scaling Up Symbiosis. Trends Ecol. Evol. 2016, 31, 539–549. [Google Scholar] [CrossRef]
- Colman, D.R.; Toolson, E.C.; Takacs-Vesbach, C.D. Do Diet and Taxonomy Influence Insect Gut Bacterial Communities? Mol. Ecol. 2012, 21, 5124–5137. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-H.; Roh, S.W.; Whon, T.W.; Jung, M.-J.; Kim, M.-S.; Park, D.-S.; Yoon, C.; Nam, Y.-D.; Kim, Y.-J.; Choi, J.-H.; et al. Insect Gut Bacterial Diversity Determined by Environmental Habitat, Diet, Developmental Stage, and Phylogeny of Host. Appl. Environ. Microbiol. 2014, 80, 5254–5264. [Google Scholar] [CrossRef]
- Sontowski, R.; van Dam, N.M. Functional Variation in Dipteran Gut Bacterial Communities in Relation to Their Diet, Life Cycle Stage and Habitat. Insects 2020, 11, 543. [Google Scholar] [CrossRef]
- Baklanova, V.; Kuprin, A.; Baklanov, I.; Kumeiko, V. Isolation and Characterization of Cultivable Microbes from the Gut of Zophobas Atratus (Coleoptera: Tenebrionidae) Larvae Reared on Two Types of Artificial Diets. Biology 2025, 14, 824. [Google Scholar] [CrossRef] [PubMed]
- Combrink, L.; Humphreys, I.R.; Washburn, Q.; Arnold, H.K.; Stagaman, K.; Kasschau, K.D.; Jolles, A.E.; Beechler, B.R.; Sharpton, T.J. Best Practice for Wildlife Gut Microbiome Research: A Comprehensive Review of Methodology for 16S rRNA Gene Investigations. Front. Microbiol. 2023, 14, 1092216. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, W.R.L.; Trugman, A.T.; Badgley, G.; Anderson, C.M.; Bartuska, A.; Ciais, P.; Cullenward, D.; Field, C.B.; Freeman, J.; Goetz, S.J.; et al. Climate-Driven Risks to the Climate Mitigation Potential of Forests. Science 2020, 368, aaz7005. [Google Scholar] [CrossRef]
- Baldrian, P.; López-Mondéjar, R.; Kohout, P. Forest Microbiome and Global Change. Nat. Rev. Microbiol. 2023, 21, 487–501. [Google Scholar] [CrossRef]
- Onyango, G.M.; Bialosuknia, M.S.; Payne, F.A.; Mathias, N.; Ciota, T.A.; Kramer, D.L. Increase in Temperature Enriches Heat Tolerant Taxa in Aedes Aegypti Midguts. Sci. Rep. 2020, 10, 19135. [Google Scholar] [CrossRef]
- Shan, H.-W.; Xia, X.-J.; Feng, Y.-L.; Wu, W.; Li, H.-J.; Sun, Z.-T.; Li, J.-M.; Chen, J.-P. The Plant-Sucking Insect Selects Assembly of the Gut Microbiota from Environment to Enhance Host Reproduction. NPJ Biofilms Microbiomes 2024, 10, 64. [Google Scholar] [CrossRef]
- Harvey, J.A.; Heinen, R.; Gols, R.; Thakur, M.P. Climate Change-mediated Temperature Extremes and Insects: From Outbreaks to Breakdowns. Glob. Change Biol. 2020, 26, 6685–6701. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Bracalini, M.; Balacenoiu, F.; Panzavolta, T. Forest Health under Climate Change: Impact of Insect Pests. Iforest-Biogeosci. For. 2024, 17, 295. [Google Scholar] [CrossRef]
- Kaitera, J.; Piispanen, J.; Bergmann, U. Terpene and Resin Acid Contents in Scots Pine Stem Lesions Colonized by the Rust Fungus Cronartium Pini. For. Pathol. 2021, 51, e12700. [Google Scholar] [CrossRef]
- Kivimäenpää, M.; Virjamo, V.; Nissinen, K.; Pikkarainen, L.; Ghimire, R.P.; Julkunen-Tiitto, R.; Peltola, H. Responses of Needle Terpene Concentrations and Characteristics of Resin Canals to Different Warming Treatments in Scots Pine and Norway Spruce Seedlings Grown in a Field Experiment. Can. J. For. Res. 2025, 55, 1–9. [Google Scholar] [CrossRef]
- Subedi, B.; Poudel, A.; Aryal, S. The Impact of Climate Change on Insect Pest Biology and Ecology: Implications for Pest Management Strategies, Crop Production, and Food Security. J. Agric. Food Res. 2023, 14, 100733. [Google Scholar] [CrossRef]
