Engineering Bispecific Peptides for Precision Immunotherapy and Beyond
Abstract
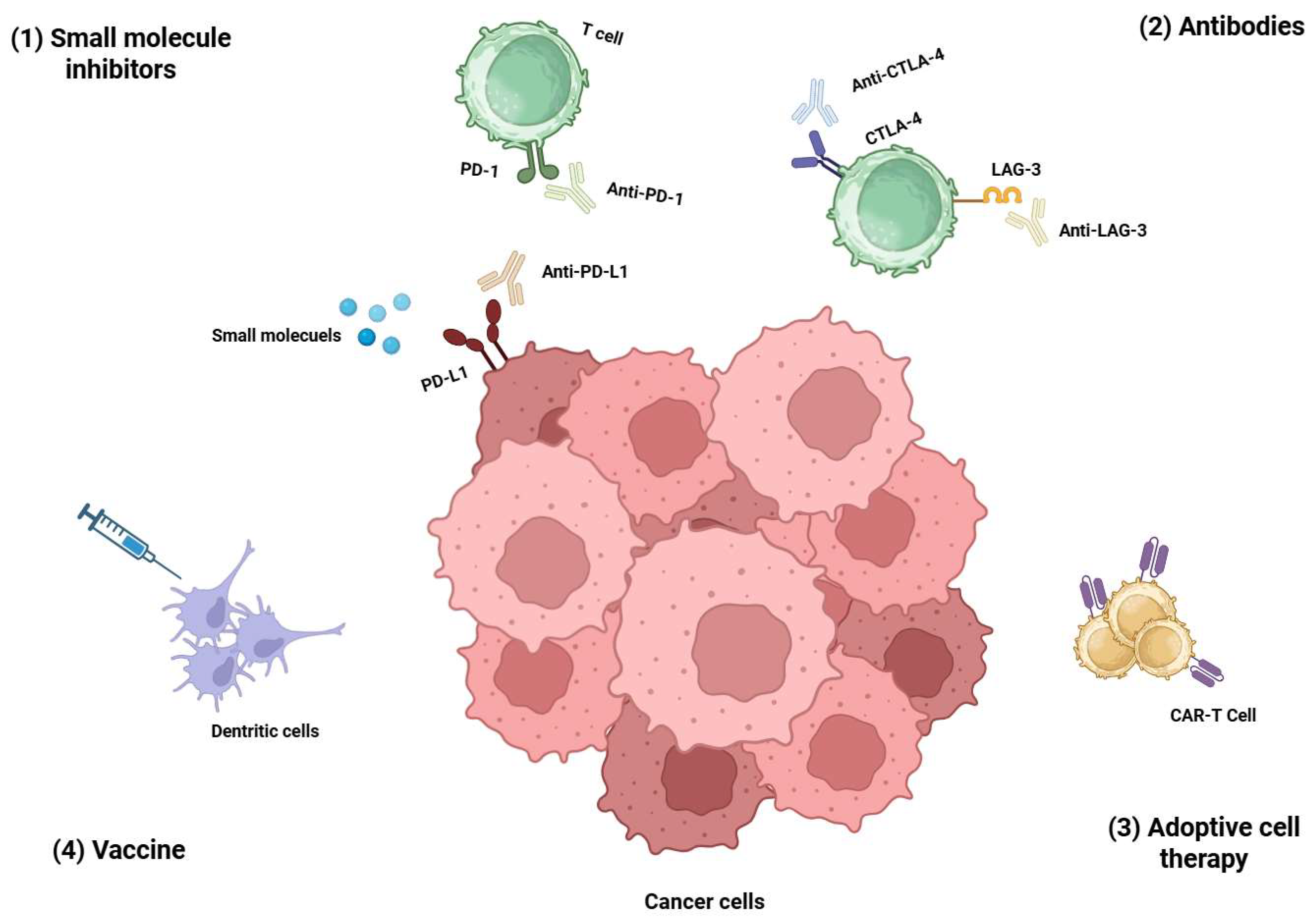
1. Introduction
2. Current Design Strategies of Bispecific Peptides
2.1. Fused Bispecific Peptide
2.2. Linked Bispecific Peptide
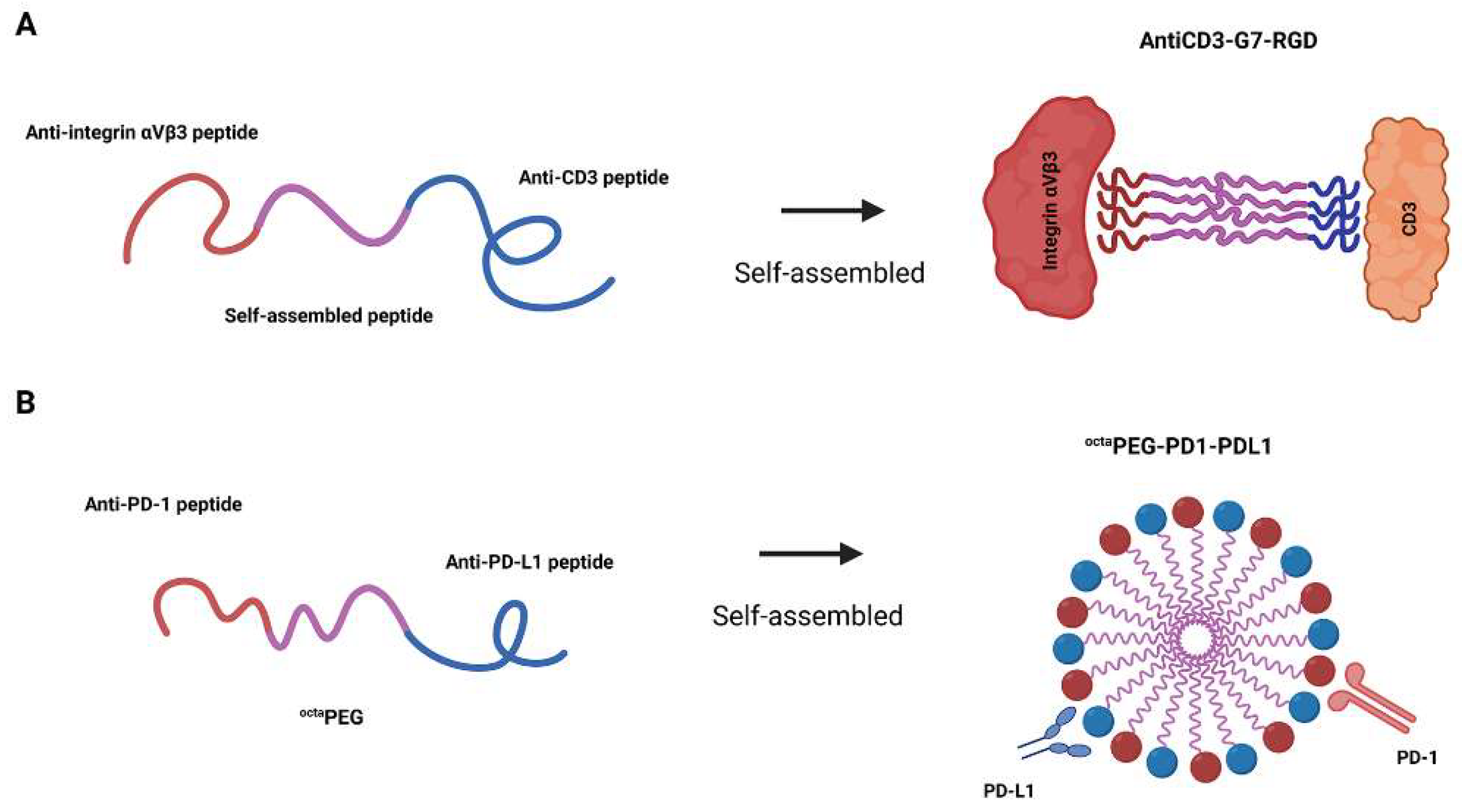
2.3. Self-Assembled Bispecific Peptides
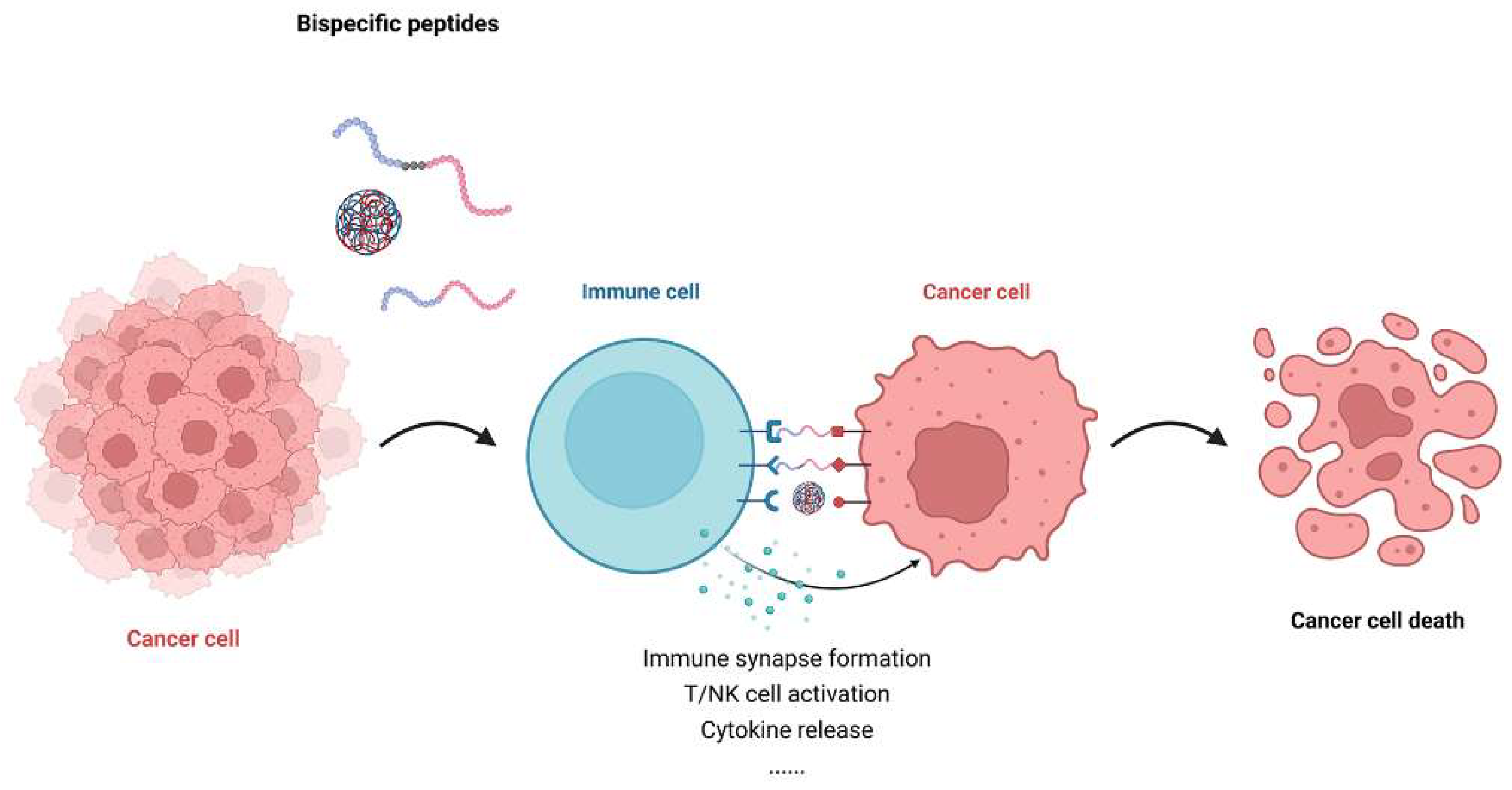
3. Mechanism of Action of Bispecific Peptides in Immunotherapy
3.1. Immune Synapse Formation and T Cell Engagement
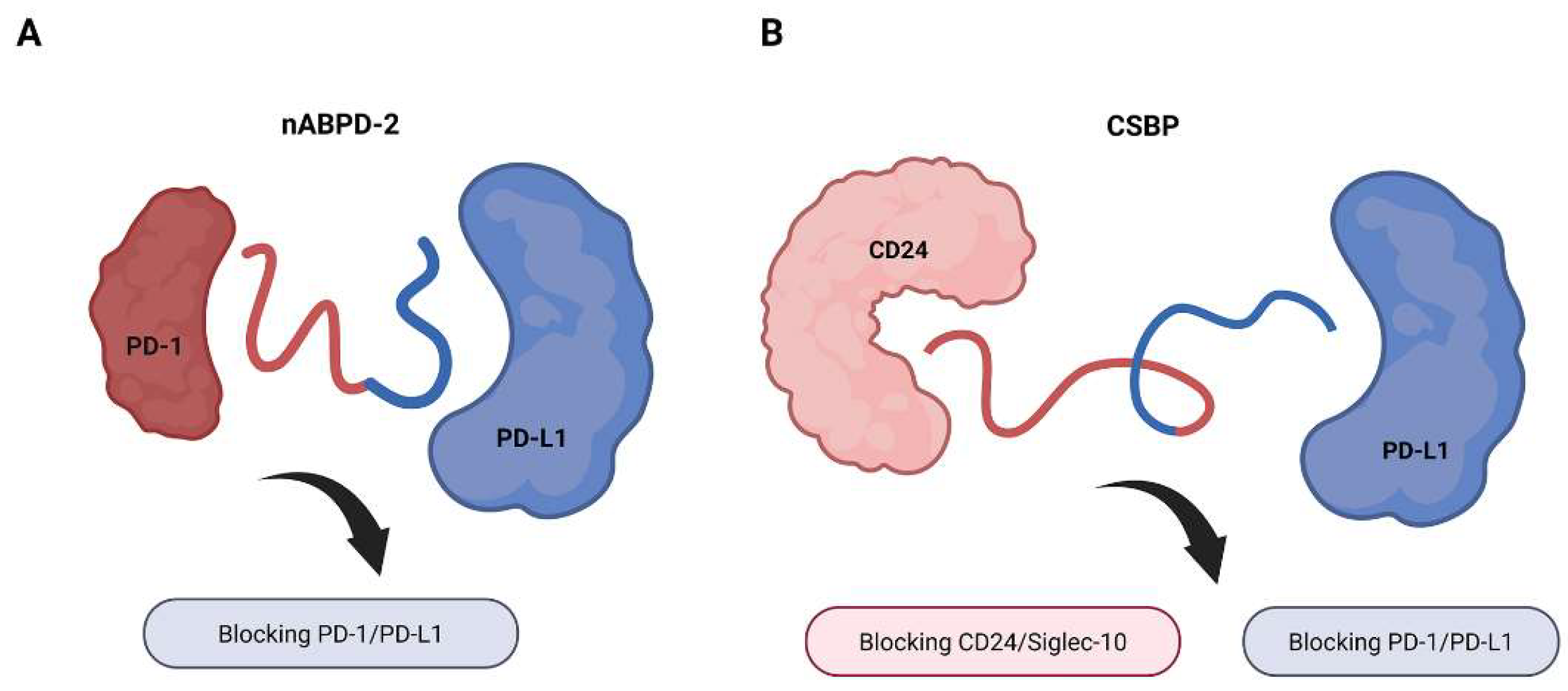
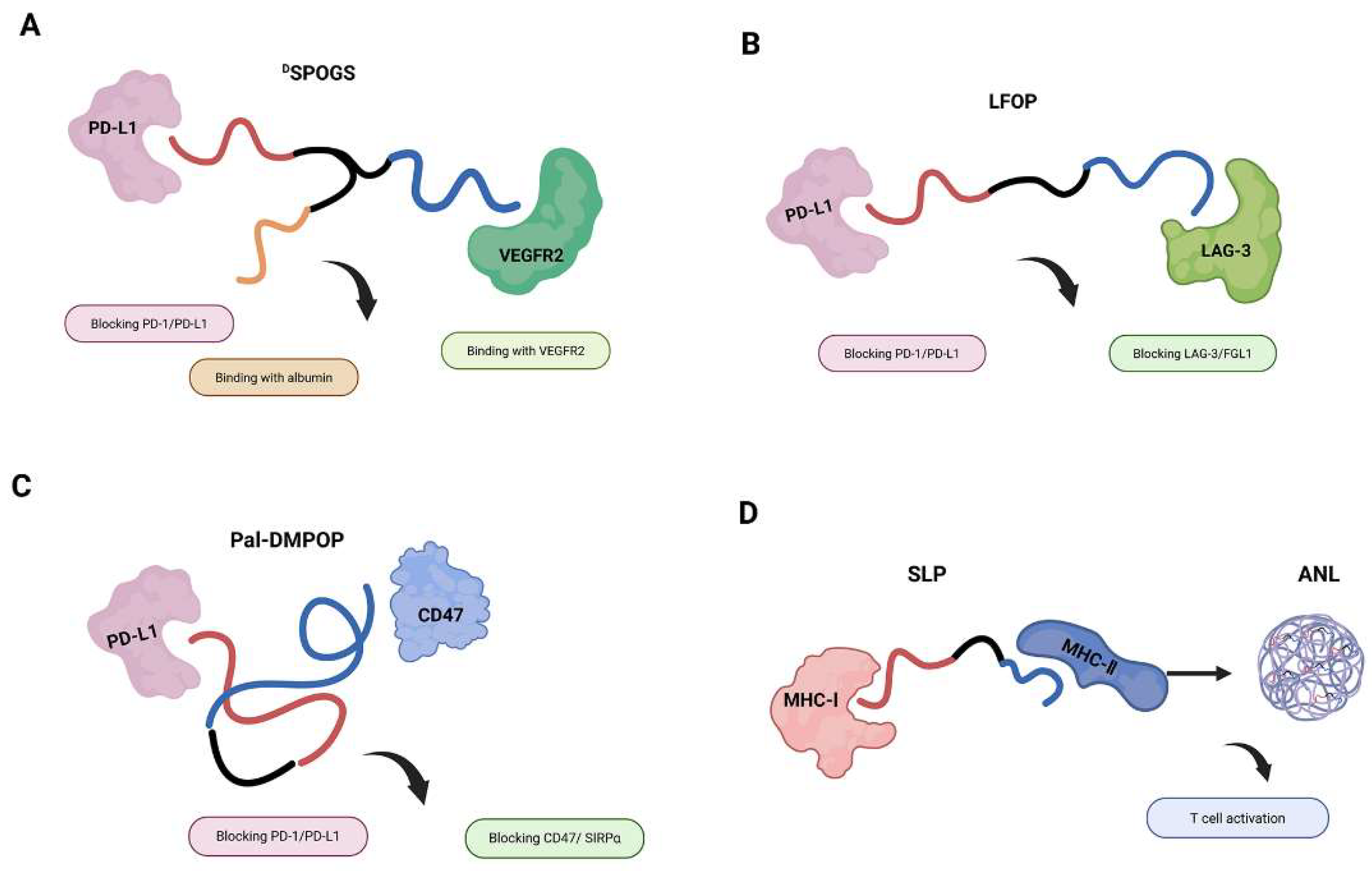
3.2. Checkpoint Inhibition and Costimulatory Reprogramming
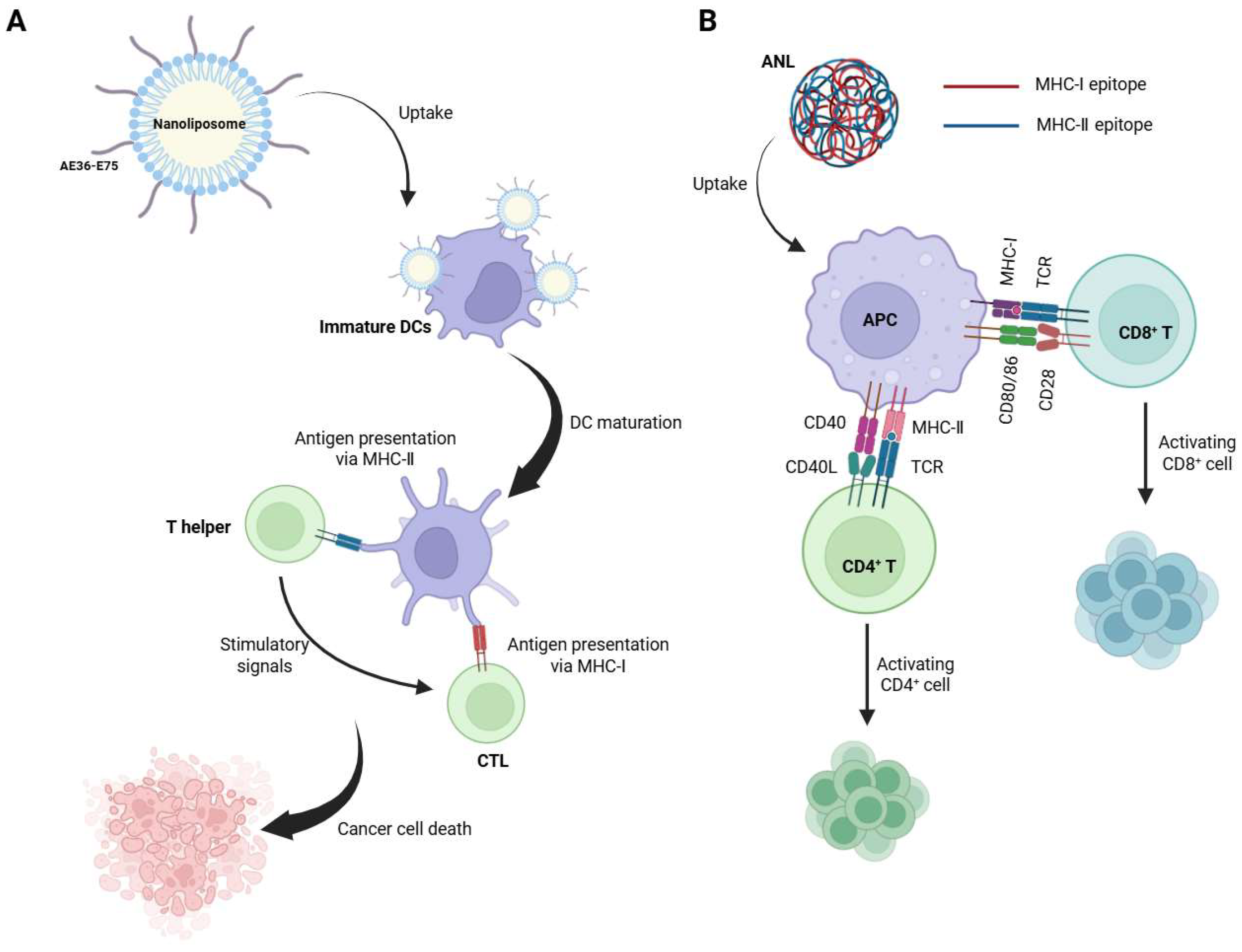
3.3. Mechanistic Enhancement of Antigen Presentation
4. Emerging Application Beyond Oncology
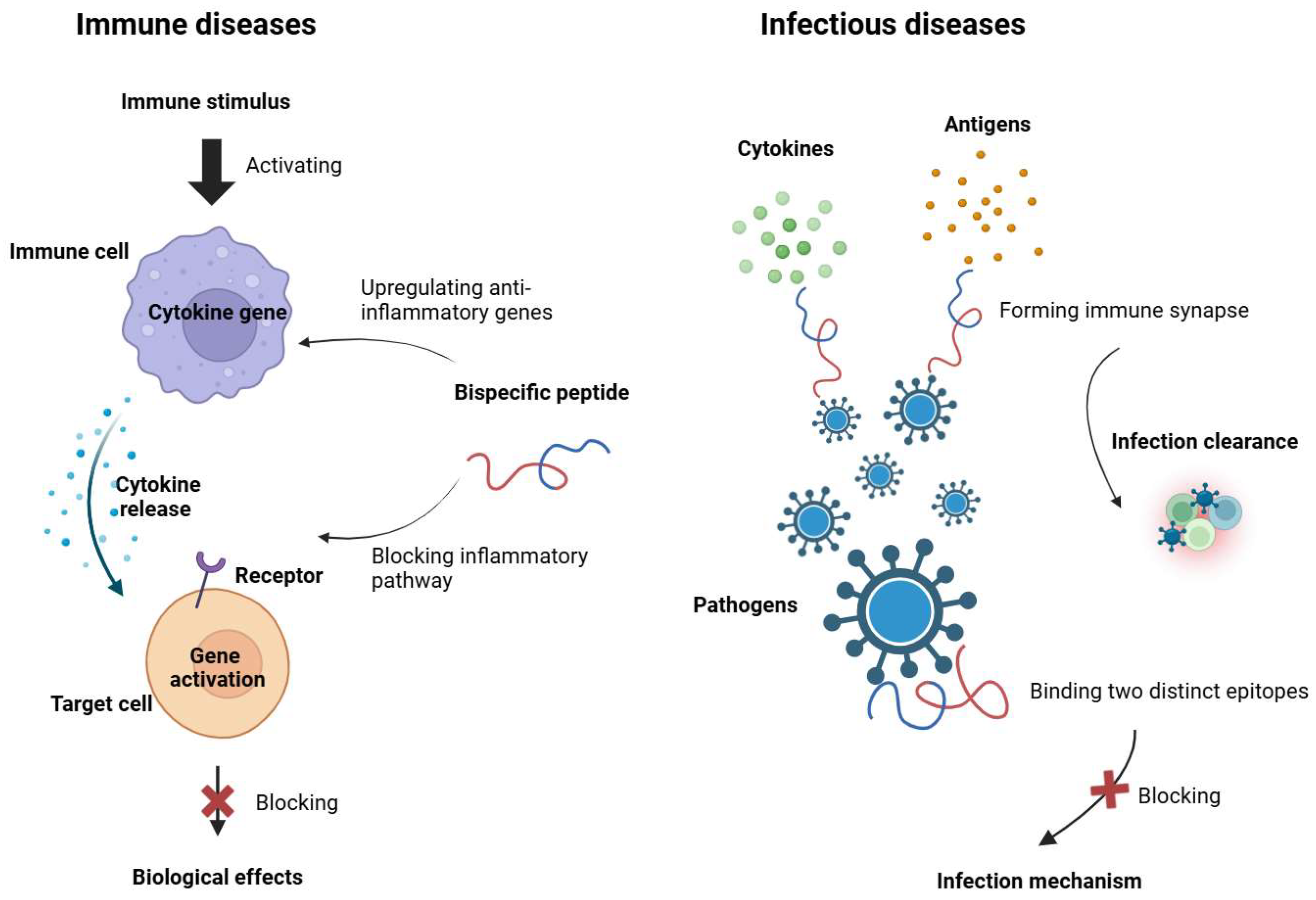
4.1. Autoimmune and Inflammatory Diseases
4.2. Infectious Diseases
5. Challenges and Limitations
5.1. Stability and Degradation Concerns
5.2. Immunogenicity and Unintended Immune Response
5.3. Delivery Challenges and Bioavailability Issues
5.4. Manufacturing and Scalability Issues
6. Future Perspectives and Emerging Trends
6.1. AI and Computational Peptide Design
6.2. Novel Conjugation Strategies and Synergistic Approaches
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cell |
| ANL | Aluminum nanoparticle-loaded long peptide |
