Study on Differences in 2-AP Synthesis and Metabolism Among Fragrant Rice Varieties
Abstract
1. Introduction
2. Results and Analysis
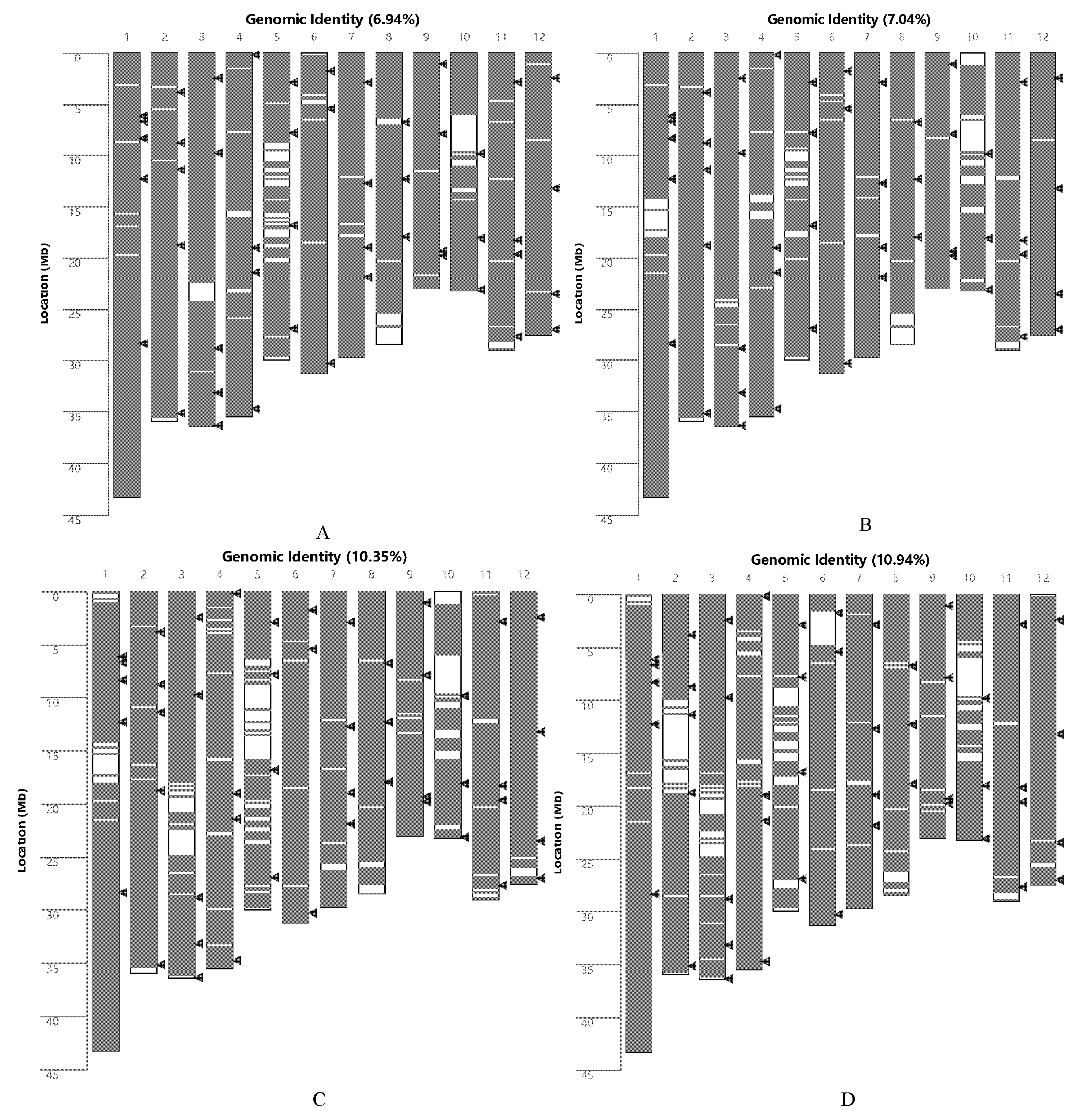
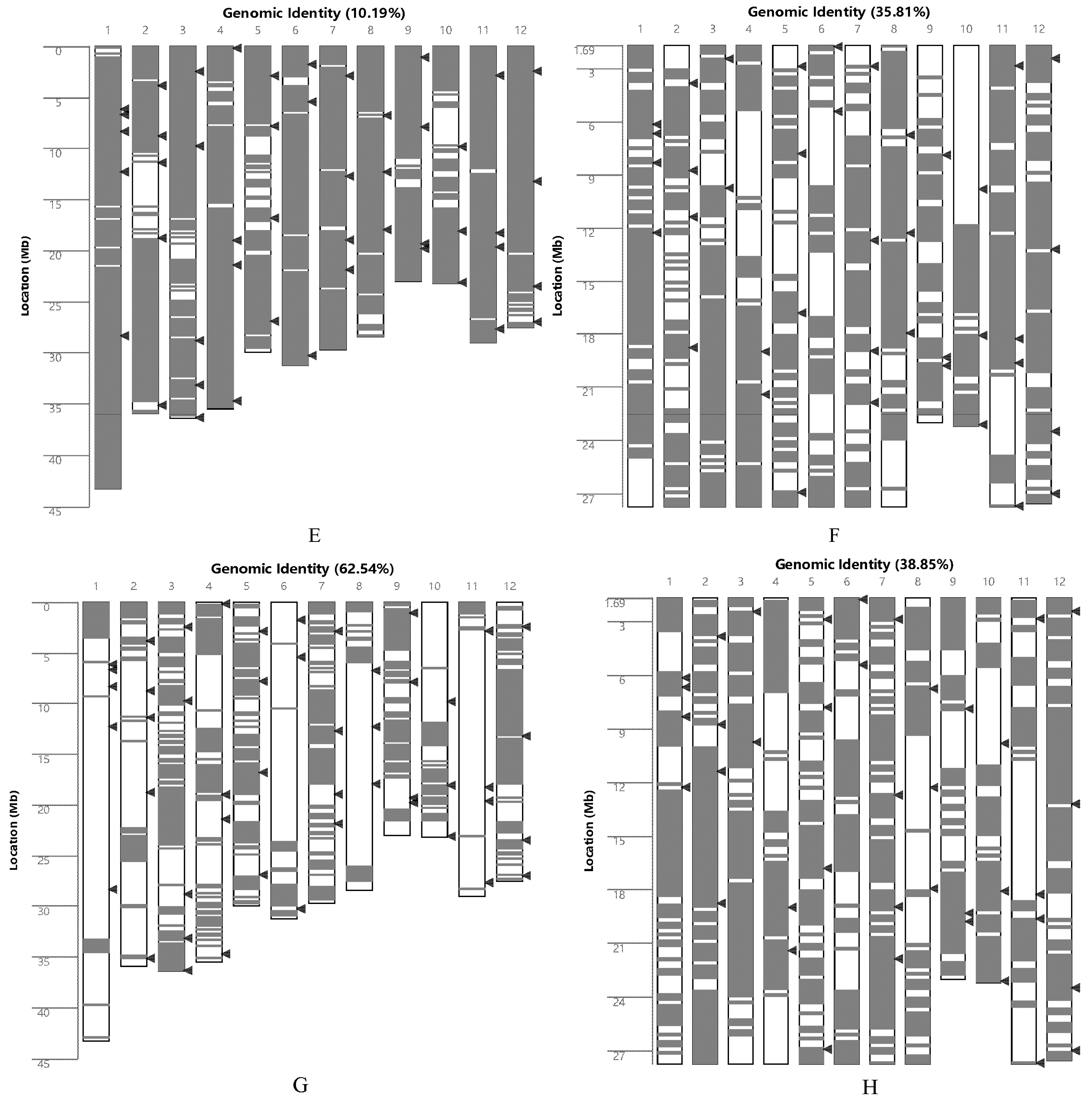
2.1. Analysis of the Genetic Background of Rice
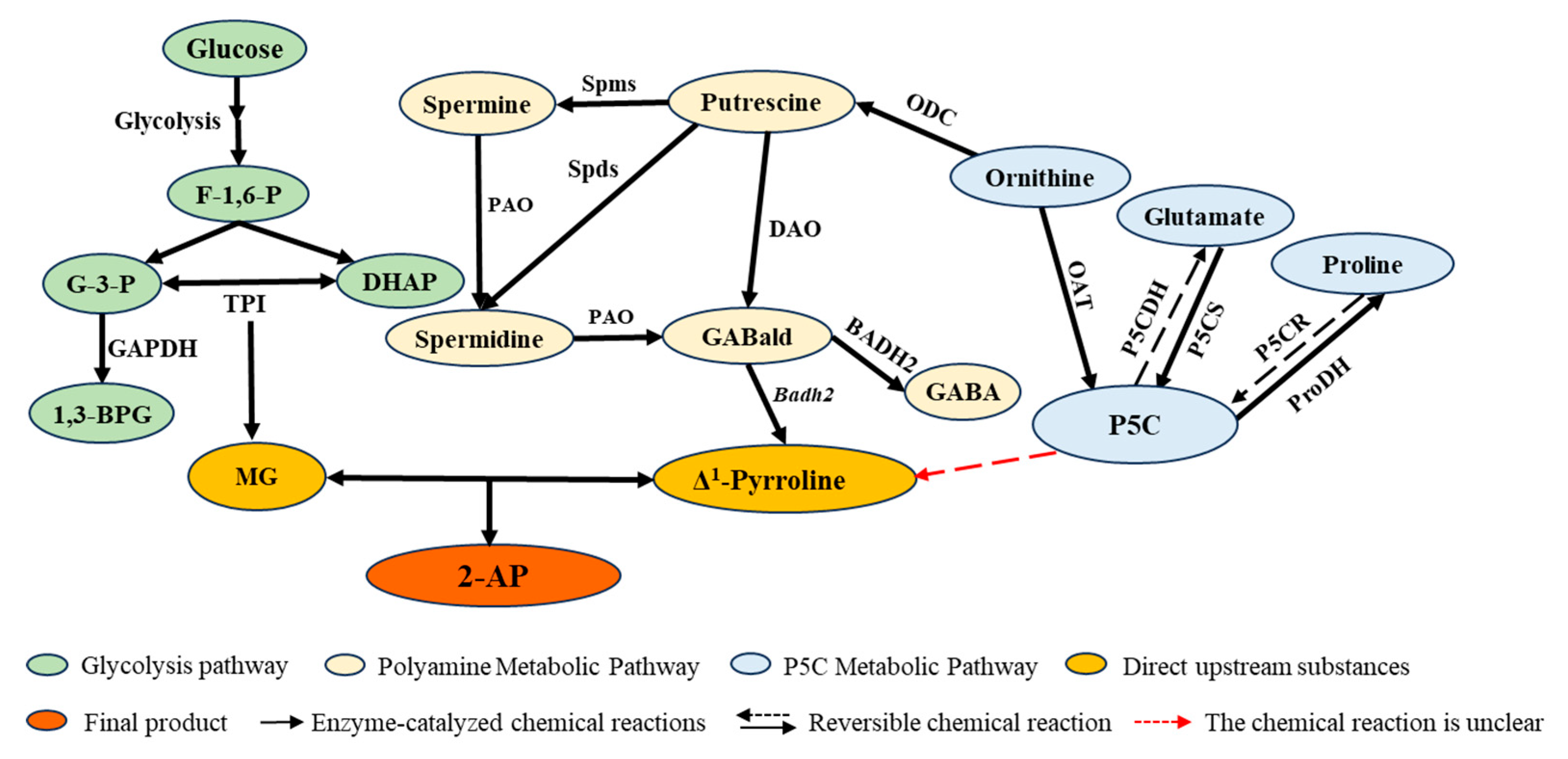
2.2. Analysis of the Metabolic Pathway for the Production of 2-AP from Putrescine and Spermine
2.3. Analysis of the 2-AP Metabolic Pathway for Proline, Glutamic Acid, and Ornithine
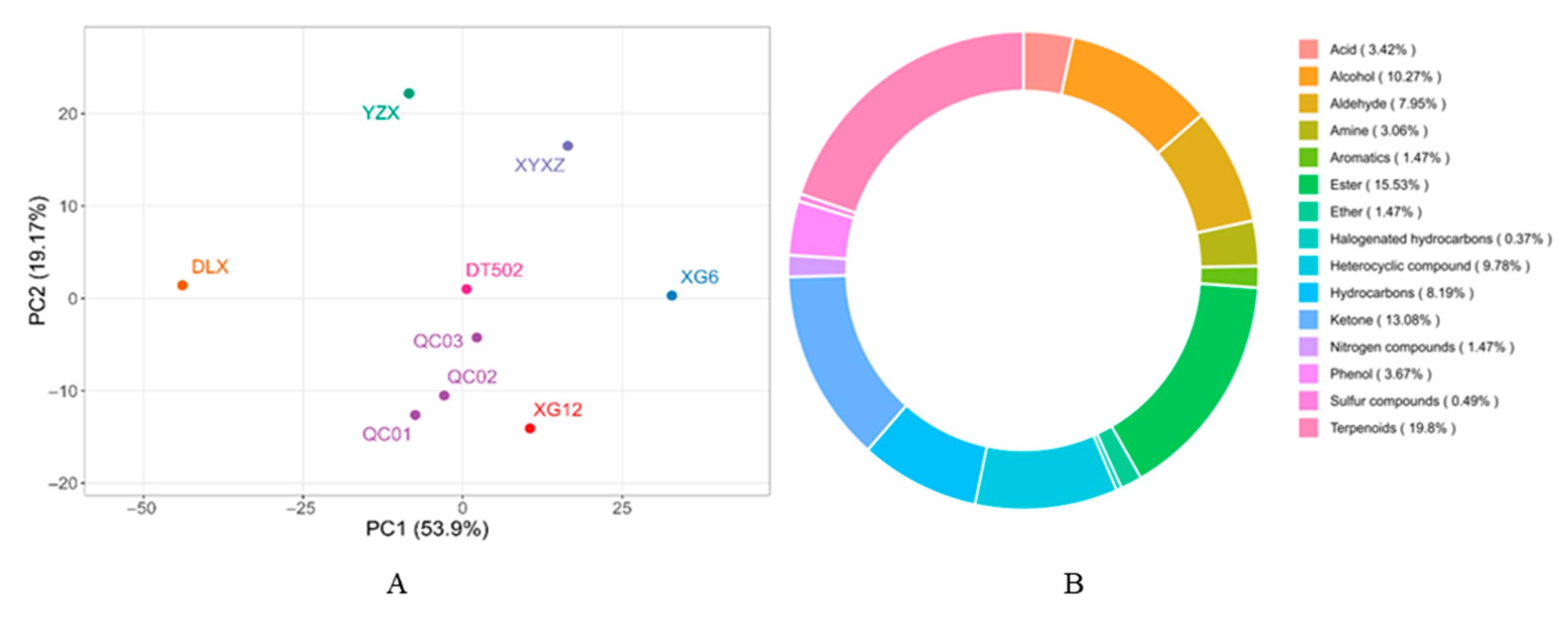
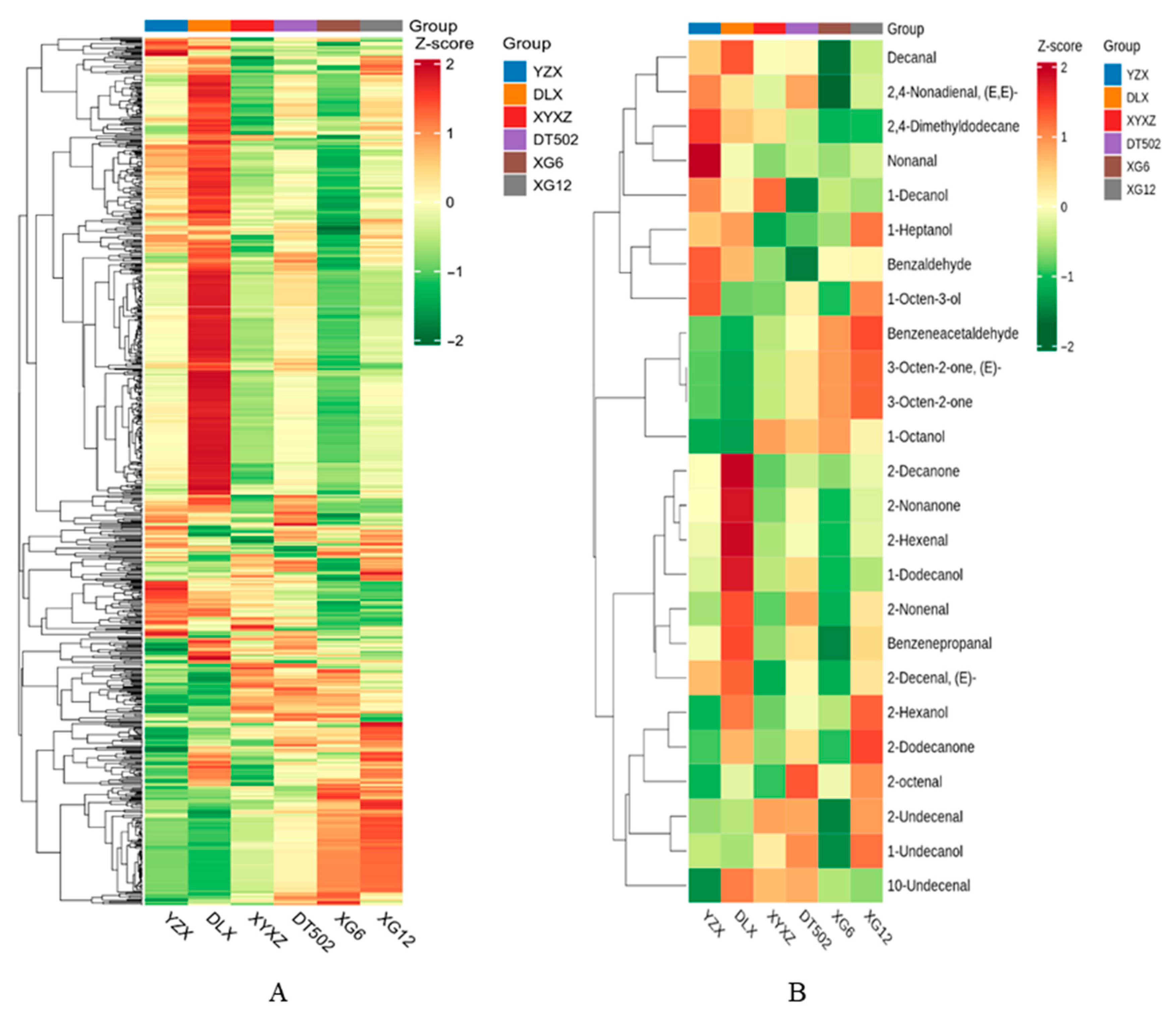
2.4. Analysis of Volatile Metabolites in Rice Aroma
3. Discussion
4. Materials and Methods
4.1. Material Reagents
4.2. Chip Detection
- GenTrain Score (The SNP cluster quality) > 0.6;
- Parental genotypes are pure (markers in which the majority of parental samples are heterozygous will be judged to be of poor quality, usually less than 5% heterozygosity is allowed);
- The number of genotypic deletions is less than 20% (except for Indel markers);
- High rate of correct typing.
4.3. Genomic Similarity
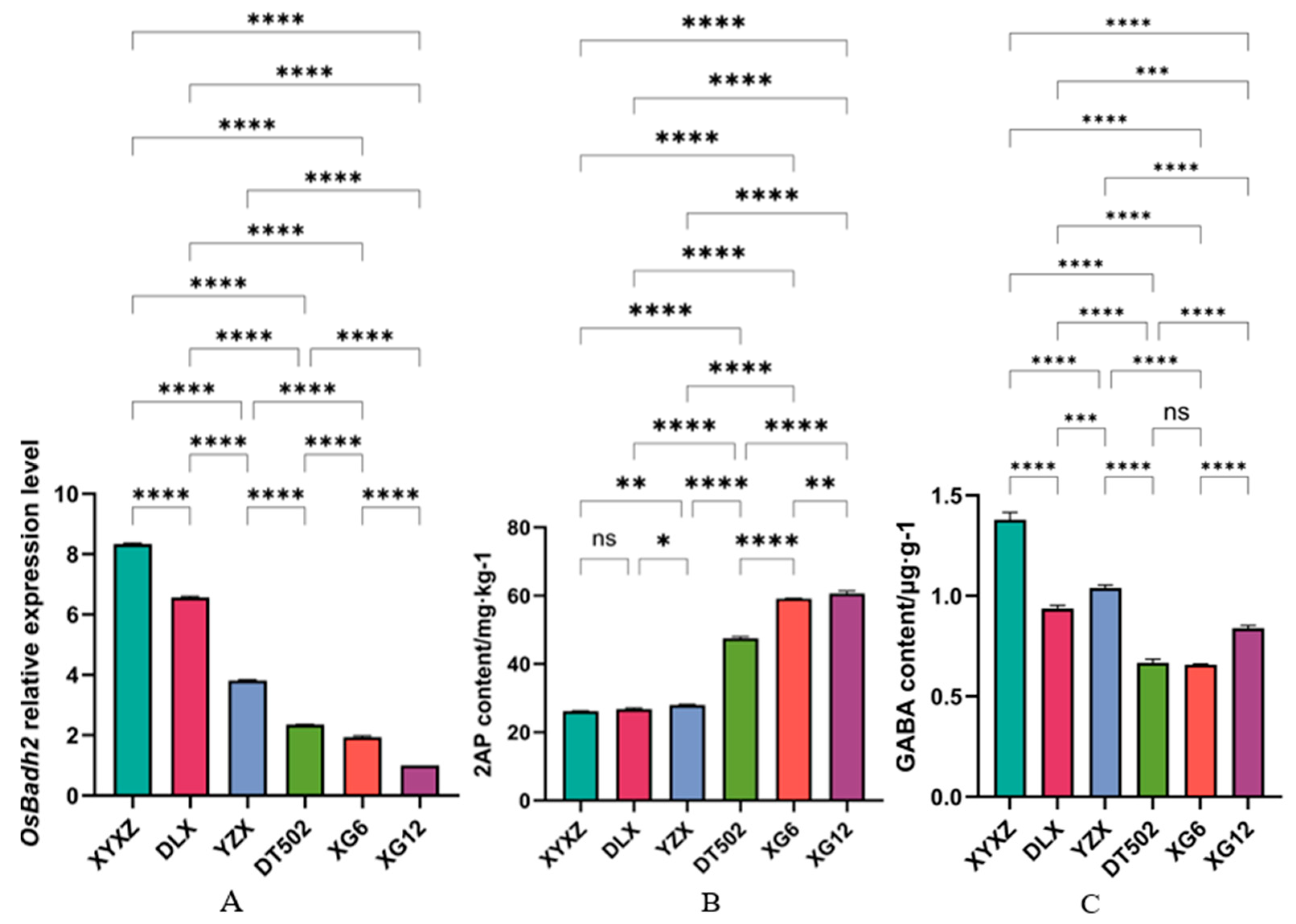
4.4. Gene Expression Level Detection
4.5. Sample Preparation and Content Detection of 2-AP, GABA, Proline, Glutamic Acid, and Ornithine
4.6. Enzyme Assays for PRODH, P5CS, and OAT
4.7. Volatile Metabolite Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.; Zhou, Q.; Wang, S.; Zhang, P.; Wang, L.; Wang, Z.; Zhang, L.; Huang, G. Effects of long-term winter cropping on paddy rice yield, soil properties and rhizosphere bacterial community in Southern China. Field Crops Res. 2025, 322, 109734. [Google Scholar] [CrossRef]
- Buttery, R.G.; Ling, L.C.; Juliano, B.O. 2-Acetyl-1pyrroline: An important aroma component of cooked rice. Chem. Ind. 1982, 12, 958–959. [Google Scholar]
- Buttery, R.G.; Ling, L.C.; Juliano, B.O.; Turnbaugh, J.G. Cooked rice aroma and 2-acetyl-1-pyrroline. J. Agric. Food Chem. 1983, 31, 823–826. [Google Scholar] [CrossRef]
- Paule, C.M.; Powers, J.J. Sensory and chemical examination of aromatic and nonaromatic rices. J. Food Sci. 1989, 54, 343–346. [Google Scholar] [CrossRef]
