1. Introduction
Thrombosis is characterized by abnormal clot formation within a blood vessel, obstructing blood flow and resulting from a substantial imbalance between coagulation and fibrinolysis. The developed thrombotic complexes mostly comprise red blood cells, fibrin, as well as platelets and leukocytes [
1,
2]. Although conditions such as an ischemic stroke (IS) or pulmonary embolism (PE) caused by thrombus development are typically seen in adults, thrombosis is also a serious health complication of pediatric populations and can be classified as venous or arterial, depending on the affected vessels, with venous thromboembolism (VTE) scoring the highest rates [
3,
4,
5].
While pediatric thrombosis is considered rare, corresponding to up to 14/10,000 annual admissions, its incidence rates have demonstrated a significant increase by 70%, with a 7-fold increase only corresponding to strokes affecting newborns and children, with newborns significantly higher thrombotic rates, due to unstable hemostasis [
1,
4,
5,
6,
7]. Thrombotic incidents in pediatric patients most frequently present as cerebral thrombosis, followed by limb incidents and entail life-threatening complications, such as pulmonary embolism and post-thrombotic syndrome [
4,
8,
9]. Early detection and thorough anticoagulant/thrombolytic treatment and subsequent chemoprophylaxis can be determinant to patients’ survival, complication occurrence, and overall therapeutic outcomes, while thorough and multifactorial evaluation of a child’s risk is crucial for their clinical management as to prevention of secondary thrombosis, post-thrombotic syndrome, and overall recurrence [
4,
6,
8,
10].
Although pediatric thrombosis is multifactorial and its manifestation is affected by possible risk factors such as hypoxia, inflammation-involving conditions, malignancy, and injury, it demonstrates a strong and well-established genetic background that increases a child’s vulnerability to thrombi formation [
9,
11,
12,
13]. Inherited thrombophilia is considered a solid risk factor in pediatric thrombosis and includes the presence of genetic variants causing functional or quantitative imbalances in factors involved in hemostasis and fibrinolysis, such as factor V (FV) Leiden, prothrombin (PTH), methylenetetrahydrofolate reductase (MTHFR), plasminogen activator inhibitor-1 (PAI-1), and angiotensin-converting enzyme (ACE) [
11,
14]. Amongst the above, PAI-1 has recently been reported as the predominant inherited genetic risk factor for pediatric thrombosis [
9].
PAI-1 is the most robust inhibitor of fibrinolysis, a process that acts at the final hemostatic stage by dissolving developed thrombi, through the enzymatic activity of plasmin, thus restoring normal blood circulation [
13,
15,
16,
17]. PAI-1, which is encoded by the
SERPINE1 gene, is predominantly secreted by endothelial cells and negatively regulates the fibrinolytic system mainly by its covalent binding to tissue plasminogen activator (tPA), obstructing the conversion of plasminogen to plasmin, which ultimately catalyzes the degradation of fibrin, the main stabilizer of blood clots [
2,
13,
18,
19]. The ratio of tPA/PAI-1 is unfavorable for effective fibrinolysis during childhood, since tPA levels are significantly reduced at birth and until late adolescence, compared to the increased levels of its primary inhibitor, PAI-1 [
19].
It has been demonstrated that increased PAI-1 levels ultimately lead to diminished fibrinolytic activity and a hypercoagulable state, therefore skyrocketing the risk of both venous and arterial thrombosis, while the generation of thrombin itself acts as an inducer of additional PAI-1 secretion [
13,
15,
16,
17,
20]. It is experimentally supported that upregulated PAI-1 levels not only promote thrombotic development but may also contribute to resistance against thrombolytics [
19,
21].
SERPINE1 expression has been demonstrated to increase prior to thrombus development, while maximum PAI-1 levels are reached during the acute phase of thrombosis and finally decrease significantly post-treatment, which includes tPA in life-threatening incidents [
5,
15,
22,
23]. However, following a thrombotic incident, PAI-1 has been shown to remain increased for prolonged periods of time, with a minimum of three months, contributing to proportional PAI-1/t-PA abnormalities and therefore decreased fibrinolytic capacity in up to 54% patients [
24].
PAI-1 levels are highly influenced by the 4G/5G (rs1799768) 1bp insertion/deletion functional polymorphism in the promoter region of the
SERPINE1 coding gene [
15,
25,
26]. The presence of the 4G polymorphic allele is strongly associated with higher levels of circulating PAI-1, resulting in hypofibrinolysis and coagulation enhancement, while the normal 5G allele has been shown to exert a protective effect against thrombotic recurrence [
25,
26]. The
PAI-
1 4G/5G polymorphism is reported to be the most frequently detected genetic variant within children who have undergone thrombosis, compared to other predisposing factors [
9]. Moreover, PAI-1 levels are also regulated by the renin-angiotensin system (RAS), which indirectly suppresses fibrinolysis via angiotensin II (angII) that triggers a significant PAI-1 increase [
27,
28,
29]. In this manner, the angiotensin-converting enzyme (ACE), which is primarily responsible for direct angII formation, serves as an upstream positive regulator of PAI-1, thus exerting a negative effect on fibrinolytic activity.
The insertion/deletion (I/D) functional polymorphism (rs1799752) of the
ACE gene is widely known for increasing circulating ACE levels in the presence of the D (deletion) allele (II < ID < DD), with the DD genotype associated with maximum concentrations and enzymatic activity for angII formation [
30,
31]. The presence of the D allele and primarily DD homozygosity has also been demonstrated to indirectly increase PAI-1 levels, resulting in fibrinolytic impairment through the functional ACE/PAI-1 axis [
32]
In addition to key proteinic regulators of thrombosis, such as PAI-1, evidence has been mounting around the implication of microRNAs (miRNAs) in the delicate regulatory interplays of adult thrombosis [
33]. MiRNAs are a class of single-stranded, small (18–25 nt), non-coding RNA molecules that act as profound negative regulators of gene expression at a post-transcriptional level, through the translational inhibition of mRNA targets. Each miRNA retains the capacity to regulate the expression of multiple gene targets at once, through the binding of its seed sequence to recognized complementary binding sites within the 3′ untranslated region (3′-UTR) of their mRNAs [
33,
34,
35]. Even after their extracellular release, miRNAs remain remarkably stable, due to their binding with Argonaute proteins and/or their encapsulation in extracellular vesicles (EVs), which provide protection against RNase-mediated degradation [
36]. Hence, given that their expressional fluctuations (up- or downregulation) can be highly reflective of a pathology, they hold great promise both as biomarkers but also as crucial elements of disease pathogenicity by the abnormal regulation of the implicated genes [
34,
37].
Despite the increasing evidence on miRNA implication in adult thrombosis, corresponding studies are completely lacking in the pediatric setting. Pediatric thrombosis exhibits a characteristic pathophysiology due to the central role of hypofibrinolysis in light of a child’s developing hemostasis, but also due to the differential impact of genetic predisposition, as well as the lack of accumulation of environmental and age-related risk factors that govern the adult form. Therefore, direct extrapolation of miRNA data from adult studies carries a substantial risk of unreliability, highlighting the need for research specifically tailored to the pediatric context of the pathology.
