Rebamipide Induces Hair Regeneration Through EP4-Driven Lipid Metabolism Remodeling
Abstract
1. Introduction
2. Results
2.1. Topical Treatment with Rebamipide Induces Hair Regeneration
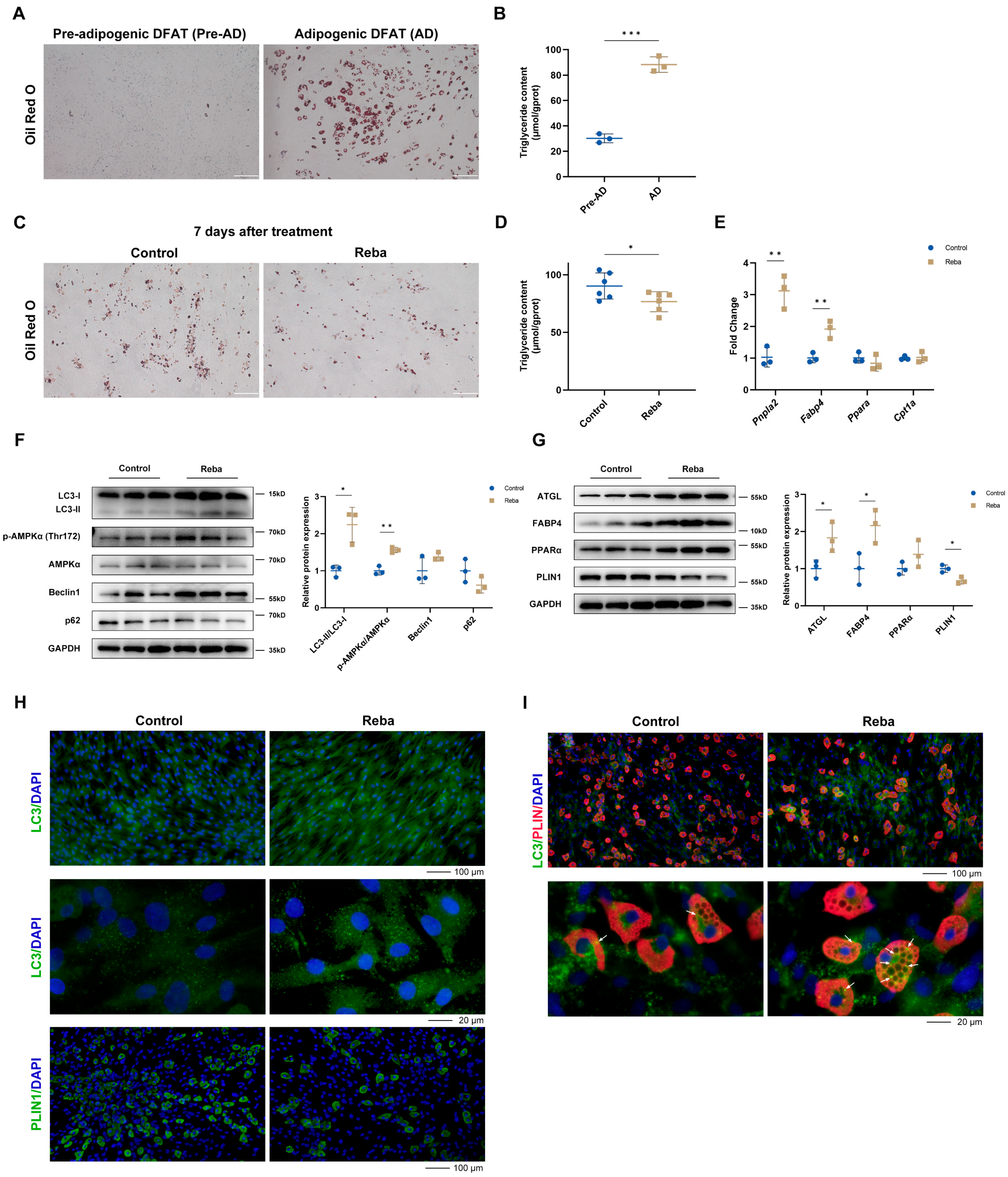
2.2. Rebamipide Induces Dermal Adipose Tissue Lipolysis
2.3. Rebamipide Induces Lipolysis in Mature Adipocytes
2.4. Rebamipide-Induced Hair Regeneration Is Reversed by Lipid Metabolism Regulators
2.5. Rebamipide Induces Adipocyte Dedifferentiation and Activates HFSCs Through Growth Factor Secretion
2.6. Rebamipide Targets EP4 to Activate Autophagy and Lipolysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Topical Treatments
4.4. Histology and Immunofluorescent Staining
4.5. RNA-Seq
4.6. RNA Isolation and Quantitative PCR
4.7. Western Blot
4.8. Double-Labeled Immunofluorescence
4.9. Primary Dedifferentiated Fat (DFAT) Cell Culture
4.10. Primary Hair Follicle Stem Cell (HFSC) Culture
4.11. Adipogenesis of DFAT Cells
4.12. Triglyceride and PDGF Assays
4.13. Conditioned Media Collection
4.14. HF Organ Culture
4.15. Cell Proliferation and Colony Formation Assays
4.16. Molecular Docking
4.17. Molecular Dynamics (MD) Simulation
4.18. Drug Affinity-Responsive Target Stability (DARTS) Experiment
4.19. Cellular Thermal Shift Assay (CETSA)
4.20. Data and Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Adenylate cyclase |
| AMPK | AMP-activated protein kinase |
| ATGL | Adipose triglyceride lipase |
| CETSA | Cellular thermal shift assay |
| CM | Conditioned medium |
| CPT1 | Carnitine palmitoyltransferase 1 |
| DARTS | Drug affinity-responsive target stability |
| DFAT cell | Dedifferentiated fat cell |
| dWAT | Dermal white adipose tissue |
| EP4 | Prostaglandin E receptor 4 |
| FABP4 | Fatty acid-binding protein 4 |
| HFSC | Hair follicle stem cell |
| PDGF | Platelet-derived growth factor |
| PGE2 | Prostaglandin E2 |
| PLIN1 | Perilipin 1 |
| TG | Triglyceride |
References
- Hamilton, J.B. Patterned loss of hair in man; types and incidence. Ann. N. Y. Acad. Sci. 1951, 53, 708–728. [Google Scholar] [CrossRef]
- Norwood, O.T. Incidence of female androgenetic alopecia (female pattern alopecia). Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. 2001, 27, 53–54. [Google Scholar]
- Wang, T.L.; Zhou, C.; Shen, Y.W.; Wang, X.Y.; Ding, X.L.; Tian, S.; Liu, Y.; Peng, G.H.; Xue, S.Q.; Zhou, J.E.; et al. Prevalence of androgenetic alopecia in China: A community-based study in six cities. Br. J. Dermatol. 2010, 162, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, D.; Wang, J.; Wang, L.; Qiu, W.; Kume, T.; Dowell, R.; Yi, R. Escape of hair follicle stem cells causes stem cell exhaustion during aging. Nat. Aging 2021, 1, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Price, V.H. Treatment of hair loss. N. Engl. J. Med. 1999, 341, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Juarez, C.F.; Plikus, M.V. Emerging nonmetabolic functions of skin fat. Nat. Rev. Endocrinol. 