- Imran, M.; Shafiq, S.; Ashraf, U.; Qi, J.; Mo, Z.; Tang, X. Biosynthesis of 2-acetyl-1-pyrroline in fragrant rice: Recent insights into agro-management, environmental factors, and functional genomics. J. Agric. Food Chem. 2023, 71, 4201–4215. [Google Scholar] [CrossRef]
- Prodhan, Z.H.; Qingyao, S. Rice aroma: A natural gift comes with price and the way forward. Rice Sci. 2020, 27, 86–100. [Google Scholar] [CrossRef]
- Bradbury, L.M.T.; Gillies, S.A.; Brushett, D.J.; Waters, D.L.E.; Henry, R.J. Inactivation of an aminoaldehyde dehydrogenase is responsible for fragrance in rice. Plant Mol. Biol. 2008, 68, 439–449. [Google Scholar] [CrossRef]
- Shan, Q.W.; Zhang, Y.; Chen, K.L.; Zhang, K.; Gao, C.X. Creation of fragrant rice by targeted knockout of the OsBadh2 gene using TALEN technology. Plant Biotechnol. 2015, 13, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Yang, Y.Y.; Shi, W.W.; Ji, Q.; He FZhang, Z.D.; Cheng, Z.K.; Liu, X.N.; Xu, M.L. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell 2008, 20, 1850–1861. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miao, Y.; Yuan, H.; Huang, F.; Sun, M.; He, L.; Liu, X.; Luo, J. Volatilome-based GWAS identifies OsWRKY19 and OsNAC021 as key regulators of rice aroma. Mol. Plant 2024, 17, 1866–1882. [Google Scholar] [CrossRef]
- Schieberle, P. The role of free amino acids present in yeast as precursors of the odorants 2-acetyl-1-pyrroline and 2-acetyltetrahydropyridine in wheat bread crust. Z. Lebensm. Unters. Forsch. 1990, 191, 206–209. [Google Scholar] [CrossRef]
- Romanczyk, L.J.; Mcclelland, C.A.; Post, L.S.; Aitken, W.M. Formation of 2-acetyl-1-pyrroline by several Bacillus cereus strains isolated from cocoa fermentation boxes. J. Agric. Food Chem. 1995, 43, 469–475. [Google Scholar] [CrossRef]
- Huang, T.C.; Huang, Y.W.; Hung, H.J.; Ho, C.T.; Wu, M.L. Delta (1)-Pyrroline-5-carboxylic acid formed by proline dehydrogenase from the Bacillus subtilis ssp natto expressed in Escherichia coli as a precursor for 2-acetyl-1-pyrroline. J. Agric. Food Chem. 2007, 55, 5097–5102. [Google Scholar] [CrossRef]