| CAR-T | Chimeric antigen receptor T cell |
| CTL | Cytotoxic T lymphocyte |
| CTLA-4 | Cytotoxic T-lymphocyte-associated protein 4 |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DC | Dendritic cells |
| EGFR | Epidermal growth factor receptor |
| Fc | Fragment crystallizable region |
| FGL1 | Fibrinogen-like protein 1 |
| HER2 | Human epidermal growth factor receptor 2 |
| IFN-γ | Interferon-gamma |
| IL-2 | Interleukin-2 |
| ICB | Immune checkpoint blocker |
| LAG-3 | Lymphocyte-activation gene 3 |
| MDSC | Myeloid-derived suppressor cell |
| MHC | Major histocompatibility complex |
| NK | Natural killer |
| PD-1 | Programmed death-1 |
| PD-L1 | Programmed death-ligand 1 |
| PEG | Polyethylene glycol |
| PPI | Protein–protein interaction |
| PRR | Pattern recognition receptors |
| ROS | Reactive oxygen species |
| scFv | Single-chain variable fragment |
| SLP | Synthetic long peptide |
| SPPS | Solid-phase peptide synthesis |
| TAM | Tumor-associated macrophage |
| TCR | T-cell receptor |
| TME | Tumor microenvironment |
| TNF-α | Tumor necrosis factor alpha |
| TSCC | Tongue squamous cell carcinoma |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
References
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Heid, M. These Are the Most Exciting Advances in Kidney Cancer Treatment. TIME 2022. Available online: https://time.com/6227200/kidney-cancer-treatment-advances/ (accessed on 23 June 2025).