- Pimentel, C.S.; Firmino, P.N.; Ayres, M.P. Interactions between Pinewood Nematodes and the Fungal Community of Pine Trees. Fungal Ecol. 2021, 51, 101046. [Google Scholar] [CrossRef]
- Zhang, B.; Ma, Y.; Duan, W.; Fan, Q.; Sun, J. Pinewood Nematode Induced Changes in the Assembly Process of Gallery Microbiomes Benefit Its Vector Beetle’s Development. Microbiol. Spectr. 2024, 12, e01412-24. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; Hall, E.K. A Trait-Based Framework for Predicting When and Where Microbial Adaptation to Climate Change Will Affect Ecosystem Functioning. Biogeochemistry 2012, 109, 35–47. [Google Scholar] [CrossRef]
- Ge, S.-X.; Li, T.-F.; Ren, L.-L.; Zong, S.-X. Host-Plant Adaptation in Xylophagous Insect-Microbiome Systems: Contributionsof Longicorns and Gut Symbionts Revealed by Parallel Metatranscriptome. iScience 2023, 26, 106680. [Google Scholar] [CrossRef]
- Lawhorn, K.A.; Yanoviak, S.P. Variation in Larval Thermal Tolerance of Three Saproxylic Beetle Species. Environ. Entomol. 2022, 51, 1218–1223. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Diehl, S.V.; Jeremic, D. Termites and Flooding Affect Microbial Communities in Decomposing Wood. Int. Biodeterior. Biodegrad. 2016, 115, 83–89. [Google Scholar] [CrossRef]
- Weiss, F.; von Wehrden, H.; Linde, A. Long-term Drought Triggers Severe Declines in Carabid Beetles in a Temperate Forest. Ecography 2024, 2024, e07020. [Google Scholar] [CrossRef]
- Avtzis, D.N.; Lakatos, F. Bark and Wood Boring Insects—Past, Present, and the Future Knowledge We Need. Insects 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Ashraf, M.Z.; Modlinger, R.; Synek, J.; Schlyter, F.; Roy, A. Unravelling the Gut Bacteriome of Ips (Coleoptera: Curculionidae: Scolytinae): Identifying Core Bacterial Assemblage and Their Ecological Relevance. Sci. Rep. 2020, 10, 18572. [Google Scholar] [CrossRef]
- Gupta, S.; Chakraborty, A.; Roy, A. Prospects for Deploying Microbes against Tree-Killing Beetles (Coleoptera) in Anthropocene. Front. For. Glob. Change 2023, 6, 1182834. [Google Scholar] [CrossRef]
- Potterf, M.; Frühbrodt, T.; Thom, D.; Lemme, H.; Hahn, A.; Seidl, R. Hotter Drought Increases Population Levels and Accelerates Phenology of the European Spruce Bark Beetle Ips Typographus. For. Ecol. Manag. 2025, 585, 122615. [Google Scholar] [CrossRef]
- Sallé, A.; Nageleisen, L.-M.; Lieutier, F. Bark and Wood Boring Insects Involved in Oak Declines in Europe: Current Knowledge and Future Prospects in a Context of Climate Change. For. Ecol. Manag. 2014, 328, 79–93. [Google Scholar] [CrossRef]
- Takov, D.; Pilarska, D.; Linde, A.; Barta, M. Infectious and Parasitic Diseases of Phytophagous Insect Pests in the Context of Extreme Environmental Conditions. Cent. Eur. For. J. 2021, 67, 72–84. [Google Scholar] [CrossRef]
- Hoback, W.W.; Stanley, D.W. Insects in Hypoxia. J. Insect Physiol. 2001, 47, 533–542. [Google Scholar] [CrossRef]
- Netherer, S.; Kandasamy, D.; Jirosová, A.; Kalinová, B.; Schebeck, M.; Schlyter, F. Interactions among Norway Spruce, the Bark Beetle Ips Typographus and Its Fungal Symbionts in Times of Drought. J. Pest Sci. 2021, 94, 591–614. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Fang, J.; Du, H.; Zhang, S.; Liu, F.; Zhang, Z.; Kong, X. Performance of Two Ips Bark Beetles and Their Associated Pathogenic Fungi on Hosts Reflects a Species-Specific Association in the Beetle-Fungus Complex. Front. Plant Sci. 2022, 13, 1029526. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Tan, M.; Zhang, A.; Jiang, D. The Exposure Risk of Heavy Metals to Insect Pests and Their Impact on Pests Occurrence and Cross-Tolerance to Insecticides: A Review. Sci. Total Environ. 2024, 916, 170274. [Google Scholar] [CrossRef]
