Mechanism and Application of Developmental Factors in Plant Genetic Transformation
Abstract
1. Introduction
2. Morphogenic Factors
2.1. WOX
2.2. BBM-WUS
2.3. GRF-GIF
2.4. DOF
2.5. Combination of Morphogenic Factors
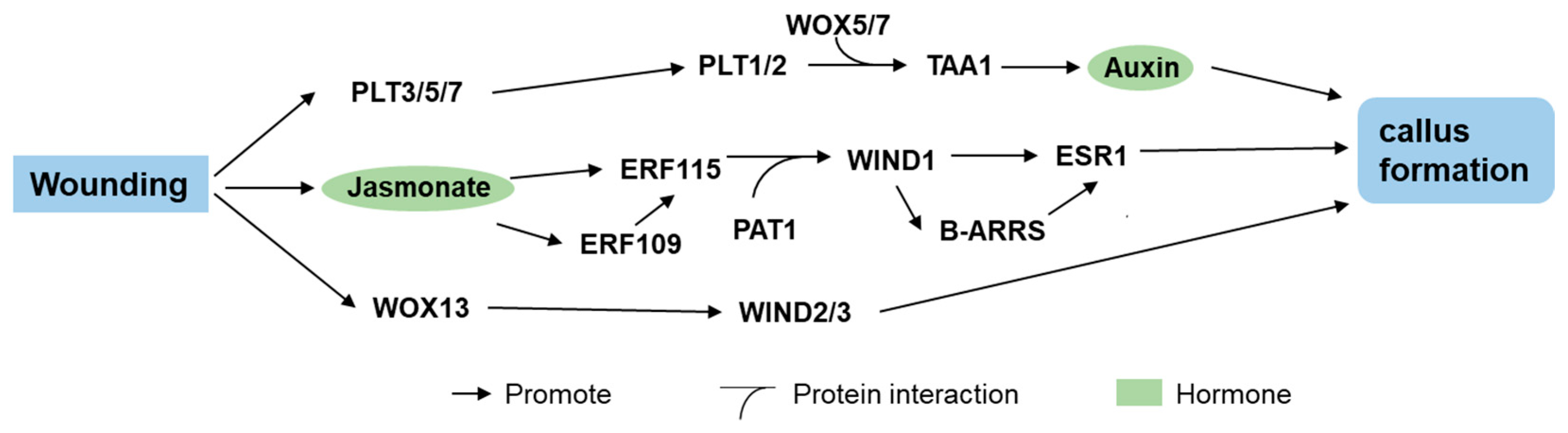
3. Wound-Inducible Factors
3.1. WIND1
3.2. REF
3.3. PLT
3.4. Other Factors
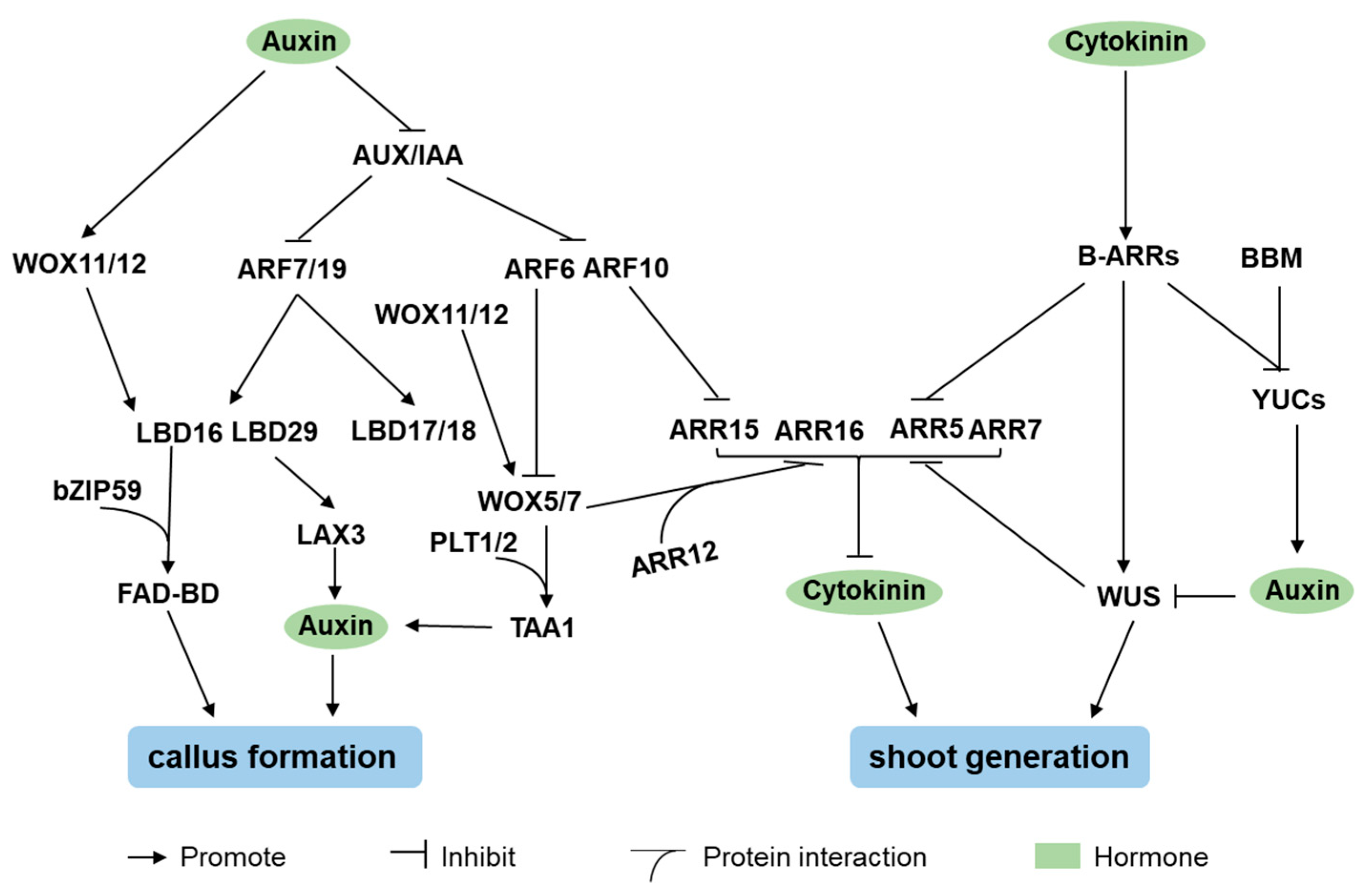
4. Hormone Signaling Factors
5. Epigenetic Modification Factors
6. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GA | Gibberellin |
| CK | Cytokinin |
| ABA | Abscisic Acid |
| BR | Brassinosteroid |
| JA | Jasmonate |
| ET | Ethylene |
References
- Aggarwal, G.; Jeena, A.S.; Mehra, K.; Kumar, B.; Kashyap, S.; Yadav, D.K.; Maurya, A.K.; Venkatesh, S.C.; Singla, P.; Bohra, A. Speed-bred crops for food security and sustainable agriculture. Planta 2025, 262, 34. [Google Scholar] [CrossRef]
- Imam, Z.; Sultana, R.; Parveen, R.; Swapnil; Singh, D.; Sinha, S.; Sahoo, J.P. Understanding the concept of speed breeding in crop improvement: Opportunities and challenges towards global food security. Trop. Plant Biol. 2024, 17, 1–23. [Google Scholar] [CrossRef]
- Li, G.; An, L.; Yang, W.; Yang, L.; Wei, T.; Shi, J.; Wang, J.; Doonan, J.H.; Xie, K.; Fernie, A.R.; et al. Integrated biotechnological and AI innovations for crop improvement. Nature 2025, 643, 925–937. [Google Scholar] [CrossRef]
- Hafeez, A.; Ali, B.; Javed, M.A.; Saleem, A.; Fatima, M.; Fathi, A.; Afridi, M.S.; Aydin, V.; Oral, M.A.; Soudy, F.A. Plant breeding for harmony between sustainable agriculture, the environment, and global food security: An era of genomics-assisted breeding. Planta 2023, 258, 97. [Google Scholar] [CrossRef]
- Bradbury, A.; Clapp, O.; Biacsi, A.S.; Kuo, P.; Gaju, O.; Hayta, S.; Zhu, J.K.; Lambing, C. Integrating genome editing with omics, artificial intelligence, and advanced farming technologies to increase crop productivity. Plant Commun. 2025, 6, 101386. [Google Scholar] [CrossRef]
- Yu, H.; Bai, S.; Li, J. Towards Breeding 5.0:Smart variety by intelligent breeding. Chin. Sci. Bull. 2024, 69, 4687–4690. [Google Scholar] [CrossRef]
- Su, W.; Xu, M.; Radani, Y.; Yang, L. Technological development and application of plant genetic transformation. Int. J. Mol. Sci. 2023, 24, 10646. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular mechanisms of plant regeneration. Annu. Rev. Plant Biol. 2019, 70, 377–406. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, D.; Kareem, A.; Durgaprasad, K.; Sreeraj, E.; Sugimoto, K.; Prasad, K. Shoot regeneration: A journey from acquisition of competence to completion. Curr. Opin. Plant Biol. 2018, 41, 23–31. [Google Scholar] [CrossRef]
- Chen, C.; Hu, Y.; Ikeuchi, M.; Jiao, Y.; Prasad, K.; Su, Y.H.; Xiao, J.; Xu, L.; Yang, W.; Zhao, Z.; et al. Plant regeneration in the new era: From molecular mechanisms to biotechnology applications. Sci. China Life Sci. 2024, 67, 1338–1367. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ren, X.; Yin, G.; Wang, K.; Li, J.; Du, L.; Xu, H.; Ye, X. Effects of environmental temperature on the regeneration frequency of the immature embryos of wheat (Triticum aestivum L.). J. Integr. Agric. 2014, 13, 722–732. [Google Scholar] [CrossRef]
- Maren, N.A.; Duan, H.; Da, K.; Yencho, G.C.; Ranney, T.G.; Liu, W. Genotype-independent plant transformation. Hortic. Res. 2022, 9, uhac047. [Google Scholar] [CrossRef]
- Ahmed, S.; Wan Azizan, W.A.S.; Akhond, M.A.Y.; Juraimi, A.S.; Ismail, S.I.; Ahmed, R.; Md Hatta, M.A. Optimization of in vitro regeneration protocol of tomato cv. MT1 for genetic transformation. Horticulturae 2023, 9, 800. [Google Scholar] [CrossRef]
- Van Eck, J.; Keen, P.; Tjahjadi, M. Agrobacterium tumefaciens-mediated transformation of tomato. Methods Mol. Biol. 2019, 1864, 225–234. [Google Scholar] [CrossRef]
- Sun, H.J.; Uchii, S.; Watanabe, S.; Ezura, H. A highly efficient transformation protocol for Micro-Tom, a model cultivar for tomato functional genomics. Plant Cell Physiol. 2006, 47, 426–431. [Google Scholar] [CrossRef]
- Atta, R.; Laurens, L.; Boucheron-Dubuisson, E.; Guivarc’h, A.; Carnero, E.; Giraudat-Pautot, V.; Rech, P.; Chriqui, D. Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 2009, 57, 626–644. [Google Scholar] [CrossRef]
- Long, Y.; Yang, Y.; Pan, G.; Shen, Y. New insights into tissue culture plant-regeneration mechanisms. Front. Plant Sci. 2022, 13, 926752. [Google Scholar] [CrossRef] [PubMed]
- Saeedpour, A.; Jahanbakhsh Godehkahriz, S.; Lohrasebi, T.; Esfahani, K.; Hatef Salmanian, A.; Razavi, K. The effect of endogenous hormones, total antioxidant and total phenol changes on regeneration of barley cultivars. Iran. J. Biotechnol. 2021, 19, e2838. [Google Scholar] [CrossRef]
