The Role of Interleukin-8 (IL-8) in Treatment-Resistant Depression: A Review of Mechanisms, Biomarker Potential, and Therapeutic Implications
Abstract
1. Introduction
2. Biology and Functions of IL-8
3. IL-8 in Depression
3.1. Evidence from Clinical Studies
3.2. Sex, Age, and Clinical Subtype Effects
3.3. Longitudinal and Interventional Studies
4. IL-8 in Treatment-Resistant Depression
4.1. Baseline IL-8 Levels and Treatment Response
4.2. IL-8 and Novel Antidepressant Interventions
4.3. Confounding Factors and Methodological Issues
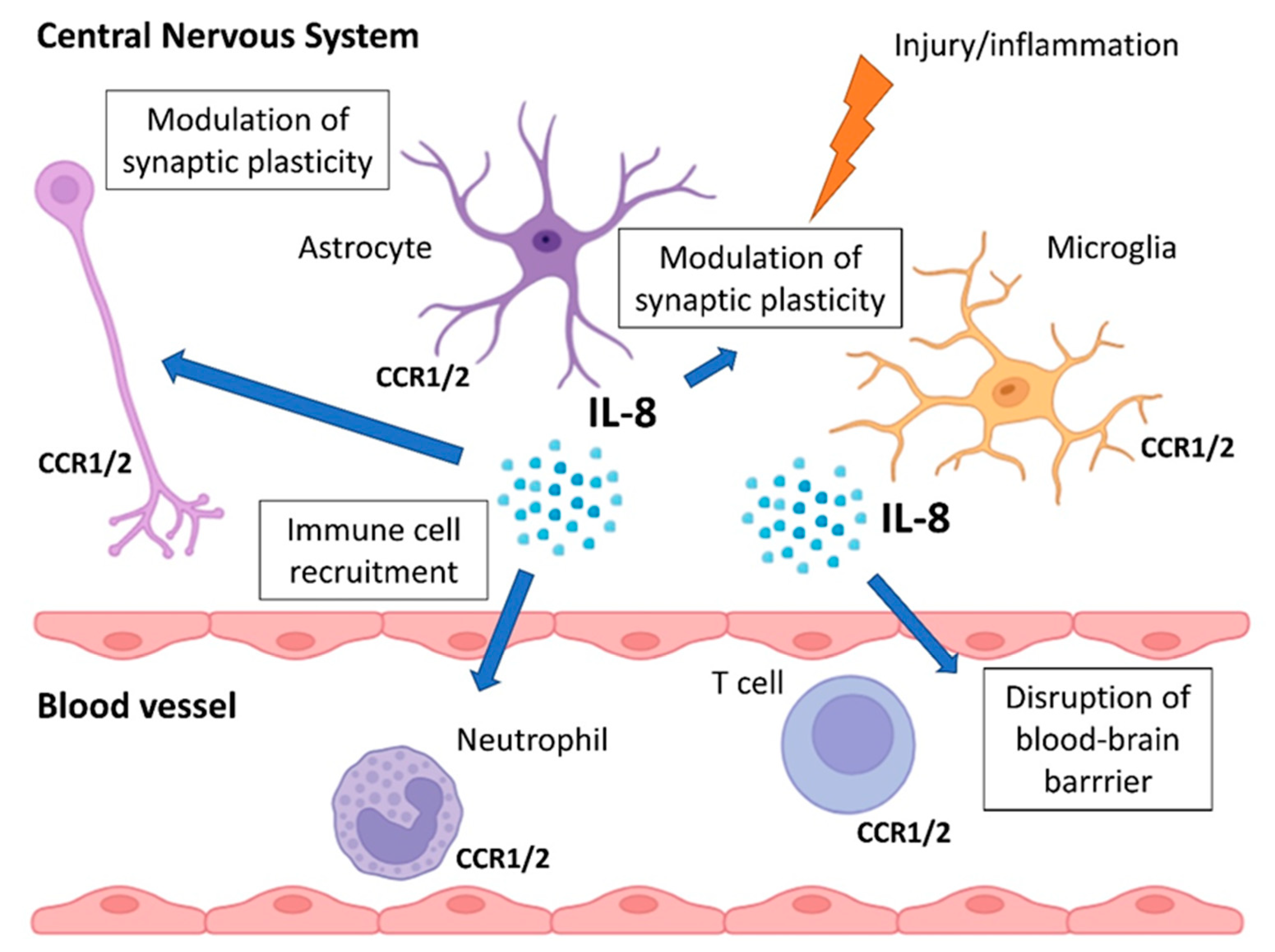
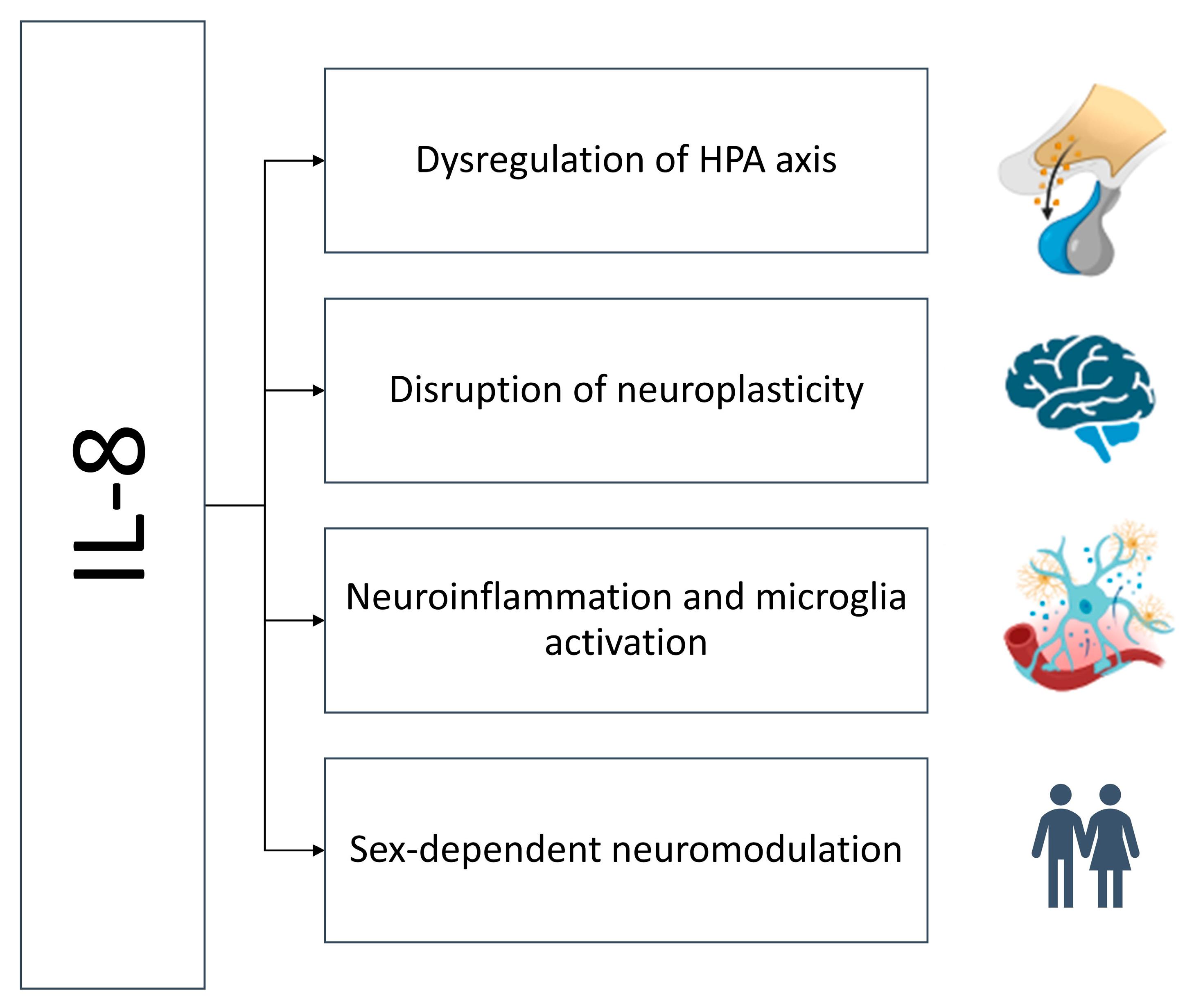
5. Mechanisms Linking IL-8 to Treatment Resistance
5.1. Dysregulation of the Hypothalamic–Pituitary–Adrenal Axis
5.2. Neuroinflammation and Microglial Activation
5.3. Disruption of Neurogenesis and Neuroplasticity
5.4. Sex-Specific Immunomodulation
6. IL-8 as a Potential Biomarker in Psychiatry
6.1. Requirements for an Effective Biomarker
6.2. Strengths and Limitations of IL-8 as a Marker
6.3. Methodological Issues
6.4. Potential for Predictive Modeling
7. IL-8 as Therapeutic Target in Depression?
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CBA | Cytometric Bead Array |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HDRS | Hamilton Depression Rating Scale |
| HPA | Hypothalamic–Pituitary–Adrenal |
| IL | Interleukin |
| MDD | Major Depressive Disorder |
| TRD | Treatment-Resistant Depression |
References
- Zhou, J.; Zhang, Y.; He, S.; Xu, S.; Sun, Q.; Zhao, T.; Dai, Y. Accelerated global burden of depressive disorders during the COVID-19 pandemic from 2019 to 2021. Sci. Rep. 2025, 15, 9529. [Google Scholar] [CrossRef]
- Caraci, F.; Calabrese, F.; Molteni, R.; Bartova, L.; Dold, M.; Leggio, G.M.; Fabbri, C.; Mendlewicz, J.; Racagni, G.; Kasper, S.; et al. International Union of Basic and Clinical Pharmacology CIV: The Neurobiology of Treatment-resistant Depression: From Antidepressant Classifications to Novel Pharmacological Targets. Pharmacol. Rev. 2018, 70, 475–504. [Google Scholar] [CrossRef]
- Szałach, Ł.P.; Lisowska, K.A.; Cubała, W.J. The Influence of Antidepressants on the Immune System. Arch. Immunol. Ther. Exp. 2019, 67, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Herrmann, N.; Swardfager, W.; Liu, H.; Sham, L.; Reim, E.K.; Lanctôt, K.L. A meta-analysis of cytokines in major depression. Biol. Psychiatry 2010, 67, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ho, R.C.; Mak, A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: A meta-analysis and meta-regression. J. Affect. Disord. 2012, 139, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Strawbridge, R.; Arnone, D.; Danese, A.; Papadopoulos, A.; Herane Vives, A.; Cleare, A.J. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol. 2015, 25, 1532–1543. [Google Scholar] [CrossRef]
- Yoshimura, T.; Matsushima, K.; Tanaka, S.; Robinson, E.A.; Appella, E.; Oppenheim, J.J.; Leonard, E.J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc. Natl. Acad. Sci. USA 1987, 84, 9233–9237. [Google Scholar] [CrossRef]
- Walz, A.; Peveri, P.; Aschauer, H.; Baggiolini, M. Purification and amino acid sequencing of NAF, a novel neutrophil-activating factor produced by monocytes. Biochem. Biophys. Res. Commun. 1987, 149, 755–761. [Google Scholar] [CrossRef]
- Schröder, J.M.; Mrowietz, U.; Morita, E.; Christophers, E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J. Immunol. 1987, 139, 3474–3483. [Google Scholar] [CrossRef]
- Schröder, J.M.; Mrowietz, U.; Christophers, E. Purification and partial biologic characterization of a human lymphocyte-derived peptide with potent neutrophil-stimulating activity. J. Immunol. 1988, 140, 3534–3540. [Google Scholar] [CrossRef]
- Dahl, J.; Ormstad, H.; Aass, H.C.; Malt, U.F.; Bendz, L.T.; Sandvik, L.; Brundin, L.; Andreassen, O.A. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 2014, 45, 77–86. [Google Scholar] [CrossRef]
- Szałach, Ł.P.; Ciesielska-Figlon, K.; Daca, A.; Cubała, W.J.; Lisowska, K.A. The effect of ketamine on the immune system in patients with treatment-resistant depression. Int. J. Mol. Sci. 2025, 26, 7500. [Google Scholar] [CrossRef]