- Jiang, D.; Tan, M.; Guo, Q.; Yan, S. Transfer of Heavy Metal along Food Chain: A Mini-review on Insect Susceptibility to Entomopathogenic Microorganisms under Heavy Metal Stress. Pest Manag. Sci. 2021, 77, 1115–1120. [Google Scholar] [CrossRef]
- Borowska, J.; Pyza, E. Effects of Heavy Metals on Insect Immunocompetent Cells. J. Insect Physiol. 2011, 57, 760–770. [Google Scholar] [CrossRef]
- Azam, I.; Afsheen, S.; Zia, A.; Javed, M.; Saeed, R.; Sarwar, M.K.; Munir, B. Evaluating Insects as Bioindicators of Heavy Metal Contamination and Accumulation near Industrial Area of Gujrat, Pakistan. BioMed Res. Int. 2015, 2015, 942751. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.; Farag, S.; Kandil, K.; Moawad, H. Tolerance and Uptake of Heavy Metals by Pseudomonads. Process Biochem. 2005, 40, 955–961. [Google Scholar] [CrossRef]
- O’Brien, S.; Hodgson, D.J.; Buckling, A. Social Evolution of Toxic Metal Bioremediation in Pseudomonas Aeruginosa. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140858. [Google Scholar] [CrossRef]
- Jaffar, S.; Ahmad, S.; Lu, Y. Contribution of Insect Gut Microbiota and Their Associated Enzymes in Insect Physiology and Biodegradation of Pesticides. Front. Microbiol. 2022, 13, 979383. [Google Scholar] [CrossRef] [PubMed]
- James, R.R.; Xu, J. Mechanisms by Which Pesticides Affect Insect Immunity. J. Invertebr. Pathol. 2012, 109, 175–182. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Khan, M.M.; Bamisile, B.S.; Hafeez, M.; Qasim, M.; Rasheed, M.T.; Rasheed, M.A.; Ahmad, S.; Shahid, M.I.; Xu, Y. Role of Insect Gut Microbiota in Pesticide Degradation: A Review. Front. Microbiol. 2022, 13, 870462. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Xu, L. The Pivotal Roles of Gut Microbiota in Insect Plant Interactions for Sustainable Pest Management. NPJ Biofilms Microbiomes 2023, 9, 66. [Google Scholar] [CrossRef]
- Werren, J.H. Symbionts Provide Pesticide Detoxification. Proc. Natl. Acad. Sci. USA 2012, 109, 8364–8365. [Google Scholar] [CrossRef]
- Vacher, C.; Castagneyrol, B.; Jousselin, E.; Schimann, H. Trees and Insects Have Microbiomes: Consequences for Forest Health and Management. Curr. For. Rep. 2021, 7, 81–96. [Google Scholar] [CrossRef]
- Wiedenbeck, J.; Cohan, F.M. Origins of Bacterial Diversity through Horizontal Genetic Transfer and Adaptation to New Ecological Niches. FEMS Microbiol. Rev. 2011, 35, 957–976. [Google Scholar] [CrossRef] [PubMed]
- Husnik, F.; McCutcheon, J.P. Functional Horizontal Gene Transfer from Bacteria to Eukaryotes. Nat. Rev. Microbiol. 2018, 16, 67–79. [Google Scholar] [CrossRef]
- Arnold, B.J.; Huang, I.-T.; Hanage, W.P. Horizontal Gene Transfer and Adaptive Evolution in Bacteria. Nat. Rev. Microbiol. 2022, 20, 206–218. [Google Scholar] [CrossRef]
- Oliver, K.M.; Martinez, A.J. How Resident Microbes Modulate Ecologically-Important Traits of Insects. Curr. Opin. Insect Sci. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Catania, F.; Krohs, U.; Chittò, M.; Ferro, D.; Ferro, K.; Lepennetier, G.; Görtz, H.-D.; Schreiber, R.S.; Kurtz, J.; Gadau, J. The Hologenome Concept: We Need to Incorporate Function. Theory Biosci. 2017, 136, 89–98. [Google Scholar] [CrossRef]