- Kumar, R.; Mamrutha, H.M.; Kaur, A.; Venkatesh, K.; Grewal, A.; Kumar, R.; Tiwari, V. Development of an efficient and reproducible regeneration system in wheat (Triticum aestivum L.). Physiol. Mol. Biol. Plants 2017, 23, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, H.; Du, L.; Ye, X. Generation of marker-free transgenic hexaploid wheat via an Agrobacterium-mediated co-transformation strategy in commercial Chinese wheat varieties. Plant Biotechnol. J. 2017, 15, 614–623. [Google Scholar] [CrossRef]
- Wang, K.; Shi, L.; Liang, X.; Zhao, P.; Wang, W.; Liu, J.; Chang, Y.; Hiei, Y.; Yanagihara, C.; Du, L.; et al. The gene TaWOX5 overcomes genotype dependency in wheat genetic transformation. Nat. Plants 2022, 8, 110–117, Erratum in Nat. Plants 2022, 8, 717–720. [Google Scholar] [CrossRef]
- Chen, L.; Cai, Y.; Liu, X.; Yao, W.; Guo, C.; Sun, S.; Wu, C.; Jiang, B.; Han, T.; Hou, W. Improvement of soybean Agrobacterium-mediated transformation efficiency by adding glutamine and asparagine into the culture media. Int. J. Mol. Sci. 2018, 19, 3039. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, P.; Liu, Y.; Liu, C.; Hu, Z.; Xin, D.; Wu, X.; Yang, M.; Chen, Q. A highly efficient soybean transformation system using GRF3-GIF1 chimeric protein. J. Integr. Plant Biol. 2025, 67, 3–6. [Google Scholar] [CrossRef]
- Zhang, A.; Kong, T.; Sun, B.; Qiu, S.; Guo, J.; Ruan, S.; Guo, Y.; Guo, J.; Zhang, Z.; Liu, Y.; et al. A telomere-to-telomere genome assembly of Zhonghuang 13, a widely-grown soybean variety from the original center of Glycine max. Crop. J. 2024, 12, 142–153. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, L.; Yu, H.; Wang, J.; Chen, Q.; Wang, H. The T2T genome assembly of soybean cultivar ZH13 and its epigenetic landscapes. Mol. Plant 2023, 16, 1715–1718. [Google Scholar] [CrossRef]
- Shen, Y.; Du, H.; Liu, Y.; Ni, L.; Wang, Z.; Liang, C.; Tian, Z. Update soybean Zhonghuang 13 genome to a golden reference. Sci. China Life Sci. 2019, 62, 1257–1260. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Han, J.; Jiang, S.; Su, B.; Zhang, B.; Liu, Z.; Yan, H.; Qiu, L. Shattering-resistance of an elite soybean variety ‘Heihe 43’ and identification of shattering-resistant genes. Euphytica 2021, 217, 120. [Google Scholar] [CrossRef]
- Wang, P.; Si, H.; Li, C.; Xu, Z.; Guo, H.; Jin, S.; Cheng, H. Plant genetic transformation: Achievements, current status and future prospects. Plant Biotechnol. J. 2025, 23, 2034–2058. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, K.; Xiao, J. Recent advances in understanding of the epigenetic regulation of plant regeneration. Abiotech 2023, 4, 31–46. [Google Scholar] [CrossRef]
- Jin, L.; Yarra, R.; Zhou, L.; Zhao, Z.; Cao, H. miRNAs as key regulators via targeting the phytohormone signaling pathways during somatic embryogenesis of plants. 3 Biotech 2020, 10, 495. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.S.; Rhee, J.H.; Chu, H.; Frost, J.M.; Choi, Y. Insights into plant regeneration: Cellular pathways and DNA methylation dynamics. Plant Cell Rep. 2024, 43, 120. [Google Scholar] [CrossRef]
- Su, Y.; Tang, L.; Zhao, X.; Zhang, X. Plant cell totipotency: Insights into cellular reprogramming. J. Integr. Plant Biol. 2021, 63, 228–243. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, M.; Horstman, A.; Zethof, J.; Koes, R.; Rijpkema, A.S.; Gerats, T. Differential recruitment of WOX transcription factors for lateral development and organ fusion in Petunia and Arabidopsis. Plant Cell 2009, 21, 2269–2283. [Google Scholar] [CrossRef]
- Costanzo, E.; Trehin, C.; Vandenbussche, M. The role of WOX genes in flower development. Ann. Bot. 2014, 114, 1545–1553. [Google Scholar] [CrossRef]
- Nakata, M.; Matsumoto, N.; Tsugeki, R.; Rikirsch, E.; Laux, T.; Okada, K. Roles of the middle domain-specific WUSCHEL-RELATED HOMEOBOX genes in early development of leaves in Arabidopsis. Plant Cell 2012, 24, 519–535. [Google Scholar] [CrossRef]
- Du, F.; Chang, Z.; Kong, X.; Xia, L.; Zhou, W.; Laux, T.; Zhang, L. Functional conservation and divergence of the WOX gene family in regulating meristem activity: From Arabidopsis to crops. Plant Physiol. 2025, 199, kiaf374. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, C.; Yu, Y.; Ran, G.; Zhai, N.; Pi, L. WUSCHEL RELATED HOMEOBOX5 and 7 maintain callus development by promoting cell division in Arabidopsis. Plant Sci. 2024, 346, 112133. [Google Scholar] [CrossRef]
- Zhai, N.; Xu, L. Pluripotency acquisition in the middle cell layer of callus is required for organ regeneration. Nat. Plants 2021, 7, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, X.S.; Su, Y.H. Application of Wox2a in transformation of recalcitrant maize genotypes. aBIOTECH 2023, 4, 386–388. [Google Scholar] [CrossRef]
- McFarland, F.L.; Collier, R.; Walter, N.; Martinell, B.; Kaeppler, S.M.; Kaeppler, H.F. A key to totipotency: Wuschel-like homeobox 2a unlocks embryogenic culture response in maize (Zea mays L.). Plant Biotechnol. J. 2023, 21, 1860–1872. [Google Scholar] [CrossRef]
