Surrogate Biomarkers in Gene Therapy for Orphan Diseases: Validation, Application, and Regulatory Aspects
Abstract
1. Introduction
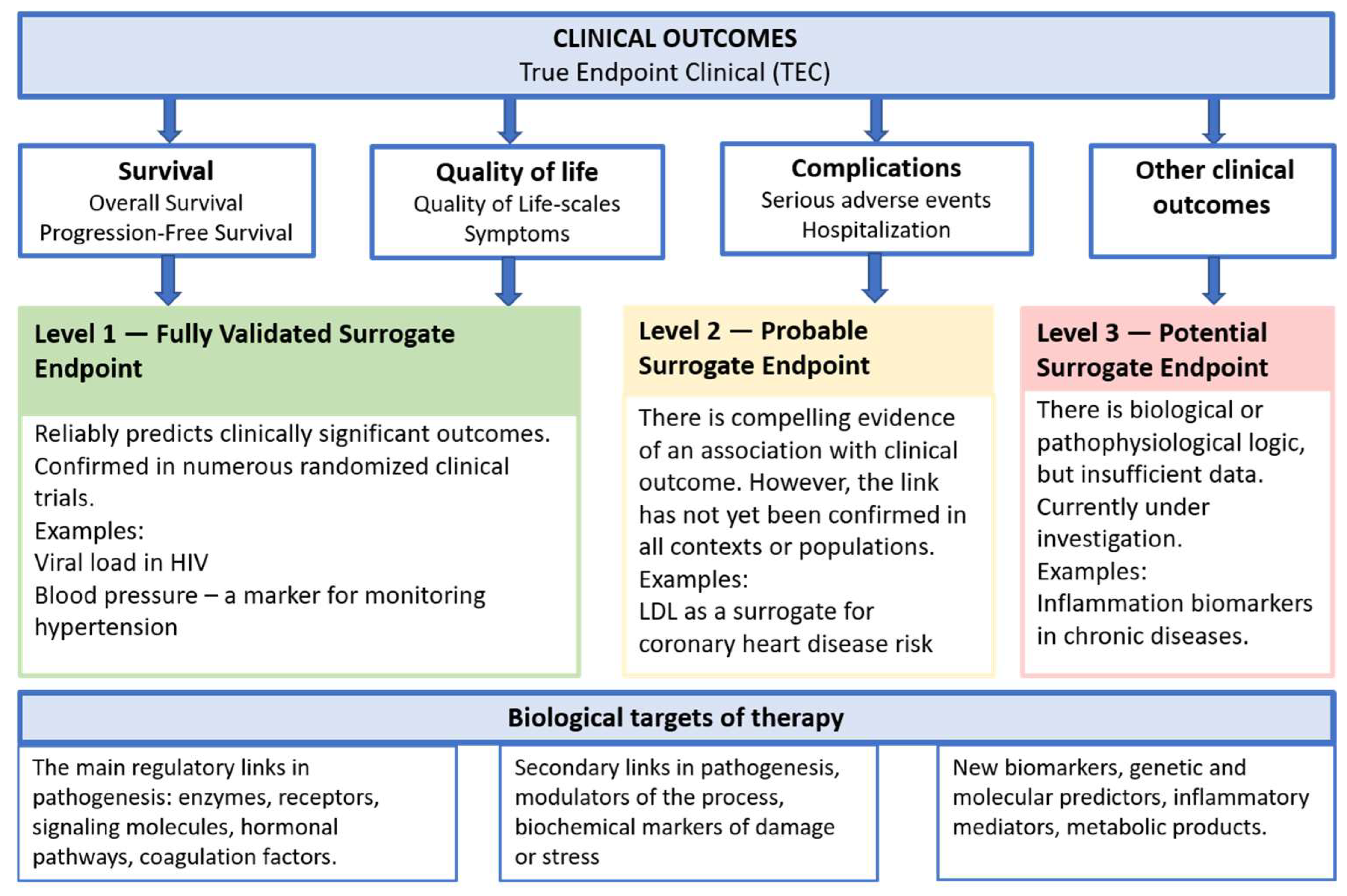
2. Surrogate Markers and Their Classification
2.1. Definition of a Surrogate Endpoint (Biomarker)
2.2. Types of Biomarkers
2.3. Criteria for a Quality Surrogate Marker
3. Regulatory Framework and Requirements for Surrogate Biomarkers
3.1. Requirements for Surrogate Markers in the United States
3.2. Requirements for Surrogate Markers in Europe
3.3. The Use of Surrogate Endpoints as a Tool for Accelerated Drug Registration in the Russian Federation
3.4. Requirements for Surrogate Markers in Japan
3.5. Requirements for Surrogate Markers in China
3.6. Requirements for Surrogate Markers in Canada
4. Preclinical Studies and Translational Biomarkers
4.1. Transgenic and Knockout Animals for Modeling Rare Diseases
4.2. Correlation with Clinical Pathology
4.3. Regulatory Requirements for Preclinical Biomarker-Based Studies
5. Surrogate Biomarkers and Clinical Studies of Gene Therapy Products
5.1. Selection and Justification of a Surrogate Marker in Phase I–III Protocols
5.2. The Role of Biomarkers in Accelerated/Conditional Approval
5.3. Post-Marketing Studies
6. Key Examples of the Use of Metabolic Surrogate Biomarkers
6.1. Metachromatic Leukodystrophy
6.2. Mucopolysaccharidosis Type I
6.3. Tay–Sachs Disease
6.4. Hemophilia B
7. Potential Challenges and Limitations
7.1. Insufficient Validation of Surrogate Endpoints
7.2. Complexity of Extrapolation
7.3. Rare and Ultra-Rare Diseases
8. Trends and Future Prospects
8.1. Novel Technologies for Biomarker Identification and Validation
8.2. Expansion of Regulatory Support
8.3. Integrated Approaches
8.4. Multi-Omics Biomarkers
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Accelerated Approval (FDA program) |
| AAV | Adeno-Associated Virus |
| ABR | Annualized Bleeding Rate |
| AD | Alzheimer’s Disease |
| AI | Artificial Intelligence |
| AIDS | Acquired Immunodeficiency Syndrome |
| ARSA | Arylsulfatase A gene/enzyme |
| BMD | Bone Mineral Density |
| CDE | Center for Drug Evaluation (China) |
| CHMP | Committee for Medicinal Products for Human Use (EMA) |
| CKD | Chronic Kidney Disease |
| CMA | Conditional Marketing Authorisation (EMA) |
| CNS | Central Nervous System |
| CSF | Cerebrospinal Fluid |
| DDT | Drug Development Tools (FDA initiative) |
| EAEU | Eurasian Economic Union |
| EEC | Eurasian Economic Commission |
| EGFR | Epidermal Growth Factor Receptor |
| EMA | European Medicines Agency |
| ERT | Enzyme Replacement Therapy |
| FDA | Food and Drug Administration (USA) |
| FIX/F9 | Coagulation Factor IX/its gene |
| GAG | Glycosaminoglycans |
| Gb3 | Globotriaosylceramide |
| GBA1 | β-glucocerebrosidase gene/enzyme |
| GCP | Good Clinical Practice |
| GFR-slope | rate of decline of Glomerular Filtration Rate |
| GLA | α-galactosidase A gene/enzyme |
| GlcCer | Glucocerebroside |
| GLP | Good Laboratory Practice |
| GM2 | Ganglioside GM2 |
| GMFM | Gross Motor Function Measure |
| GTMP | Gene Therapy Medicinal Product |
| HbA1c | Glycated Hemoglobin |
| HDL-C | High-Density Lipoprotein Cholesterol |
| HEX | β-hexosaminidase |
| HEXA | β-hexosaminidase A subunit gene/enzyme |
| HEXB | β-hexosaminidase B subunit gene/enzyme |
| HIV | Human Immunodeficiency Virus |
| HPFB | Health Products and Food Branch (Canada) |
| HSCs | Hematopoietic Stem Cells |
| ICH | The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use |
| IDUA | α-L-iduronidase gene/enzyme |
| LDL-C | Low-Density Lipoprotein Cholesterol |
| LSD | Lysosomal Storage Disorder |
| LTFU | Long-Term Follow-up |
| lyso-Gb1 | Glucosylsphingosine (Gaucher disease biomarker) |
| lyso-Gb3 | Deacylated Gb3 (Fabry disease biomarker) |
| lyso-GM2 | Deacylated GM2 (Tay-Sachs disease biomarker) |
| MHLW | Ministry of Health, Labour and Welfare (Japan) |
| MLD | Metachromatic Leukodystrophy |
| MPS I | Mucopolysaccharidosis Type I |
| MRD | Minimal Residual Disease |