- Huang, T.C.; Teng, C.S.; Chang, J.L.; Chuang, H.S.; Ho, C.T.; Wu, M.L. Biosynthetic mechanism of 2-acetyl-1-pyrroline and its relationship with delta (1)-pyrroline-5-carboxylic acid and methylglyoxal in aromatic rice (Oryza sativa L.) callus. J. Agric. Food Chem. 2008, 56, 7399–7404. [Google Scholar] [CrossRef]
- Hinge, V.; Patil, H.; Nadaf, A. Aroma volatile analyses and 2-AP characterization at various developmental stages in Basmati and Non-Basmati scented rice (Oryza sativa L.) cultivars. Rice 2016, 9, 38. [Google Scholar] [CrossRef]
- Tadashi, Y.; Thu, H.N.T.; Hideo, I. Precursors of 2-Acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety. J. Agric. Food Chem. 2022, 50, 2001–2004. [Google Scholar]
- Li, Y.; Zhang, W.; Li, M.; Ling, X.; Guo, D.; Yang, Y.; Liu, Q.; Zhang, B.; Wang, J. Discovery of OsODC as a key enhancer of aroma and development of highly fragrant rice. Plant Commun. 2025, 6, 101141. [Google Scholar] [CrossRef]
- Bradbury, L.M.T.; Fitzgerald, T.L.; Henry, R.J.; Jin, Q.S.; Waters, D.L.E. The gene for fragrance in rice. Plant Biotechnol. J. 2005, 3, 363–370. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Park, Y.J. Discovery of a novel fragrant allele and development of functional markers for fragrance in rice. Mol. Breed. 2015, 35, 217. [Google Scholar] [CrossRef]
- Mo, Z.; Li, Y.; Nie, J.; He, L.; Pan, S.; Duan, M.; Tian, H.; Xiao, L.; Zhong, K.; Tang, X. Nitrogen application and different water regimes at heading stage improved yield and 2-acetyl-1-pyrroline (2-AP) formation in fragrant rice. Rice 2019, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Okpalaa, N.E.; Mo, Z.W.; Duana, M.Y.; Tang, T.R. The genetics and biosynthesis of 2-acetyl-1-pyrroline in fragrant rice. Plant Physiol. Biochem. 2019, 135, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P. Hypothesis/review: Contribution of putrescine to 4aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193–194, 130–135. [Google Scholar] [CrossRef]
