Narciclasine as a Novel Treatment for Lung Cancer and Malignant Pleural Mesothelioma: Insights from 3D Tumor Spheroid Models
Abstract
1. Introduction
2. Results
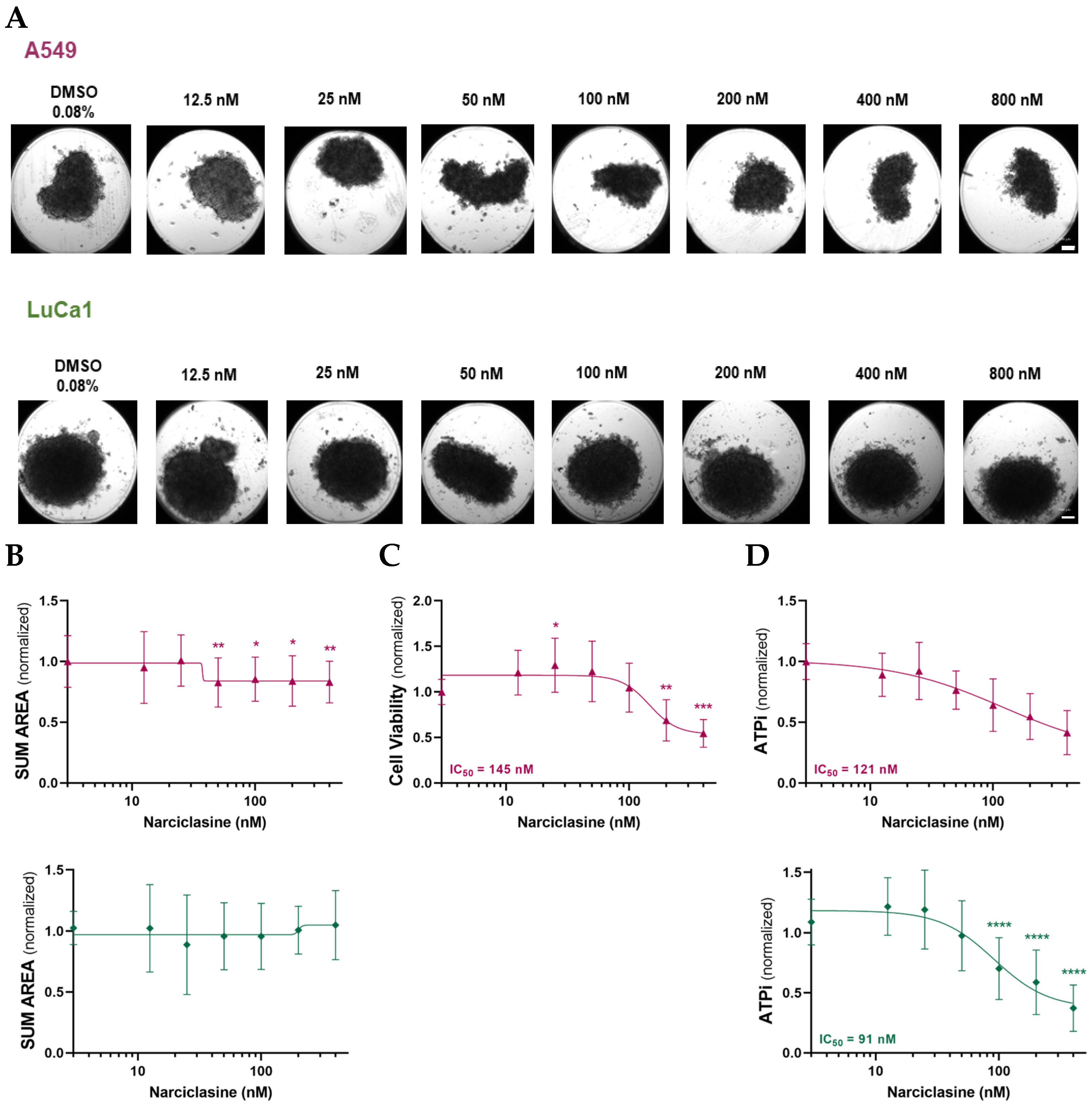
2.1. Narciclasine Induces Morphological Changes in Lung Adenocarcinoma and Malignant Mesothelioma Spheroids
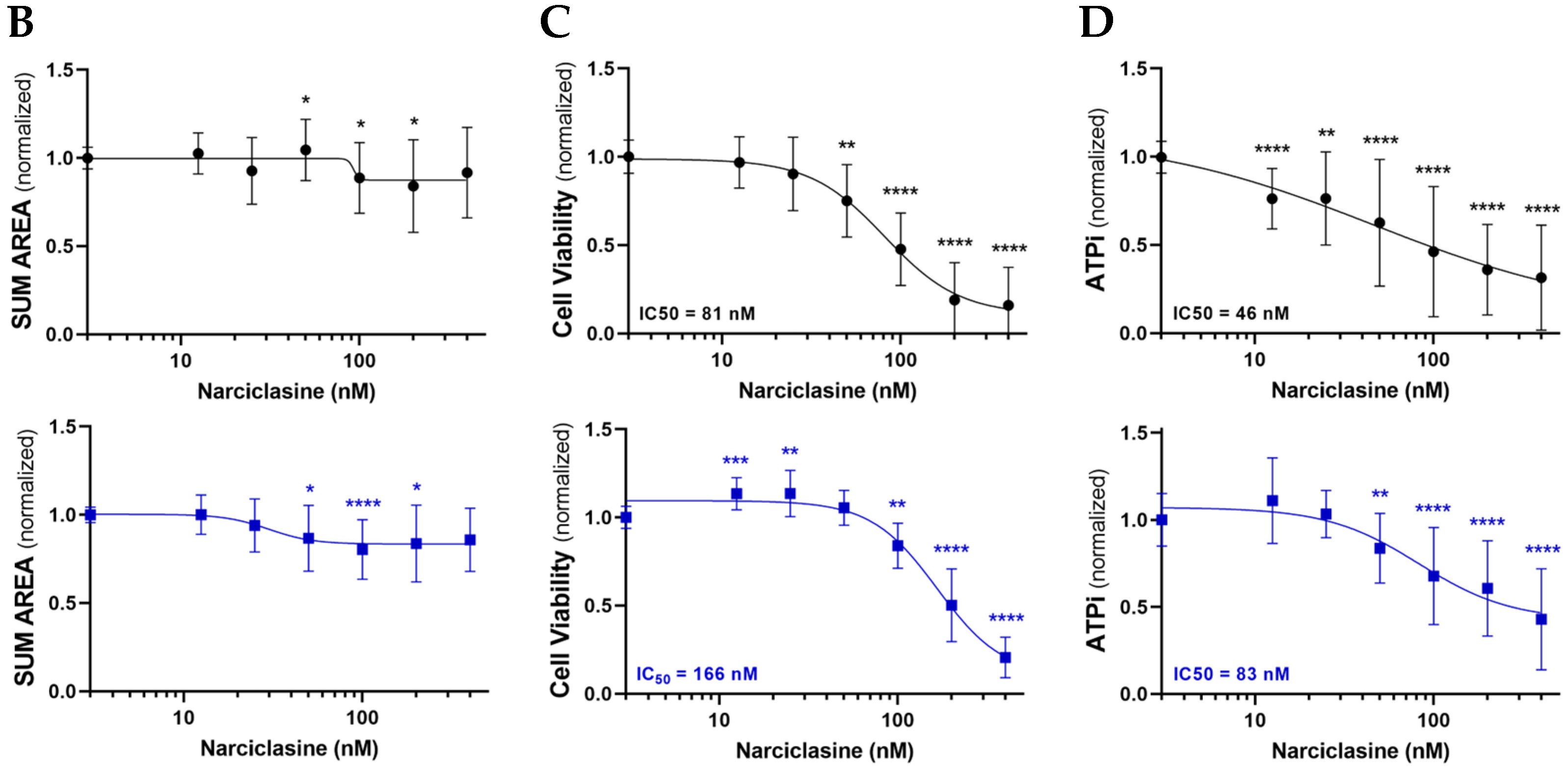
2.2. Narciclasine Affects the Viability of Lung Adenocarcinoma and Pleural Mesothelioma Cells
2.3. Narciclasine Affects Cell Proliferation and Cell Death in Lung Adenocarcinoma and Pleural Mesothelioma Spheroids
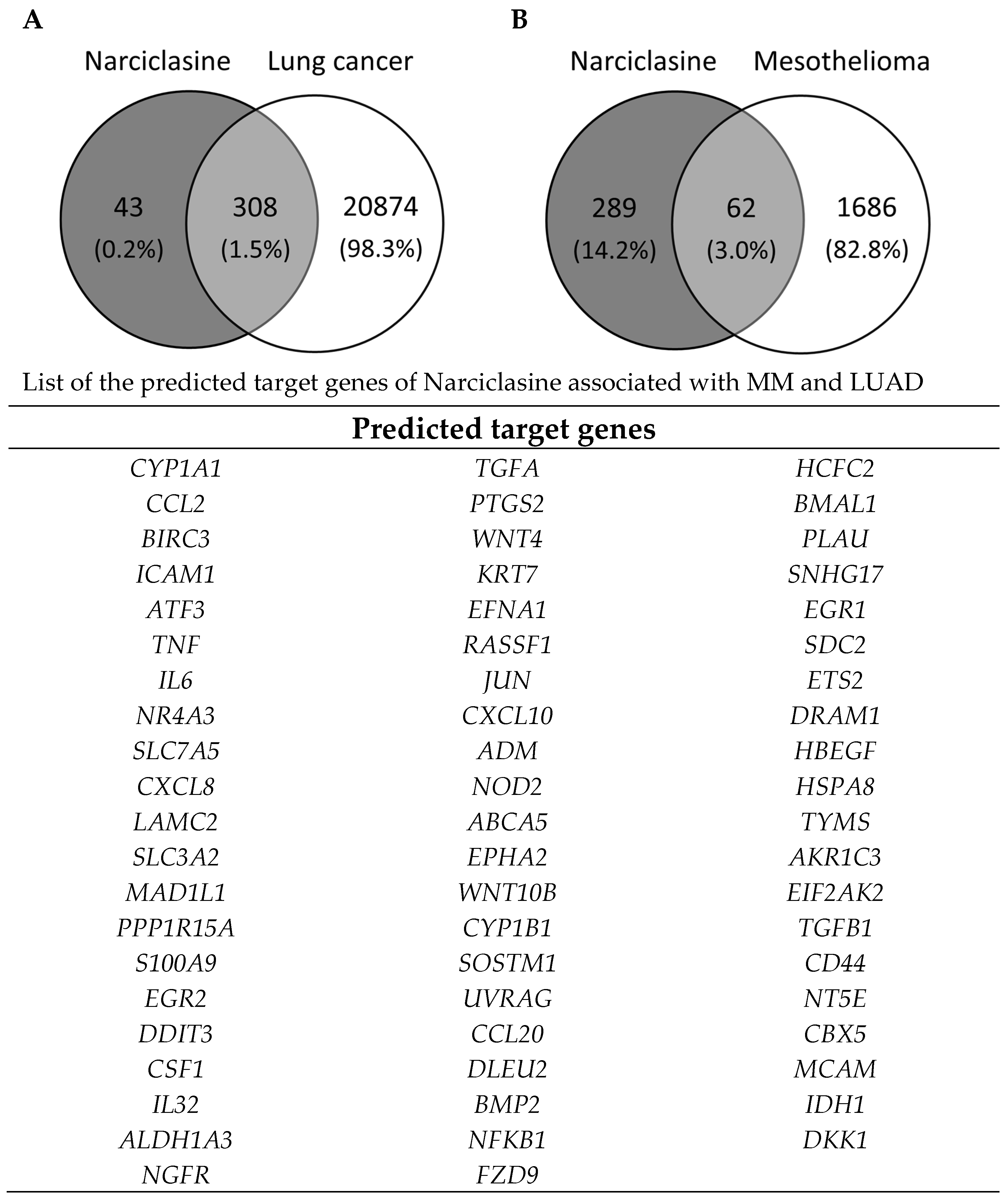
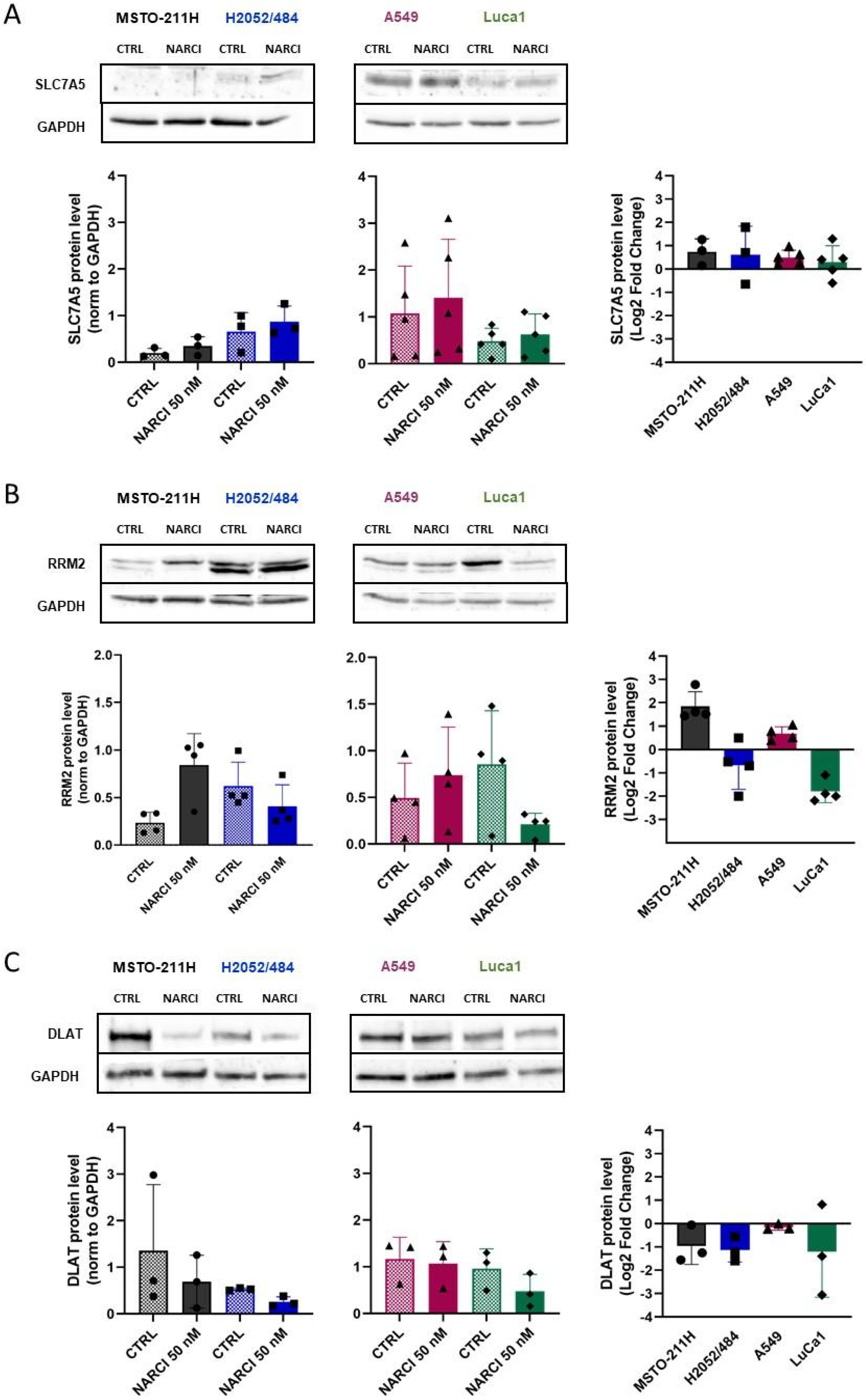
2.4. Identification of Narciclasine Gene Targets
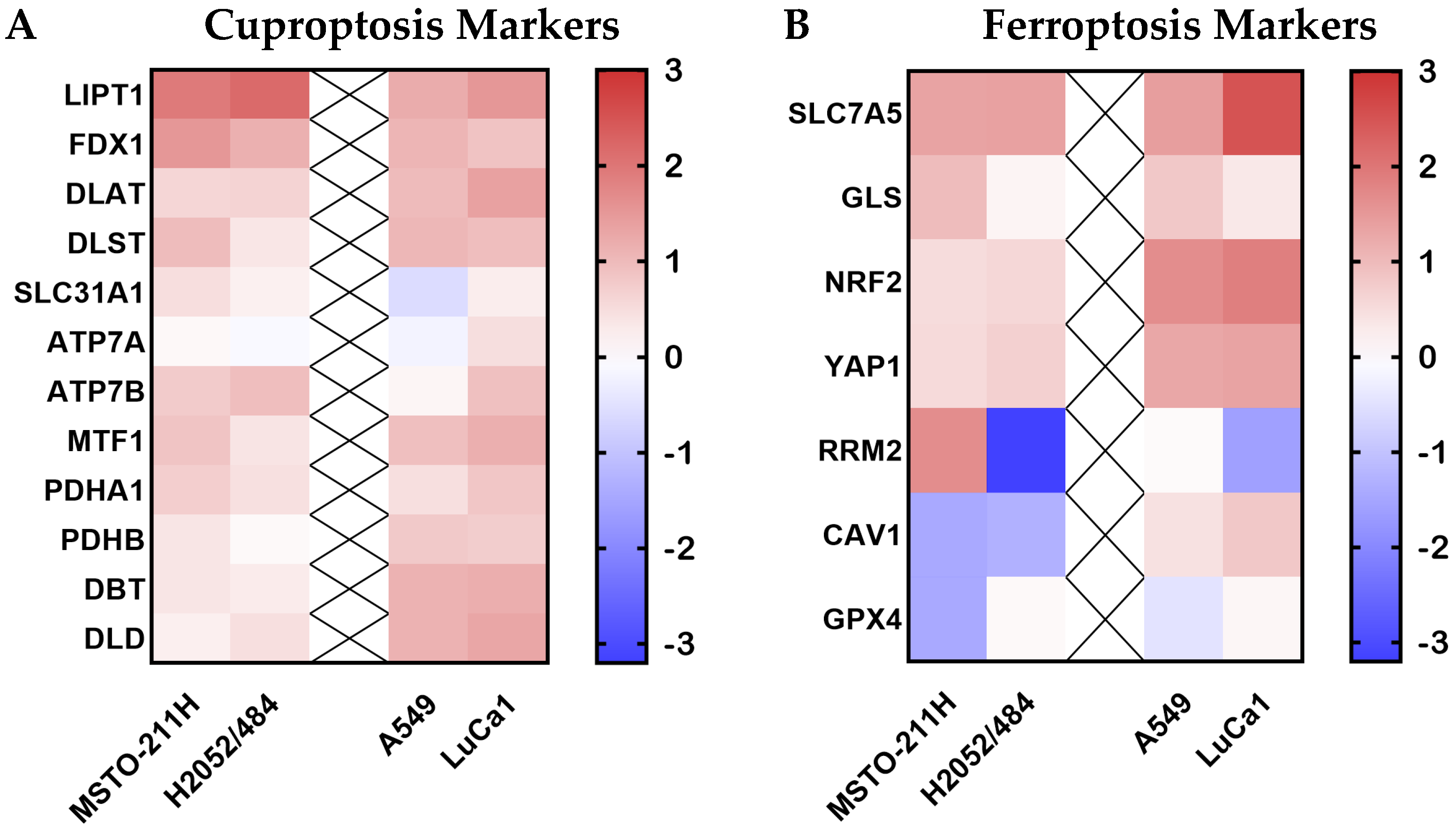
2.5. Narciclasine Effects on the Ferroptosis and Cuproptosis Pathways
2.6. Narciclasine Affects the EMT State of Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell and Spheroid Culture
4.2. Narciclasine Treatment
4.3. Cell Viability and Cytotoxicity Assays
4.4. Quantification of Cell Proliferation and Apoptosis
4.5. RNA Extraction and Quantitative RT-PCR (qPCR)
4.6. Network Pharmacology Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATPi | Intracellular adenosine triphosphate |
| 3D | Three-dimensional |
| DMSO | Dimethyl sulfoxide |
| EMT | Epithelial-to-mesenchymal transition |
| FBS | Fetal Bovine serum |
| LDH | Lactate dehydrogenase |
| LUAD | Lung adenocarcinoma |
| MM | Malignant pleural mesothelioma |
| NSCLC | Non-small cell lung cancer |
| RPMI | Roswell Park Memorial Institute |
| SCLC | Small-cell lung cancer |
| SD | Standard deviation |
| TCM | Traditional Chinese Medicine |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labeling |
References
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef]
- Parra, E.R.; Zhang, J.; Duose, D.Y.; Gonzalez-Kozlova, E.; Redman, M.W.; Chen, H.; Manyam, G.C.; Kumar, G.; Zhang, J.; Song, X.; et al. Multi-omics Analysis Reveals Immune Features Associated with Immunotherapy Benefit in Patients with Squamous Cell Lung Cancer from Phase III Lung-MAP S1400I Trial. Clin. Cancer Res. 2024, 30, 1655–1668. [Google Scholar] [CrossRef]