- Mao, J.; Ma, D.; Niu, C.; Ma, X.; Li, K.; Tahir, M.M.; Chen, S.; Liu, X.; Zhang, D. Transcriptome analysis reveals the regulatory mechanism by which MdWOX11 suppresses adventitious shoot formation in apple. Hortic. Res. 2022, 9, uhac080. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, K.; Offringa, R.; Sharma, V.K.; Kieft, H.; Ouellet, T.; Zhang, L.; Hattori, J.; Liu, C.M.; van Lammeren, A.A.; Miki, B.L.; et al. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 2002, 14, 1737–1749. [Google Scholar] [CrossRef]
- Laux, T.; Mayer, K.F.; Berger, J.; Jürgens, G. The WUSCHEL gene is required for shoot and floral meristem integrity in Arabidopsis. Development 1996, 122, 87–96. [Google Scholar] [CrossRef]
- Jha, P.; Ochatt, S.J.; Kumar, V. WUSCHEL: A master regulator in plant growth signaling. Plant Cell Rep. 2020, 39, 431–444. [Google Scholar] [CrossRef]
- Zuo, J.; Niu, Q.W.; Frugis, G.; Chua, N.H. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002, 30, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Zhao, X.Y.; Liu, Y.B.; Zhang, C.L.; O’Neill, S.D.; Zhang, X.S. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009, 59, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J.; et al. Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef]
- Lowe, K.; La Rota, M.; Hoerster, G.; Hastings, C.; Wang, N.; Chamberlin, M.; Wu, E.; Jones, T.; Gordon-Kamm, W. Rapid genotype “independent” Zea mays L. (maize) transformation via direct somatic embryogenesis. In Vitro Cell. Dev. Biol.-Plant 2018, 54, 240–252. [Google Scholar] [CrossRef]
- Wang, N.; Arling, M.; Hoerster, G.; Ryan, L.; Wu, E.; Lowe, K.; Gordon-Kamm, W.; Jones, T.J.; Chilcoat, N.D.; Anand, A. An efficient gene excision system in maize. Front. Plant Sci. 2020, 11, 1298. [Google Scholar] [CrossRef]
- Hoerster, G.; Wang, N.; Ryan, L.; Wu, E.; Anand, A.; McBride, K.; Lowe, K.; Jones, T.; Gordon-Kamm, B. Use of non-integrating Zm-Wus2 vectors to enhance maize transformation. In Vitro Cell. Dev. Biol.-Plant 2020, 56, 265–279. [Google Scholar] [CrossRef]
- Che, P.; Wu, E.; Simon, M.K.; Anand, A.; Lowe, K.; Gao, H.; Sigmund, A.L.; Yang, M.; Albertsen, M.C.; Gordon-Kamm, W.; et al. Wuschel2 enables highly efficient CRISPR/Cas-targeted genome editing during rapid de novo shoot regeneration in sorghum. Commun. Biol. 2022, 5, 344. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Jin, M.; Pan, H.; Han, B.; Li, M.; Yan, S.; Hu, G.; Yan, J. Establishment of genotype-independent high-efficiency transformation system in maize. Acta Agron. Sin. 2022, 48, 2987–2993. [Google Scholar] [CrossRef]
- Zhou, Z.; Yang, Y.; Ai, G.; Zhao, M.; Han, B.; Zhao, C.; Chen, Y.; Zhang, Y.; Pan, H.; Lan, C.; et al. Overcoming genotypic dependency and bypassing immature embryos in wheat transformation by using morphogenic regulators. Sci. China Life Sci. 2024, 67, 1535–1538. [Google Scholar] [CrossRef]
- Wang, N.; Ryan, L.; Sardesai, N.; Wu, E.; Lenderts, B.; Lowe, K.; Che, P.; Anand, A.; Worden, A.; van Dyk, D.; et al. Leaf transformation for efficient random integration and targeted genome modification in maize and sorghum. Nat. Plants 2023, 9, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H. Biological roles and an evolutionary sketch of the GRF-GIF transcriptional complex in plants. BMB Rep. 2019, 52, 227–238. [Google Scholar] [CrossRef]
- Liebsch, D.; Palatnik, J.F. MicroRNA miR396, GRF transcription factors and GIF co-regulators: A conserved plant growth regulatory module with potential for breeding and biotechnology. Curr. Opin. Plant Biol. 2020, 53, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Palmgren, M. GRF-GIF chimeras boost plant regeneration. Trends Plant Sci. 2021, 26, 201–204. [Google Scholar] [CrossRef]
- Li, Y.; Wang, N.; Feng, J.; Liu, Y.; Wang, H.; Deng, S.; Dong, W.; Liu, X.; Lv, B.; Sun, J.; et al. Enhancing genetic transformation efficiency in cucurbit crops through AtGRF5 overexpression: Mechanistic insights and applications. J. Integr. Plant Biol. 2025, 67, 1843–1860. [Google Scholar] [CrossRef]
- Rodriguez, R.E.; Mecchia, M.A.; Debernardi, J.M.; Schommer, C.; Weigel, D.; Palatnik, J.F. Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development 2010, 137, 103–112. [Google Scholar] [CrossRef]
- Debernardi, J.M.; Tricoli, D.M.; Ercoli, M.F.; Hayta, S.; Ronald, P.; Palatnik, J.F.; Dubcovsky, J. A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 2020, 38, 1274–1279. [Google Scholar] [CrossRef]
- Yang, Z.; Zhao, M.; Zhang, X.; Gu, L.; Li, J.; Ming, F.; Wang, M.; Wang, Z. MIR396-GRF/GIF enhances in planta shoot regeneration of Dendrobium catenatum. BMC Genom. 2024, 25, 543. [Google Scholar] [CrossRef]
- Feng, Q.; Xiao, L.; He, Y.; Liu, M.; Wang, J.; Tian, S.; Zhang, X.; Yuan, L. Highly efficient, genotype-independent transformation and gene editing in watermelon (Citrullus lanatus) using a chimeric ClGRF4-GIF1 gene. J. Integr. Plant Biol. 2021, 63, 2038–2042. [Google Scholar] [CrossRef]
- Segatto, R.; Paggi, G.M.; Taylor, N.J. Overexpression of GRF-GIF genes enhances plant regeneration in cassava (Manihot esculenta). In Vitro Cell. Dev. Biol.-Plant 2024, 61, 593–607. [Google Scholar] [CrossRef]
