The Heteromeric Dopamine Receptor D2:D3 Controls the Gut Recruitment and Suppressive Activity of Regulatory T-Cells
Abstract
1. Introduction
2. Results
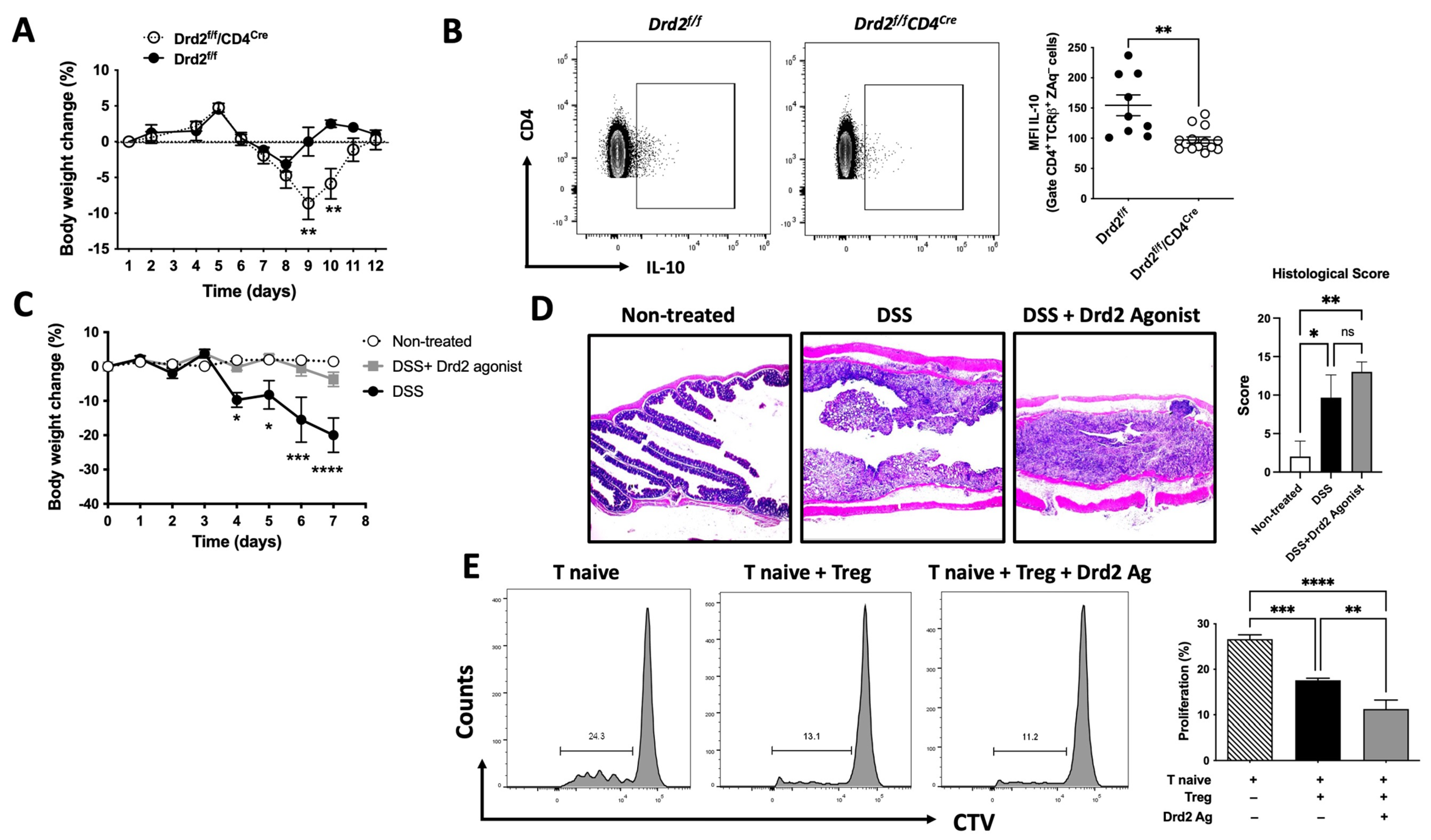
2.1. Drd2-Mediated Signalling Favours the Suppressive Activity of Treg and Limits Gut Inflammation
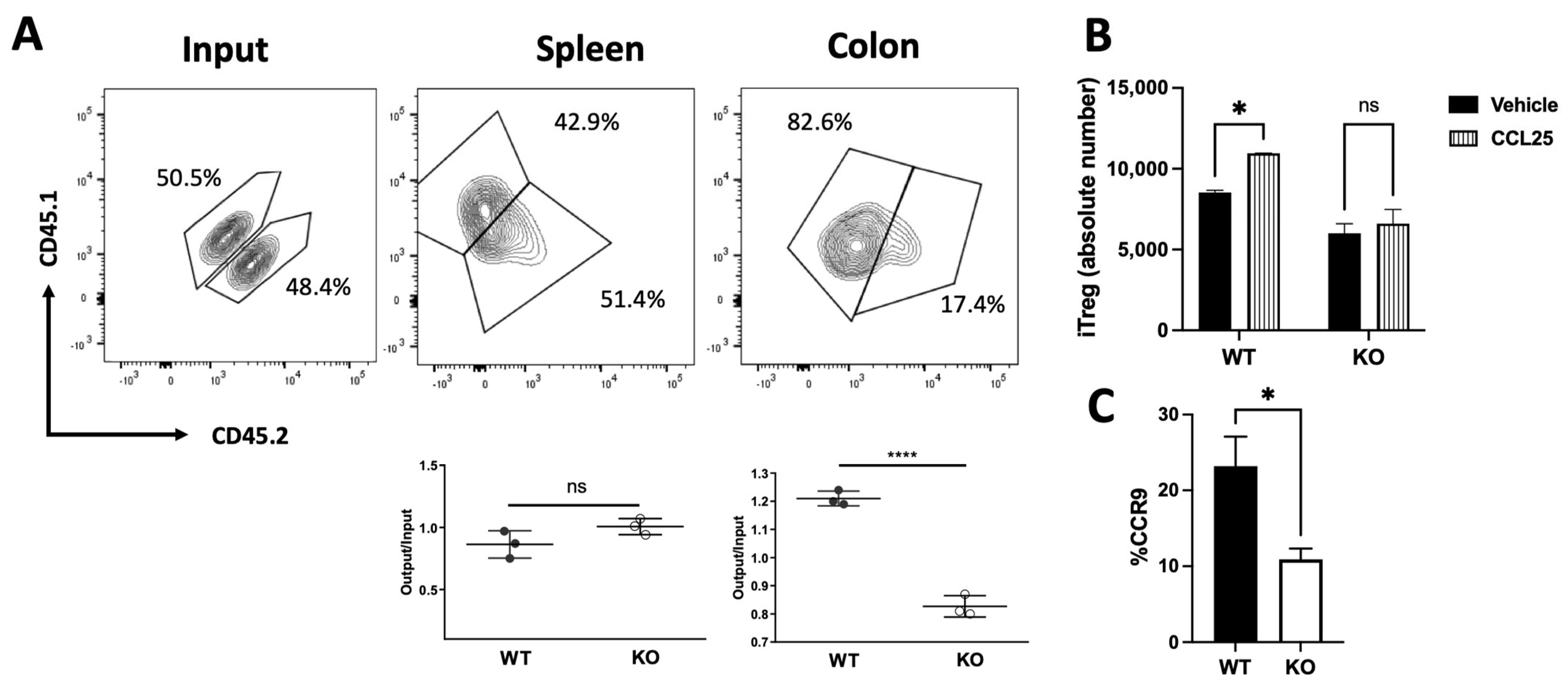
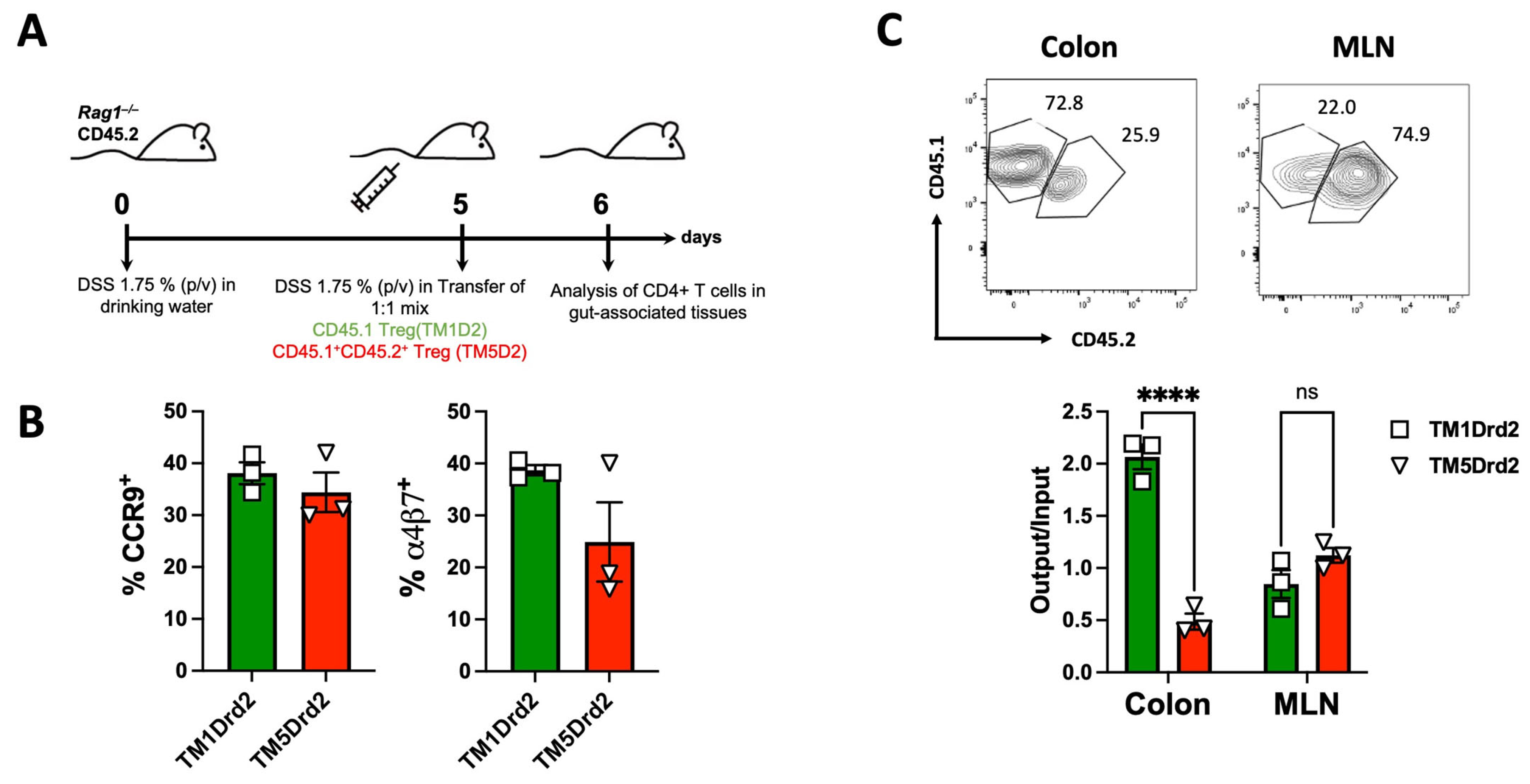
2.2. Drd2-Mediated Signalling Promotes Colonic Tropism of Treg upon Gut Inflammation
2.3. IBD Patients Display Increased DRD3 and Reduced DRD2 Expression in Mucosal Lesions
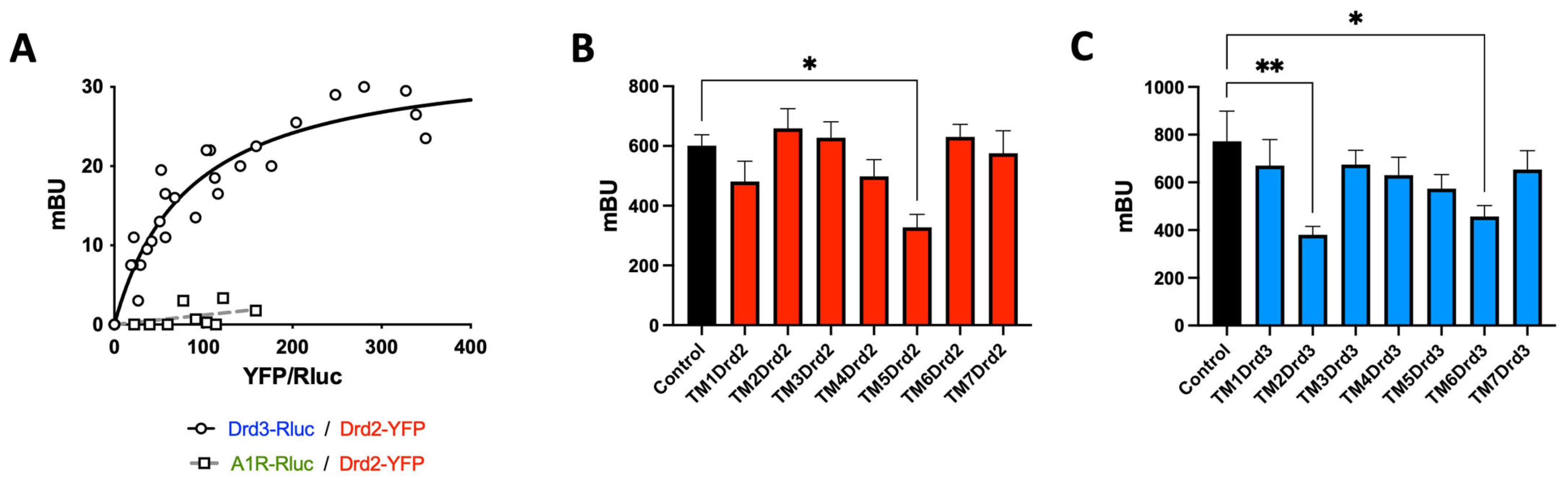
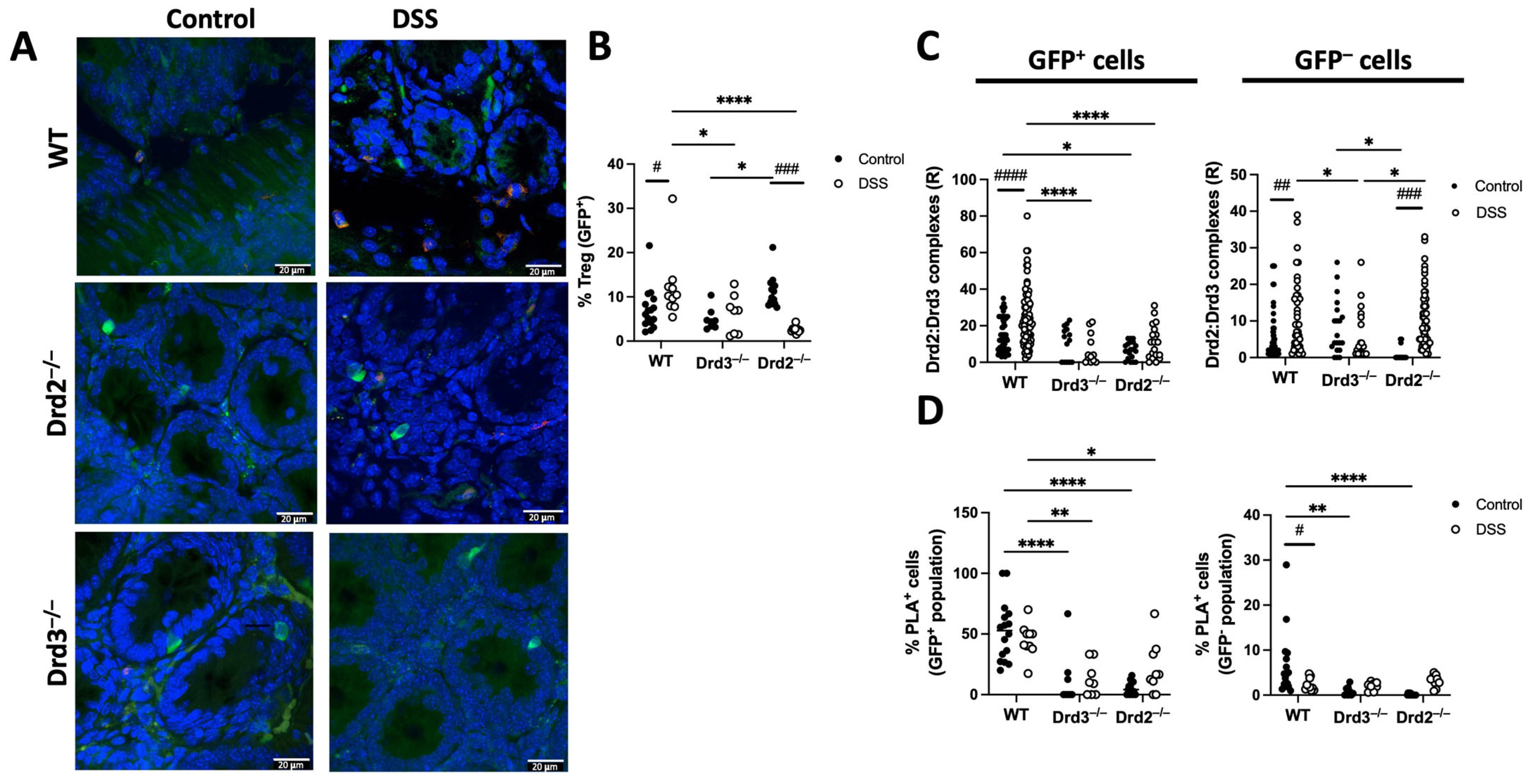
2.4. Drd2 and Drd3 Form a Heteromeric Complex on Colonic Treg Cells
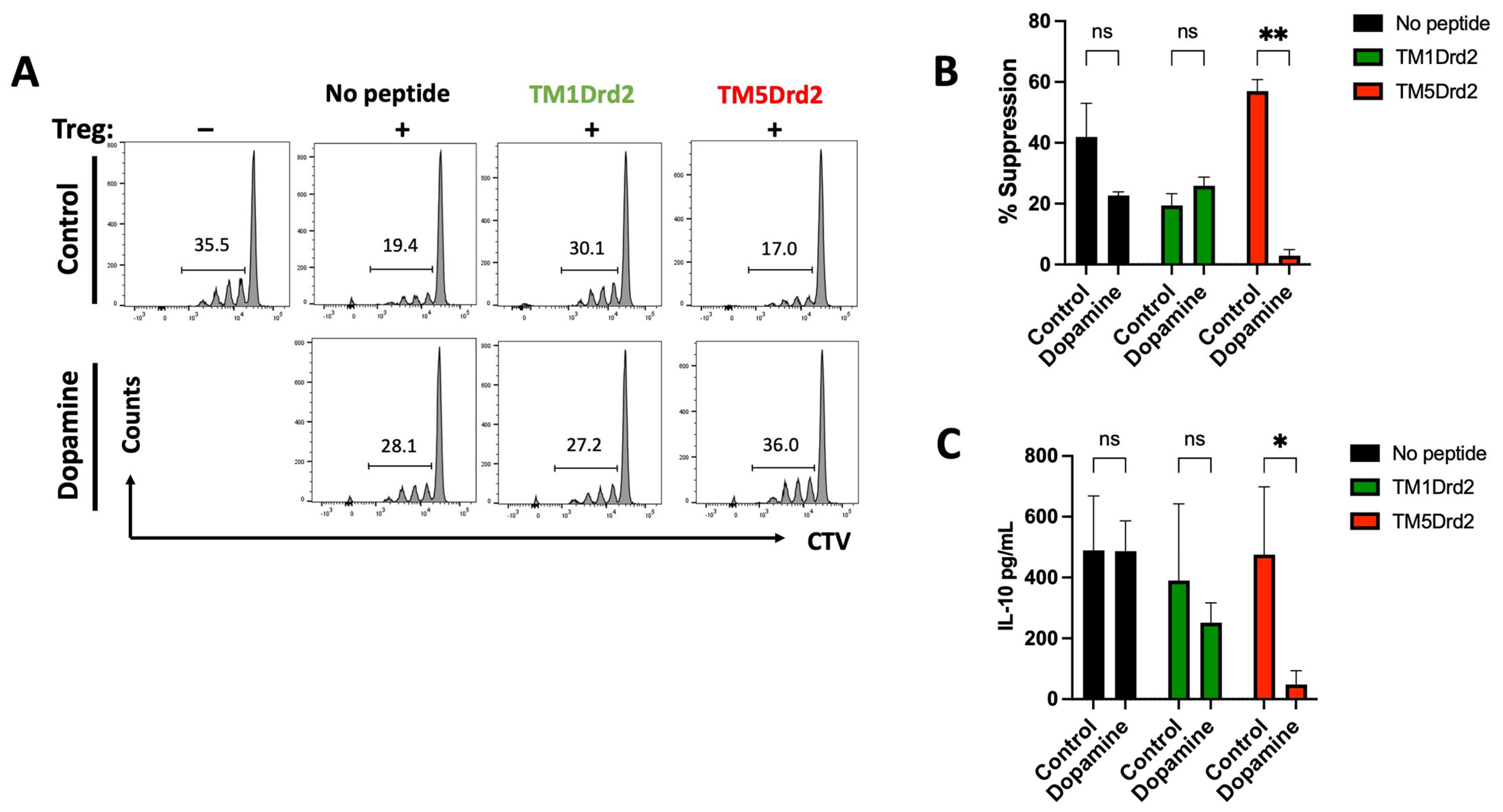
2.5. The Drd2:Drd3 Heteromer Controls the Suppressive Activity and Colonic Tropism of Treg
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents
4.3. Dextran Sodium Sulphate Induced Acute Inflammatory Colitis
4.4. Histological Analysis
4.5. In Vitro Suppression Assay
4.6. In Vivo Migration Assay
4.7. In Vitro Migration Assay
4.8. Flow Cytometry Analysis
4.9. Bulk RNA-Seq Analysis
4.10. Bioluminescence Resonance Energy Transfer Assay
4.11. Bimolecular Fluorescence Complementation Assay
4.12. In Situ Proximity Ligation Assay
4.13. cAMP Levels Determination
4.14. Determination of ERK1/2 Phosphorylation
4.15. Determination of AKT Phosphorylation
4.16. Statistical Analyses and Sample Size Estimation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AnR | adenosine receptor An |
| BiFC | bimolecular fluorescence complementation |
| BRET | bioluminescence resonance energy transfer |
| BSA | bovine serum albumin |
| CD | Crohn’s disease |
| CCR9 | chemokine receptor 9 |
| cLP | colonic lamina propria |
| CTV | cell trace violet |
| DCs | dendritic cells |
| DSS | dextran sodium sulfate |
| Drdn | dopamine receptor n |
| GFP | green fluorescent protein |
| GPCR | G-protein coupled receptor |
| IBD | inflammatory bowel disease |
| IL-n | interleukin n |
| Teff | effector T-cells |
| MadCAM-1 | mucosal vascular addressing Cell Adhesion Molecule 1 |
| MLNs | mesenteric lymph nodes |
| MNCs | mononuclear cells |
| PLA | proximity ligation assay |
| PPs | Peyer’s patches |
| RLuc | renilla luciferase |
| PMA | phorbol 12-myristate 13-acetate |
| TAT | transactivator of transcription |
| Thn | T-helper n |
| Treg | regulatory T-cells |
| TMs | transmembrane segments |
| UC | ulcerative colitis |
| WT | wild-type |
| YFP | yellow fluorescent protein |
| ZAq | Zombie Aqua |
References