- Szałach, Ł.P.; Cubała, W.J.; Lisowska, K.A. Changes in T-Cell Subpopulations and Cytokine Levels in Patients with Treatment-Resistant Depression—Preliminary Study. Int. J. Mol. Sci. 2022, 24, 479. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; O’Neill, L.A.J.; Gearing, A.J.H.; Callard, R.E. (Eds.) The Cytokine FactsBook and Webfacts. In Factsbook, 2nd ed.; Academic Press: Cambridge, MA, USA, 2001; pp. 80–84. [Google Scholar]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef]
- Mukaida, N.; Shiroo, M.; Matsushima, K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J. Immunol. 1989, 143, 1366–1371. [Google Scholar] [CrossRef]
- Bosch, I.; Xhaja, K.; Estevez, L.; Raines, G.; Melichar, H.; Warke, R.V.; Fournier, M.V.; Ennis, F.A.; Rothman, A.L. Increased production of interleukin-8 in primary human monocytes and in human epithelial and endothelial cell lines after dengue virus challenge. J. Virol. 2002, 76, 5588–5597. [Google Scholar] [CrossRef]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.Y.; Lee, J.W.; Ryu, H.C.; Wei, J.D.; Seong, C.M.; Kim, J.H. Proinflammatory cytokine IL-1beta stimulates IL-8 synthesis in mast cells via a leukotriene B4 receptor 2-linked pathway, contributing to angiogenesis. J. Immunol. 2010, 184, 3946–3954. [Google Scholar] [CrossRef] [PubMed]
- Wolff, B.; Burns, A.R.; Middleton, J.; Rot, A. Endothelial cell “memory” of inflammatory stimulation: Human venular endothelial cells store interleukin 8 in Weibel-Palade bodies. J. Exp. Med. 1998, 188, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- Eckmann, L.; Kagnoff, M.F.; Fierer, J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 1993, 61, 4569–4574. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Bhattacharyya, A.; Bai, J.; Mifflin, R.C.; Ernst, P.B.; Mitra, S.; Crowe, S.E. Tumor necrosis factor (TNF)-alpha-induced IL-8 expression in gastric epithelial cells: Role of reactive oxygen species and AP endonuclease-1/redox factor (Ref)-1. Cytokine 2009, 46, 359–369. [Google Scholar] [CrossRef]
- Osawa, Y.; Nagaki, M.; Banno, Y.; Brenner, D.A.; Asano, T.; Nozawa, Y.; Moriwaki, H.; Nakashima, S. Tumor necrosis factor alpha-induced interleukin-8 production via NF-kappaB and phosphatidylinositol 3-kinase/Akt pathways inhibits cell apoptosis in human hepatocytes. Infect. Immun. 2002, 70, 6294–6301. [Google Scholar] [CrossRef]
- Liu, X.; Yin, S.; Chen, Y.; Wu, Y.; Zheng, W.; Dong, H.; Bai, Y.; Qin, Y.; Li, J.; Feng, S.; et al. LPS-induced proinflammatory cytokine expression in human airway epithelial cells and macrophages via NF-κB, STAT3 or AP-1 activation. Mol. Med. Rep. 2018, 17, 5484–5491. [Google Scholar] [CrossRef]
- Ahuja, S.K.; Shetty, A.; Tiffany, H.L.; Murphy, P.M. Comparison of the genomic organization and promoter function for human interleukin-8 receptors A and B. J. Biol. Chem. 1994, 269, 26381–26389. [Google Scholar] [CrossRef]
- Chuntharapai, A.; Lee, J.; Hébert, C.A.; Kim, K.J. Monoclonal antibodies detect different distribution patterns of IL-8 receptor A and IL-8 receptor B on human peripheral blood leukocytes. J. Immunol. 1994, 153, 5682–5688. [Google Scholar] [CrossRef] [PubMed]
- Bolanowski, M.; Baganoff, M.; Deppeler, C.; Meyer, D.; Widomski, D.; Fretland, D.; Zhang, Y.; Jakschik, B. Interleukin 8 Plays a Fundamental Role in Inflammatory Processes in Vivo. In The Chemokines; Lindley, I.J.D., Westwick, J., Kunkel, S., Eds.; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Okamoto, S.; Mukaida, N.; Yasumoto, K.; Horiguchi, H.; Matsushima, K. Molecular Mechanism of Interleukin-8 Gene Expression. In The Chemokines; Lindley, I.J.D., Westwick, J., Kunkel, S., Eds.; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Mukaida, N.; Mahe, Y.; Matsushima, K. Cooperative interaction of nuclear factor-kappa B- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J. Biol. Chem. 1990, 265, 21128–21133. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.; Lapointe, G.R.; Feucht, P.H.; Hilt, S.; Gallegos, C.A.; Gordon, C.A.; Giedlin, M.A.; Mullenbach, G.; Tekamp-Olson, P. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J. Immunol. 1995, 155, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Guichard, C.; Pedruzzi, E.; Dewas, C.; Fay, M.; Pouzet, C.; Bens, M.; Vandewalle, A.; Ogier-Denis, E.; Gougerot-Pocidalo, M.A.; Elbim, C. Interleukin-8-induced priming of neutrophil oxidative burst requires sequential recruitment of NADPH oxidase components into lipid rafts. J. Biol. Chem. 2005, 280, 37021–37032. [Google Scholar] [CrossRef]