- Sinn, K.; Mosleh, B.; Hoda, M.A. Malignant pleural mesothelioma: Recent developments. Curr. Opin. Oncol. 2021, 33, 80–86. [Google Scholar] [CrossRef]
- Tsao, A.S.; Pass, H.I.; Rimner, A.; Mansfield, A.S. New Era for Malignant Pleural Mesothelioma: Updates on Therapeutic Options. J. Clin. Oncol. 2022, 40, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Benzerdjeb, N.; Dartigues, P.; Kepenekian, V.; Damiola, F.; Sequeiros, R.; Galateau-Salle, F.; Begueret, H.; Mery, E.; Damotte, D.; Verriele, V.; et al. Update on gene fusions and the emerging clinicopathological landscape of peritoneal and pleural mesotheliomas and other neoplasms. ESMO Open 2024, 9, 103644. [Google Scholar] [CrossRef] [PubMed]
- Ramundo, V.; Zanirato, G.; Aldieri, E. The Epithelial-to-Mesenchymal Transition (EMT) in the Development and Metastasis of Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2021, 22, 12216. [Google Scholar] [CrossRef]
- Janes, S.M.; Alrifai, D.; Fennell, D.A. Perspectives on the Treatment of Malignant Pleural Mesothelioma. N. Engl. J. Med. 2021, 385, 1207–1218. [Google Scholar] [CrossRef]
- Ji, Q.; Luo, Y.Q.; Wang, W.H.; Liu, X.; Li, Q.; Su, S.B. Research advances in traditional Chinese medicine syndromes in cancer patients. J. Integr. Med. 2016, 14, 12–21. [Google Scholar] [CrossRef]
- Liu, Q.; Guo, L.; Qi, H.; Lou, M.; Wang, R.; Hai, B.; Xu, K.; Zhu, L.; Ding, Y.; Li, C.; et al. A MYBL2 complex for RRM2 transactivation and the synthetic effect of MYBL2 knockdown with WEE1 inhibition against colorectal cancer. Cell Death Dis. 2021, 12, 683. [Google Scholar] [CrossRef]
- Wei, S.J.; Yang, I.H.; Mohiuddin, I.S.; Kshirsagar, G.J.; Nguyen, T.H.; Trasti, S.; Maurer, B.J.; Kang, M.H. DNA-PKcs as an upstream mediator of OCT4-induced MYC activation in small cell lung cancer. Biochim. Biophys. Acta Gene Regul. Mech. 2023, 1866, 194939. [Google Scholar] [CrossRef]
- Li, Z.; Feiyue, Z.; Gaofeng, L. Traditional Chinese medicine and lung cancer–From theory to practice. Biomed. Pharmacother. 2021, 137, 111381. [Google Scholar] [CrossRef]
