Intestinal Ischemia/Reperfusion Injury Influences Hyaluronan Homeostasis in the Rat Brain
Abstract
1. Introduction
2. Results
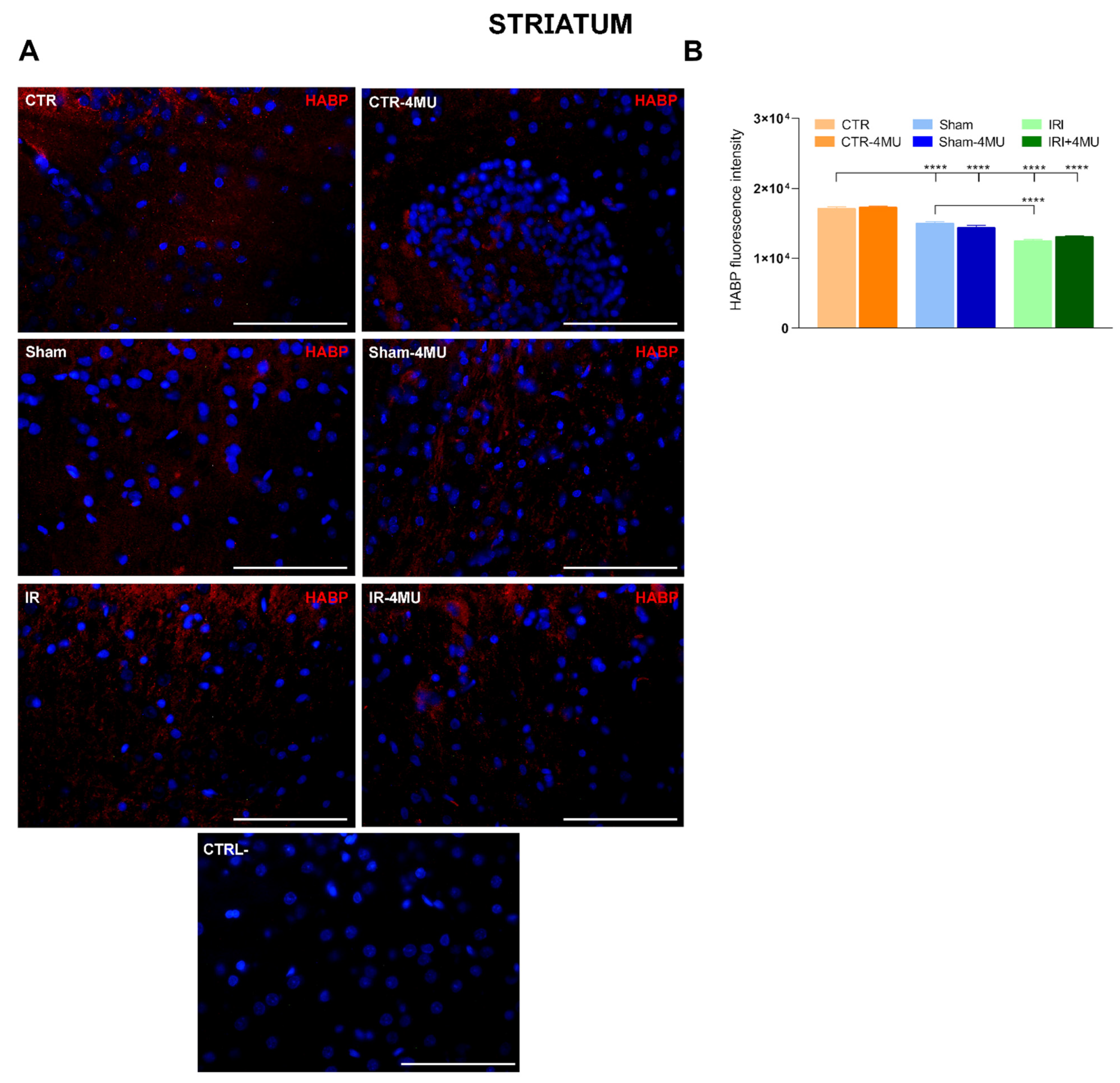
