Cytokinins Are Age- and Injury-Responsive Molecules That Regulate Skeletal Myogenesis
Abstract
1. Introduction
2. Results
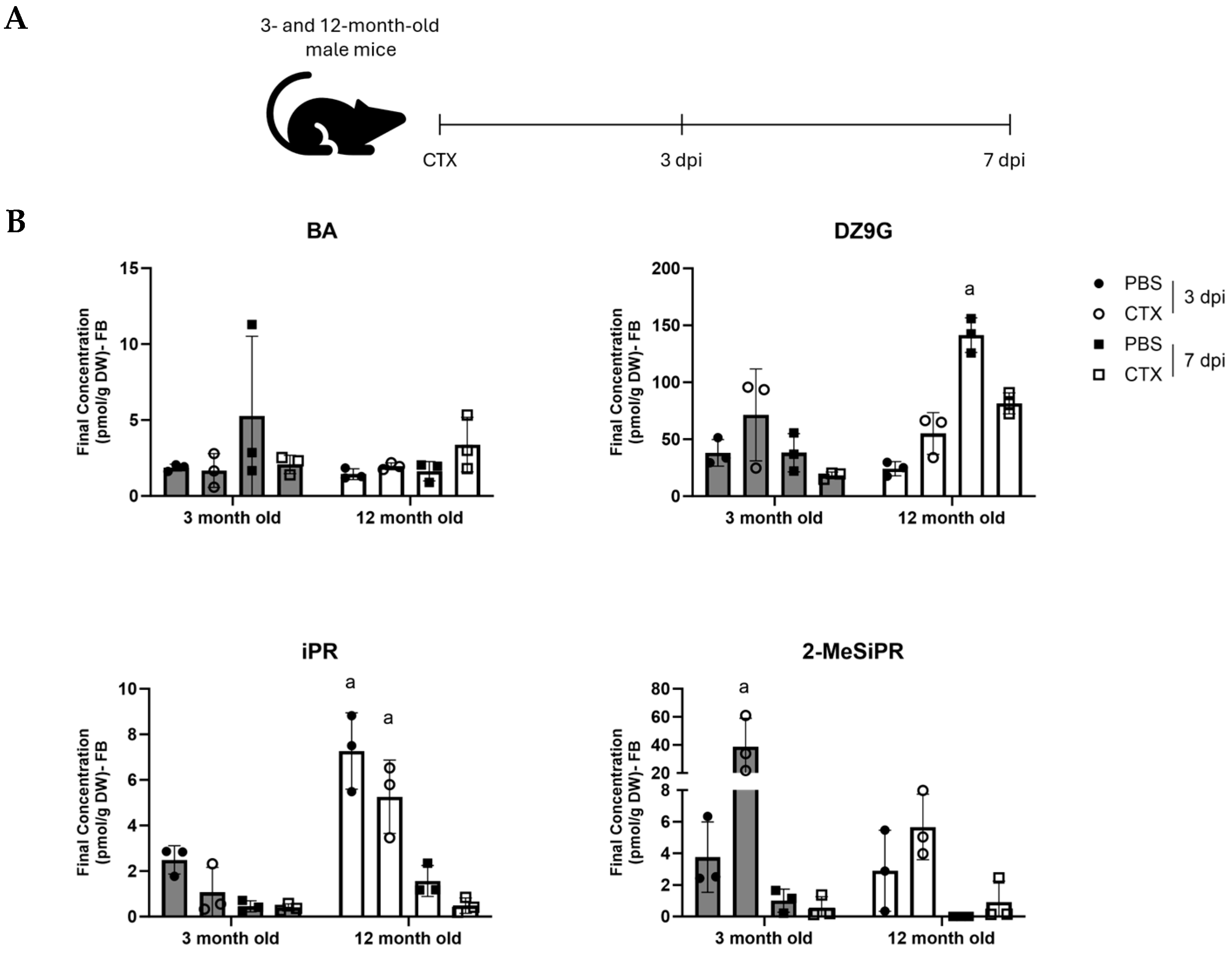
2.1. Cytokinins Are Present in Mouse Skeletal Muscle Tissue and Demonstrate Age- and Injury-Dependent Profiles
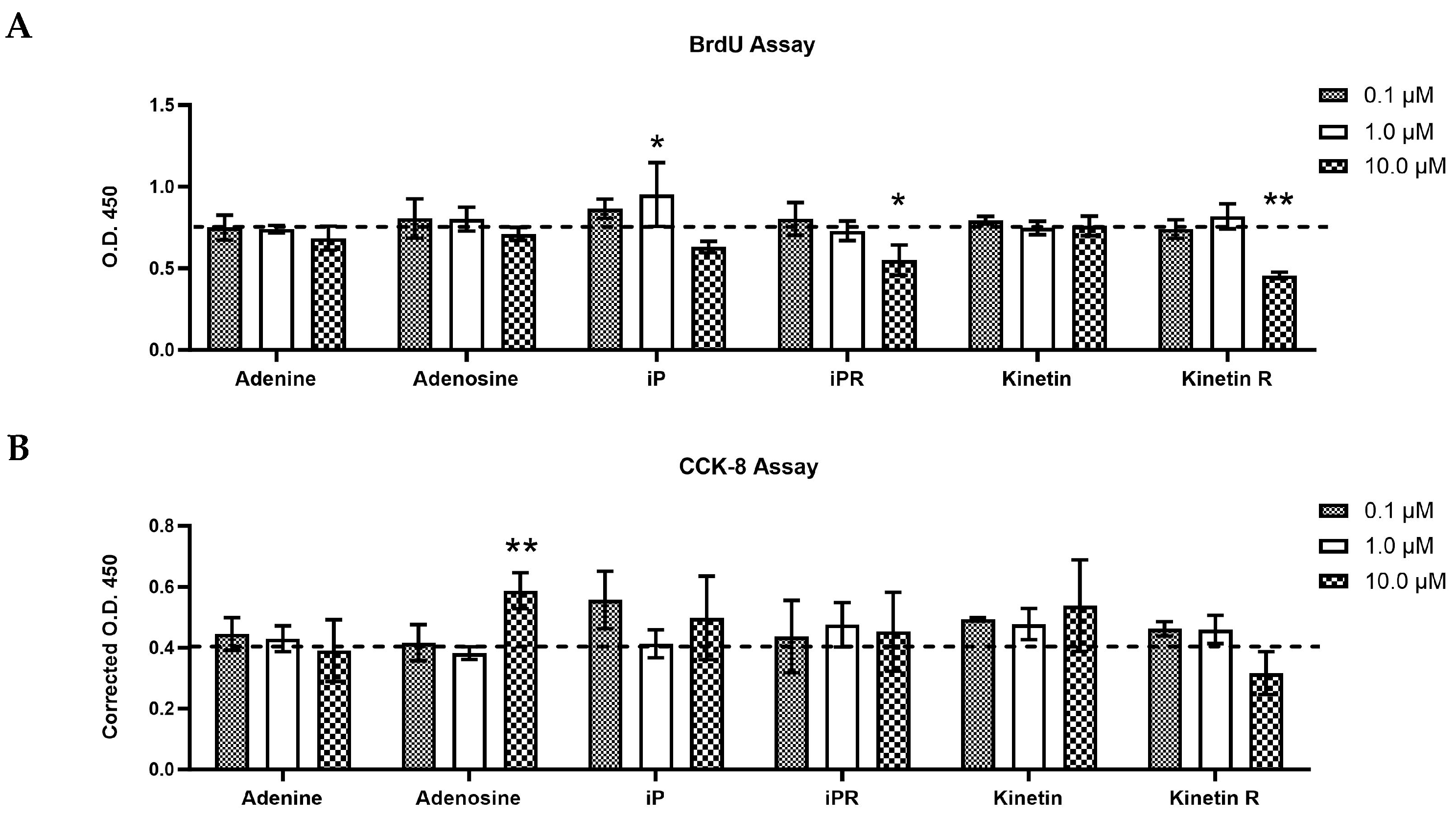
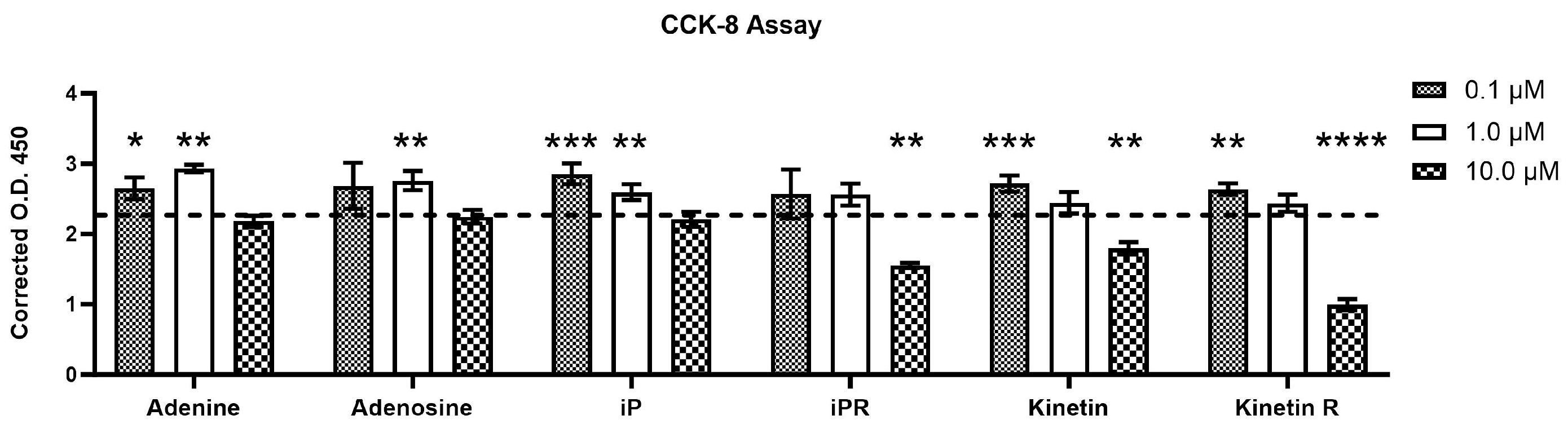
2.2. Cytokinins Have Concentration- and Side Chain-Dependent Effects on C2C12 Myoblast Proliferation and Viability
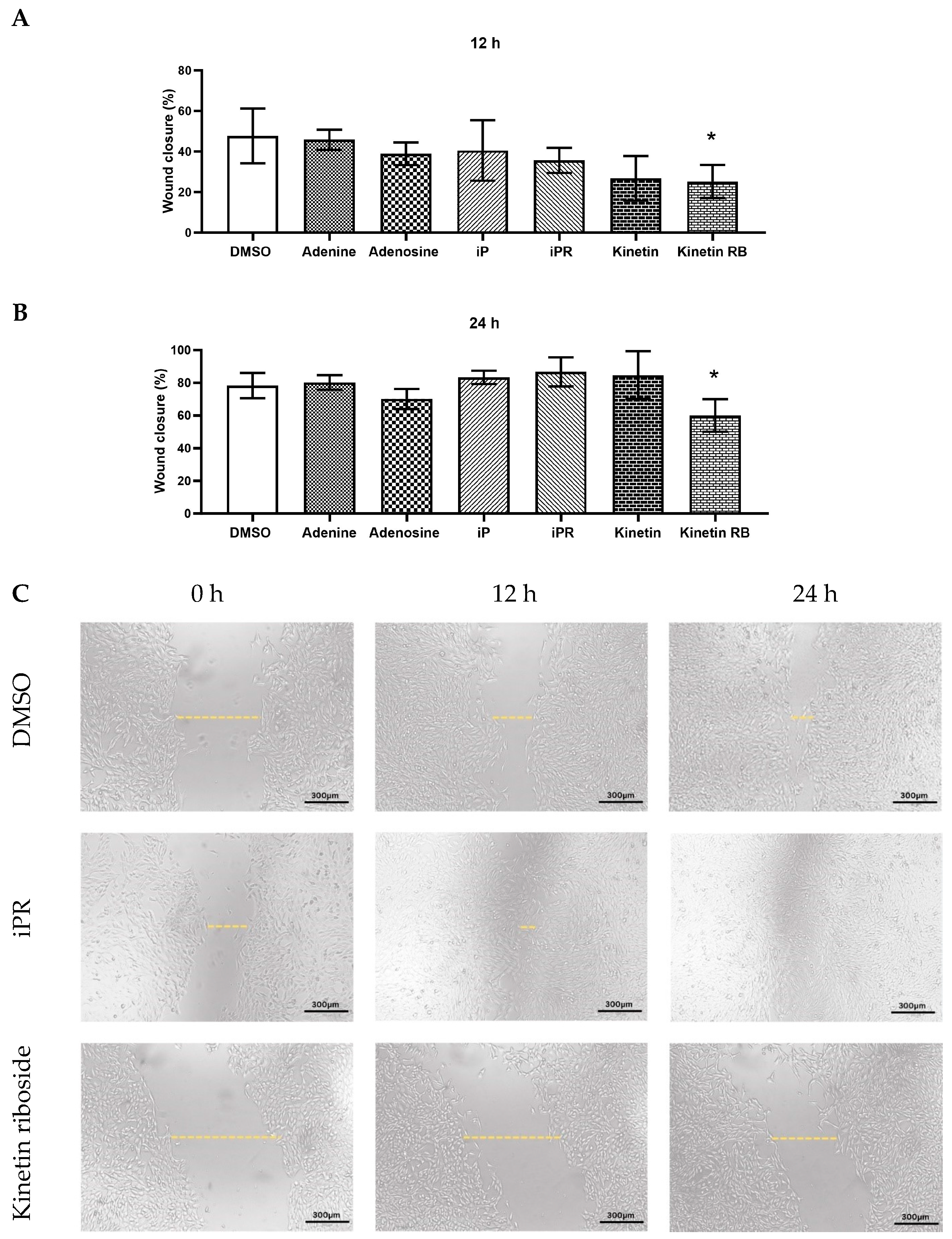
2.3. Effects of iP, Kinetin, and Their Riboside Forms on Myoblast Migration and Wound Healing
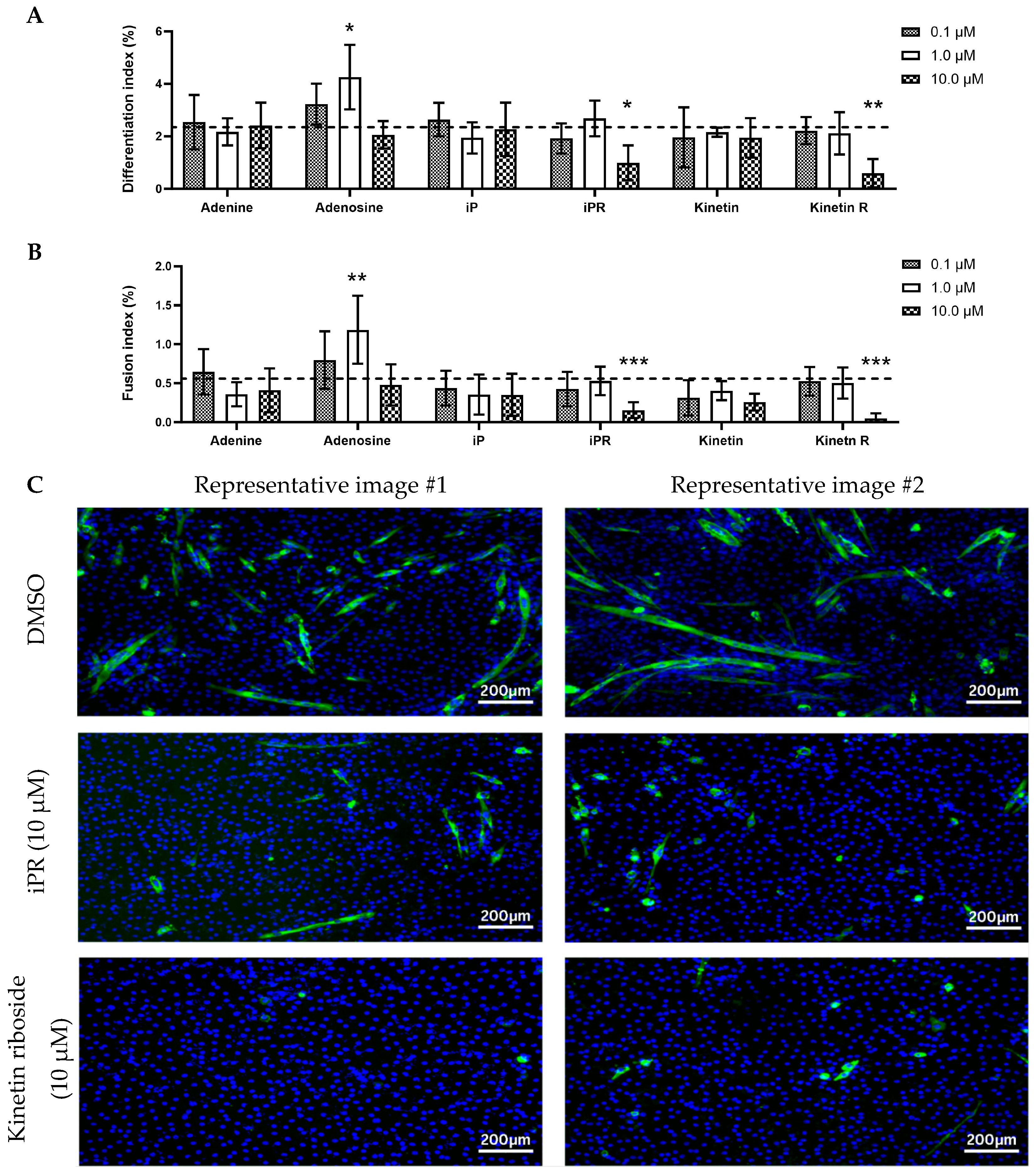
2.4. Isopentenyladenosine (iPR) and Kinetin Riboside Reduce Myotube Viability and Differentiation of C2C12 Cells
3. Discussion
3.1. CTKs Are Present in Regenerating Muscle Tissue and Show Temporal- and Age-Dependent Responses to Injury
3.2. The Effect of Exogenous iP Types on Cell Growth, Viability, and Differentiation of C2C12 Myoblasts
3.3. The Impact of Exogenous Kinetin Types on Growth, Viability, and Differentiation of Myogenic C2C12
4. Materials and Methods
4.1. Reagents
4.2. Muscle Injury
4.3. Cytokinin Extraction, Purification, and Quantification
4.4. Cell Culture
4.5. C2C12 Myoblast Proliferation
4.6. C2C12 Myoblast Differentiation
4.7. Measurements of Cell Proliferation
4.8. Measurements of Cell Viability