- Su, X.L.; Wang, J.W.; Che, H.; Wang, C.F.; Jiang, H.; Lei, X.; Zhao, W.; Kuang, H.X.; Wang, Q.H. Clinical application and mechanism of traditional Chinese medicine in treatment of lung cancer. Chin. Med. J. 2020, 133, 2987–2997. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Xu, L.; Li, H.G.; Tian, J.H.; Jiao, L.J.; You, S.F.; Han, Z.F.; Jiang, Y.; Guo, H.R.; Liu, H. Chemotherapy in conjunction with traditional Chinese medicine for survival of elderly patients with advanced non-small-cell lung cancer: Protocol for a randomized double-blind controlled trial. J. Integr. Med. 2014, 12, 175–181. [Google Scholar] [CrossRef]
- Weng, T.Y.; Wu, H.F.; Li, C.Y.; Hung, Y.H.; Chang, Y.W.; Chen, Y.L.; Hsu, H.P.; Chen, Y.H.; Wang, C.Y.; Chang, J.Y.; et al. Homoharringtonine induced immune alteration for an Efficient Anti-tumor Response in Mouse Models of Non-small Cell Lung Adenocarcinoma Expressing Kras Mutation. Sci. Rep. 2018, 8, 8216. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Hsieh, L.T.; Saarberg, W.; Erdelmeier, C.A.; Wichelhaus, T.A.; Schaefer, L.; Koch, E.; Fürst, R. Haemanthus coccineus extract and its main bioactive component narciclasine display profound anti-inflammatory activities in vitro and in vivo. J. Cell Mol. Med. 2015, 19, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, R.; Matta, H.; Choi, S.; Chaudhary, P.M. Narciclasine, an isocarbostyril alkaloid, has preferential activity against primary effusion lymphoma. Sci. Rep. 2020, 10, 5712. [Google Scholar] [CrossRef]
- Lv, C.; Huang, Y.; Huang, R.; Wang, Q.; Zhang, H.; Jin, J.; Lu, D.; Zhou, Y.; Shen, Y.; Zhang, W.; et al. Narciclasine targets STAT3 via distinct mechanisms in tamoxifen-resistant breast cancer cells. Mol. Ther. Oncolytics 2022, 24, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Fang, B.; Thuy, N.T.T.; Li, A.; Yoo, H.M.; Zheng, X.; Cho, N. Narciclasine suppresses esophageal cancer cell proliferation and migration by inhibiting the FAK signaling pathway. Eur. J. Pharmacol. 2022, 921, 174669. [Google Scholar] [CrossRef]