- Luo, H.; Duan, M.; Kong, L.; He, L.; Chen, Y.; Wang, Z.; Tang, X. The regulatory mechanism of 2-acetyl-1-pyrroline biosynthesis in fragrant rice (Oryza sativa L.) under different soil moisture contents. Front. Plant Sci. 2021, 12, 772728. [Google Scholar] [CrossRef]
- Luo, H.; Zhang, Q.; Lai, R.; Zhang, S.; Yi, W.; Tang, X. Regulation of 2-acetyl-1-pyrroline content in fragrant rice under different temperatures at the grain-filling stage. J. Agric. Food Chem. 2024, 72, 10521–10530. [Google Scholar] [CrossRef]
- Bao, G.; Ashraf, U.; Li, L.; Qiao, J.; Wang, C.; Zheng, Y. Transcription factor OsbZIP60 like regulating OsP5CS1 gene and 2-acetyl-1-pyrroline (2-AP) biosynthesis in aromatic rice. Plants 2023, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Srivastav, P.P. Extraction, identification and quantification methods of rice aroma compounds with emphasis on 2-acetyl-1-pyrroline (2-AP) and its relationship with rice quality: A comprehensive review. Food Rev. Int. 2022, 38, 111–162. [Google Scholar] [CrossRef]
- Sun, P.Y.; Zhang, W.H.; Shu, F.; He, Q.; Zhang, L.; Peng, Z.R.; Deng, H.F. Analysis of mutation sites of OsBadh2 gene in fragrant rice and development of related functional marker. Biotechnol. Bull. 2021, 37, 1–7. (In Chinese) [Google Scholar]
- Hui, S.; Li, H.; Mawia, A.M.; Zhou, L.; Cai, J.Y.; Ahmad, S.; Lai, C.K.; Wang, J.X.; Jiao, G.A.; Xie, L.H.; et al. Production of aromatic three-line hybrid rice using novel alleles of BADH2. Plant Biotechnol. J. 2022, 20, 59–74. [Google Scholar] [CrossRef]
- Luo, H.; Liu, J.; Xing, P.; Lai, R.; Zhang, T.; Wang, Z.; He, L.; Tang, X. Application of saline to seeds enhances the biosynthesis of 2-acetyl-1-pyrroline in aromatic rice seedlings (Oryza sativa L.). Acta Physiol. Plant 2020, 42, 42. [Google Scholar] [CrossRef]
- Luo, H.W.; Zhang, T.T.; Zheng, A.X.; He, L.X.; Lai, R.F.; Liu, J.H.; Xing, P.P.; Tang, X.R. Exogenous proline induces regulation in 2-acetyl-1-pyrroline (2-AP) biosynthesis and quality characters in fragrant rice (Oryza sativa L.). Sci. Rep. 2020, 10, 13971. [Google Scholar] [CrossRef]