- Gougis, P.; Jochum, F.; Abbar, B.; Dumas, E.; Bihan, K.; Lebrun-Vignes, B.; Moslehi, J.; Spano, J.-P.; Laas, E.; Hotton, J.; et al. Clinical spectrum and evolution of immune-checkpoint inhibitors toxicities over a decade—A worldwide perspective. eClinicalMedicine 2024, 70, 102536. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Sig. Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Simeon, R.; Chen, Z. In vitro-engineered non-antibody protein therapeutics. Protein Cell 2018, 9, 3–14. [Google Scholar] [CrossRef]
- Peptide Therapeutics Market CAGR, Size, Share, Trends, Growth, Value, Key Players Analysis|Stratistics MRC Report. Stratistics MRC. Available online: https://www.strategymrc.com/report/peptide-therapeutics (accessed on 23 June 2025).
- Chu, W.; Prodromou, R.; Day, K.N.; Schneible, J.D.; Bacon, K.B.; Bowen, J.D.; Kilgore, R.E.; Catella, C.M.; Moore, B.D.; Mabe, M.D.; et al. Peptides and pseudopeptide ligands: A powerful toolbox for the affinity purification of current and next-generation biotherapeutics. J. Chromatogr. A 2021, 1635, 461632. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Uhlig, T.; Kyprianou, T.; Martinelli, F.G.; Oppici, C.A.; Heiligers, D.; Hills, D.; Calvo, X.R.; Verhaert, P. The emergence of peptides in the pharmaceutical business: From exploration to exploitation. EuPA Open Proteom. 2014, 4, 58–69. [Google Scholar] [CrossRef]