| MRI | Magnetic Resonance Imaging |
| NAA | N-acetylaspartate |
| NDA/BLA | New Drug Application/Biologics License Application |
| NMPA | National Medical Products Administration (China) |
| NOC/c | Notice of Compliance with Conditions (Canada) |
| ORR | Objective Response Rate |
| OS | Overall Survival |
| PET | Positron Emission Tomography |
| PFS | Progression-Free Survival |
| PMD Act | Pharmaceuticals and Medical Device Act (Japan) |
| PMDA | Pharmaceuticals and Medical Devices Agency (Japan) |
| PNS | Peripheral Nervous System |
| PRIME | Priority Medicines (EMA program) |
| PSA | Prostate-Specific Antigen |
| RCT | Randomized Controlled Trial |
| RECIST | Response Evaluation Criteria in Solid Tumors |
| RWD | Real-World Data |
| RWE | Real-World Evidence |
| TSD | Tay–Sachs Disease |
References
- Wang, D.; Stevens, G.; Flotte, T.R. Gene Therapy Then and Now: A Look Back at Changes in the Field over the Past 25 Years. Mol. Ther. 2025, 33, 1889–1902. [Google Scholar] [CrossRef] [PubMed]
- Mellerio, J.E. The Challenges of Clinical Trials in Rare Diseases. Br. J. Dermatol. 2022, 187, 453–454. [Google Scholar] [CrossRef]
- Wickström, K.; Moseley, J. Biomarkers and Surrogate Endpoints in Drug Development: A European Regulatory View. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO27–BIO33. [Google Scholar] [CrossRef] [PubMed]
- Prentice, R.L. Surrogate Endpoints in Clinical Trials: Definition and Operational Criteria. Stat. Med. 1989, 8, 431–440. [Google Scholar] [CrossRef]
- Fleming, T.R.; DeMets, D.L. Surrogate End Points in Clinical Trials: Are We Being Misled? Ann. Intern. Med. 1996, 125, 605–613. [Google Scholar] [CrossRef]
- Buyse, M.; Sargent, D.J.; Grothey, A.; Matheson, A.; de Gramont, A. Biomarkers and Surrogate End Points—The Challenge of Statistical Validation. Nat. Rev. Clin. Oncol. 2010, 7, 309–317. [Google Scholar] [CrossRef]
- Lassere, M.N. The Biomarker-Surrogacy Evaluation Schema: A Review of the Biomarker-Surrogate Literature and a Proposal for a Criterion-Based, Quantitative, Multidimensional Hierarchical Levels of Evidence Schema for Evaluating the Status of Biomarkers as Surrogate Endpoints. Stat. Methods Med. Res. 2008, 17, 303–340. [Google Scholar] [CrossRef]
- Weir, C.J.; Walley, R.J. Statistical Evaluation of Biomarkers as Surrogate Endpoints: A Literature Review. Stat. Med. 2006, 25, 183–203. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.H.; Genest, J.; Gotto, A.M.; Kastelein, J.J.P.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Hernandez-Villafuerte, K.; Fischer, A.; Latimer, N. Challenges and Methodologies in Using Progression Free Survival as a Surrogate for Overall Survival in Oncology. Int. J. Technol. Assess. Health Care 2018, 34, 300–316. [Google Scholar] [CrossRef] [PubMed]
- Skyler, J.S.; Bergenstal, R.; Bonow, R.O.; Buse, J.; Deedwania, P.; Gale, E.A.M.; Howard, B.V.; Kirkman, M.S.; Kosiborod, M.; Reaven, P.; et al. Intensive Glycemic Control and the Prevention of Cardiovascular Events: Implications of the ACCORD, ADVANCE, and VA Diabetes Trials: A Position Statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J. Am. Coll. Cardiol. 2009, 53, 298–304. [Google Scholar] [CrossRef]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.P.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.-C.; Waters, D.D.; et al. Effects of Torcetrapib in Patients at High Risk for Coronary Events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef]
- Mayeux, R. Biomarkers: Potential Uses and Limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef]
- Aronson, J.K.; Ferner, R.E. Biomarkers-A General Review. Curr. Protoc. Pharmacol. 2017, 76, 9.23.1–9.23.17. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Yang, J.; Zhen, J.; Zhang, D. Serum microRNA-21 as a Potential Diagnostic Biomarker for Breast Cancer: A Systematic Review and Meta-Analysis. Clin. Exp. Med. 2016, 16, 29–35. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Y.; Sun, H.; Zhuang, R.; Liu, J.; Liu, T.; Cai, W. K-Ras Mutation Detection in Liquid Biopsy and Tumor Tissue as Prognostic Biomarker in Patients with Pancreatic Cancer: A Systematic Review with Meta-Analysis. Med. Oncol. 2016, 33, 61. [Google Scholar] [CrossRef]
