Clustering of Uterine Natural Killer Cells Around Uterine Glands in Women with Recurrent Implantation Failure and Recurrent Pregnancy Loss: An Immunohistochemical Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Endometrial Biopsy
4.2. Immunohistochemical Staining
4.3. Uterine NK Cell Counting and Interpretation
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ART | Assisted reproductive technologies/treatment |
| uNK | Uterine natural killer cells |
| RIF | Recurrent implantation failure |
| RPL | Recurrent pregnancy loss |
| ESHRE | European Society of Human Reproduction and Embryology |
| ASRM | American Society for Reproductive Medicine |
| RCOG | Royal College of Obstetricians and Gynaecologists |
| IVF | In vitro fertilization |
| PGT | Preimplantation genetic testing |
| HST | Hormone substitution therapy |
| LMWH | Low molecular weight heparin |
| EVT | Extravillous trophoblast |
| PlGF | Placental growth factor |
| EGF | Epidermal growth factor |
| IL-6 | Interleukin-6 |
| SDF-1 | Stromal cell-derived factor-1 |
| CXCL12 | CXC motif chemokine ligand 12 |
| CXCR4 | CXC chemokine receptor type 4 |
| CXCL8 | CXC motif chemokine ligand 8 |
| CCL14 | CC motif chemokine ligand 14 |
| CXCL6 | CXC motif chemokine ligand 6 |
| ECM | Extracellular matrix |
| CAM | Cell adhesion molecules |
| KLRG1 | killer cell lectin-like receptor G1 |
References
- Nabekura, T. Immunological memory in natural killer cells. Int. Immunol. 2025, 37, 435–443. [Google Scholar] [CrossRef]
- Roberts, J.M.; Burton, G.J.; Conrad, K.P.; Luke, B.; Mann, M.R.; Moffett, A.; Jancsura, M.K. An overview of periconceptional pathology and pathophysiology: Where do we go next? Placenta 2025, 168, 98–110. [Google Scholar] [CrossRef]
- Lapides, L.; Varga, I.; Csöbönyeiová, M.; Klein, M.; Pavlíková, L.; Visnyaiová, K.; Babál, P.; Mikušová, R. The Neglected Uterine NK Cells/Hamperl Cells/Endometrial Stromal Granular Cell, or K Cells: A Narrative Review from History through Histology and to Medical Education. Int. J. Mol. Sci. 2023, 24, 12693. [Google Scholar] [CrossRef]
- Guan, D.; Chen, Z.; Zhang, Y.; Sun, W.; Li, L.; Huang, X. Dual Role of Natural Killer Cells in Early Pregnancy: Immunopathological Implications and Therapeutic Potential in Recurrent Spontaneous Abortion and Recurrent Implantation Failure. Cell Prolif. 2025, 58, e70037. [Google Scholar] [CrossRef] [PubMed]
- Shreeve, N.; Colucci, F. Mamma MIA! Decidual NK cells involved in fetal neurodevelopment. Immunity 2025, 58, 1364–1366. [Google Scholar] [CrossRef]