- Granlund, A.; Flatberg, A.; Ostvik, A.E.; Drozdov, I.; Gustafsson, B.I.; Kidd, M.; Beisvag, V.; Torp, S.H.; Waldum, H.L.; Martinsen, T.C.; et al. Whole genome gene expression meta-analysis of inflammatory bowel disease colon mucosa demonstrates lack of major differences between Crohn’s disease and ulcerative colitis. PLoS ONE 2013, 8, e56818. [Google Scholar] [CrossRef]
- Olsen, T.; Rismo, R.; Cui, G.; Goll, R.; Christiansen, I.; Florholmen, J. TH1 and TH17 interactions in untreated inflamed mucosa of inflammatory bowel disease, and their potential to mediate the inflammation. Cytokine 2011, 56, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Sun, M.; Zhang, H.P.; Chen, T.; Wu, R.; Liu, C.; Yang, G.; Geng, X.R.; Feng, B.S.; Liu, Z.; et al. Prolactin mediates psychological stress-induced dysfunction of regulatory T-cells to facilitate intestinal inflammation. Gut 2014, 63, 1883–1892, Erratum in Gut 2020, 69, e7. [Google Scholar] [CrossRef] [PubMed]
- Powrie, F.; Mason, D. OX-22high CD4+ T-cells induce wasting disease with multiple organ pathology: Prevention by the OX-22low subset. J. Exp. Med. 1990, 172, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Mair, I.; Zandee, S.E.J.; Toor, I.S.; Saul, L.; McPherson, R.C.; Leech, M.D.; Smyth, D.J.; O’Connor, R.A.; Henderson, N.C.; Anderton, S.M. A Context-Dependent Role for alphav Integrins in Regulatory T-cell Accumulation at Sites of Inflammation. Front. Immunol. 2018, 9, 264. [Google Scholar] [CrossRef]
- Huber, S.; Schramm, C.; Lehr, H.A.; Mann, A.; Schmitt, S.; Becker, C.; Protschka, M.; Galle, P.R.; Neurath, M.F.; Blessing, M. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T-cells. J. Immunol. 2004, 173, 6526–6531. [Google Scholar] [CrossRef]
- Rubtsov, Y.P.; Rasmussen, J.P.; Chi, E.Y.; Fontenot, J.; Castelli, L.; Ye, X.; Treuting, P.; Siewe, L.; Roers, A.; Henderson, W.R., Jr.; et al. Regulatory T-cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity 2008, 28, 546–558. [Google Scholar] [CrossRef]
- Saruta, M.; Yu, Q.T.; Fleshner, P.R.; Mantel, P.Y.; Schmidt-Weber, C.B.; Banham, A.H.; Papadakis, K.A. Characterization of FOXP3+CD4+ regulatory T-cells in Crohn’s disease. Clin. Immunol. 2007, 125, 281–290. [Google Scholar] [CrossRef]
- Maul, J.; Loddenkemper, C.; Mundt, P.; Berg, E.; Giese, T.; Stallmach, A.; Zeitz, M.; Duchmann, R. Peripheral and intestinal regulatory CD4+ CD25(high) T-cells in inflammatory bowel disease. Gastroenterology 2005, 128, 1868–1878. [Google Scholar] [CrossRef]
- Magro, F.; Vieira-Coelho, M.A.; Fraga, S.; Serrao, M.P.; Veloso, F.T.; Ribeiro, T.; Soares-da-Silva, P. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig. Dis. Sci. 2002, 47, 216–224. [Google Scholar] [CrossRef]