In the present study, we have examined the expression levels of two miRNA regulators of the ACE/PAI-1 axis (miR-145-5p and miR-34a-5p) in pediatric patients with thrombosis, 1–18 months post-incident, compared to age- and gender-matched healthy controls. Expressional patterns of the studied miRNAs were furtherly evaluated within distinct subpopulations of the patient cohort, in relation to clinical and genetic factors that have been demonstrated to induce anticipated changes in PAI-1 levels. Those variables included post-thrombotic time, thrombosis site, onset type, treatment status, as well as genotypic data for the SERPINE1-4G/5G and ACE-I/D functional polymorphisms.
This study in pediatric patients was designed under the guiding hypothesis of a possible regulatory interplay between miR-145 and miR-34a expression and PAI-1 fluctuations, which are documented in the literature for hypofibrinolysis in adult thrombosis [
5,
15,
22,
23], across different stages of post-thrombotic course and treatment, as well as the different genotypes of the 4G/5G and I/D functional variants. The present research is, to our knowledge, the first globally to investigate miRNA expression profiles in the context of pediatric thrombosis. Its aim is to address the lack of evidence in the genetic/epigenetic interplays that might govern this rare and severe pediatric clinical entity, but also to potentially shed light on aspects of adult thrombosis, where impaired fibrinolysis might not be the central mechanism, but definitely represents an integral component.
3. Discussion
Childhood thrombosis, including the development of venous and arterial thrombi that obstruct circulation, is a rare condition with its etiological origins lying in the disproportionate imbalance between coagulation and fibrinolytic mechanisms [
1,
2]. Thrombotic incidents can occur at any stage during a child’s development, from the neonatal period to late adolescence, due to the interplay of environmental risk factors, including hypoxia, inflammation, and injury, alongside a robust genetically predisposing background for thrombophilia [
9,
11,
12,
13]. The above risk factors contribute to thrombosis development in a child’s normally immature hemostatic system with a markedly low fibrinolytic capacity, compared to that of adults [
1,
4,
5,
6]. Pediatric patients who develop thrombosis come across acute life-threatening or long-term complications, secondary incidents, as well as future thrombotic recurrences, typically managed through anticoagulant/fibrinolytic treatment, chemoprophylaxis, but also by a comprehensive assessment of the children’s overall risk that includes their genetic makeup [
4,
6,
8,
10].
Among the recognized genetic risk factors associated with thrombophilia,
SERPINE1 elevated gene expression has been identified as the most robust in pediatric populations affected by thrombosis [
9]. PAI-1, mainly released by endothelial cells, is the predominant inhibitor of fibrinolysis, since its binding to its antagonist, tPA, thus blocking the conversion of plasminogen to active plasmin, which in turn normally degrades fibrin, a proteinic polymer that stabilizes the thrombus [
2,
13,
18,
19]. Higher levels of PAI-1 lead to hypercoagulability by diminishing fibrinolysis, thus increasing the risk for thrombus development, while thrombin itself stimulates additional PAI-1 release in a coagulation-enhancing feedback loop [
13,
15,
16,
17,
20]. PAI-1 levels demonstrate a progressive rise a priori to the thrombotic incident, peak during the acute phase, and thereafter decline after the completion of the administered treatment regimen that corresponds to successful thrombus-resolution and additional prophylaxis [
5,
15,
22,
23]. Fibrinolytic capacity of more than 50% of patients remains impaired for the long term, for at least 3 months after the thrombotic event, due to PAI-1/t-PA disproportion, which can be attributed to prolonged
PAI-
1 upregulation [
24].
The functional 4G/5G promoter polymorphism of the
SERPINE1 gene is strongly associated with increased circulating PAI-1 levels, leading to subsequent hypofibrinolysis and hypercoagulation, in light of the 4G allele, and has been recently demonstrated as the most common predisposing variant in childhood thrombosis, which can additionally exacerbate already impaired childhood fibrinolysis [
9,
25,
26]. Further to the functional genetic variation in
SERPINE1 itself, its levels are positively mediated by ACE, through its active proteolytic product angII, which triggers PAI-1 secretion, therefore inhibiting fibrinolysis and resulting in hypercoagulability [
27,
28,
29]. The functional I/D polymorphism of the
ACE gene that elevates its expression (peaking at D homozygosity) and activity has been strongly correlated to significant PAI-1 increase, through the ACE/PAI-1 axis [
30,
31,
32].
The implication of miRNAs in adult thrombosis is being actively explored, with mounting and highly promising results [
33]. However, their role in pediatric thrombosis remains completely uninvestigated, with no relevant studies published as of yet, possibly due to the rarity of the condition, which restricts sample collection and subsequent research. Therefore, until now, pediatric thrombosis has demonstrated a vast research gap in miRNA epigenetic involvement. This is intensified since corresponding data from adult thrombosis cannot be reliably merged or extrapolated due to the fundamentally different pathophysiology of the two entities, given the central role of impaired fibrinolysis in the pediatric form, a relevant and consistent biological phenomenon from neonates to adolescents.
In the present study, we evaluated serum expression levels of miR-145-5p and miR-34a-5p miRNAs in pediatric patients aged between 0.6 and 16 years who had undergone thrombosis over the past 1–18 months (
Table 1,
Figure 1). Within the patient cohort, we assessed the expression profiles across distinct post-thrombotic time groups and in relation to treatment status, including recent completion of treatment or administration of chemoprophylaxis. Potential associations between miRNA expression and the patients’ genotypes for the
SERPINE1-4G/5G and
ACE-I/D functional polymorphisms were additionally explored. The expressional patterns of miR-34a were further evaluated in relation to family history for thrombophilia, anatomical site of thrombosis, and type of onset (provoked/unprovoked).