4.9. Scratch Wound Assay
4.10. Immunofluorescence and Myogenic Indices Determination
4.11. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building Muscle: Molecular Regulation of Myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Relaix, F.; Bencze, M.; Borok, M.J.; Der Vartanian, A.; Gattazzo, F.; Mademtzoglou, D.; Perez-Diaz, S.; Prola, A.; Reyes-Fernandez, P.C.; Rotini, A.; et al. Perspectives on Skeletal Muscle Stem Cells. Nat. Commun. 2021, 12, 692. [Google Scholar] [CrossRef]
- Holder, E.R.; Alibhai, F.J.; Caudle, S.L.; McDermott, J.C.; Tobin, S.W. The Importance of Biological Sex in Cardiac Cachexia. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H609–H627. [Google Scholar] [CrossRef]
- Fathy, M.; Saad Eldin, S.M.; Naseem, M.; Dandekar, T.; Othman, E.M. Cytokinins: Wide-Spread Signaling Hormones from Plants to Humans with High Medical Potential. Nutrients 2022, 14, 1495. [Google Scholar] [CrossRef]
- Hluska, T.; Hlusková, L.; Emery, R.J.N. The Hulks and the Deadpools of the Cytokinin Universe: A Dual Strategy for Cytokinin Production, Translocation, and Signal Transduction. Biomolecules 2021, 11, 209. [Google Scholar] [CrossRef]
- Hwang, I.; Sakakibara, H. Cytokinin Biosynthesis and Perception. Physiol. Plant 2006, 126, 528–538. [Google Scholar] [CrossRef]