- Kong, J.; Martin-Ortigosa, S.; Finer, J.; Orchard, N.; Gunadi, A.; Batts, L.A.; Thakare, D.; Rush, B.; Schmitz, O.; Stuiver, M.; et al. Overexpression of the transcription factor GROWTH-REGULATING FACTOR5 improves transformation of dicot and monocot species. Front. Plant Sci. 2020, 11, 572319. [Google Scholar] [CrossRef] [PubMed]
- Bull, T.; Debernardi, J.; Reeves, M.; Hill, T.; Bertier, L.; Van Deynze, A.; Michelmore, R. GRF-GIF chimeric proteins enhance in vitro regeneration and Agrobacterium-mediated transformation efficiencies of lettuce (Lactuca spp.). Plant Cell Rep. 2023, 42, 629–643. [Google Scholar] [CrossRef]
- Debernardi, J.M.; Rodriguez, R.E.; Mecchia, M.A.; Palatnik, J.F. Functional specialization of the plant miR396 regulatory network through distinct microRNA-target interactions. PLoS Genet. 2012, 8, e1002419. [Google Scholar] [CrossRef]
- Wang, Z.; Wong, D.C.J.; Chen, Z.; Bai, W.; Si, H.; Jin, X. Emerging roles of plant DNA-binding with one finger transcription factors in various hormone and stress signaling pathways. Front. Plant Sci. 2022, 13, 844201. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, Z.; Ai, Q.; Li, X.; Yang, J.; Zhang, N.; Si, H. DNA-binding with one finger (Dof) transcription factor gene family study reveals differential stress-responsive transcription factors in contrasting drought tolerance potato species. Int. J. Mol. Sci. 2024, 25, 3488. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S. Dof domain proteins: Plant-specific transcription factors associated with diverse phenomena unique to plants. Plant Cell Physiol. 2004, 45, 386–391. [Google Scholar] [CrossRef]
- Kang, H.; Singh, K.B. Characterization of salicylic acid-responsive, Arabidopsis Dof domain proteins: Overexpression of OBP3 leads to growth defects. Plant J. 2000, 21, 329–339. [Google Scholar] [CrossRef]
- Zhang, A.; Matsuoka, K.; Kareem, A.; Robert, M.; Roszak, P.; Blob, B.; Bisht, A.; De Veylder, L.; Voiniciuc, C.; Asahina, M.; et al. Cell-wall damage activates DOF transcription factors to promote wound healing and tissue regeneration in Arabidopsis thaliana. Curr. Biol. 2022, 32, 1883–1894.e1887. [Google Scholar] [CrossRef]
- Liu, X.; Bie, X.; Lin, X.; Li, M.; Wang, H.; Zhang, X.; Yang, Y.; Zhang, C.; Zhang, X.; Xiao, J. Uncovering the transcriptional regulatory network involved in boosting wheat regeneration and transformation. Nat. Plants 2023, 9, 908–925. [Google Scholar] [CrossRef]
- Bisht, A.; Eekhout, T.; Canher, B.; Lu, R.; Vercauteren, I.; De Jaeger, G.; Heyman, J.; De Veylder, L. PAT1-type GRAS-domain proteins control regeneration by activating DOF3.4 to drive cell proliferation in Arabidopsis roots. Plant Cell 2023, 35, 1513–1531. [Google Scholar] [CrossRef]
- Miyashima, S.; Roszak, P.; Sevilem, I.; Toyokura, K.; Blob, B.; Heo, J.-o.; Mellor, N.; Help-Rinta-Rahko, H.; Otero, S.; Smet, W.; et al. Mobile PEAR transcription factors integrate positional cues to prime cambial growth. Nature 2019, 565, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Skirycz, A.; Radziejwoski, A.; Busch, W.; Hannah, M.A.; Czeszejko, J.; Kwaśniewski, M.; Zanor, M.I.; Lohmann, J.U.; De Veylder, L.; Witt, I.; et al. The DOF transcription factor OBP1 is involved in cell cycle regulation in Arabidopsis thaliana. Plant J. 2008, 56, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Chen, H.; Ying, L.; Cai, W. AtDOF5.4/OBP4, a DOF transcription factor gene that negatively regulates cell cycle progression and cell expansion in Arabidopsis thaliana. Sci. Rep. 2016, 6, 27705. [Google Scholar] [CrossRef]
- Ramirez-Parra, E.; Perianez-Rodriguez, J.; Navarro-Neila, S.; Gude, I.; Moreno-Risueno, M.A.; Del Pozo, J.C. The transcription factor OBP4 controls root growth and promotes callus formation. New Phytol. 2017, 213, 1787–1801. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Tobimatsu, Y.; Masaomi, Y.; Sano, R.; Umezawa, T.; Demura, T.; Ohtani, M. Plant-specific Dof transcription factors VASCULAR-RELATED DOF1 and VASCULAR-RELATED DOF2 regulate vascular cell differentiation and lignin biosynthesis in Arabidopsis. Plant Mol. Biol. 2020, 104, 263–281. [Google Scholar] [CrossRef]
- Yarra, R.; Krysan, P.J. GRF-GIF duo and GRF-GIF-BBM: Novel transformation methodologies for enhancing regeneration efficiency of genome-edited recalcitrant crops. Planta 2023, 257, 60. [Google Scholar] [CrossRef]
- Vandeputte, W.; Coussens, G.; Aesaert, S.; Haeghebaert, J.; Impens, L.; Karimi, M.; Debernardi, J.M.; Pauwels, L. Use of GRF-GIF chimeras and a ternary vector system to improve maize (Zea mays L.) transformation frequency. Plant J. 2024, 119, 2116–2132. [Google Scholar] [CrossRef]
- Chen, Z.; Debernardi, J.M.; Dubcovsky, J.; Gallavotti, A. The combination of morphogenic regulators BABY BOOM and GRF-GIF improves maize transformation efficiency. Biorxiv 2022. [Google Scholar] [CrossRef]