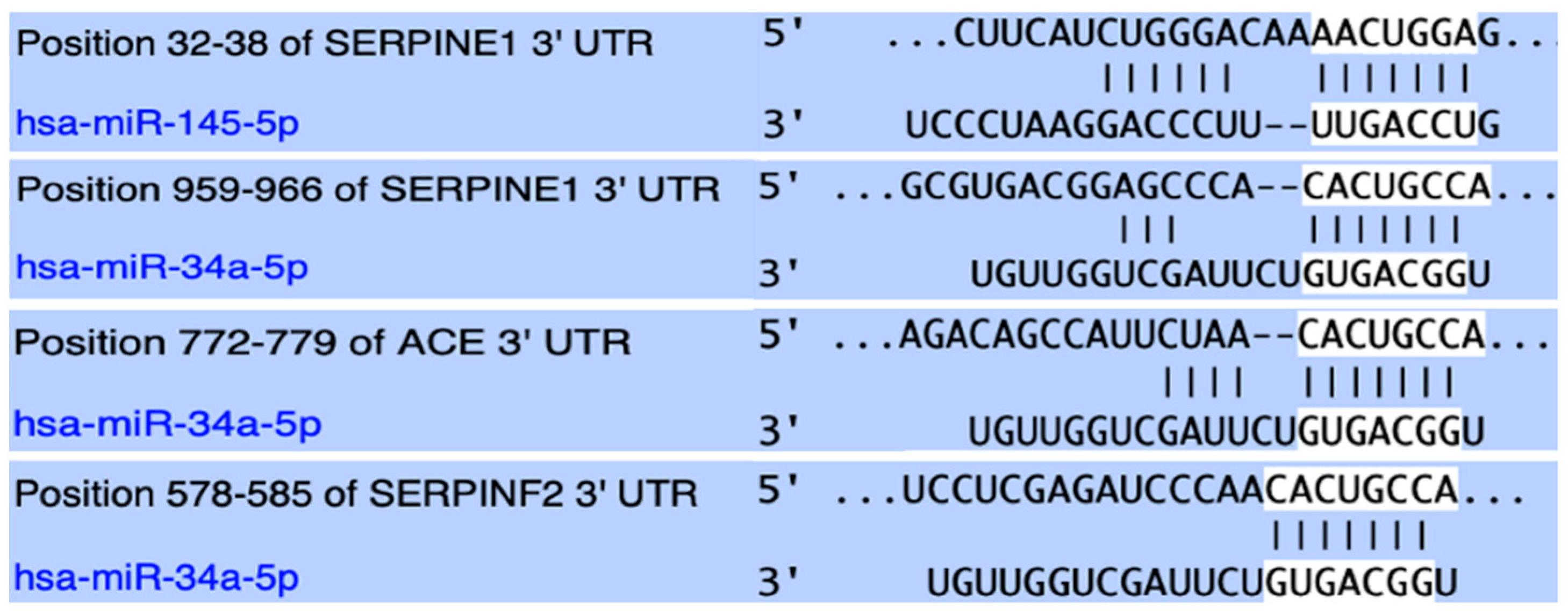
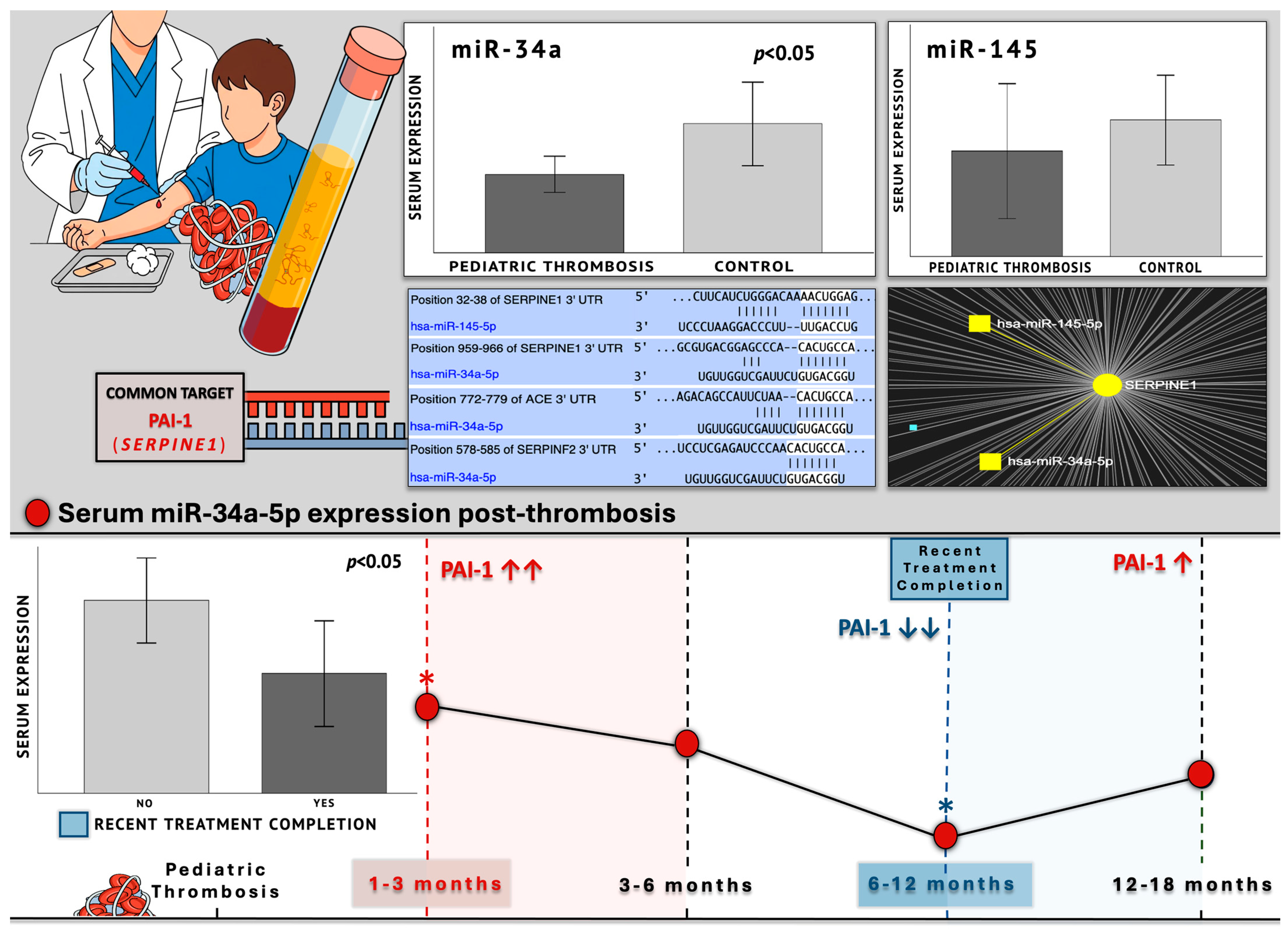
Both studied miRNAs were found to hold conserved binding sites in the 3′-UTR of the
SERPINE1 gene with perfect complementarity and have been experimentally shown to negatively regulate its expression through direct experiments. Between these two
SERPINE1 regulators, miR-34a was bioinformatically shown to form an even more stable complex and also appeared to target the mRNA of the
ACE gene and negatively regulate its expression. Lastly, miR-34a was predicted to strongly target the mRNA of the
SERPINF2 gene, which codes for α2-antiplasmin, the main inactivator of fibrinolytic plasmin [
38,
39] (
Figure 1).
Following RNA extraction and reverse-transcription, the levels of miR-145 and miR-34a were quantified by real-time qPCR through absolute quantification (copies/μL), in order to avoid bias induced from reference-gene instability across different developmental stages of the incorporated age groups. The guiding hypothesis for the present research was based on an anticipated regulatory interplay between the levels of miR-145 and miR-34a expression and PAI-1 fluctuations, as documented in the literature, across different stages of the thrombotic course, clinical parameters, but also different genotypes of PAI-1-increasing functional genetic variants.