- Faure, D.; Simon, J.-C.; Heulin, T. Holobiont: A Conceptual Framework to Explore the Eco-Evolutionary and Functional Implications of Host?Microbiota Interactions in All Ecosystems. New Phytol. 2018, 218, 1321–1324. [Google Scholar] [CrossRef]
- Chomel, M.; Guittonny-Larchevêque, M.; Fernandez, C.; Gallet, C.; DesRochers, A.; Paré, D.; Jackson, B.G.; Baldy, V. Plant Secondary Metabolites: A Key Driver of Litter Decomposition and Soil Nutrient Cycling. J. Ecol. 2016, 104, 1527–1541. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Moser, W.K.; Nowakowska, J.A.; Oszako, T.; Benia, F.; Belbahri, L. The Threat of Pests and Pathogens and the Potential for Biological Control in Forest Ecosystems. Forests 2021, 12, 1579. [Google Scholar] [CrossRef]
- Hulcr, J.; Stelinski, L.L. The Ambrosia Symbiosis: From Evolutionary Ecology to Practical Management. Annu. Rev. Entomol. 2017, 62, 285–303. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.B.; Leonardi, G.R.; Gusella, G.; Garzia, G.T.; Biondi, A.; Aiello, D.; Polizzi, G.; Gugliuzzo, A. The Invasive Ambrosia Beetle Xylosandrus Compactus as a Vector of the Novel Fungal Pathogen Thyridium Lauri. CABI Agric. Biosci. 2025, 6, 0051. [Google Scholar] [CrossRef]
- Costanzo, M.B.; Vitale, A.; Biondi, A.; Polizzi, G.; Gugliuzzo, A. Exploring the Potential of Synthetic and Biological Fungicides for Managing the Fungus-Farming Ambrosia Beetle Xylosandrus Compactus. PLoS ONE 2025, 20, e0329063. [Google Scholar] [CrossRef]
- RKirkendall, L.; Faccoli, M. Bark Beetles and Pinhole Borers (Curculionidae, Scolytinae, Platypodinae) Alien to Europe. ZooKeys 2010, 56, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Rassati, D.; Marini, L.; Malacrinò, A. Acquisition of Fungi from the Environment Modifies Ambrosia Beetle Mycobiome during Invasion. PeerJ 2019, 7, e8103. [Google Scholar] [CrossRef]
- Cerasa, G.; Guarino, S.; Moliterno, A.A.C.; Matranga, G.; Laschi, A.; Togni, M.; Peri, E. First Record of the Invasive Powderpost Beetle Lyctus Africanus Lesne (Coleoptera: Bostrichidae) Infesting Wooden Furniture in Italy. Int. J. Trop. Insect Sci. 2025, 45, 1121–1134. [Google Scholar] [CrossRef]
- Janson, E.M.; IIIStireman, J.O.; Singer, M.S.; Abbot, P. Phytophagous Insect–Microbe Mutualisms and Adaptive Evolutionary Diversification. Evolution 2008, 62, 997–1012. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, A.; Härdtle, W. Forest Ecosystems: A Functional and Biodiversity Perspective. In Perspectives for Biodiversity and Ecosystems; Hobohm, C., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 383–405. ISBN 978-3-030-57710-0. [Google Scholar]
- Muñoz-Benavent, M.; Pérez-Cobas, A.E.; García-Ferris, C.; Moya, A.; Latorre, A. Insects’ Potential: Understanding the Functional Role of Their Gut Microbiome. J. Pharm. Biomed. Anal. 2021, 194, 113787. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chang, L.; Xu, K.; Zhang, S.; Gao, F.; Fan, Y. Research Progresses on the Function and Detection Methods of Insect Gut Microbes. Microorganisms 2023, 11, 1208. [Google Scholar] [CrossRef]
- Whon, T.W.; Shin, N.-R.; Kim, J.Y.; Roh, S.W. Omics in Gut Microbiome Analysis. J. Microbiol. 2021, 59, 292–297. [Google Scholar] [CrossRef]
- Bei, Q.; Moser, G.; Wu, X.; Müller, C.; Liesack, W. Metatranscriptomics Reveals Climate Change Effects on the Rhizosphere Microbiomes in European Grassland. Soil Biol. Biochem. 2019, 138, 107604. [Google Scholar] [CrossRef]