- Li, M.; Ashraf, U.; Tian, H.; Mo, Z.; Pan, S.; Anjum, S.A.; Duan, M.; Tang, X. Manganese-induced regulations in growth, yield formation, quality characters, rice aroma and enzyme involved in 2-acetyl-1-pyrroline biosynthesis in fragrant rice. Plant Physiol. Biochem. 2016, 103, 167–175. [Google Scholar] [CrossRef]
- Xie, W.; Kong, L.; Ma, L.; Ashraf, U.; Pan, S.; Duan, M.; Tian, H.; Wu, L.; Tang, X.; Mo, Z. Enhancement of 2-acetyl-1-pyrroline (2-AP) concentration, total yield, and quality in fragrant rice through exogenous γ-aminobutyric acid (GABA) application. J. Cereal Sci. 2020, 91, 102900. [Google Scholar] [CrossRef]
- Bao, G.; Ashraf, U.; Wang, C.L.; He, L.X.; Wei, X.S.; Zheng, A.X.; Mo, Z.W.; Tang, X.R. Molecular basis for increased 2-acetyl-1-pyrroline contents under alternate wetting and drying (AWD) conditions in fragrant rice. Plant Physiol. Biochem. 2018, 133, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Kaikavoosi, K.; Kad, T.D.; Zanan, R.L.; Nadaf, A.B. 2-Acetyl-1-pyrroline augmentation in scented indica rice (Oryza sativa L.) varieties through Δ(1)-pyrroline-5-carboxylate synthetase (P5CS) gene transformation. Appl. Biochem. Biotechnol. 2015, 177, 1466–1479. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.Y.; Chen, Y.B.; Wang, C.R.; Li, H.; Huang, D.Q.; Zhou, D.G.; Wang, Z.D.; Zhao, L.; Gong, R.; Zhou, S.C. Metabolism of γ-aminobutyrate and 2-acetyl-1-pyrroline Analyses at Various Grain Developmental Stages in Rice (Oryza sativa L.). Chin. J. Rice Sci. 2021, 35, 121–129. [Google Scholar] [CrossRef]
- Yang, X.H.; Wei, Y.; Xia, X.Z.; Li, D.X.; Liang, M.L.; Su, X.J.; Yan, Y. Identifying the Genetic Diversity and Disease-pest Resistance of 326 Rice Varieties based on 40K Gene Chip. Mol. Plant Breed. 2022, 20, 3974–3987. [Google Scholar] [CrossRef]
- Shi, W.W.; Yang, Y.; Chen, S.H.; Xu, M.L. Discovery of a new fragrance allele and the development of functional markers for the breeding of fragrant rice varieties. Mol. Breed. 2008, 22, 185–192. [Google Scholar] [CrossRef]
- GB 23200.8-2016; National Food Safety Standard—Determination of 500 Pesticides and Related Chemicals Residues in Fruits and Vegetables—Gas Chromatography-Mass Spectrometry. China Agriculture Press: Beijing, China, 2016.