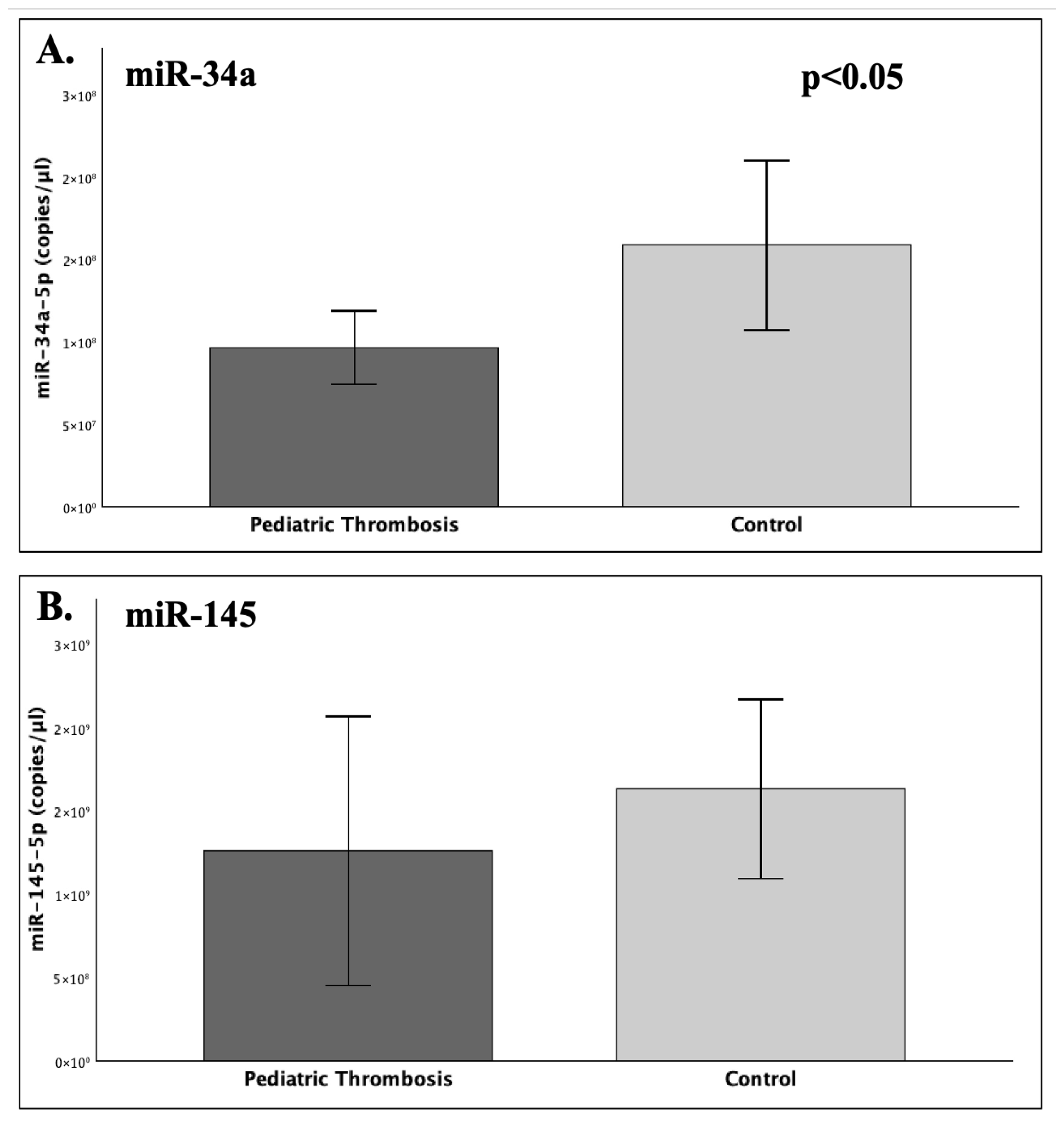
Our findings suggest that the expression of serum miR-34a was significantly reduced by approximately 40% in the patient group compared to the studied healthy children (FC = 0.61;
p = 0.029), while miR-145 did differ significantly between patients and controls (
p = 0.068). In regard to different post-thrombotic time groups (
Table 1), the 1–3- and 6–12-month groups noted a significant difference, with the latter exhibiting 45% reduced expression of miR-34a (
p = 0.031). Nevertheless, the visual inspection of the overall miR-34a expression patterns suggested a characteristic trajectory (
Figure 10). The highest levels within patients were observed during the 1–3-month period, which is the shortest after thrombosis, and
SERPINE1 expression is expected around its peak. Followingly, a gradual decline of miR-34a levels was observed, which ultimately reached its lowest point at 6–12 months. This expressional
nadir coincided with the recent completion of treatment or chemoprophylaxis (1–3 months) within this specific patient subpopulation, therefore aligning with the anticipated diminishing in PAI-1 levels. Thereafter, reaching the 12–18-month group, the expression of miR-34a demonstrated a subtle increase, which was still 39% lower than its respective expression in the control population (
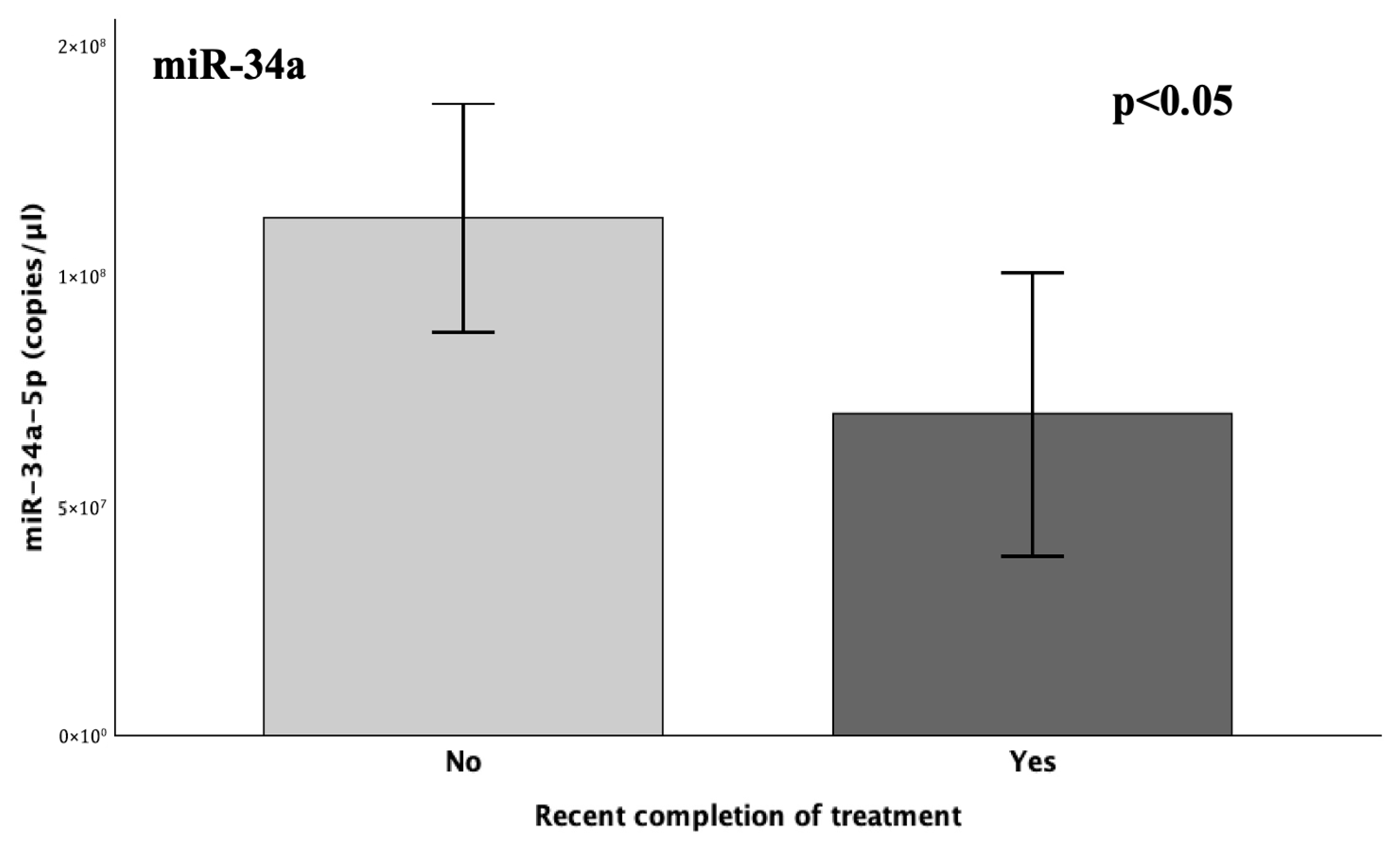
Figure 1). Indeed, the recent completion of treatment induced a significant (38%) decrease in miR-34a levels (
p = 0.048), thus suggesting a temporarily dependent effect, possibly reflecting an absence of necessity for
PAI-
1 regulation, owing to its typically diminished state following a successful treatment course [
5,
15,
22,
23].