- Wyns, C.; De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Motrenko, T.; Smeenk, J.; Bergh, C.; Tandler-Schneider, A.; Rugescu, I.A.; Vidakovic, S.; et al. ART in Europe, 2017: Results generated from European registries by ESHRE. Hum. Reprod. Open 2021, 2021, hoab026. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.M. Assisted reproductive technology after the birth of louise brown. J. Reprod. Infertil. 2013, 14, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Saito, S. Role of immune cells in the establishment of implantation and maintenance of pregnancy and immunomodulatory therapies for patients with repeated implantation failure and recurrent pregnancy loss. Reprod. Med. Biol. 2024, 23, e12600. [Google Scholar] [CrossRef]
- Vomstein, K.; Aulitzky, A.; Strobel, L.; Bohlmann, M.; Feil, K.; Rudnik-Schöneborn, S.; Zschocke, J.; Toth, B. Recurrent Spontaneous Miscarriage: A Comparison of International Guidelines. Geburtshilfe Frauenheilkd. 2021, 81, 769–779. [Google Scholar] [CrossRef]
- Stevens Brentjens, L.; Roumen, R.J.E.; Smits, L.; Derhaag, J.; Romano, A.; van Golde, R.J.T.; den Hartog, J.E. Pregnancy rate and time to pregnancy after recurrent implantation failure (RIF)-a prospective cohort follow-up study. J. Assist. Reprod. Genet. 2024, 41, 3061–3070. [Google Scholar] [CrossRef]
- Cimadomo, D.; de Los Santos, M.J.; Griesinger, G.; Lainas, G.; Le Clef, N.; McLernon, D.J.; Montjean, D.; Toth, B.; Vermeulen, N.; Macklon, N. ESHRE good practice recommendations on recurrent implantation failure. Hum. Reprod. Open 2023, 2023, hoad023. [Google Scholar] [CrossRef]
- Lapides, L.; Varga, I.; Klein, M.; Rybánska, L.; Belušáková, V.; Babál, P. When Less Is More—Pipelle Endometrial Sampling for Quantification of Uterine Natural Killer Cells in Patients with Recurrent Implantation Failure or Habitual Abortion. Physiol. Res. 2022, 71, S65–S73. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Zhang, T.; Chan, L.K.Y.; Liu, Y.; Chung, J.P.; Kwong, J.; Li, T.C. The use of multiplex staining to measure the density and clustering of four endometrial immune cells around the implantation period in women with recurrent miscarriage: Comparison with fertile controls. J. Mol. Histol. 2020, 51, 593–603. [Google Scholar] [CrossRef] [PubMed]
- King, A. Uterine leukocytes and decidualization. Hum. Reprod. Update 2000, 6, 28–36. [Google Scholar] [CrossRef]