- Sakakibara, H. Cytokinins: Activity, Biosynthesis, and Translocation. Annu. Rev. Plant Biol. 2006, 57, 431–449. [Google Scholar] [CrossRef] [PubMed]
- Frébort, I.; Kowalska, M.; Hluska, T.; Frébortová, J.; Galuszka, P. Evolution of Cytokinin Biosynthesis and Degradation. J. Exp. Bot. 2011, 62, 2431–2452. [Google Scholar] [CrossRef] [PubMed]
- Kadlecová, A.; Maková, B.; Artal-Sanz, M.; Strnad, M.; Voller, J. The Plant Hormone Kinetin in Disease Therapy and Healthy Aging. Ageing Res. Rev. 2019, 55, 100958. [Google Scholar] [CrossRef]
- Bowie, L.E.; Maiuri, T.; Alpaugh, M.; Gabriel, M.; Arbez, N.; Galleguillos, D.; Hung, C.L.K.; Patel, S.; Xia, J.; Hertz, N.T.; et al. N6-Furfuryladenine Is Protective in Huntington’s Disease Models by Signaling Huntingtin Phosphorylation. Proc. Natl. Acad. Sci. USA 2018, 115, E7081–E7090. [Google Scholar] [CrossRef]
- Naseem, M.; Othman, E.M.; Fathy, M.; Iqbal, J.; Howari, F.M.; AlRemeithi, F.A.; Kodandaraman, G.; Stopper, H.; Bencurova, E.; Vlachakis, D.; et al. Integrated Structural and Functional Analysis of the Protective Effects of Kinetin against Oxidative Stress in Mammalian Cellular Systems. Sci. Rep. 2020, 10, 13330. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Pastorino, O.; Della Monica, R.; Buonaiuto, M.; Torelli, G.; Caiazzo, P.; Bifulco, M.; et al. N6-Isopentenyladenosine Induces Cell Death through Necroptosis in Human Glioblastoma Cells. Cell Death Discov. 2022, 8, 173. [Google Scholar] [CrossRef]