- Peptide Therapeutics Market Size|Industry Report. 2030. Available online: https://www.grandviewresearch.com/industry-analysis/peptide-therapeutics-market (accessed on 2 July 2025).
- Zhang, X.; Yang, Y.; Fan, D.; Xiong, D. The development of bispecific antibodies and their applications in tumor immune escape. Exp. Hematol. Oncol. 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Sedykh, S.E.; Prinz, V.V.; Buneva, V.N.; Nevinsky, G.A. Bispecific antibodies: Design, therapy, perspectives. Drug Des. Devel. Ther. 2018, 12, 195–208. [Google Scholar] [CrossRef]
- Delou, J.M.A.; Souza, A.S.O.; Souza, L.C.M.; Borges, H.L. Highlights in Resistance Mechanism Pathways for Combination Therapy. Cells 2019, 8, 1013. [Google Scholar] [CrossRef] [PubMed]
- Szumilak, M.; Wiktorowska-Owczarek, A.; Stanczak, A. Hybrid Drugs—A Strategy for Overcoming Anticancer Drug Resistance? Molecules 2021, 26, 2601. [Google Scholar] [CrossRef]
- Marketed Bispecific Antibodies and Their Pharmacokinetic Characteristics—WuXi AppTec DMPK. Available online: https://dmpkservice.wuxiapptec.com/articles/355-marketed-bispecific-antibodies-and-their-pharmacokinetic-characteristics/ (accessed on 15 August 2025).