- Croy, B.A.; He, H.; Esadeg, S.; Wei, Q.; McCartney, D.; Zhang, J.; Borzychowski, A.; Ashkar, A.A.; Black, G.P.; Evans, S.S.; et al. Uterine natural killer cells: Insights into their cellular and molecular biology from mouse modelling. Reproduction 2003, 126, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Manaster, I.; Mandelboim, O. The unique properties of uterine NK cells. Am. J. Reprod. Immunol. 2010, 63, 434–444. [Google Scholar] [CrossRef]
- Croy, B.A.; van den Heuvel, M.J.; Borzychowski, A.M.; Tayade, C. Uterine natural killer cells: A specialized differentiation regulated by ovarian hormones. Immunol. Rev. 2006, 214, 161–185. [Google Scholar] [CrossRef] [PubMed]
- Tuckerman, E.; Mariee, N.; Prakash, A.; Li, T.C.; Laird, S. Uterine natural killer cells in peri-implantation endometrium from women with repeated implantation failure after IVF. J. Reprod. Immunol. 2010, 87, 60–66. [Google Scholar] [CrossRef]
- Clifford, K.; Flanagan, A.M.; Regan, L. Endometrial CD56+ natural killer cells in women with recurrent miscarriage: A histomorphometric study. Hum. Reprod. 1999, 14, 2727–2730. [Google Scholar] [CrossRef]
- Quenby, S.; Nik, H.; Innes, B.; Lash, G.; Turner, M.; Drury, J.; Bulmer, J. Uterine natural killer cells and angiogenesis in recurrent reproductive failure. Hum. Reprod. 2009, 24, 45–54. [Google Scholar] [CrossRef]
- Chen, X.; Mariee, N.; Jiang, L.; Liu, Y.; Wang, C.C.; Li, T.C.; Laird, S. Measurement of uterine natural killer cell percentage in the periimplantation endometrium from fertile women and women with recurrent reproductive failure: Establishment of a reference range. Am. J. Obstet. Gynecol. 2017, 217, 680.e681–680.e686. [Google Scholar] [CrossRef]
- Babayeva, G.; Purut, Y.E.; Giray, B.; Oltulu, P.; Alakuş, R.; Çolakoğlu, M.C. Endometrial CD56+ natural killer cells in women with recurrent implantation failure: An immunohistochemical study. Turk. J. Obstet. Gynecol. 2020, 17, 236–239. [Google Scholar] [CrossRef]
- Lapides, L.; Klein, M.; Belušáková, V.; Csöbönyeiová, M.; Varga, I.; Babál, P. Uterine Natural Killer Cells in the Context of Implantation: Immunohistochemical Analysis of Endometrial Samples from Women with Habitual Abortion and Recurrent Implantation Failure. Physiol. Res. 2022, 71, S99–S105. [Google Scholar] [CrossRef]