- Pisanti, S.; Picardi, P.; Ciaglia, E.; Margarucci, L.; Ronca, R.; Giacomini, A.; Malfitano, A.M.; Casapullo, A.; Laezza, C.; Gazzerro, P.; et al. Antiangiogenic Effects of N6-Isopentenyladenosine, an Endogenous Isoprenoid End Product, Mediated by AMPK Activation. FASEB J. 2014, 28, 1132–1144. [Google Scholar] [CrossRef]
- Voller, J.; Béres, T.; Zatloukal, M.; Džubák, P.; Hajdúch, M.; Doležal, K.; Schmülling, T.; Miroslav, S. Anti-Cancer Activities of Cytokinin Ribosides. Phytochem. Rev. 2019, 18, 1101–1113. [Google Scholar] [CrossRef]
- Barciszewski, J.; Siboska, G.E.; Pedersen, B.O.; Clark, B.F.C.; Rattan, S.I.S. Evidence for the Presence of Kinetin in DNA and Cell Extracts. FEBS Lett. 1996, 393, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Barciszewski, J.; Mielcarek, M.; Stobiecki, M.; Siboska, G.; Clark, B.F.C. Identification of 6-Furfuryladenine (Kinetin) in Human Urine. Biochem. Biophys. Res. Commun. 2000, 279, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Seegobin, M.; Kisiala, A.; Noble, A.; Kaplan, D.; Brunetti, C.; Emery, R.J.N. Canis Familiaris Tissues Are Characterized by Different Profiles of Cytokinins Typical of the TRNA Degradation Pathway. FASEB J. 2018, 32, 6575–6581. [Google Scholar] [CrossRef]