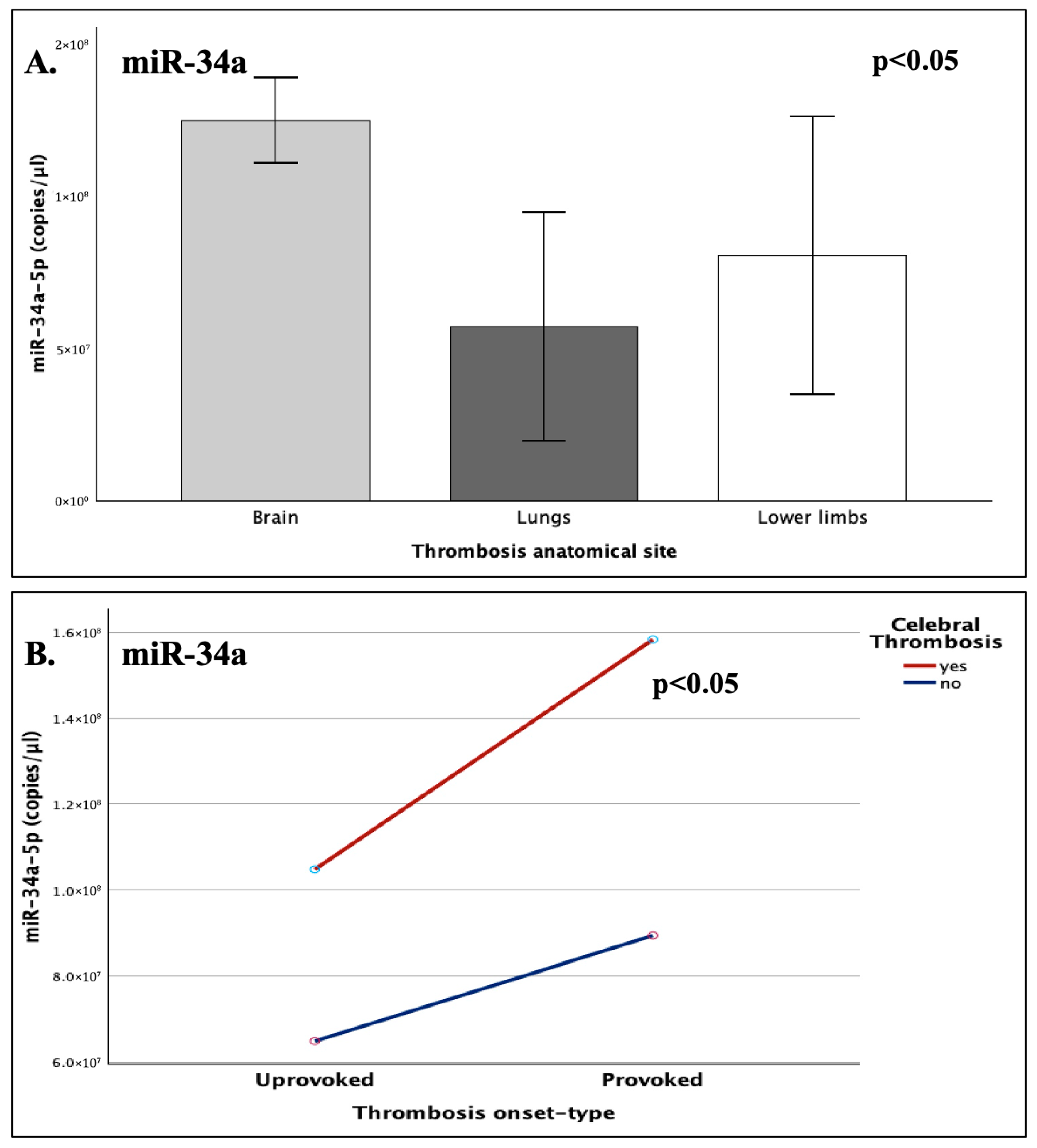
The expression of miR-34a was highly influenced by the anatomical site of the developed thrombi (p = 0.019), with a peak at cerebral thrombosis, which noted a 76% increase compared to the expressional nadir of pulmonary embolism (p = 0.007). The synergistic interaction of cerebral involvement and disease-onset type (provoked/unprovoked) was also shown to significantly influence miR-34a levels, with injury-induced cerebral strokes demonstrating a notable increase (37%) when compared to the respective cerebral unprovoked incidents, thus indicating that thrombosis onset type exerts differential effects on miRNA expression, depending on the affected organ.