- Shieu, M.K.; Ho, H.Y.; Lin, C.C.; Lo, Y.S.; Chuang, Y.C.; Hsieh, M.J.; Chen, M.K. Narciclasine suppresses oral cancer metastasis by modulating cathepsin B and extracellular signal-related kinase pathways. Biomed. Pharmacother. 2023, 158, 114159. [Google Scholar] [CrossRef]
- Yuan, Y.; He, X.; Li, X.; Liu, Y.; Tang, Y.; Deng, H.; Shi, X. Narciclasine induces autophagy-mediated apoptosis in gastric cancer cells through the Akt/mTOR signaling pathway. BMC Pharmacol. Toxicol. 2021, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Bräutigam, J.; Bischoff, I.; Schürmann, C.; Buchmann, G.; Epah, J.; Fuchs, S.; Heiss, E.; Brandes, R.P.; Fürst, R. Narciclasine inhibits angiogenic processes by activation of Rho kinase and by downregulation of the VEGF receptor 2. J. Mol. Cell Cardiol. 2019, 135, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Huang, W.; Zhang, N.; Wu, F.; Xu, T.; Pan, X.; Peng, C.; Han, B. Narciclasine induces autophagy-dependent apoptosis in triple-negative breast cancer cells by regulating the AMPK-ULK1 axis. Cell Prolif. 2018, 51, e12518. [Google Scholar] [CrossRef]
- Van Goietsenoven, G.; Mathieu, V.; Lefranc, F.; Kornienko, A.; Evidente, A.; Kiss, R. Narciclasine as well as other Amaryllidaceae isocarbostyrils are promising GTP-ase targeting agents against brain cancers. Med. Res. Rev. 2013, 33, 439–455. [Google Scholar] [CrossRef]
- Dumont, P.; Ingrassia, L.; Rouzeau, S.; Ribaucour, F.; Thomas, S.; Roland, I.; Darro, F.; Lefranc, F.; Kiss, R. The Amaryllidaceae isocarbostyril narciclasine induces apoptosis by activation of the death receptor and/or mitochondrial pathways in cancer cells but not in normal fibroblasts. Neoplasia 2007, 9, 766–776. [Google Scholar] [CrossRef]
- Kawamoto, R.; Nakano, N.; Ishikawa, H.; Tashiro, E.; Nagano, W.; Sano, K.; Irie, M.; Ikuta, M.; Kishi, F.; Nakane, T.; et al. Narciclasine is a novel YAP inhibitor that disturbs interaction between YAP and TEAD4. BBA Adv. 2021, 1, 100008. [Google Scholar] [CrossRef]
- Turanli, B. Decoding Systems Biology of Inflammation Signatures in Cancer Pathogenesis: Pan-Cancer Insights from 12 Common Cancers. Omics 2023, 27, 483–493. [Google Scholar] [CrossRef]