2018, 14, 163–173. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Zhang, Z.; Scherer, P.E. The Role of Immature and Mature Adipocytes in Hair Cycling. Trends Endocrinol. Metab. 2019, 30, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Festa, E.; Fretz, J.; Berry, R.; Schmidt, B.; Rodeheffer, M.; Horowitz, M.; Horsley, V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 2011, 146, 761–771. [Google Scholar] [CrossRef]
- Zwick, R.K.; Guerrero-Juarez, C.F.; Horsley, V.; Plikus, M.V. Anatomical, Physiological, and Functional Diversity of Adipose Tissue. Cell Metab. 2018, 27, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Kuang, S. Adipocyte dedifferentiation in health and diseases. Clin. Sci. 2019, 133, 2107–2119. [Google Scholar] [CrossRef]
- Shen, J.F.; Sugawara, A.; Yamashita, J.; Ogura, H.; Sato, S. Dedifferentiated fat cells: An alternative source of adult multipotent cells from the adipose tissues. Int. J. Oral Sci. 2011, 3, 117–124. [Google Scholar] [CrossRef]
- Dermitzakis, I.; Kampitsi, D.D.; Manthou, M.E.; Evangelidis, P.; Vakirlis, E.; Meditskou, S.; Theotokis, P. Ontogeny of Skin Stem Cells and Molecular Underpinnings. Curr. Issues Mol. Biol. 2024, 46, 8118–8147. [Google Scholar] [CrossRef]
- Chai, M.; Jiang, M.; Vergnes, L.; Fu, X.; de Barros, S.C.; Doan, N.B.; Huang, W.; Chu, J.; Jiao, J.; Herschman, H.; et al. Stimulation of Hair Growth by Small Molecules that Activate Autophagy. Cell Rep. 2019, 27, 3413–3421.e3. [Google Scholar] [CrossRef]
- Manzoor, M.; Chen, D.; Lin, J.; Wang, Y.; Xiang, L.; Qi, J. Isoquercitrin promotes hair growth through induction of autophagy and angiogenesis by targeting AMPK and IGF-1R. Phytomedicine 2025, 136, 156289. [Google Scholar] [CrossRef]
- Kang, J.I.; Choi, Y.K.; Han, S.C.; Kim, H.G.; Hong, S.W.; Kim, J.; Kim, J.H.; Hyun, J.W.; Yoo, E.S.; Kang, H.K. Limonin, a Component of Immature Citrus Fruits, Activates Anagen Signaling in Dermal Papilla Cells. Nutrients 2022, 14, 5358. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef]
- Pan, J.; Kothan, S.; Liu, L.; Moe, A.T.M.; Dong, L.; Sun, Y.; Yang, Y. Autophagy participants in the dedifferentiation of mouse 3T3-L1 adipocytes triggered by hypofunction of insulin signaling. Cell Signal. 2021, 80, 109911. [Google Scholar] [CrossRef] [PubMed]
- Genta, R.M. Review article: The role of rebamipide in the management of inflammatory disease of the gastrointestinal tract. Aliment. Pharmacol. Ther. 2003, 18 (Suppl. S1), 8–13. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, M.; Rong, Z.; Liang, H.; Xu, X.; Sun, S.; Lei, Y.; Li, P.; Meng, H.; Zheng, R.; et al. Rebamipide attenuates alcohol-induced gastric epithelial cell injury by inhibiting endoplasmic reticulum stress and activating autophagy-related proteins. Eur. J. Pharmacol. 2022, 922, 174891. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Sekino, H.; Matsuoka, O.; Saito, K.; Sekino, H.; Morikawa, A.; Uchida, K.; Koike, M.; Azuma, J. Bioequivalence of rebamipide granules and tablets in healthy adult male volunteers. Clin. Drug Investig. 2003, 23, 771–779. [Google Scholar] [CrossRef]