- Magro, F.; Fraga, S.; Ribeiro, T.; Soares-da-Silva, P. Decreased availability of intestinal dopamine in transmural colitis may relate to inhibitory effects of interferon-gamma upon L-DOPA uptake. Acta Physiol. Scand. 2004, 180, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, R.; Contreras, F.; Zouali, M. The dopaminergic system in autoimmune diseases. Front. Immunol. 2014, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P.H.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The Concise Guide to PHARMACOLOGY 2023/24: G protein-coupled receptors. Br. J. Pharmacol. 2023, 180 (Suppl. 2), S23–S144. [Google Scholar] [CrossRef] [PubMed]
- Contreras, F.; Prado, C.; Gonzalez, H.; Franz, D.; Osorio-Barrios, F.; Osorio, F.; Ugalde, V.; Lopez, E.; Elgueta, D.; Figueroa, A.; et al. Dopamine Receptor D3 Signaling on CD4+ T-cells Favors Th1- and Th17-Mediated Immunity. J. Immunol. 2016, 196, 4143–4149. [Google Scholar] [CrossRef]
- Franz, D.; Contreras, F.; Gonzalez, H.; Prado, C.; Elgueta, D.; Figueroa, C.; Pacheco, R. Dopamine receptors D3 and D5 regulate CD4(+)T-cell activation and differentiation by modulating ERK activation and cAMP production. J. Neuroimmunol. 2015, 284, 18–29. [Google Scholar] [CrossRef]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver. Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef]
- Elgueta, D.; Contreras, F.; Prado, C.; Montoya, A.; Ugalde, V.; Chovar, O.; Villagra, R.; Henriquez, C.; Abellanas, M.A.; Aymerich, M.S.; et al. Dopamine Receptor D3 Expression Is Altered in CD4+ T-Cells from Parkinson’s Disease Patients and Its Pharmacologic Inhibition Attenuates the Motor Impairment in a Mouse Model. Front. Immunol. 2019, 10, 981. [Google Scholar] [CrossRef]
- Shao, W.; Zhang, S.Z.; Tang, M.; Zhang, X.H.; Zhou, Z.; Yin, Y.Q.; Zhou, Q.B.; Huang, Y.Y.; Liu, Y.J.; Wawrousek, E.; et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alphaB-crystallin. Nature 2013, 494, 90–94. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, W.; Liu, L.; Wang, X.; Ding, C.; Tian, Z.; Zhou, R. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell 2015, 160, 62–73. [Google Scholar] [CrossRef]
- Torres-Rosas, R.; Yehia, G.; Pena, G.; Mishra, P.; del Rocio Thompson-Bonilla, M.; Moreno-Eutimio, M.A.; Arriaga-Pizano, L.A.; Isibasi, A.; Ulloa, L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat. Med. 2014, 20, 291–295. [Google Scholar] [CrossRef]