- Tobin, S.W.; Seneviratne, D.; Phan, L.; Seegobin, M.; Rico, A.L.; Westby, B.; Kisiala, A.; Martic, S.; Brunetti, C.R.; Emery, R.J.N. Profiling of Adenine-Derived Signaling Molecules, Cytokinins, in Myotubes Reveals Fluctuations in Response to Lipopolysaccharide-Induced Cell Stress. Physiol. Rep. 2023, 11, e15870. [Google Scholar] [CrossRef]
- Tobin, S.W.; Alibhai, F.J.; Wlodarek, L.; Yeganeh, A.; Millar, S.; Wu, J.; Li, S.; Weisel, R.D.; Li, R.K. Delineating the Relationship between Immune System Aging and Myogenesis in Muscle Repair. Aging Cell 2021, 20, e13312. [Google Scholar] [CrossRef]
- Kisiala, A.; Kambhampati, S.; Stock, N.L.; Aoki, M.; Emery, R.J.N. Quantification of Cytokinins Using High-Resolution Accurate-Mass Orbitrap Mass Spectrometry and Parallel Reaction Monitoring (PRM). Anal. Chem. 2019, 91, 15049–15056. [Google Scholar] [CrossRef]
- Yagasaki, K.; Shinonaga, M.A.; Funabiki, R. Effect of Isopentenyladenine, a Cytokinin, on Proliferation and Protein Synthesis in Cultured Myoblasts. Agric. Biol. Chem. 1986, 50, 2791–2794. [Google Scholar] [CrossRef]
- Mielcarek, M.; Isalan, M. Kinetin Stimulates Differentiation of C2C12 Myoblasts. PLoS ONE 2021, 16, e0258419. [Google Scholar] [CrossRef] [PubMed]
- Gibb, M.; Kisiala, A.B.; Morrison, E.N.; Emery, R.J.N. The Origins and Roles of Methylthiolated Cytokinins: Evidence from Among Life Kingdoms. Front. Cell Dev. Biol. 2020, 8, 605672. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.Y.; Zhou, B.; Suzuki, T.; Miyata, K.; Ujihara, Y.; Horiguchi, H.; Takahashi, N.; Xie, P.; Michiue, H.; Fujimura, A.; et al. Cdk5rap1-Mediated 2-Methylthio Modification of Mitochondrial TRNAs Governs Protein Translation and Contributes to Myopathy in Mice and Humans. Cell Metab. 2015, 21, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Yarham, J.W.; Lamichhane, T.N.; Pyle, A.; Mattijssen, S.; Baruffini, E.; Bruni, F.; Donnini, C.; Vassilev, A.; He, L.; Blakely, E.L.; et al. Defective I6A37 Modification of Mitochondrial and Cytosolic TRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate TRNA. PLoS Genet. 2014, 10, e1004424. [Google Scholar] [CrossRef]
- Yildirim, M.; Bektaş, Ö.; Tunçez, E.; Sü, N.Y.; Sayar, Y.; Öncül, Ü.; Teber, S. A Case of Combined Oxidative Phosphorylation Deficiency 35 Associated with a Novel Missense Variant of the TRIT1 Gene. Mol. Syndromol. 2022, 13, 139–145. [Google Scholar] [CrossRef]
- Muylle, E.; Jiang, H.; Johnsen, C.; Byeon, S.K.; Ranatunga, W.; Garapati, K.; Zenka, R.M.; Preston, G.; Pandey, A.; Kozicz, T.; et al. TRIT1 Defect Leads to a Recognizable Phenotype of Myoclonic Epilepsy, Speech Delay, Strabismus, Progressive Spasticity, and Normal Lactate Levels. J. Inherit. Metab. Dis. 2022, 45, 1039–1047. [Google Scholar] [CrossRef]