- Kinoshita, S.; Awamura, S.; Oshiden, K.; Nakamichi, N.; Suzuki, H.; Yokoi, N.; Rebamipide Ophthalmic Suspension Phase II Study Group. Rebamipide (OPC-12759) in the treatment of dry eye: A randomized, double-masked, multicenter, placebo-controlled phase II study. Ophthalmology 2012, 119, 2471–2478. [Google Scholar] [CrossRef]
- Elsaadany, B.; Anayb, S.M.; Mashhour, K.; Yossif, M.; Zahran, F. Rebamipide gargle and benzydamine gargle in prevention and management of chemo-radiotherapy and radiotherapy-induced oral mucositis in head and neck cancer patients (randomized clinical trial). BMC Oral Health 2024, 24, 645. [Google Scholar] [CrossRef] [PubMed]
- Jhun, J.; Kwon, J.E.; Kim, S.Y.; Jeong, J.H.; Na, H.S.; Kim, E.K.; Lee, S.H.; Jung, K.; Min, J.K.; Cho, M.L. Rebamipide ameliorates atherosclerosis by controlling lipid metabolism and inflammation. PLoS ONE 2017, 12, e0171674. [Google Scholar] [CrossRef]
- Jhun, J.; Moon, J.; Kim, S.Y.; Cho, K.H.; Na, H.S.; Choi, J.; Jung, Y.J.; Song, K.Y.; Min, J.K.; Cho, M.L. Rebamipide treatment ameliorates obesity phenotype by regulation of immune cells and adipocytes. PLoS ONE 2022, 17, e0277692. [Google Scholar] [CrossRef]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef]
- Deschene, E.R.; Myung, P.; Rompolas, P.; Zito, G.; Sun, T.Y.; Taketo, M.M.; Saotome, I.; Greco, V. beta-Catenin activation regulates tissue growth non-cell autonomously in the hair stem cell niche. Science 2014, 343, 1353–1356. [Google Scholar] [CrossRef]
- Shaid, S.; Brandts, C.H.; Serve, H.; Dikic, I. Ubiquitination and selective autophagy. Cell Death Differ. 2013, 20, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, M.; Hepler, C.; Zi, Z.; Zhao, S.; An, Y.A.; Zhu, Y.; Ghaben, A.L.; Wang, M.Y.; Li, N.; et al. Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J. Clin. Investig. 2019, 129, 5327–5342. [Google Scholar] [CrossRef]
- Shook, B.A.; Wasko, R.R.; Mano, O.; Rutenberg-Schoenberg, M.; Rudolph, M.C.; Zirak, B.; Rivera-Gonzalez, G.C.; Lopez-Giraldez, F.; Zarini, S.; Rezza, A.; et al. Dermal Adipocyte Lipolysis and Myofibroblast Conversion Are Required for Efficient Skin Repair. Cell Stem Cell 2020, 26, 880–895.e6. [Google Scholar] [CrossRef]
- Simiczyjew, A.; Wadzynska, J.; Pietraszek-Gremplewicz, K.; Kot, M.; Zietek, M.; Matkowski, R.; Nowak, D. Melanoma cells induce dedifferentiation and metabolic changes in adipocytes present in the tumor niche. Cell. Mol. Biol. Lett. 2023, 28, 58. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, X.; Wu, S.; Liu, Y.; Guerrero-Juarez, C.F.; Liu, W.; Huang, J.; Yao, Q.; Yin, M.; Li, J.; et al. Dynamic interplay between IL-1 and WNT pathways in regulating dermal adipocyte lineage cells during skin development and wound regeneration. Cell Rep. 2023, 42, 112647. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.; Shin, S.; Song, S.U.; Sung, J.H. Minoxidil Promotes Hair Growth through Stimulation of Growth Factor Release from Adipose-Derived Stem Cells. Int. J. Mol. Sci. 2018, 19, 691. [Google Scholar] [CrossRef] [PubMed]