- Lee, J.H.; Seo, S.H.; Shim, J.; Kim, Y.N.; Yoon, K. Narciclasine enhances cisplatin-induced apoptotic cell death by inducing unfolded protein response-mediated regulation of NOXA and MCL1. Cell Mol. Biol. Lett. 2025, 30, 59. [Google Scholar] [CrossRef] [PubMed]
- Katayama, H.; Sasai, K.; Kloc, M.; Brinkley, B.R.; Sen, S. Aurora kinase-A regulates kinetochore/chromatin associated microtubule assembly in human cells. Cell Cycle 2008, 7, 2691–2704. [Google Scholar] [CrossRef]
- Cheng, A.; Zhang, P.; Wang, B.; Yang, D.; Duan, X.; Jiang, Y.; Xu, T.; Jiang, Y.; Shi, J.; Ding, C.; et al. Aurora-A mediated phosphorylation of LDHB promotes glycolysis and tumor progression by relieving the substrate-inhibition effect. Nat. Commun. 2019, 10, 5566. [Google Scholar] [CrossRef]
- Zangari, J.; Stehling, O.; Freibert, S.A.; Bhattacharya, K.; Rouaud, F.; Serre-Beinier, V.; Maundrell, K.; Montessuit, S.; Ferre, S.M.; Vartholomaiou, E.; et al. D-cysteine impairs tumour growth by inhibiting cysteine desulfurase NFS1. Nat. Metab. 2025, 7, 1646–1662. [Google Scholar] [CrossRef]
- Zhan, Y.; Jiang, L.; Jin, X.; Ying, S.; Wu, Z.; Wang, L.; Yu, W.; Tong, J.; Zhang, L.; Lou, Y.; et al. Inhibiting RRM2 to enhance the anticancer activity of chemotherapy. Biomed. Pharmacother. 2021, 133, 110996. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.S.; Roche, T.E. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. Faseb J. 1990, 4, 3224–3233. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.; Deogharkar, A.; McKolay, J.; Smith, K.S.; Panwalkar, P.; Hoffman, S.; Tian, W.; Ji, S.; Azambuja, A.P.; Natarajan, S.K.; et al. Isocitrate dehydrogenase 1 primes group-3 medulloblastomas for cuproptosis. Cancer Cell 2025, 43, 1159–1174.e1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Lu, S.; Cao, Z.; Li, H.; Xu, J.; Zhou, Q.; Yin, H.; Qian, Q.; Zhang, X.; Tao, M.; et al. Pyruvate metabolism enzyme DLAT promotes tumorigenesis by suppressing leucine catabolism. Cell Metab. 2025, 37, 1381–1399.e1389. [Google Scholar] [CrossRef]
- Chrzan, N.; Hartman, M.L. Copper in melanoma: At the crossroad of protumorigenic and anticancer roles. Redox Biol. 2025, 81, 103552. [Google Scholar] [CrossRef]