- Willems, J.; Joniau, M.; Cinque, S.; van Damme, J. Human granulocyte chemotactic peptide (IL-8) as a specific neutrophil degranulator: Comparison with other monokines. Immunology 1989, 67, 540–542. [Google Scholar]
- Asiri, A.; Hazeldine, J.; Moiemen, N.; Harrison, P. IL-8 Induces Neutrophil Extracellular Trap Formation in Severe Thermal Injury. Int. J. Mol. Sci. 2024, 25, 7216. [Google Scholar] [CrossRef]
- Keshari, R.S.; Jyoti, A.; Dubey, M.; Kothari, N.; Kohli, M.; Bogra, J.; Barthwal, M.K.; Dikshit, M. Cytokines induced neutrophil extracellular traps formation: Implication for the inflammatory disease condition. PLoS ONE 2012, 7, e48111. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, L.; Zhang, Y.; Pu, L.; Liu, J.; Li, X.; Chen, Z.; Hao, Y.; Wang, B.; Han, J.; et al. High Level of Neutrophil Extracellular Traps Correlates with Poor Prognosis of Severe Influenza A Infection. J. Infect. Dis. 2018, 217, 428–437. [Google Scholar] [CrossRef]
- Yoshida, S.; Ono, M.; Shono, T.; Izumi, H.; Ishibashi, T.; Suzuki, H.; Kuwano, M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol. Cell Biol. 1997, 17, 4015–4023. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J. Immunol. 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.N.; Choi, N.G.; Lee, H.S.; Cho, K.S.; Roh, H.J. Induction of interleukin-8 from nasal epithelial cells during bacterial infection: The role of IL-8 for neutrophil recruitment in chronic rhinosinusitis. Mediat. Inflamm. 2010, 2010, 813610. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.S.; Fedyk, E.R.; Springer, T.A.; Mukaida, N.; Iglewski, B.H.; Phipps, R.P. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-kappa B and activator protein-2. J. Immunol. 2001, 167, 366–374. [Google Scholar] [CrossRef]
- Jung, Y.D.; Fan, F.; McConkey, D.J.; Jean, M.E.; Liu, W.; Reinmuth, N.; Stoeltzing, O.; Ahmad, S.A.; Parikh, A.A.; Mukaida, N.; et al. Role of P38 MAPK, AP-1, and NF-kappaB in interleukin-1beta-induced IL-8 expression in human vascular smooth muscle cells. Cytokine 2002, 18, 206–213. [Google Scholar] [CrossRef]
- Florczyk, U.; Czauderna, S.; Stachurska, A.; Tertil, M.; Nowak, W.; Kozakowska, M.; Poellinger, L.; Jozkowicz, A.; Loboda, A.; Dulak, J. Opposite effects of HIF-1α and HIF-2α on the regulation of IL-8 expression in endothelial cells. Free Radic. Biol. Med. 2011, 51, 1882–1892. [Google Scholar] [CrossRef]
- Dutheil, F.; Trousselard, M.; Perrier, C.; Lac, G.; Chamoux, A.; Duclos, M.; Naughton, G.; Mnatzaganian, G.; Schmidt, J. Urinary interleukin-8 is a biomarker of stress in emergency physicians, especially with advancing age—The JOBSTRESS* randomized trial. PLoS ONE 2013, 8, e71658. [Google Scholar] [CrossRef]
- Straczkowski, M.; Kowalska, I.; Nikolajuk, A.; Dzienis-Straczkowska, S.; Szelachowska, M.; Kinalska, I. Plasma interleukin 8 concentrations in obese subjects with impaired glucose tolerance. Cardiovasc. Diabetol. 2003, 2, 5. [Google Scholar] [CrossRef]
- Makiel, K.; Suder, A.; Targosz, A.; Maciejczyk, M.; Haim, A. Exercise-Induced Alternations of Adiponectin, Interleukin-8 and Indicators of Carbohydrate Metabolism in Males with Metabolic Syndrome. Biomolecules 2023, 13, 852. [Google Scholar] [CrossRef]
- Rahman, S.A.; Castanon-Cervantes, O.; Scheer, F.A.; Shea, S.A.; Czeisler, C.A.; Davidson, A.J.; Lockley, S.W. Endogenous circadian regulation of pro-inflammatory cytokines and chemokines in the presence of bacterial lipopolysaccharide in humans. Brain Behav. Immun. 2015, 47, 4–13. [Google Scholar] [CrossRef]
- Kuwada, Y.; Sasaki, T.; Morinaka, K.; Kitadai, Y.; Mukaida, N.; Chayama, K. Potential involvement of IL-8 and its receptors in the invasiveness of pancreatic cancer cells. Int. J. Oncol. 2003, 22, 765–771. [Google Scholar] [CrossRef]
- Ehrlich, L.C.; Hu, S.; Sheng, W.S.; Sutton, R.L.; Rockswold, G.L.; Peterson, P.K.; Chao, C.C. Cytokine regulation of human microglial cell IL-8 production. J. Immunol. 1998, 160, 1944–1948. [Google Scholar] [CrossRef]
- Robinson, K.F.; Narasipura, S.D.; Wallace, J.; Ritz, E.M.; Al-Harthi, L. Negative regulation of IL-8 in human astrocytes depends on β-catenin while positive regulation is mediated by TCFs/LEF/ATF2 interaction. Cytokine 2020, 136, 155252. [Google Scholar] [CrossRef] [PubMed]