- Besser, M.J.; Ganor, Y.; Levite, M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFalpha or both. J. Neuroimmunol. 2005, 169, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Matsumoto, M.; Kato, S.; Takeuchi, K. Dopamine-induced protection against indomethacin-evoked intestinal lesions in rats--role of anti-intestinal motility mediated by D2 receptors. Med. Sci. Monit. 2003, 9, BR71–BR77. [Google Scholar] [PubMed]
- Magro, F.; Cunha, E.; Araujo, F.; Meireles, E.; Pereira, P.; Dinis-Ribeiro, M.; Veloso, F.T.; Medeiros, R.; Soares-da-Silva, P. Dopamine D2 receptor polymorphisms in inflammatory bowel disease and the refractory response to treatment. Dig. Dis. Sci. 2006, 51, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Tolstanova, G.; Deng, X.; Ahluwalia, A.; Paunovic, B.; Prysiazhniuk, A.; Ostapchenko, L.; Tarnawski, A.; Sandor, Z.; Szabo, S. Role of Dopamine and D2 Dopamine Receptor in the Pathogenesis of Inflammatory Bowel Disease. Dig. Dis. Sci. 2015, 60, 2963–2975. [Google Scholar] [CrossRef]
- Mazzini, E.; Massimiliano, L.; Penna, G.; Rescigno, M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity 2014, 40, 248–261. [Google Scholar] [CrossRef]
- Elgueta, R.; Sepulveda, F.E.; Vilches, F.; Vargas, L.; Mora, J.R.; Bono, M.R.; Rosemblatt, M. Imprinting of CCR9 on CD4 T-cells requires IL-4 signaling on mesenteric lymph node dendritic cells. J. Immunol. 2008, 180, 6501–6507. [Google Scholar] [CrossRef]
- Cassani, B.; Villablanca, E.J.; Quintana, F.J.; Love, P.E.; Lacy-Hulbert, A.; Blaner, W.S.; Sparwasser, T.; Snapper, S.B.; Weiner, H.L.; Mora, J.R. Gut-tropic T-cells that express integrin alpha4beta7 and CCR9 are required for induction of oral immune tolerance in mice. Gastroenterology 2011, 141, 2109–2118. [Google Scholar] [CrossRef]
- Rivollier, A.; He, J.; Kole, A.; Valatas, V.; Kelsall, B.L. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J. Exp. Med. 2012, 209, 139–155. [Google Scholar] [CrossRef]
- Osorio-Barrios, F.; Navarro, G.; Campos, J.; Ugalde, V.; Prado, C.; Raich, I.; Contreras, F.; Lopez, E.; Espinoza, A.; Lladser, A.; et al. The Heteromeric Complex Formed by Dopamine Receptor D5 and CCR9 Leads the Gut Homing of CD4(+) T-cells Upon Inflammation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 489–506. [Google Scholar] [CrossRef]
- Campos, J.; Osorio-Barrios, F.; Villanelo, F.; Gutierrez-Maldonado, S.E.; Vargas, P.; Perez-Acle, T.; Pacheco, R. Chemokinergic and Dopaminergic Signalling Collaborates through the Heteromer Formed by CCR9 and Dopamine Receptor D5 Increasing the Migratory Speed of Effector CD4(+) T-Cells to Infiltrate the Colonic Mucosa. Int. J. Mol. Sci. 2024, 25, 10022. [Google Scholar] [CrossRef]