- Von Woon, E.; Greer, O.; Shah, N.; Nikolaou, D.; Johnson, M.; Male, V. Number and function of uterine natural killer cells in recurrent miscarriage and implantation failure: A systematic review and meta-analysis. Hum. Reprod. Update 2022, 28, 548–582. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Sunkara, S.K. Natural killer cells in female infertility and recurrent miscarriage: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.E.; Stephenson, E.; Polański, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Whettlock, E.M.; Woon, E.V.; Cuff, A.O.; Browne, B.; Johnson, M.R.; Male, V. Dynamic Changes in Uterine NK Cell Subset Frequency and Function Over the Menstrual Cycle and Pregnancy. Front. Immunol. 2022, 13, 880438. [Google Scholar] [CrossRef]
- Kuon, R.J.; Weber, M.; Heger, J.; Santillán, I.; Vomstein, K.; Bär, C.; Strowitzki, T.; Markert, U.R.; Toth, B. Uterine natural killer cells in patients with idiopathic recurrent miscarriage. Am. J. Reprod. Immunol. 2017, 78, e12721. [Google Scholar] [CrossRef]
- Russell, P.; Sacks, G.; Tremellen, K.; Gee, A. The distribution of immune cells and macrophages in the endometrium of women with recurrent reproductive failure. III: Further observations and reference ranges. Pathology 2013, 45, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Man, G.C.W.; Wang, J.; Liu, Y.; Kwong, J.; Zhang, T.; Chung, J.P.W.; Wang, C.C.; Chen, X.; Li, T.-C. The identification of endometrial immune cell densities and clustering analysis in the mid-luteal phase as predictor for pregnancy outcomes after IVF-ET treatment. J. Reprod. Immunol. 2021, 148, 103431. [Google Scholar] [CrossRef]
- Hazan, A.D.; Smith, S.D.; Jones, R.L.; Whittle, W.; Lye, S.J.; Dunk, C.E. Vascular-leukocyte interactions: Mechanisms of human decidual spiral artery remodeling in vitro. Am. J. Pathol. 2010, 177, 1017–1030. [Google Scholar] [CrossRef]
- Kusakabe, K.; Ohmoto, M.; Okada, T.; Mukamoto, M.; Sasaki, F.; Kiso, Y. Uterine NK cells produce epidermal growth factor in the murine pregnant uterus. J. Vet. Med. Sci. 1999, 61, 947–949. [Google Scholar] [CrossRef][Green Version]
- Wang, F.; Qualls, A.E.; Marques-Fernandez, L.; Colucci, F. Biology and pathology of the uterine microenvironment and its natural killer cells. Cell. Mol. Immunol. 2021, 18, 2101–2113. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.H.; Dunk, C.E.; Lye, S.J.; Aplin, J.D.; Harris, L.K.; Jones, R.L. Extravillous Trophoblast and Endothelial Cell Crosstalk Mediates Leukocyte Infiltration to the Early Remodeling Decidual Spiral Arteriole Wall. J. Immunol. 2017, 198, 4115–4128. [Google Scholar] [CrossRef] [PubMed]
- Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Bojić-Trbojević, Ž.; Dekanski, D.; Jovanović Krivokuća, M. IL-6 and IL-8: An Overview of Their Roles in Healthy and Pathological Pregnancies. Int. J. Mol. Sci. 2022, 23, 14574. [Google Scholar] [CrossRef] [PubMed]