- Löscher, W. Single-Target Versus Multi-Target Drugs Versus Combinations of Drugs with Multiple Targets: Preclinical and Clinical Evidence for the Treatment or Prevention of Epilepsy. Front. Pharmacol. 2021, 12, 730257. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.; Wang, X.; Yuan, K.; Wang, G.; Hu, L.; Zhang, G.; Pei, W.; Wang, L.; Sun, C.; et al. Bispecific antibodies in cancer therapy: Target selection and regulatory requirements. Acta Pharm. Sin. B 2023, 13, 3583–3597. [Google Scholar] [CrossRef]
- Kopp, A.; Kwon, H.; Johnston, C.; Vance, S.; Legg, J.; Galson-Holt, L.; Thurber, G.M. Impact of tissue penetration and albumin binding on design of T cell targeted bispecific agents. Neoplasia 2024, 48, 100962. [Google Scholar] [CrossRef]
- Henninot, A.; Collins, J.C.; Nuss, J.M. The current state of peptide drug discovery: Back to the future? J. Med. Chem. 2018, 61, 1382–1414. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhong, Y.; Chen, J.; Pan, X.; Fan, X.; Chen, P.; Fu, C.; Ou, C.; Chen, M. A dual-targeting peptide facilitates targeting anti-inflammation to attenuate atherosclerosis in ApoE−/− mice. Chem. Commun. 2022, 58, 8690–8693. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yang, Y.; Wang, G.; Liu, M. Current landscape and future directions of bispecific antibodies in cancer immunotherapy. Front. Immunol. 2022, 13, 1035276. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Shahbaz, A.; Hussain, N.; Ali, R.; Bashir, H.; Rizwan, K. Recent advancements in fusion protein technologies in oncotherapy: A review. Int. J. Biol. Macromol. 2023, 230, 123161. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef]
- Thomas, B.J.; Porciani, D.; Burke, D.H. Cancer immunomodulation using bispecific aptamers. Mol. Ther. Nucleic Acids 2022, 27, 894–915. [Google Scholar] [CrossRef]
- Larson, R.C.; Maus, M.V. Recent advances and discoveries in the mechanisms and functions of CAR T cells. Nat. Rev. Cancer 2021, 21, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Abken, H. TRUCKS, the fourth-generation CAR T cells: Current developments and clinical translation. Adv. Cell Gene Ther. 2020, 3, e84. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Liu, Y.; Wang, Z.; Wang, L.; Wang, Q.; Zhang, Y.; Wang, H. Engineering a High-Affinity PD-1 Peptide for Optimized Immune Cell–Mediated Tumor Therapy. Cancer Res. Treat. 2022, 54, 362–374. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, J.; Tan, Z.; Zhang, Y.; Wang, H. A novel bispecific peptide targeting PD-1 and PD-L1 with combined antitumor activity of T-cells derived from the patients with TSCC. Int. Immunopharmacol. 2024, 138, 112582. [Google Scholar] [CrossRef]
- Shen, W.; Shi, P.; Dong, Q.; Zhou, X.; Chen, C.; Sui, X.; Tian, W.; Zhu, X.; Wang, X.; Jin, S.; et al. Discovery of a novel dual-targeting D-peptide to block CD24/Siglec-10 and PD-1/PD-L1 interaction and synergize with radiotherapy for cancer immunotherapy. J. Immunother. Cancer 2023, 11, e007068. [Google Scholar] [CrossRef]
- Luo, H.; Hong, H.; Yang, S.P.; Cai, W. Design and Applications of Bispecific Heterodimers: Molecular Imaging and beyond. Mol. Pharm. 2014, 11, 1750. [Google Scholar] [CrossRef]
- Battistini, L.; Bugatti, K.; Sartori, A.; Curti, C.; Zanardi, F. RGD Peptide-Drug Conjugates as Effective Dual Targeting Platforms: Recent Advances. Eur. J. Org. Chem. 2021, 2021, 2506–2528. [Google Scholar] [CrossRef]
- Jiao, L.; Dong, Q.; Zhai, W.; Zhao, W.; Shi, P.; Wu, Y.; Zhou, X.; Gao, Y. A PD-L1 and VEGFR2 dual targeted peptide and its combination with irradiation for cancer immunotherapy. Pharmacol. Res. 2022, 182, 106343. [Google Scholar] [CrossRef]
- Qian, Y.; Sun, Y.; Shi, P.; Zhou, X.; Zhang, Q.; Dong, Q.; Jin, S.; Qiu, L.; Niu, X.; Zhou, X.; et al. Development of LAG-3/FGL1 blocking peptide and combination with radiotherapy for cancer immunotherapy. Acta Pharm. Sin. B 2024, 14, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Zhao, M.; Wang, R.; Li, H. Fibrinogen-like protein 1 (FGL1): The next immune checkpoint target. J. Hematol. Oncol. 2021, 14, 147. [Google Scholar] [CrossRef]
- Hu, Z.; Li, W.; Chen, S.; Chen, D.; Xu, R.; Zheng, D.; Yang, X.; Li, S.; Zhou, X.; Niu, X.; et al. Design of a novel chimeric peptide via dual blockade of CD47/SIRPα and PD-1/PD-L1 for cancer immunotherapy. Sci. China Life Sci. 2023, 66, 2310–2328. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Moynihan, K.D.; Zheng, Y.; Szeto, G.L.; Li, A.V.; Huang, B.; Van Egeren, D.S.; Park, C.; Irvine, D.J. Structure-based programming of lymph-node targeting in molecular vaccines. Nature 2014, 507, 519–522. [Google Scholar] [CrossRef]
- Slingluff, C.L. The Present and Future of Peptide Vaccines for Cancer: Single or Multiple, Long or Short, Alone or in Combination? Cancer J. 2011, 17, 343–350. [Google Scholar] [CrossRef]
- Zamani, P.; Teymouri, M.; Nikpoor, A.R.; Navashenaq, J.G.; Gholizadeh, Z.; Darban, S.A.; Jaafari, M.R. Nanoliposomal vaccine containing long multi-epitope peptide E75-AE36 pulsed PADRE-induced effective immune response in mice TUBO model of breast cancer. Eur. J. Cancer 2020, 129, 80–96. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, H.; Song, Y.; Zhu, Y.; Qin, M.; He, C.; Du, G.; Sun, X. Aluminum nanoparticles deliver a dual-epitope peptide for enhanced anti-tumor immunotherapy. J. Control. Release 2022, 344, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, Q.; Sun, X. Lymph node targeting strategies to improve vaccination efficacy. J. Control. Release 2017, 267, 47–56. [Google Scholar] [CrossRef]
- Igashov, I.; Stärk, H.; Vignac, C.; Schneuing, A.; Satorras, V.G.; Frossard, P.; Welling, M.; Bronstein, M.; Correia, B. Equivariant 3D-conditional diffusion model for molecular linker design. Nat. Mach. Intell. 2024, 6, 417–427. [Google Scholar] [CrossRef]
- Giese, M.; Davis, P.D.; Woodman, R.H.; Hermanson, G.; Pokora, A.; Vermillion, M. Linker Architectures as Steric Auxiliaries for Altering Enzyme-Mediated Payload Release from Bioconjugates. Bioconjug. Chem. 2021, 32, 2257–2267. [Google Scholar] [CrossRef]