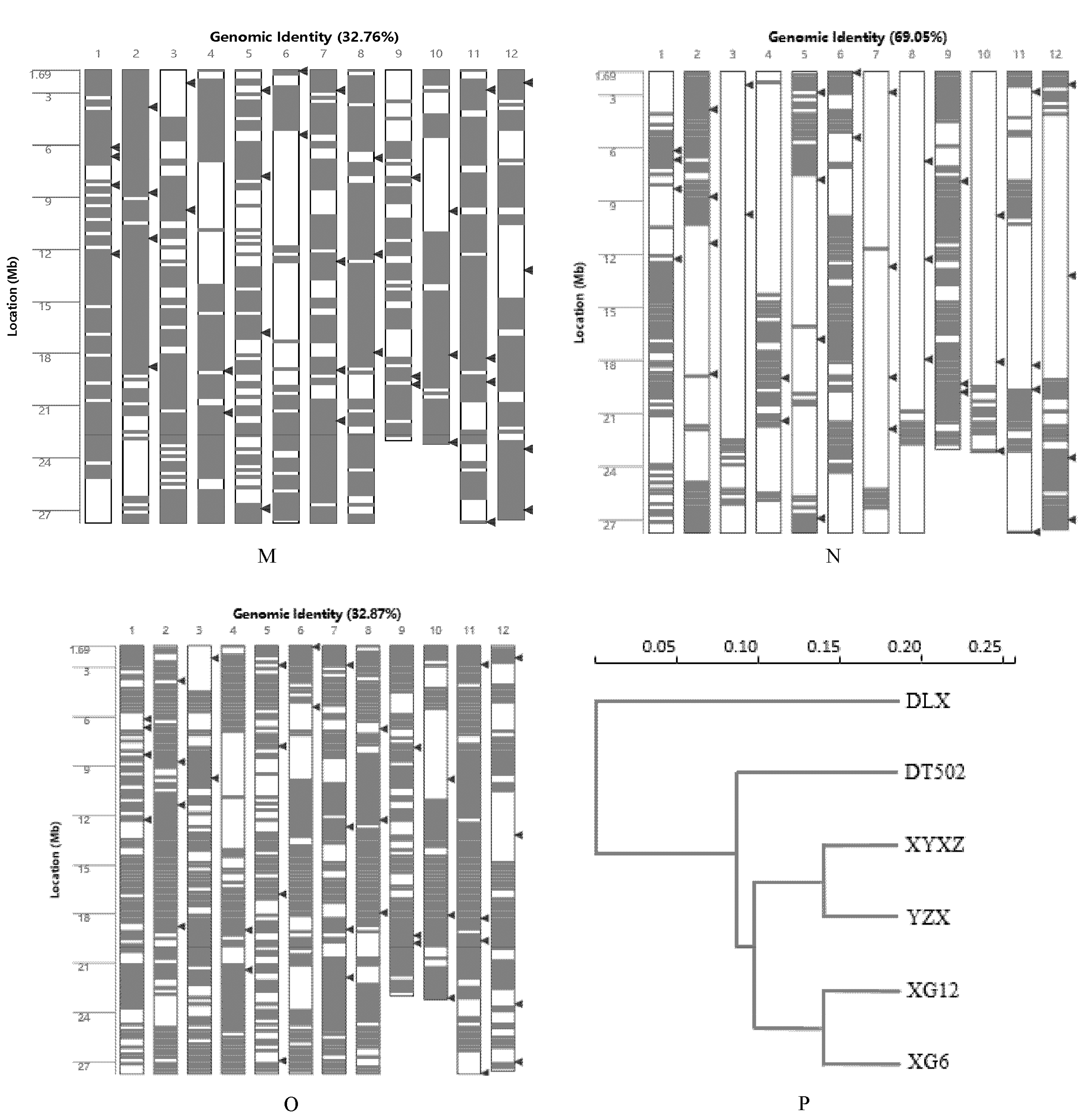
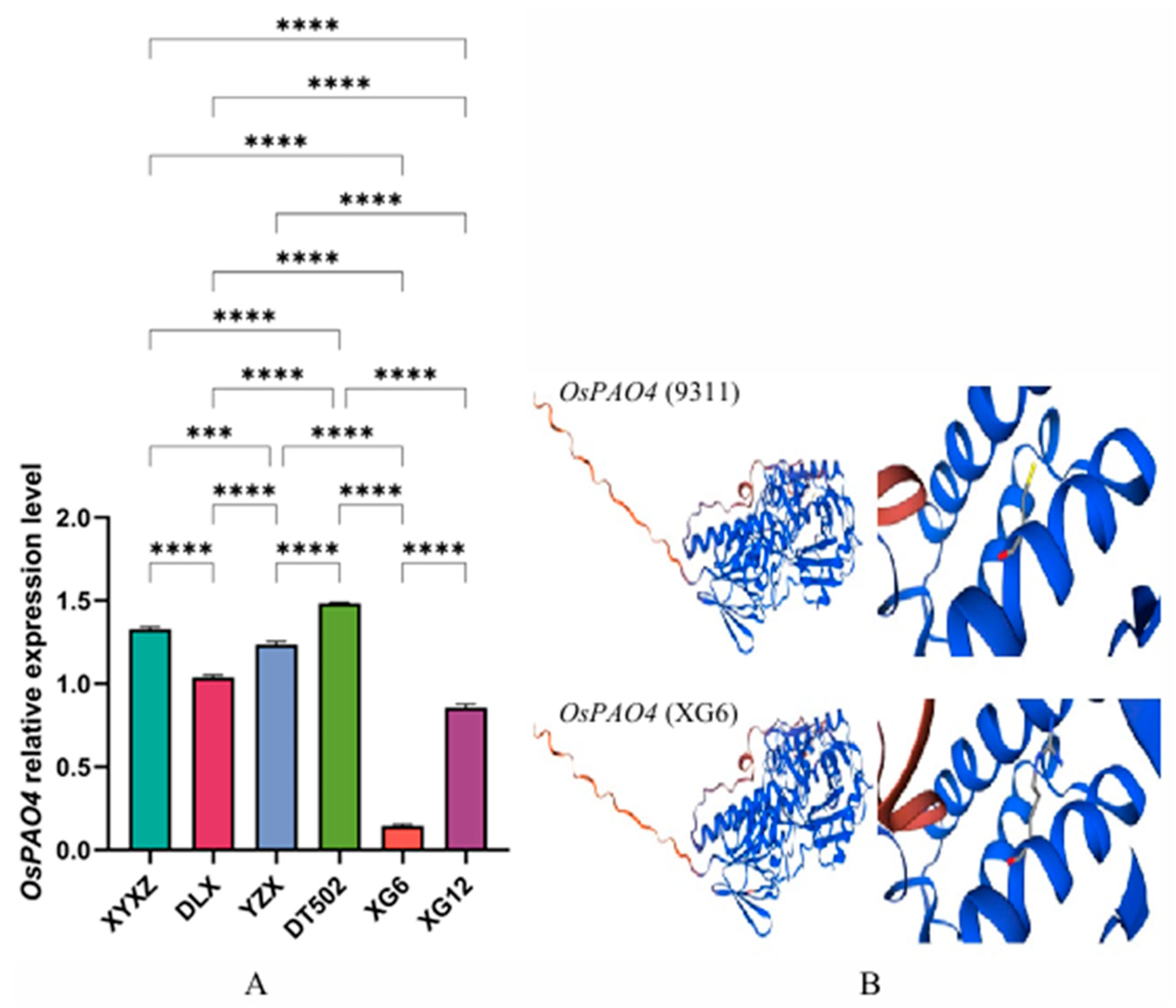

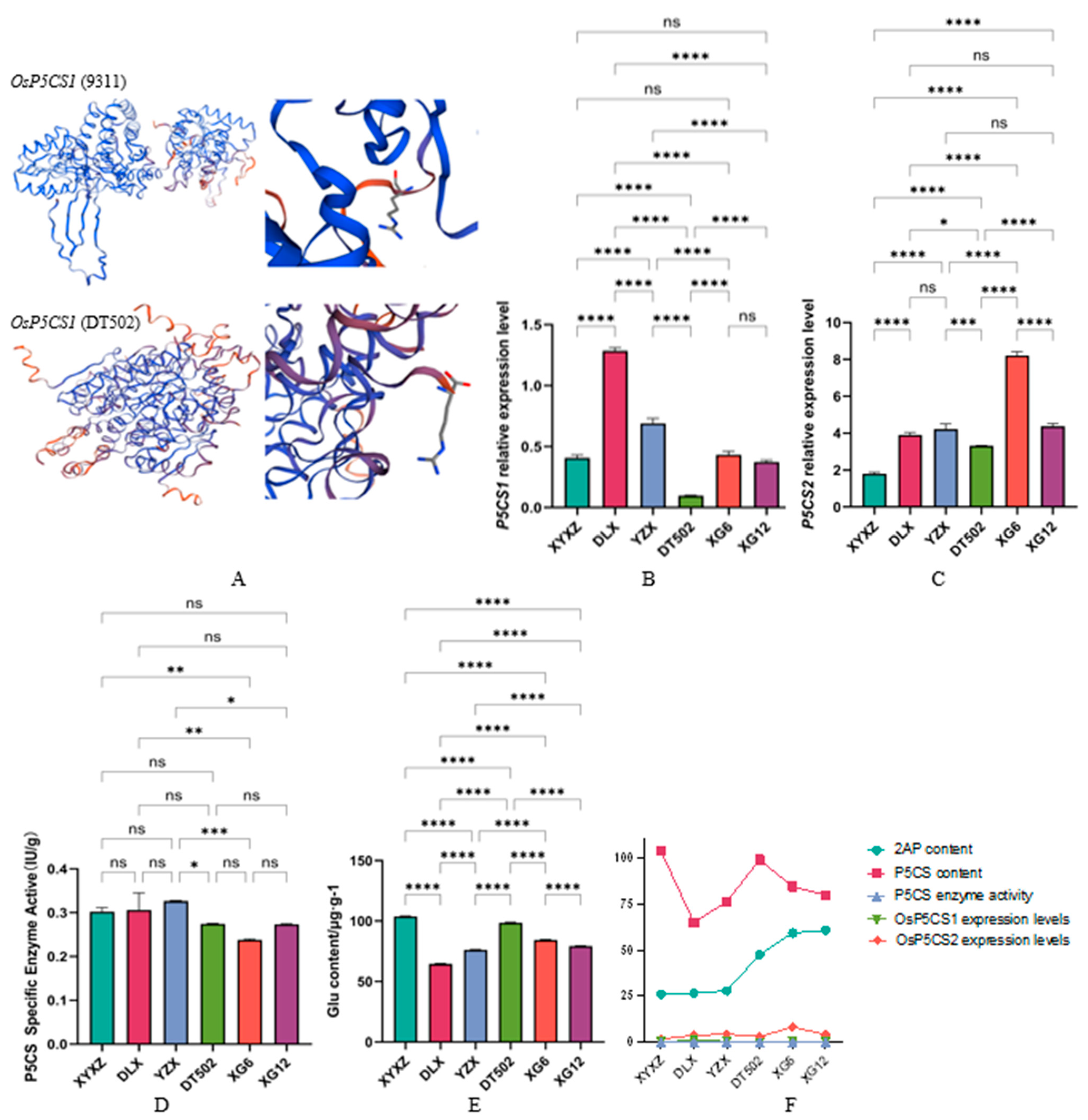
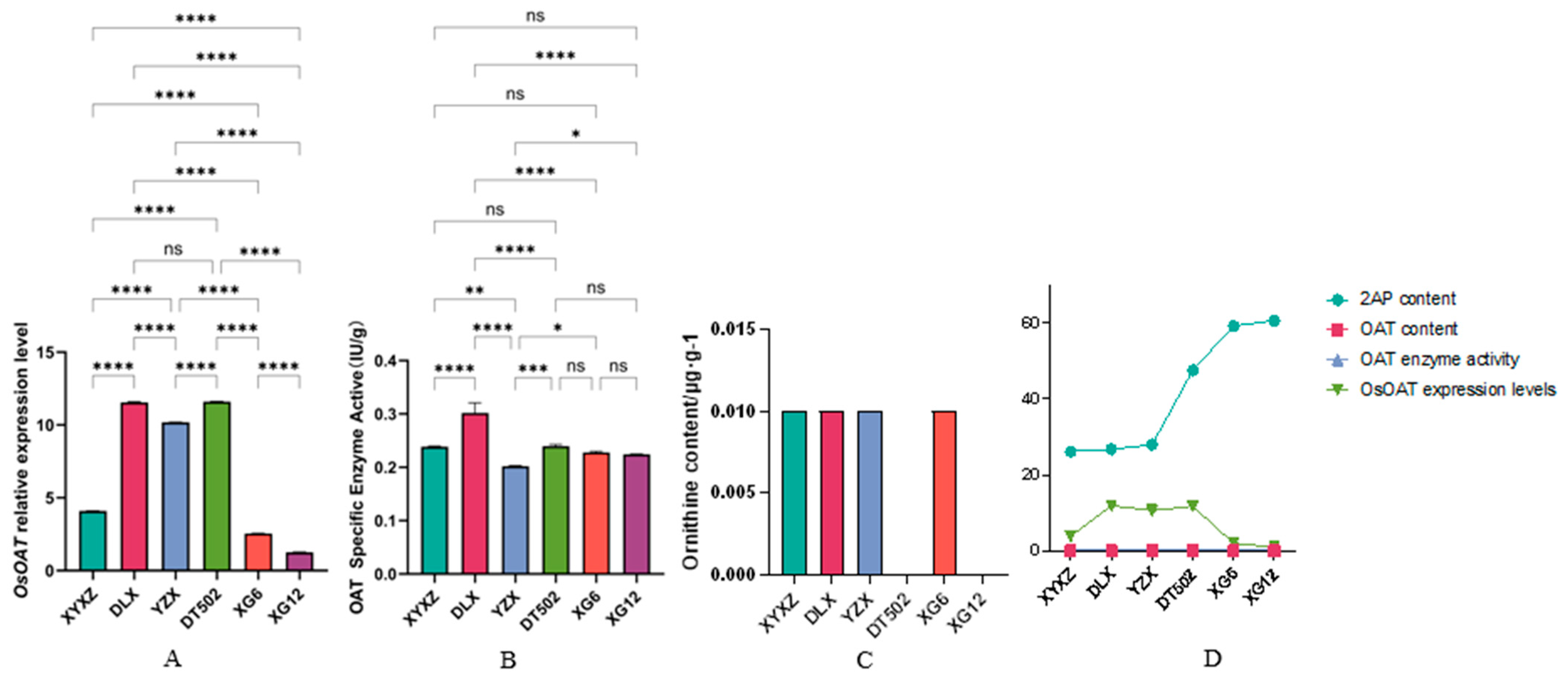
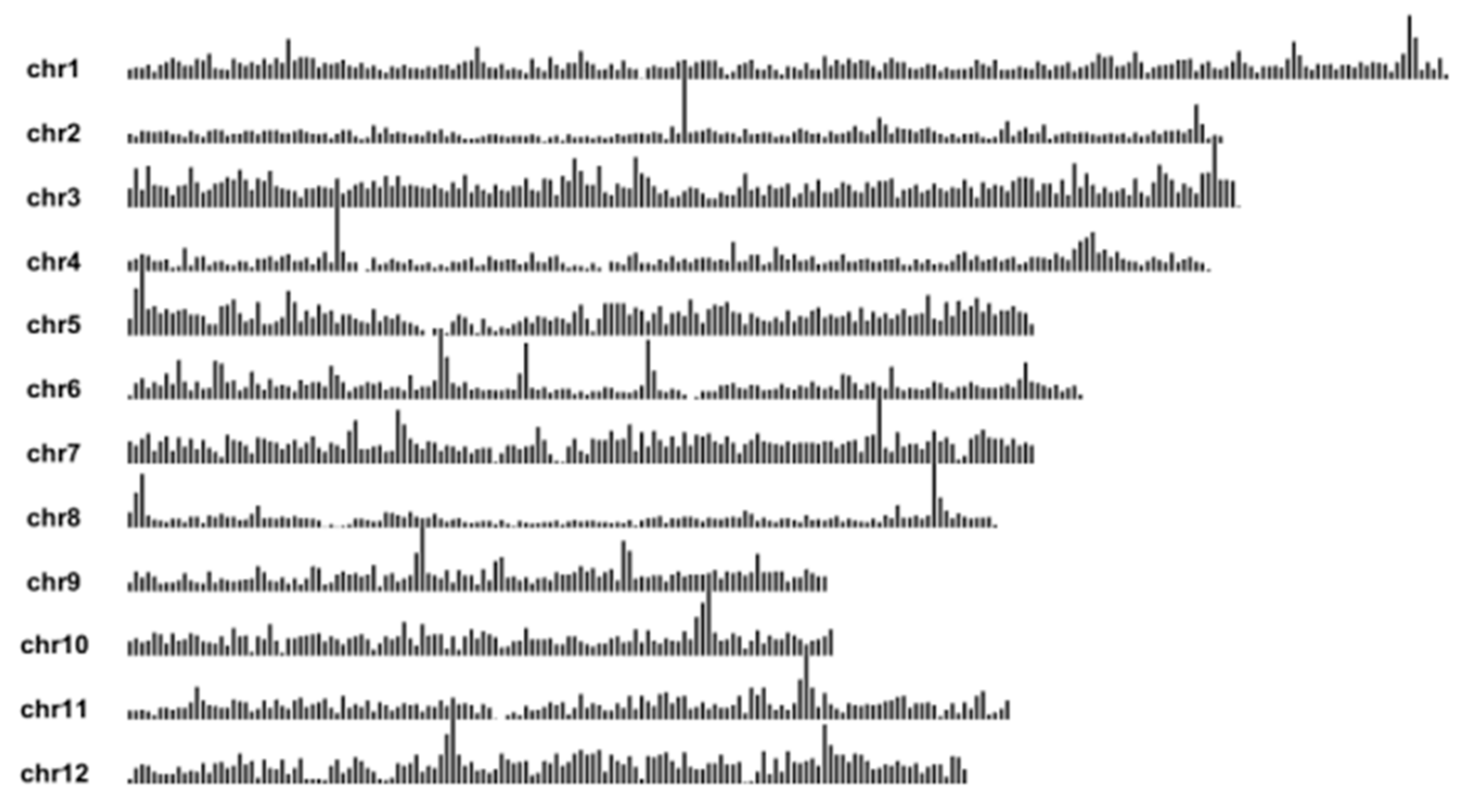
| Type | Accession | Badh2-Exon7(+727~+747) |
|---|---|---|
| Non-Fragrant | Nipponbare | GGTAAAAAGATTATGGCTTCA |
| Fragrant | YZX | GGTATATA - - - - - - - - TTTCA |
| Fragrant | XYXZ | GGTATATA - - - - - - - - TTTCA |
| Fragrant | DT502 | GGTATATA - - - - - - - - TTTCA |
| Fragrant | DLX | GGTATATA - - - - - - - - TTTCA |
| Fragrant | XG6 | GGTATATA - - - - - - - - TTTCA |
| Fragrant | XG12 | GGTATATA - - - - - - - - TTTCA |
| Type | Accession | +551~+560 |
|---|---|---|
| Non-Fragrant | Nipponbare | AATGGTGTGT |
| Fragrant | YZX | AATGGTGTGT |
| Fragrant | XYXZ | AATGGTGTGT |
| Fragrant | DT502 | AATGGTGTGT |
| Fragrant | DLX | AATGGTGTGT |
| Fragrant | XG6 | AATGGCGTGT |
| Fragrant | XG12 | AATGGTGTGT |
| OsP5CS1 (CDS) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Type | Accession | +191~+200 | +881~+890 | +921~+930 | +946~+955 | +1651~+1660 | +1671~+1680 | +1686~+1695 |
| Non-Fragrant | 9311 | TCCATATGAG | GTACTCGTGA | GCATCTACAG | GAACGAAAAA | GTTCATAAGG | GAGTCCAGGC | TATTAGTAGC |
| Fragrant | YZX | TCCATATGAG | GTGCTCGTGA | GCGTCTACAG | GAACGGAAAA | GTTCATAAGG | GAATCCAGGC | TATTAGTAGC |