- Dillenburg-Pilla, P.; Patel, V.; Mikelis, C.M.; Zárate-Bladés, C.R.; Doçi, C.L.; Amornphimoltham, P.; Wang, Z.; Martin, D.; Leelahavanichkul, K.; Dorsam, R.T.; et al. SDF-1/CXCL12 induces directional cell migration and spontaneous metastasis via a CXCR4/Gαi/mTORC1 axis. FASEB J. 2015, 29, 1056–1068. [Google Scholar] [CrossRef]
- Hanna, J.; Wald, O.; Goldman-Wohl, D.; Prus, D.; Markel, G.; Gazit, R.; Katz, G.; Haimov-Kochman, R.; Fujii, N.; Yagel, S.; et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood 2003, 102, 1569–1577. [Google Scholar] [CrossRef]
- Niessen, C.M.; Leckband, D.; Yap, A.S. Tissue organization by cadherin adhesion molecules: Dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol. Rev. 2011, 91, 691–731. [Google Scholar] [CrossRef]
- Burrows, T.D.; King, A.; Loke, Y.W. The role of integrins in adhesion of decidual NK cells to extracellular matrix and decidual stromal cells. Cell Immunol. 1995, 166, 53–61. [Google Scholar] [CrossRef]
- Wallace, A.E.; Fraser, R.; Cartwright, J.E. Extravillous trophoblast and decidual natural killer cells: A remodelling partnership. Hum. Reprod. Update 2012, 18, 458–471. [Google Scholar] [CrossRef]
- Xie, M.; Li, Y.; Meng, Y.Z.; Xu, P.; Yang, Y.G.; Dong, S.; He, J.; Hu, Z. Uterine Natural Killer Cells: A Rising Star in Human Pregnancy Regulation. Front. Immunol. 2022, 13, 918550. [Google Scholar] [CrossRef] [PubMed]
- Croy, B.A. Hasn’t the time come to replace the term metrial gland? J. Reprod. Immunol. 1999, 42, 127–129, discussion 131–134. [Google Scholar] [PubMed]
- Pijnenborg, R. The metrial gland is more than a mesometrial lymphoid aggregate of pregnancy. J. Reprod. Immunol. 2000, 46, 17–19. [Google Scholar] [CrossRef] [PubMed]
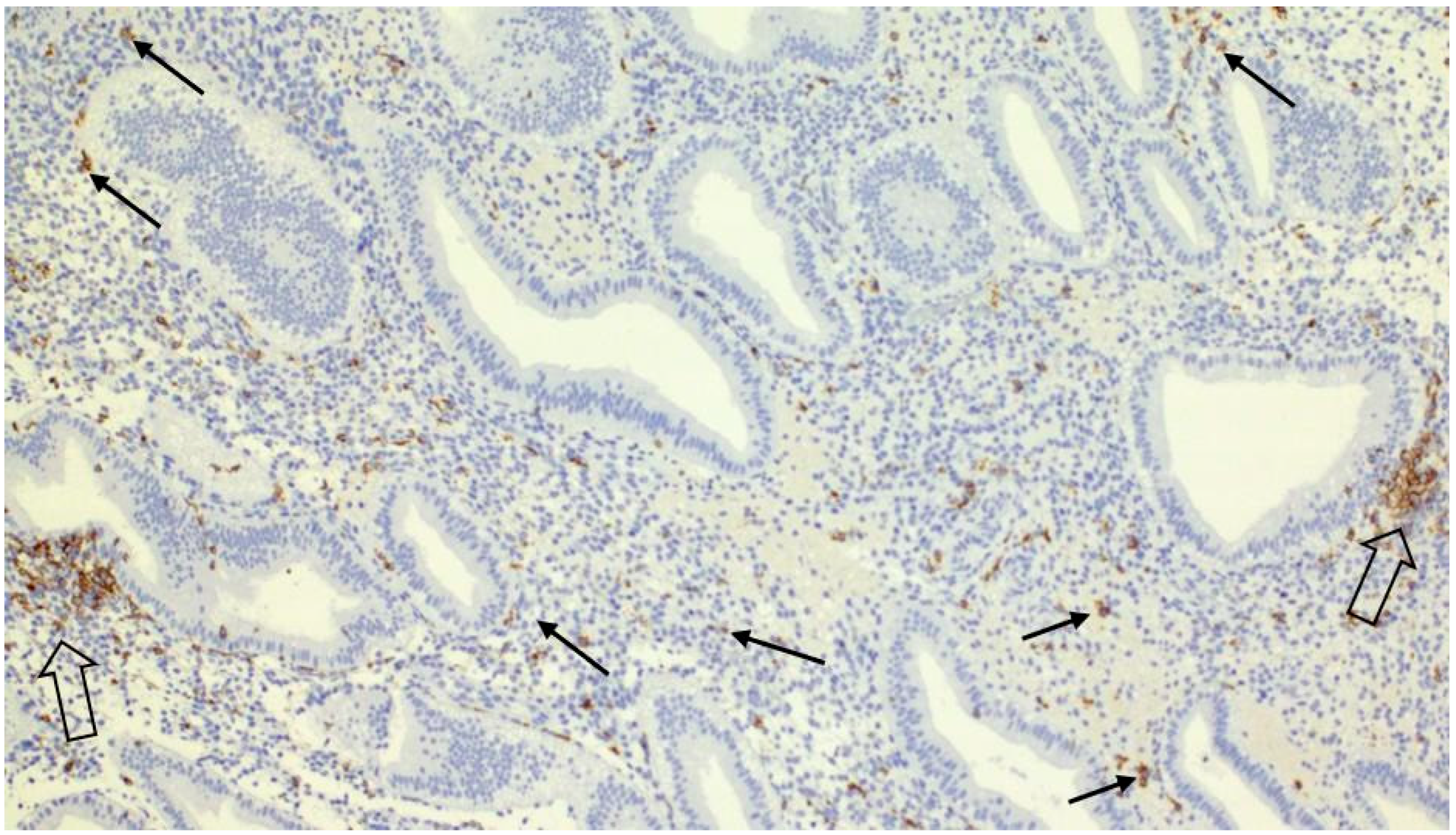
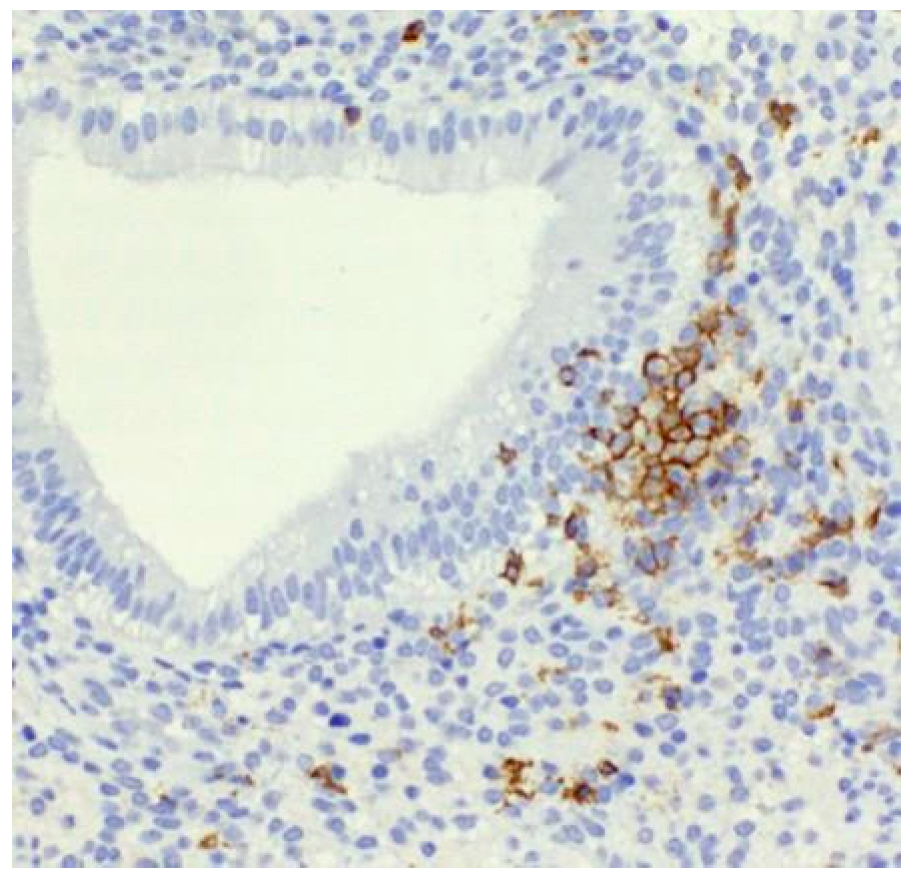
| Factor | Correlation | Significance Level (p-Value) | Notes |
|---|---|---|---|
| NK Cell Count and presence of clusters | 0.161 | 0.01 | Higher NK cell counts correlate with presence of clusters |
| NK Cell Count and L-thyroxine Treatment | 0.014 | 0.781 | No significant correlation found |
| NK Cell Count and Intralipid Treatment | −0.038 | 0.459 | No significant correlation found |
| NK Cell Count and Metformin Treatment | 0.001 | 0.980 | No significant correlation found |
| NK Cell Count and Corticosteroid Treatment | −0.011 | 0.835 | No significant correlation found |
| NK Cell Count and HRT Treatment | −0.015 | 0.761 | No significant correlation found |