- Long, J.; Shao, T.; Wang, Y.; Chen, T.; Chen, Y.; Chen, Y.-L.; Wang, Q.; Yu, X.; Yu, J.; He, K.; et al. PEGylation of Dipeptide Linker Improves Therapeutic Index and Pharmacokinetics of Antibody-Drug Conjugates. Bioconjug. Chem. 2025, 36, 179–189. [Google Scholar] [CrossRef]
- Tedeschini, T.; Campara, B.; Grigoletto, A.; Bellini, M.; Salvalaio, M.; Matsuno, Y.; Suzuki, A.; Yoshioka, H.; Pasut, G. Polyethylene glycol-based linkers as hydrophilicity reservoir for antibody-drug conjugates. J. Control. Release 2021, 337, 431–447. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Shunmugam, R. Unraveling the Effect of PEG Chain Length on the Physical Properties and Toxicant Removal Capacities of Cross-Linked Network Synthesized by Thiol–Norbornene Photoclick Chemistry. ACS Omega 2020, 5, 2800–2810. [Google Scholar] [CrossRef]
- van Rosmalen, M.; Krom, M.; Merkx, M. Tuning the Flexibility of Glycine-Serine Linkers To Allow Rational Design of Multidomain Proteins. Biochemistry 2017, 56, 6565–6574. [Google Scholar] [CrossRef]
- Arai, R. Chapter Eight—Design of helical linkers for fusion proteins and protein-based nanostructures. In Methods in Enzymology; Merkx, M., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 647, pp. 209–230. [Google Scholar] [CrossRef]
- Real-Fernandez, F.; Errante, F.; Santo, A.D.; Papini, A.M.; Rovero, P. Therapeutic proteins immunogenicity: A peptide point of view. Explor. Drug Sci. 2023, 1, 377–387. [Google Scholar] [CrossRef]
- Pontarelli, A.; Liu, J.T.; Movasat, H.; Ménard, S.; Oh, J.K.; Wilds, C.J. Synthesis of a Convertible Linker Containing a Disulfide Group for Oligonucleotide Functionalization. Org. Lett. 2022, 24, 5579–5583. [Google Scholar] [CrossRef]
- Mädler, S.; Bich, C.; Touboul, D.; Zenobi, R. Chemical cross-linking with NHS esters: A systematic study on amino acid reactivities. J. Mass Spectrom. 2009, 44, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Pan, Z.; Choudhury, M.R.; Yuan, Z.; Anifowose, A.; Yu, B.; Wang, W.; Wang, B. Making smart drugs smarter: The importance of linker chemistry in targeted drug delivery. Med. Res. Rev. 2020, 40, 2682–2713. [Google Scholar] [CrossRef]
- La Manna, S.; Di Natale, C.; Onesto, V.; Marasco, D. Self-Assembling Peptides: From Design to Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 12662. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Matson, J.B. pH-Responsive Self-Assembling Peptide-Based Biomaterials: Designs and Applications. ACS Appl. Bio Mater. 2022, 5, 4635–4651. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Yu, Y.; Gao, Y.; Jiang, S.; Ge, W.; Zeng, Y.; Wang, X.; Li, S.; Xie, X.; Guan, G. Smart self-assembled peptide-based hydrogels: Mechanism, design and biomedical applications. Colloids Surf. B Biointerfaces 2025, 253, 114704. [Google Scholar] [CrossRef]
- Wang, M.-D.; Lv, G.-T.; An, H.-W.; Zhang, N.-Y.; Wang, H. In Situ Self-Assembly of Bispecific Peptide for Cancer Immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202113649. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.; Sawaya, M.R.; Balbirnie, M.; Madsen, A.Ø.; Riekel, C.; Grothe, R.; Eisenberg, D. Structure of the cross-β spine of amyloid-like fibrils. Nature 2005, 435, 773–778. [Google Scholar] [CrossRef]
- Zhang, W.; Li, D.; Xu, X.; Shi, X.; Pan, Y.; Yao, S.; Piao, Y.; Zhou, Z.; Slater, N.K.H.; Shen, Y.; et al. A Bispecific Peptide-Polymer Conjugate Bridging Target-Effector Cells to Enhance Immunotherapy. Adv. Healthc. Mater. 2023, 12, 2202977. [Google Scholar] [CrossRef]
- Middleton, M.R.; McAlpine, C.; Woodcock, V.K.; Corrie, P.; Infante, J.R.; Steven, N.M.; Evans, T.R.J.; Anthoney, A.; Shoushtari, A.N.; Hamid, O.; et al. Tebentafusp, A TCR/Anti-CD3 Bispecific Fusion Protein Targeting gp100, Potently Activated Antitumor Immune Responses in Patients with Metastatic Melanoma. Clin. Cancer Res. 2020, 26, 5869–5878. [Google Scholar] [CrossRef]
- An, H.-W.; Hou, D.-Y.; Yang, J.; Wang, Z.-Q.; Wang, M.-D.; Zheng, R.; Zhang, N.-Y.; Hu, X.-J.; Wang, Z.-J.; Wang, L.; et al. A bispecific glycopeptide spatiotemporally regulates tumor microenvironment for inhibiting bladder cancer recurrence. Sci. Adv. 2023, 9, eabq8225. [Google Scholar] [CrossRef]
- Jacquot, P.; Muñoz-Garcia, J.; Fleury, M.; Cochonneau, D.; Gaussin, R.; Enouf, E.; Roze, C.; Ollivier, E.; Cinier, M.; Heymann, D. Engineering of a Bispecific Nanofitin with Immune Checkpoint Inhibitory Activity Conditioned by the Cross-Arm Binding to EGFR and PDL1. Biomolecules 2023, 13, 636. [Google Scholar] [CrossRef]
- June, C.H.; Sadelain, M. Chimeric Antigen Receptor Therapy. N. Engl. J. Med. 2018, 379, 64–73. [Google Scholar] [CrossRef]
- Damato, B.E.; Dukes, J.; Goodall, H.; Carvajal, R.D. Tebentafusp: T Cell Redirection for the Treatment of Metastatic Uveal Melanoma. Cancers 2019, 11, 971. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef]
- Qi, Y.-K.; Zheng, J.-S.; Liu, L. Mirror-image protein and peptide drug discovery through mirror-image phage display. Chem 2024, 10, 2390–2407. [Google Scholar] [CrossRef]
- Guan, P.; Jin, F.; Zhang, A.; Gao, S.; Liu, Z. Rationally Engineered Bispecific Nanoimmunoblocker Restores Anticancer Immunity via Dual Immune Checkpoint Blockade. ACS Nano 2025, 19, 5392–5405. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Scherpereel, A.; Cornelissen, R.; Oulkhouir, Y.; Greillier, L.; Kaplan, M.A.; Talbot, T.; Monnet, I.; Hiret, S.; Baas, P.; et al. First-line nivolumab plus ipilimumab versus chemotherapy in patients with unresectable malignant pleural mesothelioma: 3-year outcomes from CheckMate 743. Ann. Oncol. 2022, 33, 488–499. [Google Scholar] [CrossRef]
- Zamani, P.; Momtazi-Borojeni, A.A.; Nik, M.E.; Oskuee, R.K.; Sahebkar, A. Nanoliposomes as the adjuvant delivery systems in cancer immunotherapy. J. Cell. Physiol. 2018, 233, 5189–5199. [Google Scholar] [CrossRef]
- Zamani, P.; Navashenaq, J.G.; Nikpoor, A.R.; Hatamipour, M.; Oskuee, R.K.; Badiee, A.; Jaafari, M.R. MPL nano-liposomal vaccine containing P5 HER2/neu-derived peptide pulsed PADRE as an effective vaccine in a mice TUBO model of breast cancer. J. Control. Release 2019, 303, 223–236. [Google Scholar] [CrossRef]