- Whibley, C.; Pharoah, P.D.P.; Hollstein, M. P53 Polymorphisms: Cancer Implications. Nat. Rev. Cancer 2009, 9, 95–107. [Google Scholar] [CrossRef]
- Jafarzadeh, L.; Khakpoor-Koosheh, M.; Mirzaei, H.; Mirzaei, H.R. Biomarkers for Predicting the Outcome of Various Cancer Immunotherapies. Crit. Rev. Oncol. Hematol. 2021, 157, 103161. [Google Scholar] [CrossRef]
- Sakata, S.; Larson, D.W. Targeted Therapy for Colorectal Cancer. Surg. Oncol. Clin. N. Am. 2022, 31, 255–264. [Google Scholar] [CrossRef]
- Zhao, X.; Modur, V.; Carayannopoulos, L.N.; Laterza, O.F. Biomarkers in Pharmaceutical Research. Clin. Chem. 2015, 61, 1343–1353. [Google Scholar] [CrossRef]
- Bailón, L.; Puertas, M.C.; García-Guerrero, M.C.; Moraes-Cardoso, I.; Aparicio, E.; Alarcón-Soto, Y.; Rivero, A.; Rosen, E.P.; Estes, J.D.; Blanco, J.; et al. Impact of Dolutegravir Plus Lamivudine as First-Line Antiretroviral Treatment on the Human Immunodeficiency Virus Type 1 Reservoir and Inflammatory Markers in Peripheral Blood. J. Infect. Dis. 2025, 231, 600–610. [Google Scholar] [CrossRef]
- Patel, M.M.; Adrada, B.E. Hereditary Breast Cancer: BRCA Mutations and Beyond. Radiol. Clin. N. Am. 2024, 62, 627–642. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Das, S.; Hyman, B.T. APOE and Alzheimer’s Disease: Advances in Genetics, Pathophysiology, and Therapeutic Approaches. Lancet Neurol. 2021, 20, 68–80, Erratum in Lancet Neurol. 2021, 20, E2. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What Are Biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- FDA Surrogate Endpoint Resources for Drug and Biologic Development. 2018. Available online: https://www.fda.gov/drugs/development-resources/surrogate-endpoint-resources-drug-and-biologic-development (accessed on 16 September 2025).
- FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval. 2023. Available online: https://www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval (accessed on 16 September 2025).
- FDA. Pathways for Biomarker Integration in Drug Development. 2018. Available online: https://www.fda.gov/drugs/biomarker-qualification-program/pathways-biomarker-integration-drug-development#language (accessed on 16 September 2025).
- European Medicines Agency (EMA). Conditional Marketing Authorisation. 2016. Available online: https://www.ema.europa.eu/en/human-regulatory-overview/marketing-authorisation/conditional-marketing-authorisation (accessed on 16 September 2025).
- European Medicines Agency (EMA). Clinical Trials in Small Populations—Scientific Guideline. 2006. Available online: https://www.ema.europa.eu/en/clinical-trials-small-populations-scientific-guideline (accessed on 16 September 2025).
- Federal Service for Surveillance in Healthcare. 2024. Available online: https://roszdravnadzor.gov.ru/drugs/documents/66 (accessed on 16 September 2025).
- Decision of the EEC Council No. 74 of November 3, 2016, on the Approval of the Procedure for the Formation and Maintenance of the Register of Authorized Persons of Drug Manufacturers of the Eurasian Economic Union. 2016. Available online: https://docs.eaeunion.org/documents/306/2583/ (accessed on 16 September 2025).
- Kemp, R.; Prasad, V. Surrogate Endpoints in Oncology: When Are They Acceptable for Regulatory and Clinical Decisions, and Are They Currently Overused? BMC Med. 2017, 15, 134. [Google Scholar] [CrossRef]
- Mooghali, M.; Wallach, J.D.; Ross, J.S.; Ramachandran, R. Premarket Pivotal Trial End Points and Postmarketing Requirements for FDA Breakthrough Therapies. JAMA Netw. Open 2024, 7, e2430486. [Google Scholar] [CrossRef]
- Notifications and Administrative Notices. 2021. Available online: https://www.pmda.go.jp/english/safety/info-services/drugs/safety-measures/0001.html (accessed on 16 September 2025).