- Ugalde, V.; Contreras, F.; Prado, C.; Chovar, O.; Espinoza, A.; Pacheco, R. Dopaminergic signalling limits suppressive activity and gut homing of regulatory T-cells upon intestinal inflammation. Mucosal Immunol. 2021, 14, 652–666. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Bruns, T.; Ward, S.; Mai, M.; Schmidt, C.; Hirschfield, G.M.; Weston, C.J.; Adams, D.H. Intestinal CCL25 expression is increased in colitis and correlates with inflammatory activity. J. Autoimmun. 2016, 68, 98–104. [Google Scholar] [CrossRef]
- Hu, S.; Uniken Venema, W.T.; Westra, H.J.; Vich Vila, A.; Barbieri, R.; Voskuil, M.D.; Blokzijl, T.; Jansen, B.H.; Li, Y.; Daly, M.J.; et al. Inflammation status modulates the effect of host genetic variation on intestinal gene expression in inflammatory bowel disease. Nat. Commun. 2021, 12, 1122. [Google Scholar] [CrossRef]
- Callen, L.; Moreno, E.; Barroso-Chinea, P.; Moreno-Delgado, D.; Cortes, A.; Mallol, J.; Casado, V.; Lanciego, J.L.; Franco, R.; Lluis, C.; et al. Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J. Biol. Chem. 2012, 287, 20851–20865. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Ikebuchi, R.; Chtanova, T.; Kusumoto, Y.; Okuyama, H.; Moriya, T.; Honda, T.; Kabashima, K.; Watanabe, T.; Sakai, Y.; et al. Regulatory T-cells with superior immunosuppressive capacity emigrate from the inflamed colon to draining lymph nodes. Mucosal Immunol. 2018, 11, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Miyamoto, K.; Chida, A.; Okuzawa, A.T.; Yoshimatsu, Y.; Kudo, Y.; Sujino, T. Localization and movement of Tregs in gastrointestinal tract: A systematic review. Inflamm. Regen. 2022, 42, 47. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Cao, Y.; Chen, P.; Yang, Y.; Zhang, Y. Circulating and intestinal regulatory T-cells in inflammatory bowel disease: A systemic review and meta-analysis. Int. Rev. Immunol. 2024, 43, 83–94. [Google Scholar] [CrossRef]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.A.; Takayanagi, H. Pathogenic conversion of Foxp3+ T-cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef]
- Liu, Z.; Zhai, X.R.; Du, Z.S.; Xu, F.F.; Huang, Y.; Wang, X.Q.; Qiu, Y.H.; Peng, Y.P. Dopamine receptor D2 on CD4(+) T-cells is protective against neuroinflammation and neurodegeneration in a mouse model of Parkinson’s disease. Brain Behav. Immun. 2021, 98, 110–121. [Google Scholar] [CrossRef]
- Gonzalez, H.; Contreras, F.; Prado, C.; Elgueta, D.; Franz, D.; Bernales, S.; Pacheco, R. Dopamine Receptor D3 Expressed on CD4+ T-cells Favors Neurodegeneration of Dopaminergic Neurons during Parkinson’s Disease. J. Immunol. 2013, 190, 5048–5056. [Google Scholar] [CrossRef]