- Mutlu, M.Y.; Tascilar, K.; Schett, G. Rationale, current state and opportunities in combining biologic disease modifying antirheumatic drugs in rheumatoid and psoriatic arthritis. Jt. Bone Spine 2023, 90, 105578. [Google Scholar] [CrossRef]
- Sebba, A.; Bingham, C.O.; Bykerk, V.P.; Fiore, S.; Ford, K.; Janak, J.C.; Pappas, D.A.; Blachley, T.; Dave, S.S.; Kremer, J.M.; et al. Comparative effectiveness of TNF inhibitor vs IL-6 receptor inhibitor as monotherapy or combination therapy with methotrexate in biologic-experienced patients with rheumatoid arthritis: An analysis from the CorEvitas RA Registry. Clin. Rheumatol. 2023, 42, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Hammoura, I.; Fiechter, R.H.; Bryant, S.H.; Westmoreland, S.; Kingsbury, G.; Waegell, W.; Tas, S.W.; Baeten, D.L.; van de Sande, M.G.H.; van Tok, M.N.; et al. Dual Blockade of TNF and IL-17A Inhibits Inflammation and Structural Damage in a Rat Model of Spondyloarthritis. Int. J. Mol. Sci. 2022, 23, 859. [Google Scholar] [CrossRef]
- Genovese, M.C.; Weinblatt, M.E.; Aelion, J.A.; Mansikka, H.T.; Peloso, P.M.; Chen, K.; Li, Y.; Othman, A.A.; Khatri, A.; Khan, N.S.; et al. ABT-122, a Bispecific Dual Variable Domain Immunoglobulin Targeting Tumor Necrosis Factor and Interleukin-17A, in Patients with Rheumatoid Arthritis with an Inadequate Response to Methotrexate. Arthritis Rheumatol. 2018, 70, 1710–1720. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef]
- van Dorsten, R.T.; Wagh, K.; Moore, P.L.; Morris, L. Combinations of Single Chain Variable Fragments From HIV Broadly Neutralizing Antibodies Demonstrate High Potency and Breadth. Front. Immunol. 2021, 12, 734110. [Google Scholar] [CrossRef] [PubMed]
- Board, N.L.; Yuan, Z.; Wu, F.; Moskovljevic, M.; Ravi, M.; Sengupta, S.; Mun, S.S.; Simonetti, F.R.; Lai, J.; Tebas, P.; et al. Bispecific antibodies promote natural killer cell-mediated elimination of HIV-1 reservoir cells. Nat. Immunol. 2024, 25, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, M.; Manukyan, M.; Mackie, A.; Morath, S.; Hartung, T.; Heine, H.; Triantafilou, K. Lipoteichoic Acid and Toll-like Receptor 2 Internalization and Targeting to the Golgi Are Lipid Raft-dependent. J. Biol. Chem. 2004, 279, 40882–40889. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Sig. Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Pei, J.; Gao, X.; Pan, D.; Hua, Y.; He, J.; Liu, Z.; Dang, Y. Advances in the stability challenges of bioactive peptides and improvement strategies. Curr. Res. Food Sci. 2022, 5, 2162–2170. [Google Scholar] [CrossRef]
- Joo, S.H. Cyclic peptides as therapeutic agents and biochemical tools. Biomol. Ther. 2012, 20, 19–26. [Google Scholar] [CrossRef]
- Shi, J.; Sun, T.; Yang, M. Site-selective editing of peptides via backbone modification. Org. Chem. Front. 2024, 11, 1623–1640. [Google Scholar] [CrossRef]
- Kroenke, M.A.; Weeraratne, D.K.; Deng, H.; Sloey, B.; Subramanian, R.; Wu, B.; Serenko, M.; Hock, M.B. Clinical immunogenicity of the d-amino acid peptide therapeutic etelcalcetide: Method development challenges and anti-drug antibody clinical impact assessments. J. Immunol. Methods 2017, 445, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Evans, B.J.; King, A.T.; Katsifis, A.; Matesic, L.; Jamie, J.F. Methods to Enhance the Metabolic Stability of Peptide-Based PET Radiopharmaceuticals. Molecules 2020, 25, 2314. [Google Scholar] [CrossRef]
- Chen, B.-M.; Cheng, T.-L.; Roffler, S.R. Polyethylene Glycol Immunogenicity: Theoretical, Clinical, and Practical Aspects of Anti-Polyethylene Glycol Antibodies. ACS Nano 2021, 15, 14022–14048. [Google Scholar] [CrossRef] [PubMed]
- Rinauro, D.J.; Chiti, F.; Vendruscolo, M.; Limbocker, R. Misfolded protein oligomers: Mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases. Mol. Neurodegener. 2024, 19, 20. [Google Scholar] [CrossRef]
- Harris, A.G. Somatostatin and somatostatin analogues: Pharmacokinetics and pharmacodynamic effects. Gut 1994, 35, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Meibohm, B. Pharmacokinetics and Pharmacokinetic–Pharmacodynamic Correlations of Therapeutic Peptides. Clin. Pharmacokinet. 2013, 52, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Jin, Z.; Yu, T.; Guo, C.; He, Y.; Kan, Y.; Yan, L.; Wu, L. Effect of Glycosylation on the Enzymatic Degradation of D-Amino Acid-Containing Peptides. Molecules 2025, 30, 441. [Google Scholar] [CrossRef]
- Verma, S.; Goand, U.K.; Husain, A.; Katekar, R.A.; Garg, R.; Gayen, J.R. Challenges of peptide and protein drug delivery by oral route: Current strategies to improve the bioavailability. Drug Dev. Res. 2021, 82, 927–944. [Google Scholar] [CrossRef]
- Wolfman, N.M.; Hattersley, G.; Cox, K.; Celeste, A.J.; Nelson, R.; Yamaji, N.; Dube, J.L.; DiBlasio-Smith, E.; Nove, J.; Song, J.J.; et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J. Clin. Investig. 1997, 100, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Saga, T.; Neumann, R.D.; Heya, T.; Sato, J.; Kinuya, S.; Le, N.; Paik, C.H.; Weinstein, J.N. Targeting cancer micrometastases with monoclonal antibodies: A binding-site barrier. Proc. Natl. Acad. Sci. USA 1995, 92, 8999–9003. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The Future of Peptide-based Drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Perico-Franco, L.S.; Rosas-Pérez, J.E. Chapter 17—Synthetic peptides quality control and assurance. In Antimicrobial Peptides; Reyes, L.H., Cruz, J.C., Wiedman, G.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 405–416. [Google Scholar] [CrossRef]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Federal Register. Clinical Pharmacology Considerations for Peptide Drug Products; Draft Guidance for Industry; Availability. 2023. Available online: https://www.federalregister.gov/documents/2023/09/11/2023-19456/clinical-pharmacology-considerations-for-peptide-drug-products-draft-guidance-for-industry (accessed on 4 October 2025).