- Maeda, H.; Kurokawa, T. Acceptance of Surrogate End Points in Clinical Trials Supporting Approval of Drugs for Cancer Treatment by the Japanese Regulatory Agency. Ann. Oncol. 2015, 26, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S. ES10. 02 Japan Pharmaceuticals and Medical Devices Agency. J. Thorac. Oncol. 2019, 14, S38. [Google Scholar] [CrossRef]
- Research, C. For D.E. and E9 Statistical Principles for Clinical Trials. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e9-statistical-principles-clinical-trials (accessed on 16 September 2025).
- Commissioner, O. Of the FDA Facts: Biomarkers and Surrogate Endpoints. FDA 2019. Available online: https://www.fda.gov/about-fda/innovation-fda/fda-facts-biomarkers-and-surrogate-endpoints (accessed on 16 September 2025).
- Maeda, H.; Shingai, R.; Takeda, K.; Hara, A.; Murai, Y.; Ofuchi, M. Assessment of Surrogate End Point Trends in Clinical Trials to Approve Oncology Drugs From 2001 to 2020 in Japan. JAMA Netw. Open 2023, 6, e238875. [Google Scholar] [CrossRef] [PubMed]
- Notice of the National Medical Products Administration on Issuing the Technical Guidance Principles for Clinical Trial Endpoints in Advanced Non-Small Cell Lung Cancer (No. 64 of 2019) Departmental Affairs_China Government Website. 2019. Available online: https://www.gov.cn/xinwen/2019-09/18/content_5430886.htm (accessed on 16 September 2025).
- Technical Guidelines for Conditional Approval of Drugs for Marketing (Trial Implementation). Available online: https://www.cde.org.cn/zdyz/domesticinfopage?zdyzIdCODE=3ec9de4b4bfab46c8fc1cbc0211179bf (accessed on 16 September 2025).
- Notice of the Center for Drug Evaluation of the National Medical Products Administration on the Issuance of the “Technical Guidelines for Clinical Trials of Innovative Drugs for the Treatment of Postmenopausal Osteoporosis” (No. 2 of 2021). 2021. Available online: https://www.cde.org.cn/main/news/viewInfoCommon/669578efe89cadfd61421e282478e0b2 (accessed on 16 September 2025).
- Canada, H. Guidance Document: Schedule A and Section 3 to the Food and Drugs Act [Health Canada, 2010]. 2013. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/guidance-document-schedule-section-3-food-drugs-act.html (accessed on 16 September 2025).
- Structure and Content of Clinical Study Reports. 1996. Available online: https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/dhp-mps/alt_formats/hpfb-dgpsa/pdf/prodpharma/e3-eng.pdf (accessed on 16 September 2025).
- Canada, H. Guidance Document: Notice of Compliance with Conditions (NOC/c). 2016. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/notice-compliance-conditions.html (accessed on 16 September 2025).
- Visigalli, I.; Delai, S.; Politi, L.S.; Di Domenico, C.; Cerri, F.; Mrak, E.; D’Isa, R.; Ungaro, D.; Stok, M.; Sanvito, F.; et al. Gene Therapy Augments the Efficacy of Hematopoietic Cell Transplantation and Fully Corrects Mucopolysaccharidosis Type I Phenotype in the Mouse Model. Blood 2010, 116, 5130–5139. [Google Scholar] [CrossRef] [PubMed]
- Randall, D.R.; Colobong, K.E.; Hemmelgarn, H.; Sinclair, G.B.; Hetty, E.; Thomas, A.; Bodamer, O.A.; Volkmar, B.; Fernhoff, P.M.; Casey, R.; et al. Heparin Cofactor II-Thrombin Complex: A Biomarker of MPS Disease. Mol. Genet. Metab. 2008, 94, 456–461. [Google Scholar] [CrossRef]
- St Martin, T.; Seabrook, T.A.; Gall, K.; Newman, J.; Avila, N.; Hayes, A.; Kivaa, M.; Lotterhand, J.; Mercaldi, M.; Patel, K.; et al. Single Systemic Administration of a Gene Therapy Leading to Disease Treatment in Metachromatic Leukodystrophy Arsa Knock-Out Mice. J. Neurosci. 2023, 43, 3567–3581. [Google Scholar] [CrossRef]
- Shaimardanova, A.A.; Chulpanova, D.S.; Solovyeva, V.V.; Mullagulova, A.I.; Kitaeva, K.V.; Allegrucci, C.; Rizvanov, A.A. Metachromatic Leukodystrophy: Diagnosis, Modeling, and Treatment Approaches. Front. Med. 2020, 7, 576221. [Google Scholar] [CrossRef]
- Matzner, U.; Matthes, F.; Herbst, E.; Lüllmann-Rauch, R.; Callaerts-Vegh, Z.; D’Hooge, R.; Weigelt, C.; Eistrup, C.; Fogh, J.; Gieselmann, V. Induction of Tolerance to Human Arylsulfatase A in a Mouse Model of Metachromatic Leukodystrophy. Mol. Med. 2007, 13, 471–479. [Google Scholar] [CrossRef]
- Solovyeva, V.V.; Shaimardanova, A.A.; Chulpanova, D.S.; Kitaeva, K.V.; Chakrabarti, L.; Rizvanov, A.A. New Approaches to Tay-Sachs Disease Therapy. Front. Physiol. 2018, 9, 1663. [Google Scholar] [CrossRef]