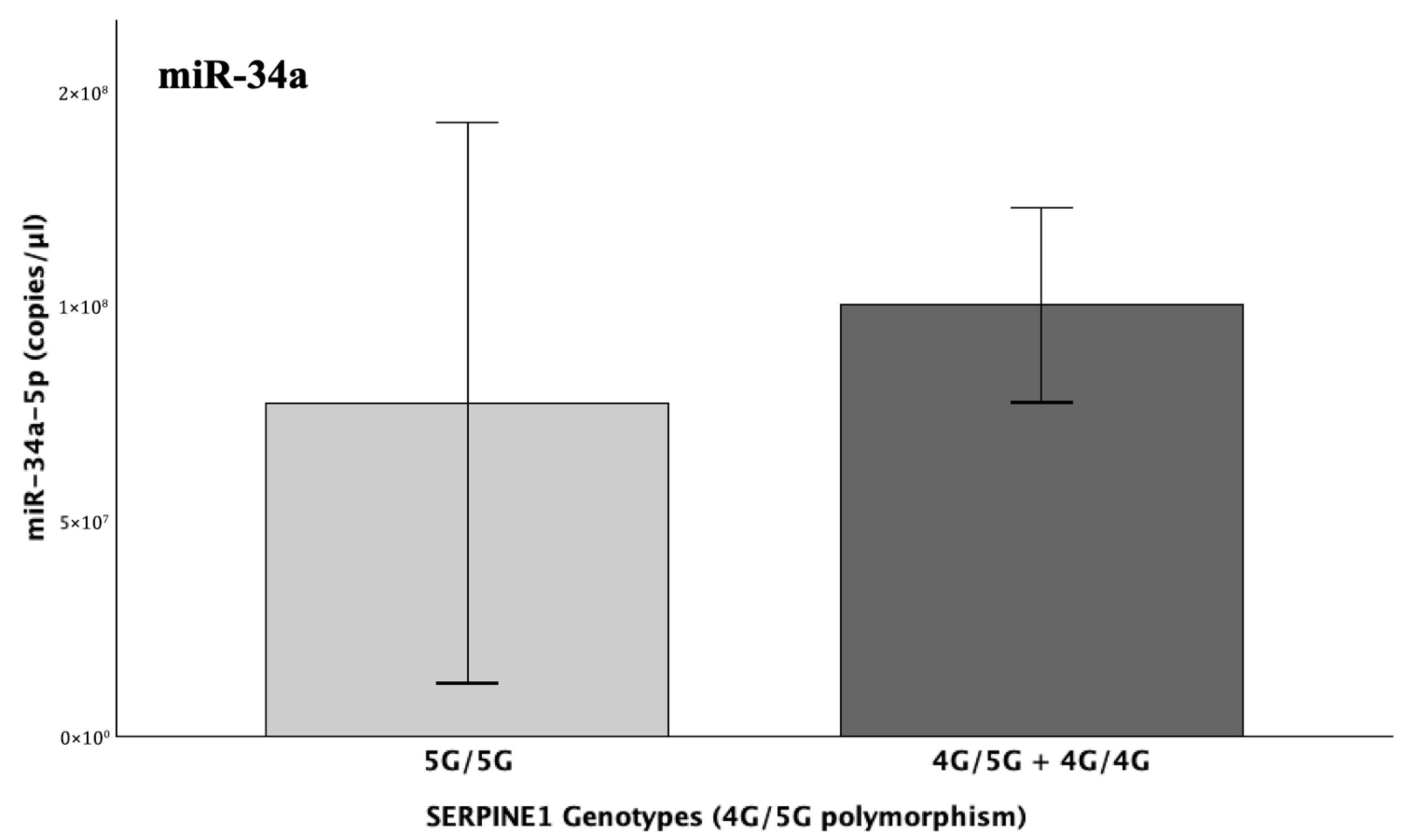
The levels of miR-34a did not significantly differ in light of the different genotypes of the SERPINE1-4G/5G and/or ACE-I/D functional polymorphisms. However, a clear tendency for upregulation was observed in the levels of miR-34a in the genotypic presence of the 4G and D alleles, which are known to ultimately increase PAI-1 levels. Although this increasing trend of miR-34a is biologically plausible, it is possible that statistical significance might have been influenced by imbalanced genotypic distributions, since 84% of patients carried the 4G allele of SERPINE1 and 95% carried the D allele of ACE.
The actual influence of the studied genetic variables was later revealed through two significant findings, which highlighted the underlying genetic/epigenetic and environmental interactions. The expression miR-34a appeared to be highly influenced by thrombosis site and onset-type, under the presence of the D allele (I/D and D/D genotypes), with cerebral trauma-induced cases that carried at least on D allele, demonstrating an almost 50% increase in miR-34a expression, compared to unprovoked cerebral ones and a 5.2-fold increase, compared to unprovoked non-cerebral thrombotic events (p = 0.035).
Taken together, the above observations are highly consistent with a regulatory role, whereby miR-34a acts in a compensational manner to reactively counterbalance the quantitative rise in its targets (
SERPINE1,
ACE, and possibly
SERPINF2) (
Figure 11). Its significant downregulation—by almost half—in the patient population compared to controls, which persists even 12–18 months post-incident and prolonged treatment discontinuation, reflects the long-term impairment of its regulatory capacity. Further support to this conclusion comes from miRNA studies in adult thrombosis, placing miR-34a among the reactively upregulated molecules during the acute phase of thrombotic events, such as ischemic stroke [
40,
41], where PAI-1 is known to steeply rise [
5,
15,
22,
23].
The robust effect of injury-induced cerebral involvement is completely in line with both the established upregulation of miR-34a during the acute phase of adult ischemic stroke, but also with its functionally demonstrated role to epigenetically counterbalance injury-induced inflammation, thus acting as a protective mechanism [
42,
43]. In fact, not only is it strongly involved in the acute response to mechanical injury, consistent with the provoked pediatric stroke cases we analyzed, but also its higher serum levels over time have been reportedly associated with successful remission in cases with spinal cord injury. The overall serum levels of injured patients were significantly downregulated compared to non-injured individuals, in accordance with our thrombosis-related findings over time, while their assessment has been suggested as a monitoring approach for spinal injury status [
44].
These observations agree with the expressional serum-level trajectory of miR-34a that was detected in our study, with its highest levels observed in the time period closest to the thrombotic event, reflecting a compensatory response, followed by its sharp, significant downregulation with treatment completion, in the setting of pharmacologically induced remission, where thrombosis-related target pathways are externally suppressed. Finally, its tendency to increase in light of the long-term absence of treatment highlights a partial yet inadequate recovery that confirms its normally protective pro-fibrinolytic role. However, the expression decrease remains both in the initial reactive response but also long-term after thrombosis, when compared to healthy children with expectedly normal miR-34a function and more efficient hemostatic mechanisms. The latter strongly points out the substantial inadequacy of the miRNA to normally function as an anti-thrombotic protective mechanism, through the effective quantitative regulation of the key pediatric thrombosis factor PAI-1 and its upstream activator, ACE, in the context of the anti-fibrinolytic ACE/PAI-1 axis, but also α2-antiplasmin that normally contributes to hypofibrinolysis [
28,
39].
This conclusion gains further strength as the overwhelming majority of studied patients (84%) carried at least one
SERPINE1 4G allele, which directly increases the mainly implicated
SERPINE1 gene at all times, while nearly all patients (95%) harbored at least one
ACE D allele that has been renown for significantly increasing both levels and activity of its positive regulator and results in even greater levels of pro-thrombotic PAI-1 [
26,
32]. Therefore, the interpretation of findings cannot be solely attributed to epigenetic regulation rather than a strong interaction between genetics, epigenetics, and environmental factors, in complete accordance with the multifactorial nature of thrombosis.