- Fiorentino, D.F.; Zlotnik, A.; Vieira, P.; Mosmann, T.R.; Howard, M.; Moore, K.W.; O’Garra, A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991, 146, 3444–3451. [Google Scholar] [CrossRef]
- Huber, S.; Gagliani, N.; Esplugues, E.; O’Connor, W., Jr.; Huber, F.J.; Chaudhry, A.; Kamanaka, M.; Kobayashi, Y.; Booth, C.J.; Rudensky, A.Y.; et al. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(-) and Foxp3+ regulatory CD4+ T-cells in an interleukin-10-dependent manner. Immunity 2011, 34, 554–565. [Google Scholar] [CrossRef]
- Ferre, S.; Baler, R.; Bouvier, M.; Caron, M.G.; Devi, L.A.; Durroux, T.; Fuxe, K.; George, S.R.; Javitch, J.A.; Lohse, M.J.; et al. Building a new conceptual framework for receptor heteromers. Nat. Chem. Biol. 2009, 5, 131–134. [Google Scholar] [CrossRef]
- Ciruela, F.; Casado, V.; Rodrigues, R.J.; Lujan, R.; Burgueno, J.; Canals, M.; Borycz, J.; Rebola, N.; Goldberg, S.R.; Mallol, J.; et al. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J. Neurosci. 2006, 26, 2080–2087. [Google Scholar] [CrossRef]
- Scarselli, M.; Novi, F.; Schallmach, E.; Lin, R.; Baragli, A.; Colzi, A.; Griffon, N.; Corsini, G.U.; Sokoloff, P.; Levenson, R.; et al. D2/D3 dopamine receptor heterodimers exhibit unique functional properties. J. Biol. Chem. 2001, 276, 30308–30314. [Google Scholar] [CrossRef] [PubMed]
- Pou, C.; Mannoury la Cour, C.; Stoddart, L.A.; Millan, M.J.; Milligan, G. Functional homomers and heteromers of dopamine D2L and D3 receptors co-exist at the cell surface. J. Biol. Chem. 2012, 287, 8864–8878. [Google Scholar] [CrossRef] [PubMed]
- Kabbani, N.; Levenson, R. A proteomic approach to receptor signaling: Molecular mechanisms and therapeutic implications derived from discovery of the dopamine D2 receptor signalplex. Eur. J. Pharmacol. 2007, 572, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Karpa, K.; Kabbani, N.; Goldman-Rakic, P.; Levenson, R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc. Natl. Acad. Sci. USA 2001, 98, 5258–5263. [Google Scholar] [CrossRef]
- Lin, R.; Canfield, V.; Levenson, R. Dominant negative mutants of filamin A block cell surface expression of the D2 dopamine receptor. Pharmacology 2002, 66, 173–181. [Google Scholar] [CrossRef]
- Ding, Y.; Yu, A.; Vujanac, M.; Copsel, S.N.; Moro, A.; Nivelo, L.; Dalzell, M.; Tchitchek, N.; Rosenzwajg, M.; Villarino, A.V.; et al. BLIMP-1 and CEACAM1 cooperatively regulate human Treg homeostasis and function to control xenogeneic GVHD. JCI Insight 2025, 10, e183676. [Google Scholar] [CrossRef]
- Watanabe, Y.; Nakayama, T.; Nagakubo, D.; Hieshima, K.; Jin, Z.; Katou, F.; Hashimoto, K.; Yoshie, O. Dopamine selectively induces migration and homing of naive CD8+ T-cells via dopamine receptor D3. J. Immunol. 2006, 176, 848–856. [Google Scholar] [CrossRef]