- Kodama, T.; Togawa, T.; Tsukimura, T.; Kawashima, I.; Matsuoka, K.; Kitakaze, K.; Tsuji, D.; Itoh, K.; Ishida, Y.-I.; Suzuki, M.; et al. Lyso-GM2 Ganglioside: A Possible Biomarker of Tay-Sachs Disease and Sandhoff Disease. PLoS ONE 2011, 6, e29074. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zoppè, M.; Hackeng, T.M.; Griffin, J.H.; Lee, K.F.; Verma, I.M. A Factor IX-Deficient Mouse Model for Hemophilia B Gene Therapy. Proc. Natl. Acad. Sci. USA 1997, 94, 11563–11566. [Google Scholar] [CrossRef] [PubMed]
- Cabasso, O.; Kuppuramalingam, A.; Lelieveld, L.; Van der Lienden, M.; Boot, R.; Aerts, J.M.; Horowitz, M. Animal Models for the Study of Gaucher Disease. Int. J. Mol. Sci. 2023, 24, 16035. [Google Scholar] [CrossRef] [PubMed]
- Enquist, I.B.; Lo Bianco, C.; Ooka, A.; Nilsson, E.; Månsson, J.-E.; Ehinger, M.; Richter, J.; Brady, R.O.; Kirik, D.; Karlsson, S. Murine Models of Acute Neuronopathic Gaucher Disease. Proc. Natl. Acad. Sci. USA 2007, 104, 17483–17488. [Google Scholar] [CrossRef]
- Comper, F.; Miranda, C.J.; Liou, B.; Dodev, T.; Jeyakumar, J.M.; Canavese, M.; Cocita, C.; Khoshrou, K.; Tiscornia, G.; Chisari, E.; et al. FLT201, a Novel Liver-Directed AAV Gene Therapy Candidate for Gaucher Disease Type 1. Mol. Ther. 2025, 33, 3789–3807. [Google Scholar] [CrossRef]
- Enquist, I.B.; Nilsson, E.; Ooka, A.; Månsson, J.-E.; Olsson, K.; Ehinger, M.; Brady, R.O.; Richter, J.; Karlsson, S. Effective Cell and Gene Therapy in a Murine Model of Gaucher Disease. Proc. Natl. Acad. Sci. USA 2006, 103, 13819–13824. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, G.; Markovic, U.; Condorelli, A.; Calafiore, V.; Nicolosi, D.; Calagna, M.; Grasso, S.; Ragusa, M.T.V.; Gentile, J.; Napolitano, M. Glucosylsphingosine (Lyso-Gb1) as a Reliable Biomarker in Gaucher Disease: A Narrative Review. Orphanet J. Rare Dis. 2023, 18, 27. [Google Scholar] [CrossRef]
- Schiffmann, R. Fabry Disease. Pharmacol. Ther. 2009, 122, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Murray, G.J.; Swaim, W.D.; Longenecker, G.; Quirk, J.M.; Cardarelli, C.O.; Sugimoto, Y.; Pastan, I.; Gottesman, M.M.; Brady, R.O.; et al. α-Galactosidase A Deficient Mice: A Model of Fabry Disease. Proc. Natl. Acad. Sci. USA 1997, 94, 2540–2544. [Google Scholar] [CrossRef]
- Jung, S.C.; Han, I.P.; Limaye, A.; Xu, R.; Gelderman, M.P.; Zerfas, P.; Tirumalai, K.; Murray, G.J.; During, M.J.; Brady, R.O.; et al. Adeno-Associated Viral Vector-Mediated Gene Transfer Results in Long-Term Enzymatic and Functional Correction in Multiple Organs of Fabry Mice. Proc. Natl. Acad. Sci. USA 2001, 98, 2676–2681. [Google Scholar] [CrossRef] [PubMed]
- Simonetta, I.; Tuttolomondo, A.; Daidone, M.; Pinto, A. Biomarkers in Anderson-Fabry Disease. Int. J. Mol. Sci. 2020, 21, 8080. [Google Scholar] [CrossRef]
- Casal, M.; Haskins, M. Large Animal Models and Gene Therapy. Eur. J. Hum. Genet. 2006, 14, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Gruntman, A.M.; Flotte, T.R. Gene Therapy and the Use of Animal Models: Why Mice Alone Are Not Sufficient. Hum. Gene Ther. 2022, 33, 477–478. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.P.F.; Bell, R.; Buckman, S.; Burckart, G.J.; Eichler, H.-G.; Fang, K.C.; Goodsaid, F.M.; Jusko, W.J.; Lesko, L.L.; Meibohm, B.; et al. Translational Biomarkers: From Preclinical to Clinical a Report of 2009 AAPS/ACCP Biomarker Workshop. AAPS J. 2011, 13, 274–283. [Google Scholar] [CrossRef]
- Podetz-Pedersen, K.M.; Laoharawee, K.; Singh, S.; Nguyen, T.T.; Smith, M.C.; Temme, A.; Kozarsky, K.; McIvor, R.S.; Belur, L.R. Neurologic Recovery in MPS I and MPS II Mice by AAV9-Mediated Gene Transfer to the CNS After the Development of Cognitive Dysfunction. Hum. Gene Ther. 2023, 34, 8–18. [Google Scholar] [CrossRef]
- Dekker, N.; van Dussen, L.; Hollak, C.E.M.; Overkleeft, H.; Scheij, S.; Ghauharali, K.; van Breemen, M.J.; Ferraz, M.J.; Groener, J.E.M.; Maas, M.; et al. Elevated Plasma Glucosylsphingosine in Gaucher Disease: Relation to Phenotype, Storage Cell Markers, and Therapeutic Response. Blood 2011, 118, e118–e127. [Google Scholar] [CrossRef]
- Doerr, J.; Böckenhoff, A.; Ewald, B.; Ladewig, J.; Eckhardt, M.; Gieselmann, V.; Matzner, U.; Brüstle, O.; Koch, P. Arylsulfatase A Overexpressing Human iPSC-Derived Neural Cells Reduce CNS Sulfatide Storage in a Mouse Model of Metachromatic Leukodystrophy. Mol. Ther. 2015, 23, 1519–1531. [Google Scholar] [CrossRef]
- Nagy, A.; Eichler, F.; Bley, A.; Bredow, J.; Fay, A.; Townsend, E.L.; Leiro, B.; Shaywitz, A.; Laforet, G.; Crippen-Harmon, D.; et al. Urine N-Acetylaspartate Distinguishes Phenotypes in Canavan Disease. Hum. Gene Ther. 2024, 36, 45–56. [Google Scholar] [CrossRef]