| Fragrant | XYXZ | TCCATATGAG | GTGCTCGTGA | GCGTCTACAG | GAACGGAAAA | GTTCATAAGG | GAATCCAGGC | TATTAGTAGC |
| Fragrant | DT502 | TTTCATATGAG | GTGCTCGTGA | GCGTCTACAG | GAACGGAAAA | GTTCATAAGG | GAATCCAGGC | TATTAGTAGC |
| Fragrant | DLX | TCCATATGAG | GTACTCGTGA | GCATCTACAG | GAACGAAAAA | GTTCATAAGG | GAGTCCAGGC | TATTAGTAGC |
| Fragrant | XG6 | TCCATATGAG | GTGCTCGTGA | GCGTCTACAG | GAACGGAAAA | GATCATAAGG | GAATCCAGGC | TATTAGTAGC |
| Fragrant | XG12 | TCCATATGAG | GTACTCGTGA | GCATCTACAG | GAACGAAAAA | GTTCATAAGG | GAGTCCAGGC | TATTAGCAGC |
| Material Name | Male Parent | Famale Parent | Breeding Unit |
|---|---|---|---|
| YZX | TLX103 | R4015 | Hunan Rice Research Institute; Hunan Jinjian Rice Industry Co. (Changde, China) |
| XYXZ | XYXZ | YZX | Hunan Golden Rice Seed Industry Co. (Changsha, China) |
| DT502 | DQ20 | HP | Seed Management Station of Indochina, Yunnan Province; Yunnan Type Hybrid Rice Institute, Yunnan Province |
| DLX | γ-194 | DLXD | Guizhou Rice Resrarch Institute |
| XG6 | Selection of a mutant strain of Xiligongmi | Guizhou Rongjiang Shengtai Agricultural Products Development Co. | |
| XG12 | Selection of a mutant strain of Xiligongmi | Guizhou Rongjiang Shengtai Agricultural Products Development Co. | |
| Gene Name | Gene Symbol | Locus ID |
|---|---|---|
| Diamine oxidase | OsDAO4 | LOC_Os04g40040 |
| Polyamine oxidase | OsPAO4 | LOC_Os04g57550 |