- Iwase, A.; Mitsuda, N.; Koyama, T.; Hiratsu, K.; Kojima, M.; Arai, T.; Inoue, Y.; Seki, M.; Sakakibara, H.; Sugimoto, K.; et al. The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr. Biol. 2011, 21, 508–514. [Google Scholar] [CrossRef]
- Iwase, A.; Mita, K.; Nonaka, S.; Ikeuchi, M.; Koizuka, C.; Ohnuma, M.; Ezura, H.; Imamura, J.; Sugimoto, K. WIND1-based acquisition of regeneration competency in Arabidopsis and rapeseed. J. Plant Res. 2015, 128, 389–397. [Google Scholar] [CrossRef]
- Jiang, Y.; Wei, X.; Zhu, M.; Zhang, X.; Jiang, Q.; Wang, Z.; Cao, Y.; An, X.; Wan, X. Developmental regulators in promoting genetic transformation efficiency in maize and other plants. Curr. Plant Biol. 2024, 40, 100383. [Google Scholar] [CrossRef]
- Iwase, A.; Harashima, H.; Ikeuchi, M.; Rymen, B.; Ohnuma, M.; Komaki, S.; Morohashi, K.; Kurata, T.; Nakata, M.; Ohme-Takagi, M.; et al. WIND1 promotes shoot regeneration through transcriptional activation of ENHANCER OF SHOOT REGENERATION1 in Arabidopsis. Plant Cell 2017, 29, 54–69. [Google Scholar] [CrossRef]
- Yang, W.; Zhai, H.; Wu, F.; Deng, L.; Chao, Y.; Meng, X.; Chen, Q.; Liu, C.; Bie, X.; Sun, C.; et al. Peptide REF1 is a local wound signal promoting plant regeneration. Cell 2024, 187, 3024–3038.e3014. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Iwase, A.; Rymen, B.; Lambolez, A.; Kojima, M.; Takebayashi, Y.; Heyman, J.; Watanabe, S.; Seo, M.; De Veylder, L.; et al. Wounding triggers callus formation via dynamic hormonal and transcriptional changes. Plant Physiol. 2017, 175, 1158–1174. [Google Scholar] [CrossRef]
- Lian, Z.; Nguyen, C.D.; Liu, L.; Wang, G.; Chen, J.; Wang, S.; Yi, G.; Wilson, S.; Ozias-Akins, P.; Gong, H.; et al. Application of developmental regulators to improve in planta or in vitro transformation in plants. Plant Biotechnol. J. 2022, 20, 1622–1635. [Google Scholar] [CrossRef]
- Kareem, A.; Durgaprasad, K.; Sugimoto, K.; Du, Y.; Pulianmackal, A.J.; Trivedi, Z.B.; Abhayadev, P.V.; Pinon, V.; Meyerowitz, E.M.; Scheres, B.; et al. PLETHORA genes control regeneration by a two-step mechanism. Curr. Biol. 2015, 25, 1017–1030. [Google Scholar] [CrossRef]
- Zhai, N.; Pan, X.; Zeng, M.; Xu, L. Developmental trajectory of pluripotent stem cell establishment in Arabidopsis callus guided by a quiescent center-related gene network. Development 2023, 150, dev200879. [Google Scholar] [CrossRef]
- Zhou, W.; Lozano-Torres, J.L.; Blilou, I.; Zhang, X.; Zhai, Q.; Smant, G.; Li, C.; Scheres, B. A Jasmonate signaling network activates root stem cells and promotes regeneration. Cell 2019, 177, 942–956.e914. [Google Scholar] [CrossRef]
- Heyman, J.; Cools, T.; Canher, B.; Shavialenka, S.; Traas, J.; Vercauteren, I.; Van den Daele, H.; Persiau, G.; De Jaeger, G.; Sugimoto, K.; et al. The heterodimeric transcription factor complex ERF115–PAT1 grants regeneration competence. Nat. Plants 2016, 2, 16165. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Iwase, A.; Ito, T.; Tanaka, H.; Favero, D.S.; Kawamura, A.; Sakamoto, S.; Wakazaki, M.; Tameshige, T.; Fujii, H.; et al. Wound-inducible WUSCHEL-RELATED HOMEOBOX 13 is required for callus growth and organ reconnection. Plant Physiol. 2021, 188, 425–441. [Google Scholar] [CrossRef]
- Lavenus, J.; Goh, T.; Guyomarc’h, S.; Hill, K.; Lucas, M.; Voß, U.; Kenobi, K.; Wilson, M.H.; Farcot, E.; Hagen, G.; et al. Inference of the Arabidopsis lateral root gene regulatory network suggests a bifurcation mechanism that defines primordia flanking and central zones. Plant Cell 2015, 27, 1368–1388. [Google Scholar] [CrossRef]
- Porco, S.; Larrieu, A.; Du, Y.; Gaudinier, A.; Goh, T.; Swarup, K.; Swarup, R.; Kuempers, B.; Bishopp, A.; Lavenus, J.; et al. Lateral root emergence in Arabidopsis is dependent on transcription factor LBD29 regulation of auxin influx carrier LAX3. Development 2016, 143, 3340–3349. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, N.Y.; Lee, D.J.; Kim, J. LBD18/ASL20 regulates lateral root formation in combination with LBD16/ASL18 downstream of ARF7 and ARF19 in Arabidopsis. Plant Physiol. 2009, 151, 1377–1389. [Google Scholar] [CrossRef]
- Fan, M.; Xu, C.; Xu, K.; Hu, Y. LATERAL ORGAN BOUNDARIES DOMAIN transcription factors direct callus formation in Arabidopsis regeneration. Cell Res. 2012, 22, 1169–1180. [Google Scholar] [CrossRef]
- Ding, Z.; Friml, J. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2010, 107, 12046–12051. [Google Scholar] [CrossRef]
- Xu, C.; Cao, H.; Zhang, Q.; Wang, H.; Xin, W.; Xu, E.; Zhang, S.; Yu, R.; Yu, D.; Hu, Y. Control of auxin-induced callus formation by bZIP59-LBD complex in Arabidopsis regeneration. Nat. Plants 2018, 4, 108–115. [Google Scholar] [CrossRef]
- Hu, X.; Xu, L. Transcription factors WOX11/12 directly activate WOX5/7 to promote root primordia initiation and organogenesis. Plant Physiol. 2016, 172, 2363–2373. [Google Scholar] [CrossRef]