- Li, Z.N.; Shu, Y.; Chen, C.G.; Li, X.Q.; Li, M.Y.; Zhao, X.H.; Wang, S.; Li, J. Acquired tamoxifen resistance is surmounted by GW8510 through ribonucleotide reductase M2 downregulation-mediated autophagy induction. Biochem. Biophys. Res. Commun. 2020, 528, 554–560. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, J.; Guo, S.; Xue, X.; Wang, Y.; Qiu, S.; Cui, J.; Ma, L.; Zhang, X.; Wang, J. RRM2 protects against ferroptosis and is a tumor biomarker for liver cancer. Cancer Cell Int. 2020, 20, 587. [Google Scholar] [CrossRef]
- Wei, X.; Li, X.; Hu, S.; Cheng, J.; Cai, R. Regulation of Ferroptosis in Lung Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 14614. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Y.; Zhong, W.; Wu, K.; Zhong, T.; Jiang, T. Targeting ferroptosis: A promising approach for treating lung carcinoma. Cell Death Discov. 2025, 11, 33. [Google Scholar] [CrossRef]
- Bingham, T.W.; Hernandez, L.W.; Olson, D.G.; Svec, R.L.; Hergenrother, P.J.; Sarlah, D. Enantioselective Synthesis of Isocarbostyril Alkaloids and Analogs Using Catalytic Dearomative Functionalization of Benzene. J. Am. Chem. Soc. 2019, 141, 657–670. [Google Scholar] [CrossRef]
- Southgate, E.H.; Holycross, D.R.; Sarlah, D. Total Synthesis of Lycoricidine and Narciclasine by Chemical Dearomatization of Bromobenzene. Angew. Chem. Int. Ed. Engl. 2017, 56, 15049–15052. [Google Scholar] [CrossRef] [PubMed]
- Gendre, D.A.J.; Ameti, E.; Karenovics, W.; Perriraz-Mayer, N.; Triponez, F.; Serre-Beinier, V. Optimization of tumor spheroid model in mesothelioma and lung cancers and anti-cancer drug testing in H2052/484 spheroids. Oncotarget 2021, 12, 2375–2387. [Google Scholar] [CrossRef] [PubMed]
- Colin, D.J.; Cottet-Dumoulin, D.; Faivre, A.; Germain, S.; Triponez, F.; Serre-Beinier, V. Experimental Model of Human Malignant Mesothelioma in Athymic Mice. Int. J. Mol. Sci. 2018, 19, 1881. [Google Scholar] [CrossRef] [PubMed]
- Mueggler, A.; Pilotto, E.; Perriraz-Mayer, N.; Jiang, S.; Addeo, A.; Bédat, B.; Karenovics, W.; Triponez, F.; Serre-Beinier, V. An Optimized Method to Culture Human Primary Lung Tumor Cell Spheroids. Cancers 2023, 15, 5576. [Google Scholar] [CrossRef] [PubMed]