- Shwartz, Y.; Gonzalez-Celeiro, M.; Chen, C.L.; Pasolli, H.A.; Sheu, S.H.; Fan, S.M.; Shamsi, F.; Assaad, S.; Lin, E.T.; Zhang, B.; et al. Cell Types Promoting Goosebumps Form a Niche to Regulate Hair Follicle Stem Cells. Cell 2020, 182, 578–593.e19. [Google Scholar] [CrossRef]
- Quan, R.; Zheng, X.; Ni, Y.; Xie, S.; Li, C. Culture and characterization of rat hair follicle stem cells. Cytotechnology 2016, 68, 621–628. [Google Scholar] [CrossRef]
- Takahashi, M.; Takada, H.; Takagi, K.; Kataoka, S.; Soma, R.; Kuwayama, H. Gastric restitution is inhibited by dexamethasone, which is reversed by hepatocyte growth factor and rebamipide. Aliment. Pharmacol. Ther. 2003, 18 (Suppl. S1), 126–132. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, F.; Zhou, M.; Chen, S.; Huang, H.; Liu, Y.; Zhao, X.; Zhang, Q.; Zhou, X.; Li, Z.; et al. Enhancement of hair growth through stimulation of hair follicle stem cells by prostaglandin E2 collagen matrix. Exp. Cell Res. 2022, 421, 113411. [Google Scholar] [CrossRef]
- Hanson, W.R.; Pelka, A.E.; Nelson, A.K.; Malkinson, F.D. Subcutaneous or topical administration of 16,16 dimethyl prostaglandin E2 protects from radiation-induced alopecia in mice. Int. J. Radiat. Oncol. Biol. Phys. 1992, 23, 333–337. [Google Scholar] [CrossRef]
- Konya, V.; Marsche, G.; Schuligoi, R.; Heinemann, A. E-type prostanoid receptor 4 (EP4) in disease and therapy. Pharmacol. Ther. 2013, 138, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kageyama, S.; Ito, T.; Hirao, H.; Kadono, K.; Aziz, A.; Dery, K.J.; Everly, M.J.; Taura, K.; Uemoto, S.; et al. Antibiotic pretreatment alleviates liver transplant damage in mice and humans. J. Clin. Investig. 2019, 129, 3420–3434. [Google Scholar] [CrossRef]
- Inazumi, T.; Yamada, K.; Shirata, N.; Sato, H.; Taketomi, Y.; Morita, K.; Hohjoh, H.; Tsuchiya, S.; Oniki, K.; Watanabe, T.; et al. Prostaglandin E(2)-EP4 Axis Promotes Lipolysis and Fibrosis in Adipose Tissue Leading to Ectopic Fat Deposition and Insulin Resistance. Cell Rep. 2020, 33, 108265. [Google Scholar] [CrossRef]
- Huang, S.M.; Xiong, M.Y.; Liu, L.; Mu, J.; Wang, M.W.; Jia, Y.L.; Cai, K.; Tie, L.; Zhang, C.; Cao, S.; et al. Single hormone or synthetic agonist induces G(s)/G(i) coupling selectivity of EP receptors via distinct binding modes and propagating paths. Proc. Natl. Acad. Sci. USA 2023, 120, e2216329120. [Google Scholar] [CrossRef]