- Basova, L.; Najera, J.A.; Bortell, N.; Wang, D.; Moya, R.; Lindsey, A.; Semenova, S.; Ellis, R.J.; Marcondes, M.C.G. Dopamine and its receptors play a role in the modulation of CCR5 expression in innate immune cells following exposure to Methamphetamine: Implications to HIV infection. PLoS ONE 2018, 13, e0199861. [Google Scholar] [CrossRef]
- Biswas, S.; Bryant, R.V.; Travis, S. Interfering with leukocyte trafficking in Crohn’s disease. Best. Pract. Res. Clin. Gastroenterol. 2019, 38–39, 101617. [Google Scholar] [CrossRef]
- Sands, B.E. Leukocyte Anti-Trafficking Strategies: Current Status and Future Directions. Dig. Dis. 2017, 35, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Rasmussen, J.P.; Williams, L.M.; Dooley, J.L.; Farr, A.G.; Rudensky, A.Y. Regulatory T-cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005, 22, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.D.; Wang, Y.M.; Miles, P.R.; Budygin, E.A.; Picetti, R.; Gainetdinov, R.R.; Caron, M.G.; Wightman, R.M. Dopamine autoreceptor regulation of release and uptake in mouse brain slices in the absence of D(3) receptors. Neuroscience 2002, 112, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Menning, A.; Loddenkemper, C.; Westendorf, A.M.; Szilagyi, B.; Buer, J.; Siewert, C.; Hamann, A.; Huehn, J. Retinoic acid-induced gut tropism improves the protective capacity of Treg in acute but not in chronic gut inflammation. Eur. J. Immunol. 2010, 40, 2539–2548. [Google Scholar] [CrossRef]
- Sierra, S.; Luquin, N.; Rico, A.J.; Gomez-Bautista, V.; Roda, E.; Dopeso-Reyes, I.G.; Vazquez, A.; Martinez-Pinilla, E.; Labandeira-Garcia, J.L.; Franco, R.; et al. Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: Changes following experimental parkinsonism. Brain Struct. Funct. 2015, 220, 2721–2738. [Google Scholar] [CrossRef]
- Law, A.M.K.; Yin, J.X.M.; Castillo, L.; Young, A.I.J.; Piggin, C.; Rogers, S.; Caldon, C.E.; Burgess, A.; Millar, E.K.A.; O’Toole, S.A.; et al. Andy’s Algorithms: New automated digital image analysis pipelines for FIJI. Sci. Rep. 2017, 7, 15717. [Google Scholar] [CrossRef]


| Functional Name | Short Name | Sequence 1 |
|---|---|---|
| TM1hDRD2-TAT | TM1Drd2 | ATLLTLLIAVIVFGNVLVSMAVSYGRKKRRQRRR |
| TAT-TM2hDRD2 | TM2Drd2 | RRRQRRKKRGYYLIVSLAVADLLVATLVMPWVVY |
| TM3hDRD2-TAT | TM3Drd2 | IFVTLDVMMSTASILNLSAISIYGRKKRRQRRR |
| TAT-TM4hDRD2 | TM4Drd2 | RRRQRRKKRGYVTVMISIVWVLSFTISSPLLF |
| TM5hDRD2-TAT | TM5Drd2 | FVVYSSIVSFYVPFIVTLLVYIKIYYGRKKRRQRRR |
| TAT-TM6hDRD2 | TM6Drd2 | RRRQRRKKRGYMLAIVLGVFIISWLPFFITHIL |