| KEGG Pathway | |||
| Entry | Pathway | Adjusted p-Value | Genes |
| hsa04668 | TNF signaling pathway | 1.48 × 10−13 | CXCL10; JUN; IL6; CSF1; CCL20; CCL2; NOD2; PTGS2; TNF; NFKB1; ICAM1; BIRC3 |
| hsa04657 | IL-17 signaling pathway | 2.36 × 10−11 | CXCL10; JUN; IL6; CXCL8; CCL20; CCL2; PTGS2; TNF; S100A9; NFKB1 |
| hsa04064 | NF-kappa B signaling pathway | 4.19 × 10−7 | CXCL8; PLAU; PTGS2; TNF; NFKB1; ICAM1; BIRC3 |
| hsa04210 | Apoptosis | 2.75 × 10−5 | NGFR; JUN; DDIT3; TNF; NFKB1; BIRC3 |
| MSigDB Hallmark | |||
| Hallmark Name | Adjusted p-Value | Genes | |
| Inflammatory Response | 1.95 × 10−11 | CXCL10; IL6; CXCL8; CSF1; CCL20; CCL2; EIF2AK2; ADM; NOD2; NFKB1; ICAM1; HBEGF | |
| p53 Pathway | 7.10 × 10−9 | PPP1R15A; JUN; BMP2; DRAM1; DDIT3; TGFA; SLC3A2; ATF3; EPHA2; HBEGF | |
| Epithelial–Mesenchymal Transition | 5.97 × 10−8 | IL32; NT5E; JUN; IL6; CXCL8; LAMC2; TGFBI; DKK1; CD44 | |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| ATP7A | TGACCCTAAACTACAGACTCCAA | CGCCGTAACAGTCAGAAACAA |
| ATP7B | GGCCGTCATCACTTATCAGCC | GGGAGCCACTTTGCTCTTGA |
| Cav1 | CATCCCGATGGCACTCATCTG | TGCACTGAATCTCAATCA |
| CDH1 | CCCGGGACAACGTTTATTAC | GCTGGCTCAAGTCAAAGTCC |
| CDH2 | GGTGGAGGAGAAGAAGACCAG | GGCATCAGGCTCCACAGT |
| DBT | CAGTTCGCCGTCTGGCAAT | CCTGTGAATACCGGAGGTTTTG |
| DLD | CTCATGGCCTACAGGGACTTT | GCATGTTCCACCAAGTGTTTCAT |
| DLAT | ACTCCCCAGCCTTTAGCTC | CAATCCCTTTCTCTACTGCCAAC |
| DLST | GAACTGCCCTCTAGGGAGAC | AACCTTCCTGCTGTTAGGGTA |
| EEF1A1 | ACTACCCCTAAAAGCCAAAATGG | GGTGGACTTGCCCGAATCTA |
| FDX1 | CCACTTTATAAACCGTGATGGTG | ACATGCACCAAAGCCATCAA |
| GAPDH | GCACAAGAGGAAGAGAGAGACC | AGGGGAGATTCAGTGTGGTG |
| GLS | TCTACAGGATTGCGAACGTCT | CTTTGTCTAGCATGACACCATCT |
| GPX4 | GAGGCAAGACCGAAGTAAACTAC | CCGAACTGGTTACACGGGAA |
| GUSB | ACGTGGTTGGAGAGCTCATT | CTCTGCCGAGTGAAGATCCC |
| HPRT1 | ACAGGACTGAACGTCTTGCTCG | TGATGTAATCCAGCAGGTCAGCA |
| LIPT1 | CCTCTGTTGTAATTGGTAGGCAT | CTGGGGTTGGACAGCATTCAG |
| MTF1 | CACAGTCCAGACAACAACATCA | GCACCAGTCCGTTTTTATCCAC |