- Smol, T.; Brunelle, P.; Caumes, R.; Boute-Benejean, O.; Thuillier, C.; Figeac, M.; Ait-Yahya, E.; Bonte, F.; Mau-Them, F.T.; Thauvin-Robinet, C.; et al. TRIT1 Deficiency: Two Novel Patients with Four Novel Variants. Eur. J. Med. Genet. 2022, 65, 104603. [Google Scholar] [CrossRef]
- Cheong, A.; Archambault, D.; Degani, R.; Iverson, E.; Tremblay, K.D.; Mager, J. Nuclear-Encoded Mitochondrial Ribosomal Proteins Are Required to Initiate Gastrulation. Development 2020, 147, dev188714. [Google Scholar] [CrossRef]
- Pokorná, E.; Hluska, T.; Galuszka, P.; Hallmark, H.T.; Dobrev, P.I.; Drábková, L.Z.; Filipi, T.; Holubová, K.; Plíhal, O.; Rashotte, A.M.; et al. Cytokinin N-Glucosides: Occurrence, Metabolism and Biological Activities in Plants. Biomolecules 2021, 11, 24. [Google Scholar] [CrossRef]
- Ranieri, R.; Ciaglia, E.; Amodio, G.; Picardi, P.; Proto, M.C.; Gazzerro, P.; Laezza, C.; Remondelli, P.; Bifulco, M.; Pisanti, S. N6-Isopentenyladenosine Dual Targeting of AMPK and Rab7 Prenylation Inhibits Melanoma Growth through the Impairment of Autophagic Flux. Cell Death Differ. 2018, 25, 353–367. [Google Scholar] [CrossRef]
- Ogawa, A.; Watanabe, S.; Ozerova, I.; Tsai, A.Y.L.; Kuchitsu, Y.; Chong, H.B.; Kawakami, T.; Fuse, J.; Han, W.; Kudo, R.; et al. Adenosine Kinase and ADAL Coordinate Detoxification of Modified Adenosines to Safeguard Metabolism. Cell 