- Liu, J.; Sheng, L.; Xu, Y.; Li, J.; Yang, Z.; Huang, H.; Xu, L. WOX11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef]
- Chen, L.; Tong, J.; Xiao, L.; Ruan, Y.; Liu, J.; Zeng, M.; Huang, H.; Wang, J.W.; Xu, L. YUCCA-mediated auxin biogenesis is required for cell fate transition occurring during de novo root organogenesis in Arabidopsis. J. Exp. Bot. 2016, 67, 4273–4284. [Google Scholar] [CrossRef]
- Wójcikowska, B.; Jaskóła, K.; Gąsiorek, P.; Meus, M.; Nowak, K.; Gaj, M.D. LEAFY COTYLEDON2 (LEC2) promotes embryogenic induction in somatic tissues of Arabidopsis, via YUCCA-mediated auxin biosynthesis. Planta 2013, 238, 425–440. [Google Scholar] [CrossRef]
- Li, M.; Wrobel-Marek, J.; Heidmann, I.; Horstman, A.; Chen, B.; Reis, R.; Angenent, G.C.; Boutilier, K. Auxin biosynthesis maintains embryo identity and growth during BABY BOOM-induced somatic embryogenesis. Plant Physiol. 2021, 188, 1095–1110. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Zhang, Y.; Li, Y.; Pei, Y.; Zhang, M. Regulation of PIN-FORMED protein degradation. Int. J. Mol. Sci. 2023, 24, 843. [Google Scholar] [CrossRef]
- Gordon, S.P.; Heisler, M.G.; Reddy, G.V.; Ohno, C.; Das, P.; Meyerowitz, E.M. Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development 2007, 134, 3539–3548. [Google Scholar] [CrossRef]
- Dai, X.; Liu, Z.; Qiao, M.; Li, J.; Li, S.; Xiang, F. ARR12 promotes de novo shoot regeneration in Arabidopsis thaliana via activation of WUSCHEL expression. J. Integr. Plant Biol. 2017, 59, 747–758. [Google Scholar] [CrossRef]
- Argueso, C.T.; Raines, T.; Kieber, J.J. Cytokinin signaling and transcriptional networks. Curr. Opin. Plant Biol. 2010, 13, 533–539. [Google Scholar] [CrossRef]
- Buechel, S.; Leibfried, A.; To, J.P.C.; Zhao, Z.; Andersen, S.U.; Kieber, J.J.; Lohmann, J.U. Role of A-type ARABIDOPSIS RESPONSE REGULATORS in meristem maintenance and regeneration. Eur. J. Cell Biol. 2010, 89, 279–284. [Google Scholar] [CrossRef]
- To, J.P.; Kieber, J.J. Cytokinin signaling: Two-components and more. Trends Plant Sci. 2008, 13, 85–92. [Google Scholar] [CrossRef]
- To, J.P.C.; Haberer, G.; Ferreira, F.J.; Deruère, J.; Mason, M.G.; Schaller, G.E.; Alonso, J.M.; Ecker, J.R.; Kieber, J.J. Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 2004, 16, 658–671. [Google Scholar] [CrossRef]
- Kiba, T.; Yamada, H.; Sato, S.; Kato, T.; Tabata, S.; Yamashino, T.; Mizuno, T. The Type-A response regulator, ARR15, acts as a negative regulator in the cytokinin-mediated signal transduction in Arabidopsis thaliana. Plant Cell Physiol. 2003, 44, 868–874. [Google Scholar] [CrossRef]
- Meng, W.; Cheng, Z.; Sang, Y.; Zhang, M.; Rong, X.; Wang, Z.; Tang, Y.; Zhang, X. Type-B ARABIDOPSIS RESPONSE REGULATORs specify the shoot stem cell niche by dual regulation of WUSCHEL. Plant Cell 2017, 29, 1357–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lian, H.; Zhou, C.; Xu, L.; Jiao, Y.; Wang, J. A two-step model for de novo activation of WUSCHEL during plant shoot regeneration. Plant Cell 2017, 29, 1073–1087. [Google Scholar] [CrossRef]
- Leibfried, A.; To, J.P.C.; Busch, W.; Stehling, S.; Kehle, A.; Demar, M.; Kieber, J.J.; Lohmann, J.U. WUSCHEL controls meristem function by direct regulation of cytokinin-inducible response regulators. Nature 2005, 438, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, Y.; Cheng, Y.; Huang, J.; Lian, J.; Yang, L.; He, R.; Lei, M.; Liu, Y.; Yuan, C.; et al. Genome-wide analysis and functional annotation of chromatin-enriched noncoding RNAs in rice during somatic cell regeneration. Genome Biol. 2022, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Seo, P.J. Dynamic epigenetic changes during plant regeneration. Trends Plant Sci. 2018, 23, 235–247. [Google Scholar] [CrossRef]
- Lee, K.; Park, O.S.; Seo, P.J. ATXR2 as a core regulator of de novo root organogenesis. Plant Signal. Behav. 2018, 13, e1449543. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, O.S.; Seo, P.J. Arabidopsis ATXR2 deposits H3K36me3 at the promoters of LBD genes to facilitate cellular dedifferentiation. Sci. Signal. 2017, 10, eaan0316. [Google Scholar] [CrossRef]
- Lee, K.; Park, O.; Go, J.Y.; Yu, J.; Han, J.H.; Kim, J.; Bae, S.; Jung, Y.J.; Seo, P.J. Arabidopsis ATXR2 represses de novo shoot organogenesis in the transition from callus to shoot formation. Cell Rep. 2021, 37, 109980. [Google Scholar] [CrossRef]
- Lee, K.; Park, O.S.; Seo, P.J. JMJ30-mediated demethylation of H3K9me3 drives tissue identity changes to promote callus formation in Arabidopsis. Plant J. 2018, 95, 961–975. [Google Scholar] [CrossRef]
- Li, W.; Liu, H.; Cheng, Z.J.; Su, Y.H.; Han, H.N.; Zhang, Y.; Zhang, X.S. DNA methylation and histone modifications regulate de novo shoot regeneration in Arabidopsis by modulating WUSCHEL expression and auxin signaling. PLoS Genet. 2011, 7, e1002243. [Google Scholar] [CrossRef]