- Kossmann, T.; Stahel, P.F.; Lenzlinger, P.M.; Redl, H.; Dubs, R.W.; Trentz, O.; Schlag, G.; Morganti-Kossmann, M.C. Interleukin-8 released into the cerebrospinal fluid after brain injury is associated with blood-brain barrier dysfunction and nerve growth factor production. J. Cereb. Blood Flow. Metab. 1997, 17, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, T.; Mukaida, N.; Yamashita, K.; Yagisawa, H.; Akahoshi, T.; Matsushima, K. IL-1 and TNF-alpha induction of IL-8 and monocyte chemotactic and activating factor (MCAF) mRNA expression in a human astrocytoma cell line. Immunology 1991, 74, 60–67. [Google Scholar] [PubMed]
- Lipovsky, M.M.; Gekker, G.; Hu, S.; Ehrlich, L.C.; Hoepelman, A.I.; Peterson, P.K. Cryptococcal glucuronoxylomannan induces interleukin (IL)-8 production by human microglia but inhibits neutrophil migration toward IL-8. J. Infect. Dis. 1998, 177, 260–263. [Google Scholar] [CrossRef][Green Version]
- Horuk, R.; Martin, A.W.; Wang, Z.; Schweitzer, L.; Gerassimides, A.; Guo, H.; Lu, Z.; Hesselgesser, J.; Perez, H.D.; Kim, J.; et al. Expression of chemokine receptors by subsets of neurons in the central nervous system. J. Immunol. 1997, 158, 2882–2890. [Google Scholar] [CrossRef]
- Coughlan, C.M.; McManus, C.M.; Sharron, M.; Gao, Z.; Murphy, D.; Jaffer, S.; Choe, W.; Chen, W.; Hesselgesser, J.; Gaylord, H.; et al. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience 2000, 97, 591–600. [Google Scholar] [CrossRef]
- Du, S.H.; Zhang, W.; Yue, X.; Luo, X.Q.; Tan, X.H.; Liu, C.; Qiao, D.F.; Wang, H. Role of CXCR1 and Interleukin-8 in Methamphetamine-Induced Neuronal Apoptosis. Front. Cell Neurosci. 2018, 12, 230. [Google Scholar] [CrossRef]
- Dorf, M.E.; Berman, M.A.; Tanabe, S.; Heesen, M.; Luo, Y. Astrocytes express functional chemokine receptors. J. Neuroimmunol. 2000, 111, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Omari, K.M.; John, G.; Lango, R.; Raine, C.S. Role for CXCR2 and CXCL1 on glia in multiple sclerosis. Glia. 2006, 53, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, M.M.J.; Desa, M.N.M. Mechanisms of Blood Brain Barrier Disruption by Different Types of Bacteria, and Bacterial-Host Interactions Facilitate the Bacterial Pathogen Invading the Brain. Cell Mol. Neurobiol. 2018, 38, 1349–1368. [Google Scholar] [CrossRef]
- Sun, Y.; Li, N.; Zhang, J.; Liu, H.; Liu, J.; Xia, X.; Sun, C.; Feng, X.; Gu, J.; Du, C.; et al. Enolase of Streptococcus Suis Serotype 2 Enhances Blood-Brain Barrier Permeability by Inducing IL-8 Release. Inflammation 2016, 39, 718–726. [Google Scholar] [CrossRef]
- Cui, G.B.; An, J.Z.; Zhang, N.; Zhao, M.G.; Liu, S.B.; Yi, J. Elevated interleukin-8 enhances prefrontal synaptic transmission in mice with persistent inflammatory pain. Mol. Pain. 2012, 8, 11. [Google Scholar] [CrossRef]
- Limatola, C.; Ciotti, M.T.; Mercanti, D.; Santoni, A.; Eusebi, F. Signaling pathways activated by chemokine receptor CXCR2 and AMPA-type glutamate receptors and involvement in granule cells survival. J. Neuroimmunol. 2002, 123, 9–17. [Google Scholar] [CrossRef]
- Wu, Z.W.; Yu, H.H.; Wang, X.; Guan, H.Y.; Xiu, M.H.; Zhang, X.Y. Interrelationships Between Oxidative Stress, Cytokines, and Psychotic Symptoms and Executive Functions in Patients with Chronic Schizophrenia. Psychosom. Med. 2021, 83, 485–491. [Google Scholar] [CrossRef]
- Ramesh, G.; MacLean, A.G.; Philipp, M.T. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediat. Inflamm. 2013, 2013, 480739. [Google Scholar] [CrossRef]
- Capogna, E.; Watne, L.O.; Sørensen, Ø.; Guichelaar, C.J.; Idland, A.V.; Halaas, N.B.; Blennow, K.; Zetterberg, H.; Walhovd, K.B.; Fjell, A.M.; et al. Associations of neuroinflammatory IL-6 and IL-8 with brain atrophy, memory decline, and core AD biomarkers-in cognitively unimpaired older adults. Brain Behav. Immun. 2023, 113, 56–65. [Google Scholar] [CrossRef]
- Petreaca, M.L.; Yao, M.; Liu, Y.; Defea, K.; Martins-Green, M. Transactivation of vascular endothelial growth factor receptor-2 by interleukin-8 (IL-8/CXCL8) is required for IL-8/CXCL8-induced endothelial permeability. Mol. Biol. Cell. 2007, 18, 5014–5023. [Google Scholar] [CrossRef] [PubMed]