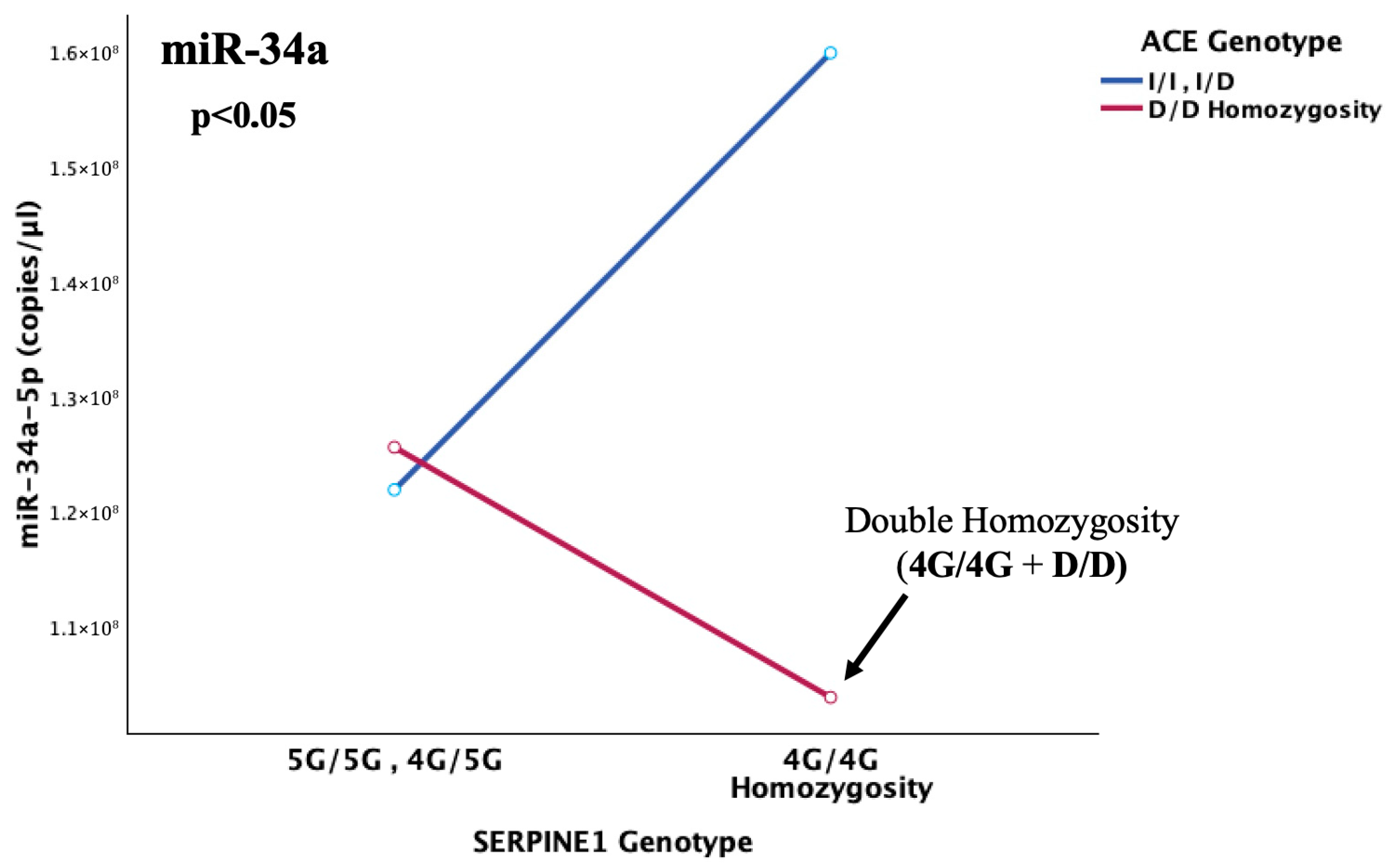
This notion was additionally confirmed by the detection of an epistatic interaction between the SERPINE1 and ACE genes that was observed to highly influence the abundance of miR-34a in cerebral thrombotic incidents (p = 0.006). More specifically, the lowest serum miR-34a levels were observed under the state of double homozygosity (4G/4G + D/D) for the SERPINE1-4G/5G and ACE-I/D functional variants. This pattern was completely reversed in 4G/4G carriers without concurrent D homozygosity, which appears to act as the genetic switch. This leads to the conclusion that, probably, in light of double 4G4G + D/D homozygosity, where the maximum possible resultant levels of PAI-1 are secreted, the already impaired miR-34a, although not quantitatively enough, is forced to regulate both increased targets that synergistically lead to PAI-1 overflooding. This inadequate response allows for ultimate fibrinolytic dysregulation in children with genetically determined thrombophilia, which, when combined with their immature hemostatic system, can pave the way for thrombosis development.
This might provide a combinatorial genetic/epigenetic predisposing profile, which is highly possible to account for pediatric thrombosis development itself, by acting as a multivariate molecular switch in light of an environmental trigger, such as injury. This conclusion is furtherly supported by the fact that
SERPINE1-4G/5G polymorphism has been recognized as the predominant risk factor for childhood thrombophilia, among many established risk factors that are considered much more important in adult thrombosis [
9]. Since, to our knowledge, the present exploratory piece of research is the first conducted in the context of pediatric thrombosis and miRNA profiling so far, we anticipate that it will lay the groundwork for the future broadening of predisposition from a solely genetic term to a genetic/epigenetic dynamic concept.
Study Limitations
A limitation of the present study is the relatively small sample size, largely attributable to the rarity of cases, which may adversely affect statistical power, thereby partly elucidating the lack of significance in certain overall comparisons, despite consistent patterns and significant pairwise differences (p < 0.05). Moreover, the wide dispersion in the expression values, commonly reported in miRNA studies, further contributes to this matter, thus possibly reducing the power of omnibus tests (one-way ANOVA, Kruskal–Wallis, and Jonckheere–Terpstra). Therefore, biological plausibility should be cautiously co-evaluated in light of results demonstrating contradiction between statistical significance and clearly observed trends, before definite conclusions are drawn.
The present study holds 85.1% statistical power to detect large effects, rather than small or incidental variations. However, power can be reduced in subgroup analyses due to even smaller cohorts, where non-borderline p-values and biologically plausible results minimize the likelihood of spurious findings and support robustness of the associations, despite limited power.
4. Materials and Methods
4.1. Sample Collection
This study was bioethically approved by the Ethics Committee of the Scientific Council of “Aghia Sophia” Children’s University Hospital (7516/01-04-19). Whole blood samples of 38 children with an age range of 0.6–16 years (19 patients and 19 gender and age-matched healthy controls) were collected at the Haemophilia Center for Children and Adolescents/Haemostasis and Thrombosis Unit of “Aghia Sophia” Children’s University Hospital. Eligible participants were neonates, children, and adolescents with radiologically confirmed thrombotic episodes who had been hospitalized for this particular reason. Patients with systemic comorbidities that have been demonstrated to induce thrombosis, such as malignancy and congenital heart disease, were excluded. The control group consisted of healthy neonates, children, and adolescents without any history of thrombotic incidents.
Patients were recruited during post-thrombosis routine follow-up visits. After thorough informative discussion, the parents of the enrolled participants provided written consent for their offspring’s blood samples to be collected and processed for subsequent molecular analyses, as well as for selected clinical data to be co-evaluated. In addition, a thorough four-generation family history of the patients was obtained. Blood samples that were intended for serum separation were drawn directly into collection tubes containing clot-activator, while samples intended for genomic analysis were collected in EDTA-containing tubes.
4.2. Serum Separation and RNA Extraction
Whole blood samples with clot-activator remained at room temperature for 15–30 min, to allow clotting to take place. The samples were subsequently centrifuged at 3000 rpm, at 4 °C for 10 min, using a swing-bucket rotor centrifuge. The supernatant serum phase was carefully collected and centrifuged again for additional sample purity.
RNA extraction was performed using the “NucleoSpin miRNA Plasma, Mini kit for circulating miRNA” (Macherey-Nagel GmbH, Dueren, Germany), according to the manufacturer’s protocol for serum samples and RNA elution in the minimum recommended volume, to obtain maximum miRNA concentrations. The concentration (ng/μL) and purity of the eluted RNA were assessed using a BioSpec-nano Spectrophotometer for Life Science (Shimadzu Corporation, Kyoto, Japan). RNA was reverse-transcribed into cDNA directly after extraction, quantification, and normalization across sample concentrations to ensure template comparability.