- Trefts, E.; Shaw, R.J. AMPK: Restoring metabolic homeostasis over space and time. Mol. Cell 2021, 81, 3677–3690. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, U.; Iwatsubo, K.; Umemura, M.; Fujita, T.; Ishikawa, Y. The prostanoid EP4 receptor and its signaling pathway. Pharmacol. Rev. 2013, 65, 1010–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.A.; Song, A.; Chen, W.; Schwalie, P.C.; Zhang, F.; Vishvanath, L.; Jiang, L.; Ye, R.; Shao, M.; Tao, C.; et al. Reversible De-differentiation of Mature White Adipocytes into Preadipocyte-like Precursors during Lactation. Cell Metab. 2018, 28, 282–288.e3. [Google Scholar] [CrossRef] [PubMed]
- Nicu, C.; Hardman, J.A.; Pople, J.; Paus, R. Do human dermal adipocytes switch from lipogenesis in anagen to lipophagy and lipolysis during catagen in the human hair cycle? Exp. Dermatol. 2019, 28, 432–435. [Google Scholar] [CrossRef]
- Grabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef]
- Forni, M.F.; Peloggia, J.; Braga, T.T.; Chinchilla, J.E.O.; Shinohara, J.; Navas, C.A.; Camara, N.O.S.; Kowaltowski, A.J. Caloric Restriction Promotes Structural and Metabolic Changes in the Skin. Cell Rep. 2017, 20, 2678–2692. [Google Scholar] [CrossRef]
- Chen, H.; Liu, C.; Cui, S.; Xia, Y.; Zhang, K.; Cheng, H.; Peng, J.; Yu, X.; Li, L.; Yu, H.; et al. Intermittent fasting triggers interorgan communication to suppress hair follicle regeneration. Cell 2025, 188, 157–174.e22. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, S.; Kim, W.; Kim, D.; Yang, Y.; Yoon, J.H.; Kim, B.J.; Min, D.S.; Jung, Y. Rebamipide induces the gastric mucosal protective factor, cyclooxygenase-2, via activation of 5’-AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2017, 483, 449–455. [Google Scholar] [CrossRef]
- Cao, Y.; Mai, W.; Li, R.; Deng, S.; Li, L.; Zhou, Y.; Qin, Q.; Zhang, Y.; Zhou, X.; Han, M.; et al. Macrophages evoke autophagy of hepatic stellate cells to promote liver fibrosis in NAFLD mice via the PGE2/EP4 pathway. Cell. Mol. Life Sci. 2022, 79, 303. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Liu, Y.; Xin, W.; Qin, S.; Gong, S.; Xiao, T.; Zhang, D.; Li, Y.; Xiong, J.; Yang, K.; et al. Activation of EP4 alleviates AKI-to-CKD transition through inducing CPT2-mediated lipophagy in renal macrophages. Front. Pharmacol. 2022, 13, 1030800. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, Y.; Tsuboi, H.; Okuno, Y.; Tamba, S.; Tsuchiya, S.; Tsujimoto, G.; Ichikawa, A. Microarray evaluation of EP4 receptor-mediated prostaglandin E2 suppression of 3T3-L1 adipocyte differentiation. Biochem. Biophys. Res. Commun. 2004, 322, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ying, F.; Song, E.; Wang, Y.; Xu, A.; Vanhoutte, P.M.; Tang, E.H. Mice lacking prostaglandin E receptor subtype 4 manifest disrupted lipid metabolism attributable to impaired triglyceride clearance. FASEB J. 2015, 29, 4924–4936. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Bai, B.; Song, G.; Zhang, J.; Yu, H.; Huang, S.; Wang, Z.; Lu, G. Topical drug delivery strategies for enhancing drug effectiveness by skin barriers, drug delivery systems and individualized dosing. Front. Pharmacol. 2023, 14, 1333986. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.H.; Cho, C.S.; Lee, S.T.; Yoo, W.H.; Kim, S.K.; Kang, Y.M.; Rew, J.S.; Park, Y.W.; Lee, S.K.; et al. Preventive efficacy and safety of rebamipide in nonsteroidal anti-inflammatory drug-induced mucosal toxicity. Gut Liver 2014, 8, 371–379. [Google Scholar] [CrossRef]
- Jang, E.; Park, M.; Jeong, J.E.; Lee, J.Y.; Kim, M.G. Frequently reported adverse events of rebamipide compared to other drugs for peptic ulcer and gastroesophageal reflux disease. Sci. Rep. 2022, 12, 7839. [Google Scholar] [CrossRef]
- Zhou, H.; He, J.; Liu, R.; Cheng, J.; Yuan, Y.; Mao, W.; Zhou, J.; He, H.; Liu, Q.; Tan, W.; et al. Microenvironment-responsive metal-phenolic network release platform with ROS scavenging, anti-pyroptosis, and ECM regeneration for intervertebral disc degeneration. Bioact. Mater. 2024, 37, 51–71. [Google Scholar] [CrossRef]
- Wei, S.; Du, M.; Jiang, Z.; Duarte, M.S.; Fernyhough-Culver, M.; Albrecht, E.; Will, K.; Zan, L.; Hausman, G.J.; Elabd, E.M.; et al. Bovine dedifferentiated adipose tissue (DFAT) cells: DFAT cell isolation. Adipocyte 2013, 2, 148–159. [Google Scholar] [CrossRef]
- Philpott, M.P. Hair Follicle Culture. In TRP Channels in Drug Discovery: Volume II; Szallasi, A., Bíró, T., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 287–299. [Google Scholar]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein-ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein-ligand interaction profiler to DNA and RNA. Nucleic Acids Res 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Dong, H.; Jiang, D.; Gao, Y.; Gu, X.; Diao, W.; Zhou, Y.; Xu, D.; Li, R.; Wu, L. Rebamipide Induces Hair Regeneration Through EP4-Driven Lipid Metabolism Remodeling. Int. J. Mol. Sci. 2025, 26, 10132. https://doi.org/10.3390/ijms262010132
Feng C, Dong H, Jiang D, Gao Y, Gu X, Diao W, Zhou Y, Xu D, Li R, Wu L. Rebamipide Induces Hair Regeneration Through EP4-Driven Lipid Metabolism Remodeling. International Journal of Molecular Sciences. 2025; 26(20):10132. https://doi.org/10.3390/ijms262010132
Chicago/Turabian StyleFeng, Chenjie, Hao Dong, Dongyue Jiang, Yuan Gao, Xinyue Gu, Weiwei Diao, Ying Zhou, Dayang Xu, Ruixin Li, and Liang Wu. 2025. "Rebamipide Induces Hair Regeneration Through EP4-Driven Lipid Metabolism Remodeling" International Journal of Molecular Sciences 26, no. 20: 10132. https://doi.org/10.3390/ijms262010132
APA StyleFeng, C., Dong, H., Jiang, D., Gao, Y., Gu, X., Diao, W., Zhou, Y., Xu, D., Li, R., & Wu, L. (2025). Rebamipide Induces Hair Regeneration Through EP4-Driven Lipid Metabolism Remodeling. International Journal of Molecular Sciences, 26(20), 10132. https://doi.org/10.3390/ijms262010132