- Puma, C.; Danik, M.; Quirion, R.; Ramon, F.; Williams, S. The chemokine interleukin-8 acutely reduces Ca2+ currents in identified cholinergic septal neurons expressing CXCR1 and CXCR2 receptor mRNAs. J. Neurochem. 2001, 78, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Leighton, S.P.; Nerurkar, L.; Krishnadas, R.; Johnman, C.; Graham, G.J.; Cavanagh, J. Chemokines in depression in health and in inflammatory illness: A systematic review and meta-analysis. Mol. Psychiatry 2018, 23, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Mikova, O.; Yakimova, R.; Bosmans, E.; Kenis, G.; Maes, M. Increased serum tumor necrosis factor alpha concentrations in major depression and multiple sclerosis. Eur. Neuropsychopharmacol. 2001, 11, 203–208. [Google Scholar] [CrossRef]
- Zhu, Z.H.; Song, X.Y.; Man, L.J.; Chen, P.; Tang, Z.; Li, R.H.; Ji, C.F.; Dai, N.B.; Liu, F.; Wang, J.; et al. Comparisons of Serum Interleukin-8 Levels in Major Depressive Patients with Drug-Free Versus SSRIs Versus Healthy Controls. Front. Psychiatry 2022, 13, 858675. [Google Scholar] [CrossRef]
- Islam, S.; Islam, T.; Nahar, Z.; Shahriar, M.; Islam, S.M.A.; Bhuiyan, M.A.; Islam, M.R. Altered serum adiponectin and interleukin-8 levels are associated in the pathophysiology of major depressive disorder: A case-control study. PLoS ONE 2022, 17, e0276619. [Google Scholar] [CrossRef]
- Eyre, H.A.; Air, T.; Pradhan, A.; Johnston, J.; Lavretsky, H.; Stuart, M.J.; Baune, B.T. A meta-analysis of chemokines in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 68, 1–8. [Google Scholar] [CrossRef]
- Birur, B.; Amrock, E.M.; Shelton, R.C.; Li, L. Sex Differences in the Peripheral Immune System in Patients with Depression. Front. Psychiatry 2017, 8, 108. [Google Scholar] [CrossRef]
- Kruse, J.L.; Olmstead, R.; Hellemann, G.; Breen, E.C.; Tye, S.J.; Brooks, J.O.; Wade, B.; Congdon, E.; Espinoza, R.; Narr, K.L.; et al. Interleukin-8 and lower severity of depression in females, but not males, with treatment-resistant depression. J. Psychiatr. Res. 2021, 140, 350–356. [Google Scholar] [CrossRef]
- Di Benedetto, M.G.; Landi, P.; Mencacci, C.; Cattaneo, A. Depression in Women: Potential Biological and Sociocultural Factors Driving the Sex Effect. Neuropsychobiology 2024, 83, 2–16. [Google Scholar] [CrossRef]
- Kruse, J.L.; Vasavada, M.M.; Olmstead, R.; Hellemann, G.; Wade, B.; Breen, E.C.; Brooks, J.O.; Congdon, E.; Espinoza, R.; Narr, K.L.; et al. Depression treatment response to ketamine: Sex-specific role of interleukin-8, but not other inflammatory markers. Transl. Psychiatry. 2021, 11, 167. [Google Scholar] [CrossRef]
- Fülöp, T.; Larbi, A.; Witkowski, J.M. Human Inflammaging. Gerontology 2019, 65, 495–504. [Google Scholar] [CrossRef]
- Versel, J.; Cantos, A.; Castillo, M.F.; Fatourou, E.; Sinacore, J.; Halaris, A. Interleukin-8 is a potential inflammation biomarker in major depressive disorder. J. Affect. Dis. Rep. 2024, 17, 100828. [Google Scholar] [CrossRef]
- Kruse, J.L.; Boyle, C.C.; Olmstead, R.; Breen, E.C.; Tye, S.J.; Eisenberger, N.I.; Irwin, M.R. Interleukin-8 and depressive responses to an inflammatory challenge: Secondary analysis of a randomized controlled trial. Sci. Rep. 2022, 12, 12627. [Google Scholar] [CrossRef] [PubMed]
- Versel, J.L.; Cantos, A.; Castillo, M.F.; Fatourou, E.; Halaris, A. Effects of Anti-Depressant Treatment on Interleukin-8 in Major Depressive Disorder. Biol. Psychiatry 2023, 93, S329. [Google Scholar] [CrossRef]
- Cai, Y.; Zhu, Z.H.; Li, R.H.; Yin, X.Y.; Chen, R.F.; Man, L.J.; Hou, W.L.; Zhu, H.L.; Wang, J.; Zhang, H.; et al. Association between increased serum interleukin-8 levels and improved cognition in major depressive patients with SSRIs. BMC Psychiatry 2023, 23, 122. [Google Scholar] [CrossRef]
- Guimarães, M.E.A.; Derhon, V.; Signori, L.U.; Seiffer, B.A.; Wolf, S.; Schuch, F.B. Acute and chronic effects of physical exercise in inflammatory biomarkers in people with depression: A systematic review with meta-analysis. J. Psychiatr. Res. 2024, 179, 26–32. [Google Scholar] [CrossRef]
- Yoon, H.K.; Kim, Y.K.; Lee, H.J.; Kwon, D.Y.; Kim, L. Role of cytokines in atypical depression. Nord. J. Psychiatry 2012, 66, 183–188. [Google Scholar] [CrossRef]
- Wang, H.; Li, P.; Zhang, Y.; Zhang, C.; Li, K.; Song, C. Cytokine changes in different types of depression: Specific or general? Neurol. Psychiatry Brain Res. 2020, 36, 39–51. [Google Scholar] [CrossRef]