- Mattsson-Carlgren, N.; Palmqvist, S.; Blennow, K.; Hansson, O. Increasing the Reproducibility of Fluid Biomarker Studies in Neurodegenerative Studies. Nat. Commun. 2020, 11, 6252, Erratum in Nat. Commun. 2021, 4, 196. [Google Scholar] [CrossRef]
- Cuzick, J. The Importance of Long-Term Follow up of Participants in Clinical Trials. Br. J. Cancer 2023, 128, 432–438. [Google Scholar] [CrossRef]
- Mahase, E. Sickle Cell Drug Is Withdrawn over Safety Concerns Just Months after Rollout. BMJ 2024, 387, q2147. [Google Scholar] [CrossRef]
- Whitehouse, P.J.; Saini, V. Making the Case for the Accelerated Withdrawal of Aducanumab. J. Alzheimers Dis. 2022, 87, 999–1001. [Google Scholar] [CrossRef] [PubMed]
- Mullagulova, A.; Shaimardanova, A.; Solovyeva, V.; Mukhamedshina, Y.; Chulpanova, D.; Kostennikov, A.; Issa, S.; Rizvanov, A. Safety and Efficacy of Intravenous and Intrathecal Delivery of AAV9-Mediated ARSA in Minipigs. Int. J. Mol. Sci. 2023, 24, 9204. [Google Scholar] [CrossRef] [PubMed]
- Shaimardanova, A.A.; Chulpanova, D.S.; Solovyeva, V.V.; Mullagulova, A.I.; Kitaeva, K.V.; Rizvanov, A.A. New therapeutic strategies for the treatment of metachromatic leukodystrophy. Genes Cells 2020, 15, 41–50. [Google Scholar] [CrossRef]
- Audouard, E.; Khefif, N.; Mansat, C.; Nelcha, O.; Banchi, E.-G.; Lupiet, C.; Farabos, D.; Lamaziere, A.; Sevin, C.; Piguet, F. Dose-Response Evaluation of Intravenous Gene Therapy in a Symptomatic Mouse Model of Metachromatic Leukodystrophy. Mol. Ther. Methods Clin. Dev. 2024, 32, 101248. [Google Scholar] [CrossRef]
- Indication. Libmeldy. Available online: https://www.libmeldy.eu/indication/# (accessed on 15 October 2025).
- Commissioner, O. Of the FDA Approves First Gene Therapy for Children with Metachromatic Leukodystrophy. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-children-metachromatic-leukodystrophy (accessed on 16 September 2025).
- European Medicines Agency (EMA). New Gene Therapy to Treat Rare Genetic Disorder Metachromatic Leukodystrophy. 2020. Available online: https://www.ema.europa.eu/en/news/new-gene-therapy-treat-rare-genetic-disorder-metachromatic-leukodystrophy (accessed on 16 September 2025).
- Fumagalli, F.; Calbi, V.; Natali Sora, M.G.; Sessa, M.; Baldoli, C.; Rancoita, P.M.V.; Ciotti, F.; Sarzana, M.; Fraschini, M.; Zambon, A.A.; et al. Lentiviral Haematopoietic Stem-Cell Gene Therapy for Early-Onset Metachromatic Leukodystrophy: Long-Term Results from a Non-Randomised, Open-Label, Phase 1/2 Trial and Expanded Access. Lancet 2022, 399, 372–383. [Google Scholar] [CrossRef]
- Fahim, S.M.; Lin, G.; Suh, K.; Carlson, J.J.; Richardson, M.; Herce-Hagiwara, B.; Dickerson, R.; Pearson, S.D.; Rind, D.M.; Agboola, F. Atidarsagene Autotemcel for Metachromatic Leukodystrophy. J. Manag. Care Spec. Pharm. 2024, 30, 201–205. [Google Scholar] [CrossRef]
- Í Dali, C.; Sevin, C.; Krägeloh-Mann, I.; Giugliani, R.; Sakai, N.; Wu, J.; Wasilewski, M. Safety of Intrathecal Delivery of Recombinant Human Arylsulfatase A in Children with Metachromatic Leukodystrophy: Results from a Phase 1/2 Clinical Trial. Mol. Genet. Metab. 2020, 131, 235–244. [Google Scholar] [CrossRef]
- Al Zaabi, N.N.; Sirajum, M.; Al-Wawi, M.Z.; Al Suwaiji, M. Alpha-L-Iduronidase Deficiency: A Novel Mutation Resulting in Severe Early Presentation of Mucopolysaccharidosis Type I and Literature Review of the Molecular Basis. Clin. Case Rep. 2022, 10, e05904. [Google Scholar] [CrossRef]
- Sohn, Y.B.; Wang, R.; Ashworth, J.; Broqua, P.; Tallandier, M.; Abitbol, J.-L.; Jozwiak, E.; Pollard, L.; Wood, T.C.; Aslam, T.; et al. Biomarkers of Glycosaminoglycans (GAG) Accumulation in Patients with Mucopolysaccharidosis Type VI-LeukoGAG, Corneal Opacification (COM) and Carotid Intima Media Thickening (CIMT). Mol. Genet. Metab. Rep. 2024, 38, 101041. [Google Scholar] [CrossRef] [PubMed]
- Jameson, E.; Jones, S.; Remmington, T. Enzyme Replacement Therapy with Laronidase (Aldurazyme®) for Treating Mucopolysaccharidosis Type I. Cochrane Database Syst. Rev. 2019, 6, CD009354. [Google Scholar] [CrossRef] [PubMed]
- Orchard Therapeutics. Orchard Therapeutics Announces First Patient Randomized in Registrational Trial of OTL-203 for MPS-I Hurler Syndrome. 2024. Available online: https://ir.orchard-tx.com/news-releases/news-release-details/orchard-therapeutics-announces-first-patient-randomized/ (accessed on 16 September 2025).