- Nesatyy, V.J.; Suter, M.J.-F. Proteomics for the Analysis of Environmental Stress Responses in Organisms. Environ. Sci. Technol. 2007, 41, 6891–6900. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Rivas-Ubach, A. Ecological Metabolomics: Overview of Current Developments and Future Challenges. Chemoecology 2011, 21, 191–225. [Google Scholar] [CrossRef]
- Poretsky, R.; Rodriguez-R, L.M.; Luo, C.; Tsementzi, D.; Konstantinidis, K.T. Strengths and Limitations of 16S rRNA Gene Amplicon Sequencing in Revealing Temporal Microbial Community Dynamics. PLoS ONE 2014, 9, e93827. [Google Scholar] [CrossRef] [PubMed]
- Bharti, R.; Grimm, D.G. Current Challenges and Best-Practice Protocols for Microbiome Analysis. Brief. Bioinform. 2021, 22, 178–193. [Google Scholar] [CrossRef]
- Shakya, M.; Lo, C.-C.; Chain, P.S.G. Advances and Challenges in Metatranscriptomic Analysis. Front. Genet. 2019, 10, 904. [Google Scholar] [CrossRef]
- Shi, W.; Syrenne, R.; Sun, J.-Z.; Yuan, J.S. Molecular Approaches to Study the Insect Gut Symbiotic Microbiota at the ‘Omics’ Age. Insect Sci. 2010, 17, 199–219. [Google Scholar] [CrossRef]
- Pelletier, F.; Garant, D.; Hendry, A.P. Eco-Evolutionary Dynamics. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1483–1489. [Google Scholar] [CrossRef]
- Reznick, D.N.; Bassar, R.D.; Handelsman, C.A.; Ghalambor, C.K.; Arendt, J.; Coulson, T.; Potter, T.; Ruell, E.W.; Torres-Dowdall, J.; Bentzen, P.; et al. Eco-Evolutionary Feedbacks Predict the Time Course of Rapid Life-History Evolution. Am. Nat. 2019, 194, 671–692. [Google Scholar] [CrossRef]
- Stencel, A.; Wloch-Salamon, D.M. Some Theoretical Insights into the Hologenome Theory of Evolution and the Role of Microbes in Speciation. Theory Biosci. 2018, 137, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Huitzil, S.; Huepe, C.; Aldana, M.; Frank, A. The Missing Link: How the Holobiont Concept Provides a Genetic Framework for Rapid Evolution and the Inheritance of Acquired Characteristics. Front. Ecol. Evol. 2023, 11, 1279938. [Google Scholar] [CrossRef]
- Auger, L.; Tegtmeier, D.; Caccia, S.; Klammsteiner, T.; De Smet, J. BugBook: How to Explore and Exploit the Insect-Associated Microbiome. J. Insects Food Feed. 2025, 1, 1–35. [Google Scholar] [CrossRef]
- Carpentier, J.; Abenaim, L.; Luttenschlager, H.; Dessauvages, K.; Liu, Y.; Samoah, P.; Francis, F.; Caparros Megido, R. Microorganism Contribution to Mass-Reared Edible Insects: Opportunities and Challenges. Insects 2024, 15, 611. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, J.S.T.; Bauer, E.; Okude, G.; Fukatsu, T.; Kaltenpoth, M.; Engl, T. Cuticle Supplementation and Nitrogen Recycling by a Dual Bacterial Symbiosis in a Family of Xylophagous Beetles. ISME J. 2023, 17, 1029–1039. [Google Scholar] [CrossRef]


| Group | Taxa | Feeding and Characteristics | Ecological Role | Sources |
|---|---|---|---|---|
| Coleoptera | Curculionidae: Scolytinae, Cerambycidae, Ptinidae: Anobiinae, Buprestidae | Various stages of wood: phloem, sapwood, dense wood. Larvae create tunnels in wood; adults sometimes feed on leaves or bark. | Decomposition of dead and weakened wood, creating habitats for other organisms, influence carbon cycle; some species are forest and structural pests. | [6,7,8,24,25,26,27,28,29,30,31] |