| NK Cell Count and Aspirin Treatment | −0.049 | 0.340 | No significant correlation found |
| NK Cell Count and LMWH Treatment | −0.010 | 0.851 | No significant correlation found |
| NK Cell Count and Bromocriptine Treatment | 0.012 | 0.819 | No significant correlation found |
| Impact of Clusters on Pregnancy/Miscarriages | 0.031 | 0.549 | No significant correlation found |
| NK Cell Count by Location (Martin, Bratislava) | −0.053 | 0.296 | No significant association found |
| NK Cell Count by Season | −0.044 | 0.393 | No significant association found |
| Impact of food intolerances on NK Cell Count | 0.007 | 0.890 | No significant association found |
| Age Group (Years) | Number of Patients | Average uNK Cells/mm2 |
|---|---|---|
| 20–29 | 31 | 94.97 |
| 30–39 | 257 | 115.55 |
| ≥40 | 99 | 112.29 |
| Age Group (Years) | Patients with Positive Clusters |
|---|---|
| 20–29 | 9 |
| 30–39 | 70 |
| ≥40 | 39 |
| Clinical Subgroup | Number of Patients |
|---|---|
| Patients with RIF | 218 |
| of which younger than 37 | 107 |
| Patients with RPL | 28 |
| of which younger than 37 | 16 |
| Patients with idiopathic sterility | 158 |
| of which younger than 37 | 82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapides, L.; Klein, M.; Varga, I.; Voller, J.; Babal, P. Clustering of Uterine Natural Killer Cells Around Uterine Glands in Women with Recurrent Implantation Failure and Recurrent Pregnancy Loss: An Immunohistochemical Study. Int. J. Mol. Sci. 2025, 26, 10109. https://doi.org/10.3390/ijms262010109
Lapides L, Klein M, Varga I, Voller J, Babal P. Clustering of Uterine Natural Killer Cells Around Uterine Glands in Women with Recurrent Implantation Failure and Recurrent Pregnancy Loss: An Immunohistochemical Study. International Journal of Molecular Sciences. 2025; 26(20):10109. https://doi.org/10.3390/ijms262010109
Chicago/Turabian StyleLapides, Lenka, Martin Klein, Ivan Varga, Jaroslav Voller, and Pavel Babal. 2025. "Clustering of Uterine Natural Killer Cells Around Uterine Glands in Women with Recurrent Implantation Failure and Recurrent Pregnancy Loss: An Immunohistochemical Study" International Journal of Molecular Sciences 26, no. 20: 10109. https://doi.org/10.3390/ijms262010109
APA StyleLapides, L., Klein, M., Varga, I., Voller, J., & Babal, P. (2025). Clustering of Uterine Natural Killer Cells Around Uterine Glands in Women with Recurrent Implantation Failure and Recurrent Pregnancy Loss: An Immunohistochemical Study. International Journal of Molecular Sciences, 26(20), 10109. https://doi.org/10.3390/ijms262010109