- Shaimardanova, A.A.; Chulpanova, D.S.; Solovyeva, V.V.; Issa, S.S.; Mullagulova, A.I.; Titova, A.A.; Mukhamedshina, Y.O.; Timofeeva, A.V.; Aimaletdinov, A.M.; Nigmetzyanov, I.R.; et al. Increasing β-Hexosaminidase A Activity Using Genetically Modified Mesenchymal Stem Cells. Neural Regen. Res. 2024, 19, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Neuwelt, E.A.; Johnson, W.G.; Blank, N.K.; Pagel, M.A.; Maslen-McClure, C.; McClure, M.J.; Wu, P.M. Characterization of a New Model of GM2-Gangliosidosis (Sandhoff’s Disease) in Korat Cats. J. Clin. Investig. 1985, 76, 482–490. [Google Scholar] [CrossRef]
- Lawson, C.A.; Martin, D.R. Animal Models of GM2 Gangliosidosis: Utility and Limitations. Appl. Clin. Genet. 2016, 9, 111–120. [Google Scholar] [CrossRef]
- Flotte, T.R.; Cataltepe, O.; Puri, A.; Batista, A.R.; Moser, R.; McKenna-Yasek, D.; Douthwright, C.; Gernoux, G.; Blackwood, M.; Mueller, C.; et al. AAV Gene Therapy for Tay-Sachs Disease. Nat. Med. 2022, 28, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Fidanacogene Elaparvovec: First Approval. Drugs 2024, 84, 479–486. [Google Scholar] [CrossRef]
- Anguela, X.M.; High, K.A. Hemophilia B and Gene Therapy: A New Chapter with Etranacogene Dezaparvovec. Blood Adv. 2024, 8, 1796–1803. [Google Scholar] [CrossRef]
- Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary Report: Effect of Encainide and Flecainide on Mortality in a Randomized Trial of Arrhythmia Suppression after Myocardial Infarction. N. Engl. J. Med. 1989, 321, 406–412. [Google Scholar] [CrossRef]
- Newson, L.R.; Lass, A. Effectiveness of Transdermal Oestradiol and Natural Micronised Progesterone for Menopausal Symptoms. Br. J. Gen. Pract. 2018, 68, 499–500. [Google Scholar] [CrossRef]
- Santhanam, P.; Rowe, S.P.; Solnes, L.B.; Javadi, M.S. Plasma Fluoride Level and Femoral Bone Mineral Density in Post-Menopausal Women. Int. J. Occup. Environ. Med. 2017, 8, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, B.; Heijl, A. Lack of Visual Field Improvement After Initiation of Intraocular Pressure Reducing Treatment in the Early Manifest Glaucoma Trial. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5611–5615. [Google Scholar] [CrossRef]
- Shi, Q.; Sargent, D.J. Meta-Analysis for the Evaluation of Surrogate Endpoints in Cancer Clinical Trials. Int. J. Clin. Oncol. 2009, 14, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, I.T.T.; Reynolds, G.; Kesselheim, A.S.; Cliff, E.R.S. Regulatory and Clinical Outcomes of Nononcology Accelerated Approvals. JAMA 2025, 334, 1194–1196. [Google Scholar] [CrossRef]
- Alipour-Haris, G.; Liu, X.; Acha, V.; Winterstein, A.G.; Burcu, M. Real-World Evidence to Support Regulatory Submissions: A Landscape Review and Assessment of Use Cases. Clin. Transl. Sci. 2024, 17, e13903. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Cedazo-Minguez, A. Successful Therapies for Alzheimer’s Disease: Why so Many in Animal Models and None in Humans? Front. Pharmacol. 2014, 5, 146. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of Clinical Drug Development Fails and How to Improve It? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The Current State of Animal Models in Research: A Review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef]
- Augustine, E.F.; Adams, H.R.; Mink, J.W. Clinical Trials in Rare Disease: Challenges and Opportunities. J. Child Neurol. 2013, 28, 1142–1150. [Google Scholar] [CrossRef]
- Hilgers, R.-D.; König, F.; Molenberghs, G.; Senn, S. Design and Analysis of Clinical Trials for Small Rare Disease Populations. J. Rare Dis. Res. Treat. 2016, 1, 53–60. [Google Scholar] [CrossRef]
- Gülbakan, B.; Özgül, R.K.; Yüzbaşıoğlu, A.; Kohl, M.; Deigner, H.-P.; Özgüç, M. Discovery of Biomarkers in Rare Diseases: Innovative Approaches by Predictive and Personalized Medicine. EPMA J. 2016, 7, 24. [Google Scholar] [CrossRef]
- Arango-Argoty, G.; Bikiel, D.E.; Sun, G.J.; Kipkogei, E.; Smith, K.M.; Carrasco Pro, S.; Choe, E.Y.; Jacob, E. AI-Driven Predictive Biomarker Discovery with Contrastive Learning to Improve Clinical Trial Outcomes. Cancer Cell 2025, 43, 875–890.e8. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Li, W.; Zhang, S.; Wang, L.; Yang, J.; Zhao, D. A Novel Biomarker Selection Method Combining Graph Neural Network and Gene Relationships Applied to Microarray Data. BMC Bioinform. 2022, 23, 303. [Google Scholar] [CrossRef] [PubMed]
- Kamya, P.; Ozerov, I.V.; Pun, F.W.; Tretina, K.; Fokina, T.; Chen, S.; Naumov, V.; Long, X.; Lin, S.; Korzinkin, M.; et al. PandaOmics: An AI-Driven Platform for Therapeutic Target and Biomarker Discovery. J. Chem. Inf. Model. 2024, 64, 3961–3969. [Google Scholar] [CrossRef]
- FDA. Biomarker Qualification Program. 2025. Available online: https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/biomarker-qualification-program (accessed on 17 September 2025).