| NRF2 | AGGTTGCCCACATTCCCAAA | ACGTAGCCGAAGAAACCTCA |
| PDHA1 | TGGTAGCATCCCGTAATTTTGC | ATTCGGCGTACAGTCTGCATC |
| PDHB | AAGAGGCGCTTTCACTGGAC | ACTAACCTTGTATGCCCCATCA |
| RPLP0 | GAAGACAGGGCGACCTGGAAG | GCGCATCATGGTGTTCTTGCC |
| RRM2 | CTGGCTCAAGAAACGAGGACT | ACATCAGGCAAGCAAAATCACA |
| SLC7A5 | CCGTGCCGTCCCTCG | AGAAGGCGTAGAGCAGCGTC |
| SLC31A1 | GGGGATGAGCTATATGGACTCC | TCACCAAACCGGAAAACAGTAG |
| SNAIL-1 | GCTGCAGGACTCTAATCCAGA | ATCTCCGGAGGTGGGAT |
| SNAIL-2 | TGGTTGCTTCAAGGACACAT | GTTGCAGTGAGGGCAAGAA |
| TBP | GCCCGAAACGCCGAATATA | CGTGGCTCTCTTATCCTCATGA |
| TWIST | CGGCCAGGTACATCGACT | CATCTTGGAGTCCAGCTCGT |
| VIM | AGATGGCCCTTGACATTGAG | TGGAAGAGGCAGAGAAATCC |
| YAP1 | AGAGGCTGCGGCTGAAAC | TTGCTGTGCTGGGATTGATA |
| ZEB-1 | GCCAACAGACCAGACAGTGTT | CAGGAAAGGAAGGGCAAGA |
| ZEB-2 | CAAGAGGCGCAAACAAG | AACCTGTGTCCACTACATTGTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Berkcan, S.; Perriraz-Mayer, N.; Triponez, F.; Serre-Beinier, V. Narciclasine as a Novel Treatment for Lung Cancer and Malignant Pleural Mesothelioma: Insights from 3D Tumor Spheroid Models. Int. J. Mol. Sci. 2025, 26, 10127. https://doi.org/10.3390/ijms262010127
Jiang S, Berkcan S, Perriraz-Mayer N, Triponez F, Serre-Beinier V. Narciclasine as a Novel Treatment for Lung Cancer and Malignant Pleural Mesothelioma: Insights from 3D Tumor Spheroid Models. International Journal of Molecular Sciences. 2025; 26(20):10127. https://doi.org/10.3390/ijms262010127
Chicago/Turabian StyleJiang, Sicong, Serkan Berkcan, Nadja Perriraz-Mayer, Frédéric Triponez, and Véronique Serre-Beinier. 2025. "Narciclasine as a Novel Treatment for Lung Cancer and Malignant Pleural Mesothelioma: Insights from 3D Tumor Spheroid Models" International Journal of Molecular Sciences 26, no. 20: 10127. https://doi.org/10.3390/ijms262010127
APA StyleJiang, S., Berkcan, S., Perriraz-Mayer, N., Triponez, F., & Serre-Beinier, V. (2025). Narciclasine as a Novel Treatment for Lung Cancer and Malignant Pleural Mesothelioma: Insights from 3D Tumor Spheroid Models. International Journal of Molecular Sciences, 26(20), 10127. https://doi.org/10.3390/ijms262010127