| Ornithine decarboxylase | OsODC | LOC_Os02g28110 |
| Ornithine aminotransferase | OsOAT | LOC_Os03g44150 |
| Proline dehydrogenase | OsProDH | LOC_Os10g40360 |
| Δ1-pyrroline-5-carboxylate synthetase | OsP5CS1 | LOC_Os05g38150 |
| Δ1-pyrroline-5-carboxylate synthetase | OsP5CS2 | LOC_Os01g62900 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Long, W.; Wu, X.; Wu, C.; Gong, Y.; Wang, Z.; Zhu, S. Study on Differences in 2-AP Synthesis and Metabolism Among Fragrant Rice Varieties. Int. J. Mol. Sci. 2025, 26, 10102. https://doi.org/10.3390/ijms262010102
Wang Q, Long W, Wu X, Wu C, Gong Y, Wang Z, Zhu S. Study on Differences in 2-AP Synthesis and Metabolism Among Fragrant Rice Varieties. International Journal of Molecular Sciences. 2025; 26(20):10102. https://doi.org/10.3390/ijms262010102
Chicago/Turabian StyleWang, Qian, Wuhua Long, Xian Wu, Chaoxin Wu, Yanlong Gong, Zhongni Wang, and Susong Zhu. 2025. "Study on Differences in 2-AP Synthesis and Metabolism Among Fragrant Rice Varieties" International Journal of Molecular Sciences 26, no. 20: 10102. https://doi.org/10.3390/ijms262010102
APA StyleWang, Q., Long, W., Wu, X., Wu, C., Gong, Y., Wang, Z., & Zhu, S. (2025). Study on Differences in 2-AP Synthesis and Metabolism Among Fragrant Rice Varieties. International Journal of Molecular Sciences, 26(20), 10102. https://doi.org/10.3390/ijms262010102