| Blattodea | Various genera within Blattodea | Social insects; feed on dry or decayed wood, soil detritus. Build large nests. | Efficient wood decomposition, improving soil structure, bio-enrichment, important in food chains. | [9,32,33,34] |
| Diptera | Mycetophilidae, Syrphidae, Stratiomyidae | Larvae feed on fungal mycelium and decomposing organic matter. | Additional decomposition of wood and organic matter, participation in trophic networks, often underestimated wood decomposers. | [10,11,35] |
| Lepidoptera | Cossidae | Specialized larvae bore deep tunnels inside trunks and branches of living and dead trees. | Creation of cavities and acceleration of wood decomposition, weakening trees and increasing susceptibility to diseases. | [12] |
| Environmental Factor | Effects on Xylophagous Insect Microbiota | Impact on Insect Adaptation and Condition | Consequences for Forest Ecosystems |
|---|---|---|---|
| Temperature rise | Shift in dominant taxa, increase in thermophiles, decrease in microbial diversity; changes in enzymatic functions | Reduced enzymatic activity, immune suppression | Slowed wood decomposition, accumulation of dead organic matter, decreased biodiversity |
| Drought | Proliferation of pathogens, microbial community imbalance, weakening of symbionts | Increased susceptibility to infections, reduced viability | Pest outbreaks, forest degradation, reduced soil fertility |
| Flooding | Mortality of aerobes, increase in anaerobes, restructuring of microbial community | Decreased digestive activity, reduced survival | Disruption of decomposition, enhanced wood decay, spread of diseases |
| Pollution (heavy metals, pesticides) | Reduced diversity, increase in resistant forms, suppression of beneficial microbes | Decline in detoxification and nitrogen fixation, increased susceptibility to pathogens, dysbiosis | Decline in forest health, intensified epiphytotics, altered soil composition, bioremediation |
| Changes in flora and fauna | Emergence of new associations, horizontal gene transfer among microbes | Microbiota plasticity, enhanced invasiveness, adaptation to new resources | Spread of invasive species, disruption of biocenoses, emergence of new pathogens |
| Disruption of symbiotic relationships | Reduction in synergy among bacteria, fungi, and protists | Impaired digestion and immunity, increased vulnerability to parasites | Decreased decomposition efficiency, impaired ecosystem recovery after disturbances/fires |
| Combined influence | Synergistic dysbiosis, loss of most key microbiota functions | Severe impairment of adaptive mechanisms, disruption of metabolic and immune processes | Catastrophic biodiversity loss, collapse of carbon and nutrient cycling |
| Research Focus Area | Key Evidence from Primary Studies (Examples) | Identified Gaps and Proposed Experimental Approaches | Sources |
|---|---|---|---|
| Thermal Adaptation | In bark beetles (Dendroctonus spp.), a shift in gut bacterial communities under elevated temperature was correlated with improved fitness on a novel host tree. In mosquito models, heat-induced microbiome shifts (e.g., enrichment in Bacillus) were linked to altered host thermal tolerance. | Gap: causal evidence that microbiome shifts directly mediate insect host thermotolerance is scarce. Approach: perform microbiome transplantation experiments between heat-exposed and control insects, followed by fitness and physiological assays (e.g., critical thermal maximum, CTmax). | [102,113] |