- Syed, S.A.; Beurel, E.; Loewenstein, D.A.; Lowell, J.A.; Craighead, W.E.; Dunlop, B.W.; Mayberg, H.S.; Dhabhar, F.; Dietrich, W.D.; Keane, R.W.; et al. Defective Inflammatory Pathways in Never-Treated Depressed Patients Are Associated with Poor Treatment Response. Neuron 2018, 99, 914–924.e3. [Google Scholar] [CrossRef]
- Kruse, J.L.; Congdon, E.; Olmstead, R.; Njau, S.; Breen, E.C.; Narr, K.L.; Espinoza, R.; Irwin, M.R. Inflammation and Improvement of Depression Following Electroconvulsive Therapy in Treatment-Resistant Depression. J. Clin. Psychiatry 2018, 79, 17m11597. [Google Scholar] [CrossRef] [PubMed]
- Oppong, E.; Cato, A.C. Effects of Glucocorticoids in the Immune System. Adv. Exp. Med. Biol. 2015, 872, 217–233. [Google Scholar] [PubMed]
- Licinio, J.; Wong, M.L.; Gold, P.W. Neutrophil-activating peptide-1/interleukin-8 mRNA is localized in rat hypothalamus and hippocampus. Neuroreport 1992, 3, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Yektaei-Karin, E.; Moshfegh, A.; Lundahl, J.; Berggren, V.; Hansson, L.O.; Marchini, G. The stress of birth enhances in vitro spontaneous and IL-8-induced neutrophil chemotaxis in the human newborn. Pediatr. Allergy Immunol. 2007, 18, 643–651. [Google Scholar] [CrossRef]
- Martinez, J.M.; Garakani, A.; Yehuda, R.; Gorman, J.M. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress. Anxiety 2012, 29, 32–38. [Google Scholar] [CrossRef]
- Haapakoski, R.; Mathieu, J.; Ebmeier, K.P.; Alenius, H.; Kivimäki, M. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav. Immun. 2015, 49, 206–215. [Google Scholar] [CrossRef]
- Kappelmann, N.; Lewis, G.; Dantzer, R.; Jones, P.B.; Khandaker, G.M. Antidepressant activity of anti-cytokine treatment: A systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry 2018, 23, 335–343. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Qin, J.; Ma, Z.; Chen, X.; Shu, S. Microglia activation in central nervous system disorders: A review of recent mechanistic investigations and development efforts. Front. Neurol. 2023, 14, 1103416. [Google Scholar] [CrossRef]
- Goczalik, M.I.; Raap, M.; Weick, M.; Milenkovic, I.; Heidmann, J.; Enzmann, V.; Wiedemann, P.; Reichenbach, A.; Francke, M. The activation of IL-8 receptors in cultured guinea pig Müller glial cells is modified by signals from retinal pigment epithelium. J. Neuroimmunol. 2005, 161, 49–60. [Google Scholar] [CrossRef]
- Araujo, D.M.; Cotman, C.W. Trophic effects of interleukin-4, -7 and -8 on hippocampal neuronal cultures: Potential involvement of glial-derived factors. Brain Res. 1993, 600, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Dranovsky, A.; Hen, R. Hippocampal neurogenesis: Regulation by stress and antidepressants. Biol. Psychiatry 2006, 59, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Videbech, P.; Ravnkilde, B. Hippocampal volume and depression: A meta-analysis of MRI studies. Am. J. Psychiatry 2004, 161, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Shkundin, A.; Halaris, A. IL-8 (CXCL8) Correlations with Psychoneuroimmunological Processes and Neuropsychiatric Conditions. J. Pers. Med. 2024, 14, 488. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Charlton, T.; Prowse, N.; McFee, A.; Heiratifar, N.; Fortin, T.; Paquette, C.; Hayley, S. Brain-derived neurotrophic factor (BDNF) has direct anti-inflammatory effects on microglia. Front. Cell Neurosci. 2023, 17, 1188672. [Google Scholar] [CrossRef]
- Dietrick, B.; Molloy, E.; Massaro, A.N.; Strickland, T.; Zhu, J.; Slevin, M.; Donoghue, V.; Sweetman, D.; Kelly, L.; O’Dea, M.; et al. Plasma and Cerebrospinal Fluid Candidate Biomarkers of Neonatal Encephalopathy Severity and Neurodevelopmental Outcomes. J. Pediatr. 2020, 226, 71–79.e5. [Google Scholar] [CrossRef]
- Xiu, M.H.; Wang, D.M.; Du, X.D.; Chen, N.; Tan, S.P.; Tan, Y.L.; Yang, F.; Cho, R.Y.; Zhang, X.Y. Interaction of BDNF and cytokines in executive dysfunction in patients with chronic schizophrenia. Psychoneuroendocrinology 2019, 108, 110–117. [Google Scholar] [CrossRef]
- Liou, Y.J.; Wang, T.Y.; Lee, S.Y.; Chang, Y.H.; Tsai, T.Y.; Chen, P.S.; Huang, S.Y.; Tzeng, N.S.; Lee, I.H.; Chen, K.C.; et al. Effects of comorbid alcohol use disorder on bipolar disorder: Focusing on neurocognitive function and inflammatory markers. Psychoneuroendocrinology 2023, 152, 106083. [Google Scholar] [CrossRef]