- Bakker, E.; Hendrikse, N.M.; Ehmann, F.; van der Meer, D.S.; Llinares Garcia, J.; Vetter, T.; Starokozhko, V.; Mol, P.G.M. Biomarker Qualification at the European Medicines Agency: A Review of Biomarker Qualification Procedures From 2008 to 2020. Clin. Pharmacol. Ther. 2022, 112, 69–80. [Google Scholar] [CrossRef]
- Marius CDISC and C-Path Continue CFAST Partnership with Recently Awarded FDA Grants. C-Path 2015. Available online: https://c-path.org/cdisc-and-c-path-continue-cfast-partnership-with-recently-awarded-fda-grants/ (accessed on 15 October 2025).
- Global Genes and RARE-X Partner to Enable Patient Data Collection for Rare Disease Groups—RARE-X. 2015. Available online: https://rare-x.org/2021/10/20/global-genes-and-rare-x-partner-to-enable-patient-data-collection-for-rare-disease-groups/ (accessed on 10 October 2025).
- Niu, L.; Sulek, K.; Vasilopoulou, C.G.; Santos, A.; Wewer Albrechtsen, N.J.; Rasmussen, S.; Meier, F.; Mann, M. Defining NASH from a Multi-Omics Systems Biology Perspective. J. Clin. Med. 2021, 10, 4673. [Google Scholar] [CrossRef]
| Regulator | Validated Surrogate | Candidate Surrogate | Approach to Use | Accelerated/Conditional Programs | Examples |
|---|---|---|---|---|---|
| FDA (USA) | Endpoint proven by multiple datasets as a reliable predictor of clinical benefit. | A marker with a justified hypothesis but not fully confirmed. Used in AA. | Validated surrogates are accepted in traditional NDAs/BLAs; “reasonably likely” surrogates are used for AA, with post-marketing confirmation. Actively consults on new markers. | AA, Fast Track, Breakthrough, Priority Review; Qualification Program (DDT ). | Lecanemab (AD, amyloid reduction by PET); multiple oncology drugs (ORR, PFS). |
| EMA (EU) | Does not formally define the term, but requires evidence of the marker’s association with clinical benefit. | Analogous to “candidate”, considered through a qualification procedure. | Surrogates may be used in conditional approval if benefit–risk is favorable and early access is needed. Biomarkers undergo CHMP qualification opinions. Early Scientific Advice on endpoints is encouraged. | CMA, Accelerated Assessment, PRIME; biomarker-method qualification procedure. | CMA for rare/severe diseases: drugs for oncology or rare genetic diseases. Qualified marker: GFR-slope for CKD. |
| Ministry of Health/Roszdravnadzor (Russia) | No formal surrogate definitions in regulations; primary requirement is efficacy based on clinical outcomes. | No clear classification; markers are considered within clinical trials. | Preference for “hard” clinical outcomes (survival, clinical status, etc.). Surrogates may be used if sufficient evidence of association with efficacy exists, often relying on international practice. Work is ongoing on simplified registration procedures. | Currently, there is no accelerated program equivalent to FDA/EMA. Elements of accelerated registration introduced in emergencies or drug shortages, (draft norms for “accelerated” or “conditional” registration under development). | Proposals for fast-track registration of foreign medicines in case of market shortages. In exceptional cases: approval of vaccines/therapies based on immune response (surrogate). |
| PMDA (Japan) | Requires strict biological rationale and statistical association with the clinical outcome. | Allowed if there is a reasonable link to the true endpoint. | Requires inclusion of secondary endpoints, including true outcomes, and comparative data in the Japanese cohort. Conditional approval possible for severe diseases lacking alternatives. | Conditional approval with mandatory post-marketing verification. Re-examination in 6–8 years. | Tumor response rate in oncology. |
| NMPA (China) | Must have biological plausibility and statistical association with clinical benefit. | Allowed for conditional approval in life-threatening diseases. | Requires mandatory post-marketing confirmation. Relies on international standards (ICH, FDA, EMA). | Conditional approval with confirmatory studies required. | MRD levels in multiple myeloma; change in BMD in osteoporosis. |
| Health Canada | A validated surrogate must predict clinical benefit. | Non-validated surrogates require confirmatory studies. | Evaluates the totality of evidence. Recommends discussing surrogate use with the regulator prior to study initiation. | NOC/c conditional approval with obligations for further studies. | Viral load suppression in HIV; PFS in oncology; antibody titers after vaccination. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayupova, A.I.; Solovyeva, V.V.; Issa, S.S.; Fayoud, H.J.; Rizvanov, A.A. Surrogate Biomarkers in Gene Therapy for Orphan Diseases: Validation, Application, and Regulatory Aspects. Int. J. Mol. Sci. 2025, 26, 10107. https://doi.org/10.3390/ijms262010107
Ayupova AI, Solovyeva VV, Issa SS, Fayoud HJ, Rizvanov AA. Surrogate Biomarkers in Gene Therapy for Orphan Diseases: Validation, Application, and Regulatory Aspects. International Journal of Molecular Sciences. 2025; 26(20):10107. https://doi.org/10.3390/ijms262010107
Chicago/Turabian StyleAyupova, Aisylu I., Valeriya V. Solovyeva, Shaza S. Issa, Haidar J. Fayoud, and Albert A. Rizvanov. 2025. "Surrogate Biomarkers in Gene Therapy for Orphan Diseases: Validation, Application, and Regulatory Aspects" International Journal of Molecular Sciences 26, no. 20: 10107. https://doi.org/10.3390/ijms262010107
APA StyleAyupova, A. I., Solovyeva, V. V., Issa, S. S., Fayoud, H. J., & Rizvanov, A. A. (2025). Surrogate Biomarkers in Gene Therapy for Orphan Diseases: Validation, Application, and Regulatory Aspects. International Journal of Molecular Sciences, 26(20), 10107. https://doi.org/10.3390/ijms262010107