| Drought and Humidity Stress | In termites, flooding events led to a restructuring of gut microbial communities and a significant loss of cellulolytic activity. For bark beetles (Ips typographus), drought stress in host trees is associated with an enrichment of xerotolerant fungal symbionts in beetle galleries. | Gap: understanding of how gut microbiota helps insects maintain water balance and digest drier wood is limited. Approach: manipulate humidity in mesocosms, track microbiome dynamics via metatranscriptomics, and measure insect water retention and digestive efficiency. | [115,124] |
| Pollution Detoxification | Gut bacteria of wood-feeding insects (e.g., Pseudomonas) demonstrate capabilities for heavy metal bioremediation in vitro. Insecticide-degrading genes have been identified in the gut metagenomes of various pest insects. | Gap: the in vivo contribution of these microbes to host detoxification and survival under chronic pollution is not quantified. Approach: use gnotobiotic insects colonized with specific degradative bacteria and challenge them with pollutants to measure survival, detoxification metabolite profiles, and microbiome stability. | [130,131,134] |
| Microbiome Transmission and Plasticity | Mycangia in ambrosia beetles maintain specific fungal symbionts across generations, yet allow for environmental acquisition. Horizontal gene transfer among gut bacteria is a proposed mechanism for rapid adaptation in longicorn beetles. | Gap: the relative contribution of vertical vs. horizontal transmission to adaptive potential under climate change is unknown for most taxa. Approach: conduct multi-generational selection experiments in controlled environments, tracking microbiome heritability and adaptive trait gain using metagenomic sequencing. | [93,113] |
| Host-Microbiome Coevolution | In cerambycid beetles, metatranscriptomes reveal coordinated expression of host and microbial genes in response to different host plants. Dual bacterial symbionts in a xylophagous beetle family show evolutionary stability and complementary nutritional roles. | Gap: lack of predictive models on how host genetics and microbiome composition interact to determine resilience. Approach: integrate genome-wide association studies (GWASs) of the host with microbiome profiling across populations under environmental clines to identify holobiont adaptation signatures. | [113,170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuprin, A.; Baklanova, V. The Microbiome as a Protagonist of Xylophagous Insects in Adaptation to Environmental Conditions and Climate Change. Int. J. Mol. Sci. 2025, 26, 10143. https://doi.org/10.3390/ijms262010143
Kuprin A, Baklanova V. The Microbiome as a Protagonist of Xylophagous Insects in Adaptation to Environmental Conditions and Climate Change. International Journal of Molecular Sciences. 2025; 26(20):10143. https://doi.org/10.3390/ijms262010143
Chicago/Turabian StyleKuprin, Alexander, and Vladislava Baklanova. 2025. "The Microbiome as a Protagonist of Xylophagous Insects in Adaptation to Environmental Conditions and Climate Change" International Journal of Molecular Sciences 26, no. 20: 10143. https://doi.org/10.3390/ijms262010143
APA StyleKuprin, A., & Baklanova, V. (2025). The Microbiome as a Protagonist of Xylophagous Insects in Adaptation to Environmental Conditions and Climate Change. International Journal of Molecular Sciences, 26(20), 10143. https://doi.org/10.3390/ijms262010143