- Shim, S.; Lee, H.G.; Seo, P.J. MET1-dependent DNA methylation represses light signaling and influences plant regeneration in Arabidopsis. Mol. Cells 2021, 44, 746–757. [Google Scholar] [CrossRef]
- Zhang, T.; Lian, H.; Tang, H.; Dolezal, K.; Zhou, C.; Yu, S.; Chen, J.; Chen, Q.; Liu, H.; Ljung, K.; et al. An intrinsic microRNA timer regulates progressive decline in shoot regenerative capacity in plants. Plant Cell 2015, 27, 349–360. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Zhou, C.; Li, X.M.; Zhang, X. The microRNA167 controls somatic embryogenesis in Arabidopsis through regulating its target genes ARF6 and ARF8. Plant Cell Tissue Organ Cult. 2016, 124, 405–417. [Google Scholar] [CrossRef]
- Arora, S.; Singh, A.K.; Chaudhary, B. Target-mimicry based miRNA167-diminution ameliorates cotton somatic embryogenesis via transcriptional biases of auxin signaling associated miRNAs and genes. Plant Cell Tissue Organ Cult. 2020, 141, 511–531. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Wang, L.; Li, Q.; Lu, Q.; Yu, Y.; Li, S.; Bai, M.-y.; Hu, Y.; Xiang, F. Repression of callus initiation by the miRNA-directed interaction of auxin–cytokinin in Arabidopsis thaliana. Plant J. 2016, 87, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Choi, M.H.; Noh, B.; Noh, Y.S. De novo shoot regeneration controlled by HEN1 and TCP3/4 in Arabidopsis. Plant Cell Physiol. 2020, 61, 1600–1613. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Cheng, Z.; Han, Z.; Yang, H.; Zhang, W.; Zhang, H. Efficient genetic transformation and CRISPR/Cas9-mediated genome editing of watermelon assisted by genes encoding developmental regulators. J. Zhejiang Univ. Sci. B 2022, 23, 339–344. [Google Scholar] [CrossRef]
- Rengasamy, B.; Manna, M.; Jonwal, S.; Sathiyabama, M.; Thajuddin, N.B.; Sinha, A.K. A simplified and improved protocol of rice transformation to cater wide range of rice cultivars. Protoplasma 2024, 261, 641–654. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Sugimoto, K.; Iwase, A. Plant callus: Mechanisms of induction and repression. Plant Cell 2013, 25, 3159–3173. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.-Y.; Wang, J.-W. Analysis of meristems and plant regeneration at single-cell resolution. Curr. Opin. Plant Biol. 2023, 74, 102378. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, L.; Wang, S.; Wang, X.; Li, S.; Gong, P.; Bai, M.; Paul, A.; Tvedt, N.; Ren, H.; et al. AlphaFold-guided bespoke gene editing enhances field-grown soybean oil contents. Adv. Sci. 2025, 12, 2500290. [Google Scholar] [CrossRef] [PubMed]
| Developmental Regulators | Molecular Functions | Name | Species of Origin | Species of Application | Explants | Biological Functions | References |
|---|---|---|---|---|---|---|---|
| WUSCHEL-related homeobox 5 (WOX5) | maintaining stem cell identity in SAM and RAM | TaWOX5 | wheat (Triticum aestivum) | wheat (Triticum aestivum) | immature embryo | 2.1–21.4-fold increase in transformation efficiency | [21] |
| TaWOX5 | wheat (Triticum aestivum) | wheat (Triticum aestivum) | immature embryo | 2.7-fold increase in transformation efficiency | [54] | ||
| ZmWOX2a | maize (Zea mays) | maize (Zea mays) | immature embryo | improving transformation in recalcitrant variety B73 | [40,41] | ||
| MdWOX11 | apple (Malus domestica) | apple (Malus domestica) | leaves | suppressing adventitious shoot formation | [42] | ||
| BABY BOOM-WUSCHEL (BBM-WUS) | BBM promotes cell proliferation and morphogenesis during embryogenesis; WUS maintains stem cell identity in SAM and RAM | ZmBBM-WUS2 | maize (Zea mays) | wheat (Triticum aestivum) | immature embryo | 2.8-fold increase in transformation efficiency | [54] |
| ZmBBM-WUS2 | maize (Zea mays) | maize (Zea mays) | immature embryo | 2.5-fold increase in transformation efficiency | [53] | ||
| ZmBBM-WUS2 | maize (Zea mays) | maize (Zea mays) | mature embryo | improving transformation with mature embryo | [48] | ||
| ZmBBM-WUS2 | maize (Zea mays) | sorghum (Sorghum bicolor) | immature embryo | improving transformation efficiency | [48,52] | ||
| GROWTH-REGULATING FACTOR- GRF-INTERACTING FACTOR (GIF-GIF) | promoting cell proliferation during organogenesis | AtGRF5 | Arabidopsis | cassava (Manihot esculenta) | leaf petiole | improving shoot regeneration | [64] |
| AtGRF5 | Arabidopsis | sugar beet (Beta vulgaris ssp. vulgaris) | leaves | improving shoot regeneration | [65] | ||
| BvGRF5-LIKE | sugar beet (Beta vulgaris ssp. vulgaris) | sugar beet (Beta vulgaris ssp. vulgaris) | leaves | no | [65] | ||
| AtGRF5 | Arabidopsis | soybean (Glycine max) | mature embryo | improving shoot regeneration | [65] | ||
| AtGRF5 | Arabidopsis | sunflower (Helianthus annuus) | cotyledon | improving shoot regeneration | [65] | ||
| AtGRF5 | Arabidopsis | canola (Brassica napus) | hypocotyl | improving callus formation | [65] | ||
| AtGRF6 | Arabidopsis | canola (Brassica napus) | hypocotyl | improving callus formation | [65] | ||
| AtGRF9 | Arabidopsis | canola (Brassica napus) | hypocotyl | improving callus formation | [65] | ||