- Cavaleri, D.; Moretti, F.; Bartoccetti, A.; Mauro, S.; Crocamo, C.; Carrà, G.; Bartoli, F. The role of BDNF in major depressive disorder, related clinical features, and antidepressant treatment: Insight from meta-analyses. Neurosci. Biobehav. Rev. 2023, 149, 105159. [Google Scholar] [CrossRef]
- Conner, J.M.; Franks, K.M.; Titterness, A.K.; Russell, K.; Merrill, D.A.; Christie, B.R.; Sejnowski, T.J.; Tuszynski, M.H. NGF is essential for hippocampal plasticity and learning. J. Neurosci. 2009, 29, 10883–10889. [Google Scholar] [CrossRef]
- Jaiswal, A.; Shreekantiah, U.; Goyal, N. Nerve Growth Factor in Psychiatric Disorders: A Scoping Review. Indian. J. Psychol. Med. 2023, 45, 555–564. [Google Scholar] [CrossRef]
- Martino, M.; Rocchi, G.; Escelsior, A.; Contini, P.; Colicchio, S.; de Berardis, D.; Amore, M.; Fornaro, P.; Fornaro, M. NGF serum levels variations in major depressed patients receiving duloxetine. Psychoneuroendocrinology 2013, 38, 1824–1828. [Google Scholar] [CrossRef]
- Mishra, B.R.; Maiti, R.; Nath, S.; Sahoo, P.; Jena, M.; Mishra, A. Effect of Sertraline, Dosulepin, and Venlafaxine on Non-BDNF Neurotrophins in Patients With Depression: A Cohort Study. J. Clin. Psychopharmacol. 2019, 39, 220–225. [Google Scholar] [CrossRef]
- Guo, H.; Ren, Y.; Huang, B.; Wang, J.; Yang, X.; Wang, Y. Psychological Status, Compliance, Serum Brain-Derived Neurotrophic Factor, and Nerve Growth Factor Levels of Patients with Depression after Augmented Mindfulness-Based Cognitive Therapy. Genet. Res. 2022, 2022, 1097982. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Machado-Vieira, R.; Zarate, C.A., Jr.; Vieira, E.L.; Valiengo, L.; Benseñor, I.M.; Lotufo, P.A.; Gattaz, W.F.; Teixeira, A.L. Assessment of non-BDNF neurotrophins and GDNF levels after depression treatment with sertraline and transcranial direct current stimulation in a factorial, randomized, sham-controlled trial (SELECT-TDCS): An exploratory analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 56, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.S.; Hu, S.; Feng, A.; Rock, R.B. Reactive oxygen species from human astrocytes induced functional impairment and oxidative damage. Neurochem. Res. 2013, 38, 2148–2159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gao, J.H.; Yan, Z.F.; Huang, X.Y.; Guo, P.; Sun, L.; Liu, Z.; Hu, Y.; Zuo, L.J.; Yu, S.Y.; et al. Minimally Toxic Dose of Lipopolysaccharide and α-Synuclein Oligomer Elicit Synergistic Dopaminergic Neurodegeneration: Role and Mechanism of Microglial NOX2 Activation. Mol. Neurobiol. 2018, 55, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.; Kim, S.U. Human astrocytes: Secretome profiles of cytokines and chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar] [CrossRef]
- Liao, B.; Zhao, W.; Beers, D.R.; Henkel, J.S.; Appel, S.H. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp. Neurol. 2012, 237, 147–152. [Google Scholar] [CrossRef]
- Ngo, S.T.; Steyn, F.J.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocrinol. 2014, 35, 347–369. [Google Scholar] [CrossRef]
- Aulock, S.V.; Deininger, S.; Draing, C.; Gueinzius, K.; Dehus, O.; Hermann, C. Gender difference in cytokine secretion on immune stimulation with LPS and LTA. J. Interferon Cytokine Res. 2006, 26, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Coyle, S.M.; Calvano, S.E.; Lowry, S.F. Gender influences in vivo human responses to endotoxin. Shock 2006, 26, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Karshikoff, B.; Lekander, M.; Soop, A.; Lindstedt, F.; Ingvar, M.; Kosek, E.; Olgart Höglund, C.; Axelsson, J. Modality and sex differences in pain sensitivity during human endotoxemia. Brain Behav. Immun. 2015, 46, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kadetoff, D.; Lampa, J.; Westman, M.; Andersson, M.; Kosek, E. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. J. Neuroimmunol. 2012, 242, 33–38. [Google Scholar] [CrossRef]
- Nati, I.D.; Malutan, A.; Ciortea, R.; Oancea, M.; Bucuri, C.; Roman, M.; Ormindean, C.; Milon, A.G.; Mihu, D. Exploring the Influence of IL-8, IL-10, Patient-Reported Pain, and Physical Activity on Endometriosis Severity. Diagnosis 2024, 14, 1822. [Google Scholar] [CrossRef]
- Schmidt, H.D.; Shelton, R.C.; Duman, R.S. Functional biomarkers of depression: Diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 2011, 36, 2375–2394. [Google Scholar] [CrossRef]
- Insel, T.R.; Cuthbert, B.N. Medicine. Brain disorders? Precisely. Science 2015, 348, 499–500. [Google Scholar] [CrossRef]