2025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabiri, F.; Azimychetabi, Z.; Seneviratne, D.; Phan, L.N.; Kavanagh, H.M.; Smith, H.C.; Emery, R.J.N.; Brunetti, C.R.; Yee, J.; Tobin, S.W. Cytokinins Are Age- and Injury-Responsive Molecules That Regulate Skeletal Myogenesis. Int. J. Mol. Sci. 2025, 26, 10136. https://doi.org/10.3390/ijms262010136
Kabiri F, Azimychetabi Z, Seneviratne D, Phan LN, Kavanagh HM, Smith HC, Emery RJN, Brunetti CR, Yee J, Tobin SW. Cytokinins Are Age- and Injury-Responsive Molecules That Regulate Skeletal Myogenesis. International Journal of Molecular Sciences. 2025; 26(20):10136. https://doi.org/10.3390/ijms262010136
Chicago/Turabian StyleKabiri, Farnoush, Zeynab Azimychetabi, Dev Seneviratne, Lorna N. Phan, Hannah M. Kavanagh, Hannah C. Smith, R. J. Neil Emery, Craig R. Brunetti, Janet Yee, and Stephanie W. Tobin. 2025. "Cytokinins Are Age- and Injury-Responsive Molecules That Regulate Skeletal Myogenesis" International Journal of Molecular Sciences 26, no. 20: 10136. https://doi.org/10.3390/ijms262010136
APA StyleKabiri, F., Azimychetabi, Z., Seneviratne, D., Phan, L. N., Kavanagh, H. M., Smith, H. C., Emery, R. J. N., Brunetti, C. R., Yee, J., & Tobin, S. W. (2025). Cytokinins Are Age- and Injury-Responsive Molecules That Regulate Skeletal Myogenesis. International Journal of Molecular Sciences, 26(20), 10136. https://doi.org/10.3390/ijms262010136