| BnGRF5-LIKE | canola (Brassica napus) | canola (Brassica napus) | hypocotyl | improving callus formation | [65] | ||
| ZmGRF5 | maize (Zea mays) | maize (Zea mays) | immature embryo | improving shoot regeneration | [65] | ||
| AtGRF5 | Arabidopsis | maize (Zea mays) | immature embryo | improving shoot regeneration | [65] | ||
| TaGRF4-GIF1 | wheat (Triticum aestivum) | wheat (Triticum aestivum) | immature embryo | 3.7-fold increase in transformation efficiency | [54] | ||
| GRF-GIF | grape (Vitis vinifera) | citrus | epicotyl | 4.7-fold increase in shoot regeneration | [61] | ||
| GRF-GIF | citrus | citrus | epicotyl | 4.7-fold increase in shoot regeneration | [61] | ||
| GRF4/8-GIF1 | tomato (Solanum lycopersicum) | lettuce (Lactuca spp.) | cotyledon | improving shoot regeneration | [66] | ||
| GRF4-GIF1 | pepper (Capsicum annuum) | lettuce (Lactuca spp.) | cotyledon | improving shoot regeneration | [66] | ||
| GRF4-GIF1 | citrus | lettuce (Lactuca spp.) | cotyledon | improving shoot regeneration | [66] | ||
| GRF4-GIF1 | grape (Vitis vinifera) | lettuce (Lactuca spp.) | cotyledon | improving shoot regeneration | [66] | ||
| GRF4-GIF1 | grape (Vitis vinifera) | cassava (Manihot esculenta) | leaf-petiole | improving shoot regeneration | [64] | ||
| GRF4-GIF1 | wheat (Triticum aestivum) | rice (Oryza sativa) | mature embryo | 2.1-fold increase in transformation efficiency | [61] | ||
| GmGRF3-GIF1 | soybean (Glycine max) | soybean (Glycine max) | mature embryo | 2.7-fold increase in transformation efficiency | [23] | ||
| ClGRF4-GIF1 | watermelon (Citrullus lanatus) | watermelon (Citrullus lanatus) | cotyledon | 9.0-fold increase in transformation efficiency | [63] | ||
| TaGRF4-GIF1 | wheat (Triticum aestivum) | Dendrobium catenatum | young seedling | improving shoot regeneration | [62] | ||
| DcGRF4-GIF1 | Dendrobium catenatum | Dendrobium catenatum | young seedling | improving shoot regeneration | [62] | ||
| TaGRF4-GIF1 | wheat (Triticum aestivum) | wheat (Triticum aestivum) | immature embryo | 7.8-fold increase in transformation efficiency | [61] | ||
| GRF4-GIF1 + ZmBBM-WUS2 | wheat (Triticum aestivum) | immature embryo | 5.2-fold increase in transformation efficiency | [54] | |||
| GRF4-GIF1 + ZmBBM-WUS2 | wheat (Triticum aestivum) | mature embryo | transformation efficiency from 0% to 19.4% | [54] | |||
| GRF-GIF-BBM | maize (Zea mays) | maize (Zea mays) | immature embryo | 7.0-fold increase in transformation efficiency | [82] | ||
| DNA binding with one finger (DOF) | promoting cell proliferation | TaDOF5.6 | wheat (Triticum aestivum) | wheat (Triticum aestivum) | immature embryo | 1.9-fold increase in transformation efficiency | [73] |
| TaDOF3.4 | wheat (Triticum aestivum) | wheat (Triticum aestivum) | immature embryo | 2.1-fold increase in transformation efficiency | [73] | ||
| WOUND-INDUCED DEDIFFERENTIATION1 (WIND1) | promoting cell dedifferentiation and proliferation | ZmWIND1 | maize (Zea mays) | maize (Zea mays) | immature embryo | 3.2-4.0-fold increase in transformation efficiency | [85] |
| AtWIND1 | Arabidopsis | canola (Brassica napus) | hypocotyl | improving shoot regeneration | [84] | ||
| AtWIND1 | Arabidopsis | Arabidopsis | young seedlings | improving shoot regeneration | [84] | ||
| REGENERATION FACTOR1 (REF1) | activating WIND1 expression | SIREF1 | tomato (Solanum lycopersicum) | tomato (Solanum lycopersicum) | hypocotyl | 12-fold increase in transformation efficiency | [87] |
| GmREF1 | soybean (Glycine max) | soybean (Glycine max) | mature embryo | 5-fold increase in transformation efficiency | [87] | ||
| ZmREF1 | maize (Zea mays) | maize (Zea mays) | immature embryo | 4-fold increase in transformation efficiency | [87] | ||
| TaREF1 | wheat (Triticum aestivum) | wheat (Triticum aestivum) | immature embryo | 4-fold increase in transformation efficiency | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, F.; Luo, B.; Chen, N.; Wang, Y.; Zhang, X. Mechanism and Application of Developmental Factors in Plant Genetic Transformation. Int. J. Mol. Sci. 2025, 26, 10135. https://doi.org/10.3390/ijms262010135
Zhang L, Wang F, Luo B, Chen N, Wang Y, Zhang X. Mechanism and Application of Developmental Factors in Plant Genetic Transformation. International Journal of Molecular Sciences. 2025; 26(20):10135. https://doi.org/10.3390/ijms262010135
Chicago/Turabian StyleZhang, Lixin, Fang Wang, Biao Luo, Na Chen, Yan Wang, and Xianwen Zhang. 2025. "Mechanism and Application of Developmental Factors in Plant Genetic Transformation" International Journal of Molecular Sciences 26, no. 20: 10135. https://doi.org/10.3390/ijms262010135
APA StyleZhang, L., Wang, F., Luo, B., Chen, N., Wang, Y., & Zhang, X. (2025). Mechanism and Application of Developmental Factors in Plant Genetic Transformation. International Journal of Molecular Sciences, 26(20), 10135. https://doi.org/10.3390/ijms262010135