- Breit, S.; Mazza, E.; Poletti, S.; Benedetti, F. White matter integrity and pro-inflammatory cytokines as predictors of antidepressant response in MDD. J. Psychiatr. Res. 2023, 159, 22–32. [Google Scholar] [CrossRef]
- Osimo, E.F.; Pillinger, T.; Rodriguez, I.M.; Khandaker, G.M.; Pariante, C.M.; Howes, O.D. Inflammatory markers in depression: A meta-analysis of mean differences and variability in 5166 patients and 5083 controls. Brain Behav. Immun. 2020, 87, 901–909. [Google Scholar] [CrossRef]
- Platchek, M.; Lu, Q.; Tran, H.; Xie, W. Comparative Analysis of Multiple Immunoassays for Cytokine Profiling in Drug Discovery. SLAS Discov. 2020, 25, 1197–1213. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Di, Y.P. Fast and Efficient Measurement of Clinical and Biological Samples Using Immunoassay-Based Multiplexing Systems. Methods Mol. Biol. 2020, 2102, 129–147. [Google Scholar]
- Nenning, K.H.; Langs, G. Machine learning in neuroimaging: From research to clinical practice. Radiologie 2022, 62, 1–10. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Mayberg, H.S. Neuroimaging Advances for Depression. Cerebrum 2017, 2017, cer-16-17. [Google Scholar]
- Vu, T.; Dawadi, R.; Yamamoto, M.; Tay, J.T.; Watanabe, N.; Kuriya, Y.; Oya, A.; Tran, P.N.H.; Araki, M. Prediction of depressive disorder using machine learning approaches: Findings from the NHANES. BMC Med. Inf. Decis. Mak. 2025, 25, 83. [Google Scholar] [CrossRef]

| Author (year) | Type of Research | Number of Participants | IL-8 Level | Material/Measuring Method |
|---|---|---|---|---|
| Islam et al. (2022) [69] | A case–control study | 63 MDD/94 HC | IL-8 higher in MDD | Serum/ELISA |
| Zhu et al. (2022) [68] | Comparison of groups | 87 MDD/101 HC | IL-8 lower in MDD patients | Serum/CBA |
| Leighton et al. (2018) [66] | Comparison of cytokine levels between groups | 80 MDD/40 HC | IL-8 higher in MDD patients | Plasma/ELISA |
| Eyre et al. (2016) [70] | A meta-analysis | 316 MDD/265 HC | No difference in IL-8 | Serum or plasma/ELISA or multiplex |
| Dahl et al. (2014) [11] | Comparison of cytokine levels between groups | 50 MDD/34 HC | IL-8 higher in MDD patients | Plasma/multiplex |
| Dowlati et al. (2010) [4] | A meta-analysis | 205 MDD/177 HC from 4 studies | No difference in IL-8 | Serum or plasma/ELISA or multiplex |
| Mikova et al. (2001) [67] | Comparison of cytokine levels between groups | 47 MDD/20 HC | IL-8 lower in MDD patients | Serum/ELISA |
| Author (Year) | Number of Participants | Type of Treatment | Relationship Between IL-8 Level and Response to Treatment | Material/Measuring Method |
|---|---|---|---|---|
| Szałach et al. (2025) [12] | 18 TRD | Ketamine | Response to treatment associated with decreasing IL-8 levels | Serum/CBA |
| Kruse et al. (2021) [74] | 46 TRD | Ketamine | Response to treatment associated with increasing IL-8 in women and decreasing IL-8 in men | Plasma/multiplex |
| Kruse et al. (2018) [84] | 29 TRD | Electroconvulsive therapy | No changes in IL-8 during treatment | Plasma/multiplex |
| Syed et al. (2018) [83] | 171 MDD | Escitalopram, duloxetine, CBT | No changes in IL-8 during treatment | Plasma/multiplex |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisowska, K.A. The Role of Interleukin-8 (IL-8) in Treatment-Resistant Depression: A Review of Mechanisms, Biomarker Potential, and Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 10092. https://doi.org/10.3390/ijms262010092
Lisowska KA. The Role of Interleukin-8 (IL-8) in Treatment-Resistant Depression: A Review of Mechanisms, Biomarker Potential, and Therapeutic Implications. International Journal of Molecular Sciences. 2025; 26(20):10092. https://doi.org/10.3390/ijms262010092
Chicago/Turabian StyleLisowska, Katarzyna Aleksandra. 2025. "The Role of Interleukin-8 (IL-8) in Treatment-Resistant Depression: A Review of Mechanisms, Biomarker Potential, and Therapeutic Implications" International Journal of Molecular Sciences 26, no. 20: 10092. https://doi.org/10.3390/ijms262010092
APA StyleLisowska, K. A. (2025). The Role of Interleukin-8 (IL-8) in Treatment-Resistant Depression: A Review of Mechanisms, Biomarker Potential, and Therapeutic Implications. International Journal of Molecular Sciences, 26(20), 10092. https://doi.org/10.3390/ijms262010092