- Diller, D.J.; Swanson, J.; Bayden, A.S.; Jarosinski, M.; Audie, J. Rational, Computer-Enabled Peptide Drug Design: Principles, Methods, Applications and Future Directions. Future Med. Chem. 2015, 7, 2173–2193. [Google Scholar] [CrossRef]
- Huber, F.; Arnaud, M.; Stevenson, B.J.; Michaux, J.; Benedetti, F.; Thevenet, J.; Bobisse, S.; Chiffelle, J.; Gehert, T.; Müller, M.; et al. A comprehensive proteogenomic pipeline for neoantigen discovery to advance personalized cancer immunotherapy. Nat. Biotechnol. 2024, 43, 1360–1372. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Yin, H.; Chen, X.-T.; Yao, J.-F.; Ma, Y.-N.; Song, M.; Xu, H.; Yu, Q.-Y.; Du, S.-S.; Qi, Y.-K.; et al. Three Rounds of Stability-Guided Optimization and Systematical Evaluation of Oncolytic Peptide LTX-315. J. Med. Chem. 2024, 67, 3885–3908. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Chen, X.; Chi, Q.; Ma, Y.; Fu, X.; Du, S.; Qi, Y.; Wang, K. The hybrid oncolytic peptide NTP-385 potently inhibits adherent cancer cells by targeting the nucleus. Acta Pharmacol. Sin. 2023, 44, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Fu, X.-Y.; Gao, H.-Y.; Ma, Y.-N.; Yao, J.-F.; Du, S.-S.; Qi, Y.-K.; Wang, K.-W. Design, synthesis and anticancer evaluation of novel oncolytic peptide-chlorambucil conjugates. Bioorganic Chem. 2023, 138, 106674. [Google Scholar] [CrossRef]
- Sioud, M.; Westby, P.; Olsen, J.K.E.; Mobergslien, A. Generation of new peptide-Fc fusion proteins that mediate antibody-dependent cellular cytotoxicity against different types of cancer cells. Mol. Ther. Methods Clin. Dev. 2015, 2, 15043. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Q.; Li, L.; Gao, F.; Zhang, Y.; Zhu, Y. The peptide-based bispecific CAR T cells target EGFR and tumor stroma for effective cancer therapy. Int. J. Pharm. 2024, 663, 124558. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, J.L.; Giulianotti, M.A.; Welmaker, G.S.; Houghten, R.A. Shifting from the single- to the multitarget paradigm in drug discovery. Drug Discov. Today 2013, 18, 495–501. [Google Scholar] [CrossRef] [PubMed]

| Class | Subtypes/Examples | Features | Representative References |
|---|---|---|---|
| Antibodies | BiTEs, DARTs, CrossMab, KiH | Protein-based, varied half-life and effector function | [21] |
| Peptides | Cyclic, linear, self-assembling | Small, modifiable, access to shallow epitopes | [8] |
| Fusion Proteins | Cytokine fusions, decoys | Modular, tunable | [22] |
| Small Molecules | PROTACs, molecular glues | Orally available, intracellular access | [4] |
| Aptamers | Dual-aptamer, aptamer-antibody | Nucleic acid-based, reversible binding | [23,24] |
| Cellular Platforms | CAR-T, TRUCKs | Live-cell therapeutics with bispecific features | [25,26] |
| Class | Compound | Target | Mechanisms of Action | Reference |
|---|---|---|---|---|
| Immune Synapse Formation and T Cell Engagement | CSBP | CD24 × PD-L1 | Blocks CD24/Siglec-10 and PD-1/PD-L1 axes; activates macrophages and CD8+ T cells | [29] |
| LFOP | LAG-3 × PD-L1 | Dual checkpoint blockade enhances T-cell proliferation and IFN-γ production | [33] | |
| Pal-DMPOP | CD47 × PD-L1 | Mobilizes T cells and macrophages; amplifies antitumor immunity via dual signaling | [35] | |
| antiCD3-G7-RGD | CD3 × αVβ3 | Induces CD3 oligomerization and T-cell-mediated cytolysis via self-assembly | [55] | |
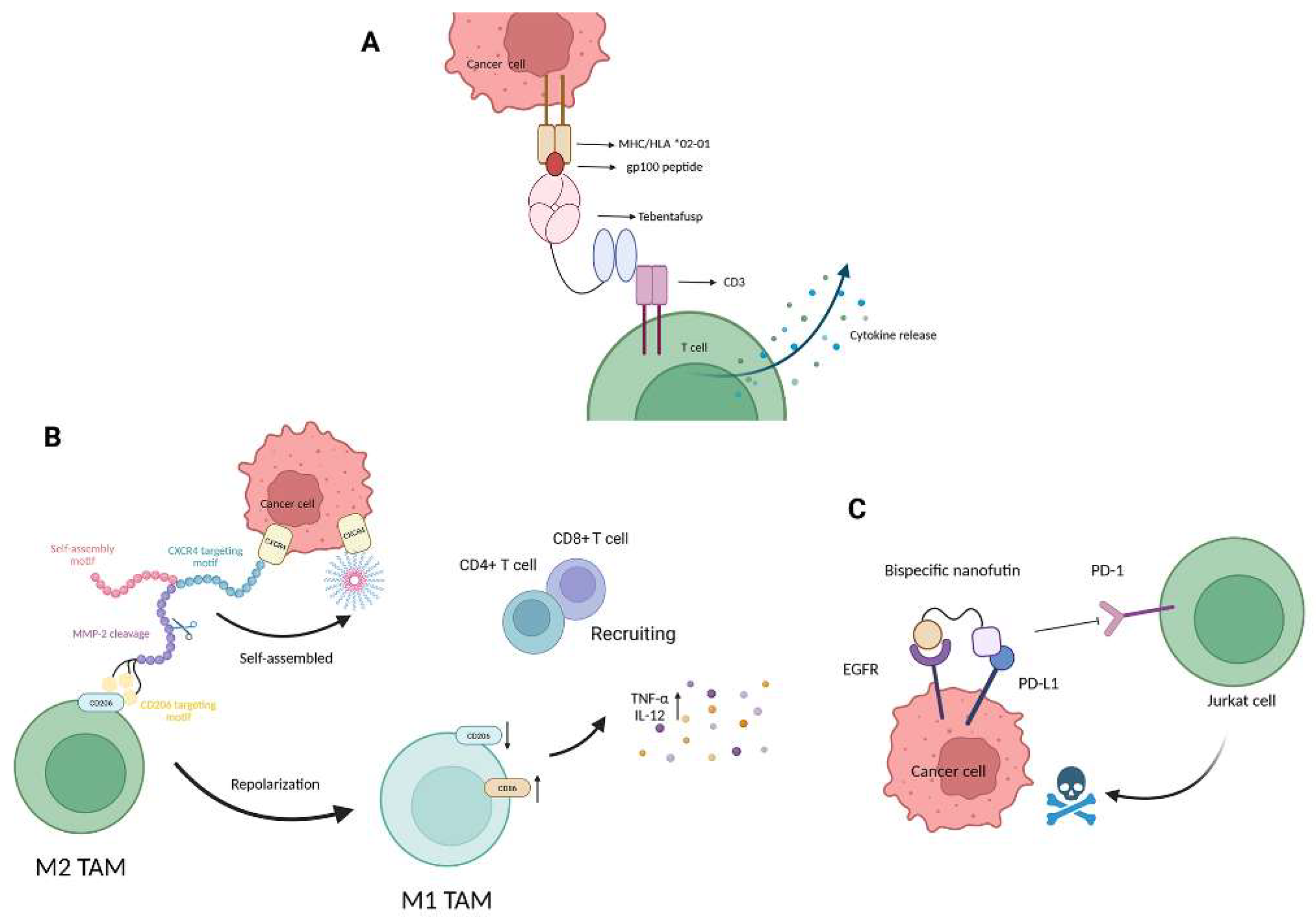
| Tebentafusp | CD3 × gp100 | Redirects T cells to gp100+ tumor cells; increases IFN-γ and CXCL10 in TME | [58] | |
| bsGP | CD206 × CXCR4 | Reprograms M2 macrophages to M1 phenotype; recruits CD8+ T cells | [59] | |
| Checkpoint Inhibition and Costimulatory Reprogramming | nABPD-2 | PD-1 × PD-L1 | Simultaneously blocks PD-1 and PD-L1; enhances cytotoxic T-cell function | [28] |
| DSPOGS | VEGFR × PD-L1 | Dual targeting facilitates CD8+ T-cell infiltration and IFN-γ secretion | [32] | |
| octaPEG-PD1-PDL1 | PD-1 × PD-L1 | PEG-based scaffold bridges tumor cells and T cells and boosts immune synapse formation | [57] | |
| B10-B11 | EGFR × PD-L1 | Conditionally activates PDL1 blockade while minimizing off-target immune toxicity | [60] | |
| Mechanistic Enhancement of Antigen Presentation | Nanoliposome | MHC-I × MHC-II | Increased tumor infiltration of both CD8+ and CD4+ T cells | [38] |
| ANL | MHC-I × MHC-II | promotes antigen presentation and activates CD8+ T-cell responses | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Li, Y. Engineering Bispecific Peptides for Precision Immunotherapy and Beyond. Int. J. Mol. Sci. 2025, 26, 10082. https://doi.org/10.3390/ijms262010082
Ding X, Li Y. Engineering Bispecific Peptides for Precision Immunotherapy and Beyond. International Journal of Molecular Sciences. 2025; 26(20):10082. https://doi.org/10.3390/ijms262010082
Chicago/Turabian StyleDing, Xumeng, and Yi Li. 2025. "Engineering Bispecific Peptides for Precision Immunotherapy and Beyond" International Journal of Molecular Sciences 26, no. 20: 10082. https://doi.org/10.3390/ijms262010082
APA StyleDing, X., & Li, Y. (2025). Engineering Bispecific Peptides for Precision Immunotherapy and Beyond. International Journal of Molecular Sciences, 26(20), 10082. https://doi.org/10.3390/ijms262010082