4.3. Bioinformatic Target Prediction Analyses
Target prediction and retrieval of experimentally validated miRNA/mRNA interactions were combinatorially performed via the utilization of TargetScanHuman, miRNet 2.0, as well as TarBase.v.9 (incorporating DIANA-microT-CDS) computational algorithms. Since pediatric thrombosis is mainly driven by hypofibrinolysis, we focused on identifying miRNAs that might contribute to this condition through their expression dysregulation. Hence, the selection started by targeting the mRNA of
SERPINE1, which codes for the main inhibitor of fibrinolysis, PAI-1, the key player in the pediatric setting. Only miRNA candidates with perfect seed complementarity (7/7 nucleotides) and evolutionary conserved 3′-UTR binding sites were considered for subsequent analysis, and only those with an experimentally validated regulatory relationship with
SERPINE1 were finally considered (>5 functional experiments, including direct validation). Additional priority was given to additional conserved (7/7) targeting and validated regulation of the
ACE mRNA, given the regulatory role of ACE in PAI-1 levels. To ensure high-confidence miRNA/mRNA complexes, only interactions presenting with microT scores >0.7 were considered. Finally, selection was performed among miRNAs that have been demonstrated to be implicated in adult thrombosis, where impaired fibrinolysis (although not the central mechanism compared to hypercoagulability) also plays a crucial role. On that basis, miR-145 and miR-34a were selected, as both target and have been experimentally shown to regulate the expression of
SERPINE1, with miR-34a additionally acting as a negative regulator of
ACE, but also targeting the mRNA of
SERPINF2, which codes for another crucial anti-fibrinolytic agent (α2-antiplasmin) [
38,
39].
4.4. Reverse Transcription and Quantitative Real-Time PCR
Reverse Transcription (RT) of miRNAs was performed using the miRCURY LNA RT Kit (Qiagen, Hilden, Germany) for miRNA polyadenylation and first-strand cDNA synthesis in a single-tube reaction of 10 μL (2 μL 5xRT SYBR-Green Reaction Buffer, 2 μL RNA template, 1 μL 10xRT Enzyme-Mix, and 5 μL RNase-free water). The RT reaction was performed using a Gradient Thermal Cycler (Takara Bio, Shiga, Japan) and involved incubation at 40 °C for 60 min, an inactivation step at 95 °C for 5 min, followed by subsequent cooling of the reaction at 4 °C. The cDNA samples were stored at −20 °C overnight and processed the following day.
Since the studied cohorts included children from an age range of 0.6–16 years (
Table 1), which corresponds to various developmental stages, the use of a conventional reference gene for relative quantification was deemed unreliable due to possible lack of stability. Therefore, absolute quantification of the expression (copies/μL) of the two studied miRNAs (miRBase accession numbers: MIMAT0000255 and MIMAT0000437) was performed using standard curves, produced from serial 2-fold dilutions of reverse-transcribed synthetic RNA oligonucleotides corresponding to the mature miRNAs (including matching length, GC content, and molecular weight). The produced curves demonstrated high linearity (R
2 ≈ 0.99), and efficacy within the acceptable range of 90–110%. As an illustrative example, for miR-34a, the efficacy was 96%, with an R
2 of 0.9915. Reverse transcription of the synthetic RNA molecules was performed under the same protocol as the target serum-miRNAs, which were analyzed in this study (LNA series, Qiagen, Hilden, Germany).
Quantification was conducted by SYBR® Green-based, real-time quantitative PCR (qPCR), performed on a LightCycler 480ii real-time PCR instrument (Roche, Basel, Switzerland). Each reaction of 10 μL contained 5 μL of 2XmiRCURY SYBR-Green Master Mix, 4 μL cDNA, and 1 μL miRCURY LNA miRNA PCR Assay (Qiagen, Hilden, Germany) for the respective miRNA. The qPCR protocol involved heat activation at 95 °C for 10 min, followed by 45 cycles of 95 °C for 15 s and 60 °C for 1 min, and finally a melting-curve analysis cycle ranging from 60 °C to 95 °C, with a ramp rate of 0.03 °C/s and continuous fluorescence acquisition.
Threshold cycles (Ct values) were obtained through the instrument’s software by employing the “Absolute Quantification/2nd Derivative Max” analysis. Although the experimental protocol incorporated 45 cycles of amplification, a threshold of 40 cycles was set for reliable detection.
4.5. PAI-1 Genotyping for the PAI-1 (SERPINE1) 4G/5G Polymorphism
Genomic DNA was extracted from white blood cells of the patients’ EDTA-containing whole blood samples, using the NucleoSpin Blood kit (MACHEREY-NAGEL, Germany). Genotyping of the
PAI-
1 4G/5G polymorphism was conducted by PCR amplification, performed on a Gradient Thermal Cycler (Takara Bio, Japan), using the following primers: 5′-CACAGAGAGAGTCTGGCCACGT-3′ and R: 5′-CCAACAGAGGACTCTTGGTCT-3′. Thermocycling included initial denaturation at 94 °C for 4 min, followed by 35 cycles consisting of a denaturation step at 94 °C for 0.5 min, subsequent annealing at 57 °C for 1 min, and an elongation step at 72 °C for 1 min. The final 5 min elongation step was set at 72 °C, ultimately resulting in an amplified fragment of 99 bp [
45].
The obtained PCR products underwent overnight incubation with the BslI restriction enzyme at 37 °C, which resulted in a 77 bp and a 22 bp fragment in the presence of the 5G allele. Genotyping of the studied samples was carried out through the visualization of the DNA fragments under UV-light, following agarose gel electrophoresis and nucleic acid staining with GelRed fluorescent stain (Biotium, Fremont, CA, USA).
4.6. ACE Genotyping for the ACE I/D Polymorphism
Genotyping of the ACE I/D polymorphism was conducted by PCR amplification, performed on a Gradient Thermal Cycler (Takara Bio, Japan), using the following primers for I or D allele amplification: F: 5′-CTGGAGACCACTCCCATCCTTTCT-3′ and R: 5′-GATGTGGCCATCACATTCGTCAGAT-3′.
The thermocycling protocol included initial denaturation at 94 °C for 4 min, followed by 35 cycles consisting of a denaturation step at 94 °C for 0.5 min, subsequent annealing at 60 °C for 1 min, and a 1 min elongation step at 72 °C. The final 5 min elongation step was set at 72 °C. The PCR amplification resulted in two DNA fragments of 490 and 190 bp, respectively, corresponding to the I and D alleles [
45]. Genotyping was performed under UV light, following agarose gel electrophoresis and nucleic acid staining.
4.7. Statistical Analysis
The total of statistical analyses was performed using IBM SPSS Statistics, Version 29.0.2.0. Normality was assessed using the Shapiro–Wilk test for continuous values, according to sample size. Two-sample comparisons were conducted by employing the independent-sample T-test or Mann–Whitney U test, with respect to the distribution of values. Likewise, comparisons between more than two groups were conducted using “one-way ANOVA” or the Kruskal–Wallis test, as appropriate. Additionally, the Jonckheere–Terpstra test was applied for ordered group comparisons and subsequent pairwise analyses. Finally, to examine the effect of the interaction between time and treatment on miRNA expression, general linear models (GLMs) were employed. Error bars are included in the presented result figures in order to illustrate the variability of observed values around the central tendency (mean). An a priori power analysis (two-sided α = 0.05) indicated that with the included sample sizes (19 patients and 19 controls), the present study had 85.1% power to detect large effects and biologically meaningful differences (Cohen’s d = 1.0). The level of statistical significance was set at p < 0.05 (two-tailed).