| TM7hDRD2-TAT | TM7Drd2 | AFTWLGYVNSAVNPIIYTTFNIYGRKKRRQRRR |
| TM1hDRD3-TAT | TM1Drd3 | ALSYSALILAIVFGNGLVSMAVLYGRKKRRQRRR |
| TAT-TM2hDRD3 | TM2Drd3 | RRRQRRKKRGYYLVVSLAVADLLVATLVMPWVVY |
| TM3hDRD3-TAT | TM3Drd3 | VFVTLDVMMSTASILNLSAISIYGRKKRRQRRR |
| TAT-TM4hDRD3 | TM4Drd3 | RRRQRRKKRGYVALMITAVWVLAFAVSSPLLF |
| TM5hDRD3-TAT | TM5Drd3 | FVIYSSVVSFYLPFGVTVLVYARIYYGRKKRRQRRR |
| TAT-TM6hDRD3 | TM6Drd3 | RRRQRRKKRGYMVAIVLGAFIVSWLPFFLTHVL |
| TM7hDRD3-TAT | TM7Drd3 | ATTWLGYVNSALNPVIYTTFNIYGRKKRRQRRR |
| Feature | Score | Description |
|---|---|---|
| Inflammation | 0 | None |
| 1 | Slight | |
| 2 | Moderate | |
| 3 | Severe | |
| Extent of injury | 0 | None |
| 1 | Mucosal | |
| 2 | Mucosal and sub-mucosal | |
| 3 | Transmural | |
| Crypt damage | 0 | None |
| 1 | Basal 1/2 damage | |
| 2 | Basal 2/3 damage | |
| 3 | Only surface epithelium intact | |
| 4 | Entire crypt and epithelium lost | |
| The score of each parameter above is multiplied by a factor reflecting the percentage of tissue involved as indicated below | ||
| Percentage involved | Factor | |
| 0–25% | 1 | |
| 26–50% | 2 | |
| 51–75% | 3 | |
| 76–100% | 4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, J.; Raïch, I.; Ugalde, V.; Navarro, G.; Prado, C.; Vidal, P.M.; Leal, P.; Espinoza, A.; Liu, M.; Weersma, R.; et al. The Heteromeric Dopamine Receptor D2:D3 Controls the Gut Recruitment and Suppressive Activity of Regulatory T-Cells. Int. J. Mol. Sci. 2025, 26, 10069. https://doi.org/10.3390/ijms262010069
Mora J, Raïch I, Ugalde V, Navarro G, Prado C, Vidal PM, Leal P, Espinoza A, Liu M, Weersma R, et al. The Heteromeric Dopamine Receptor D2:D3 Controls the Gut Recruitment and Suppressive Activity of Regulatory T-Cells. International Journal of Molecular Sciences. 2025; 26(20):10069. https://doi.org/10.3390/ijms262010069
Chicago/Turabian StyleMora, Jacob, Iu Raïch, Valentina Ugalde, Gemma Navarro, Carolina Prado, Pia M. Vidal, Pedro Leal, Alexandra Espinoza, Moting Liu, Rinse Weersma, and et al. 2025. "The Heteromeric Dopamine Receptor D2:D3 Controls the Gut Recruitment and Suppressive Activity of Regulatory T-Cells" International Journal of Molecular Sciences 26, no. 20: 10069. https://doi.org/10.3390/ijms262010069
APA StyleMora, J., Raïch, I., Ugalde, V., Navarro, G., Prado, C., Vidal, P. M., Leal, P., Espinoza, A., Liu, M., Weersma, R., Gacesa, R., Hermoso, M. A., Franco, R., & Pacheco, R. (2025). The Heteromeric Dopamine Receptor D2:D3 Controls the Gut Recruitment and Suppressive Activity of Regulatory T-Cells. International Journal of Molecular Sciences, 26(20), 10069. https://doi.org/10.3390/ijms262010069