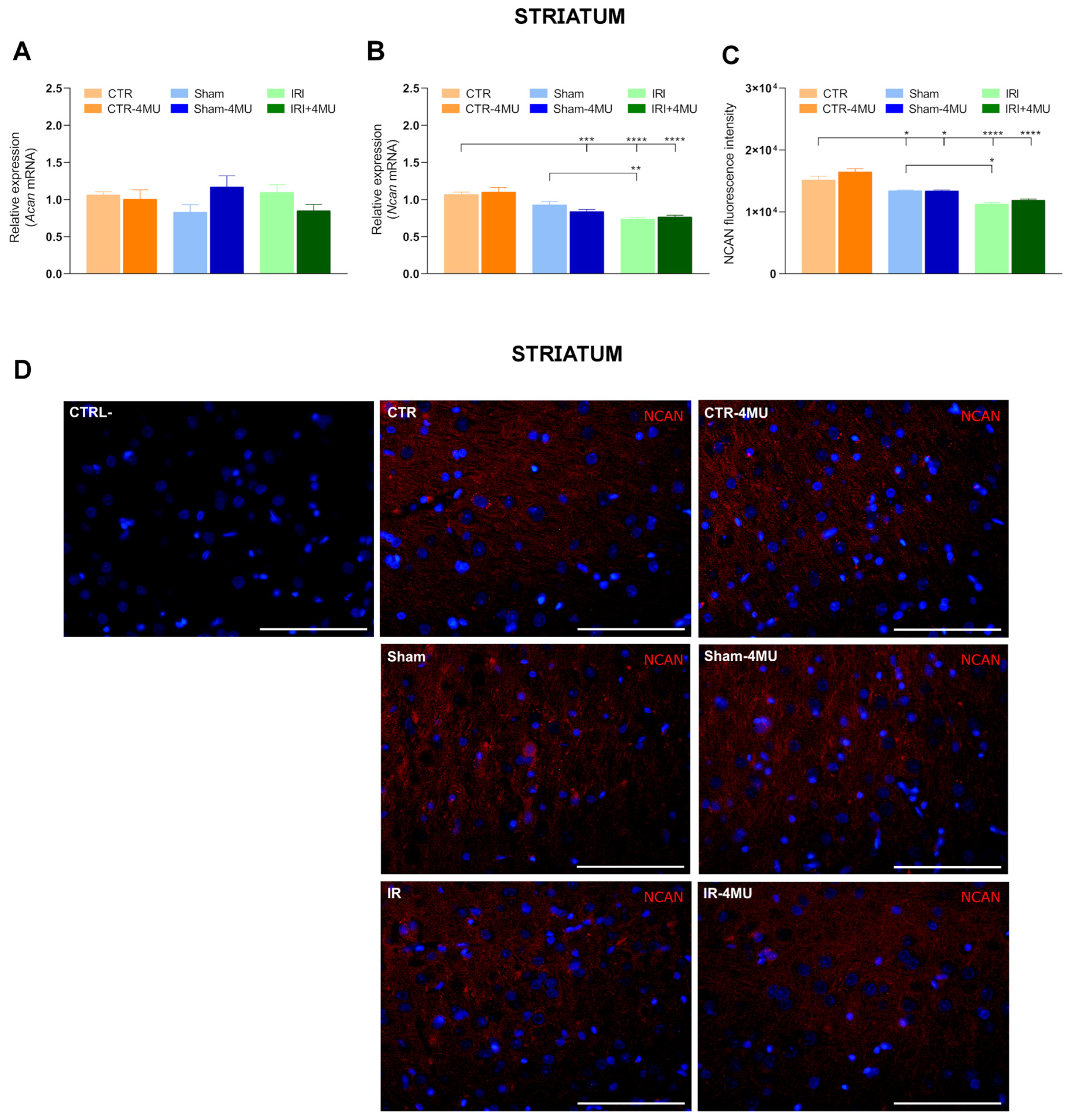
2.1. Intestinal IRI Influences Hyaluronan Homeostasis and Perineuronal Net Molecular Components in the Rat Brain
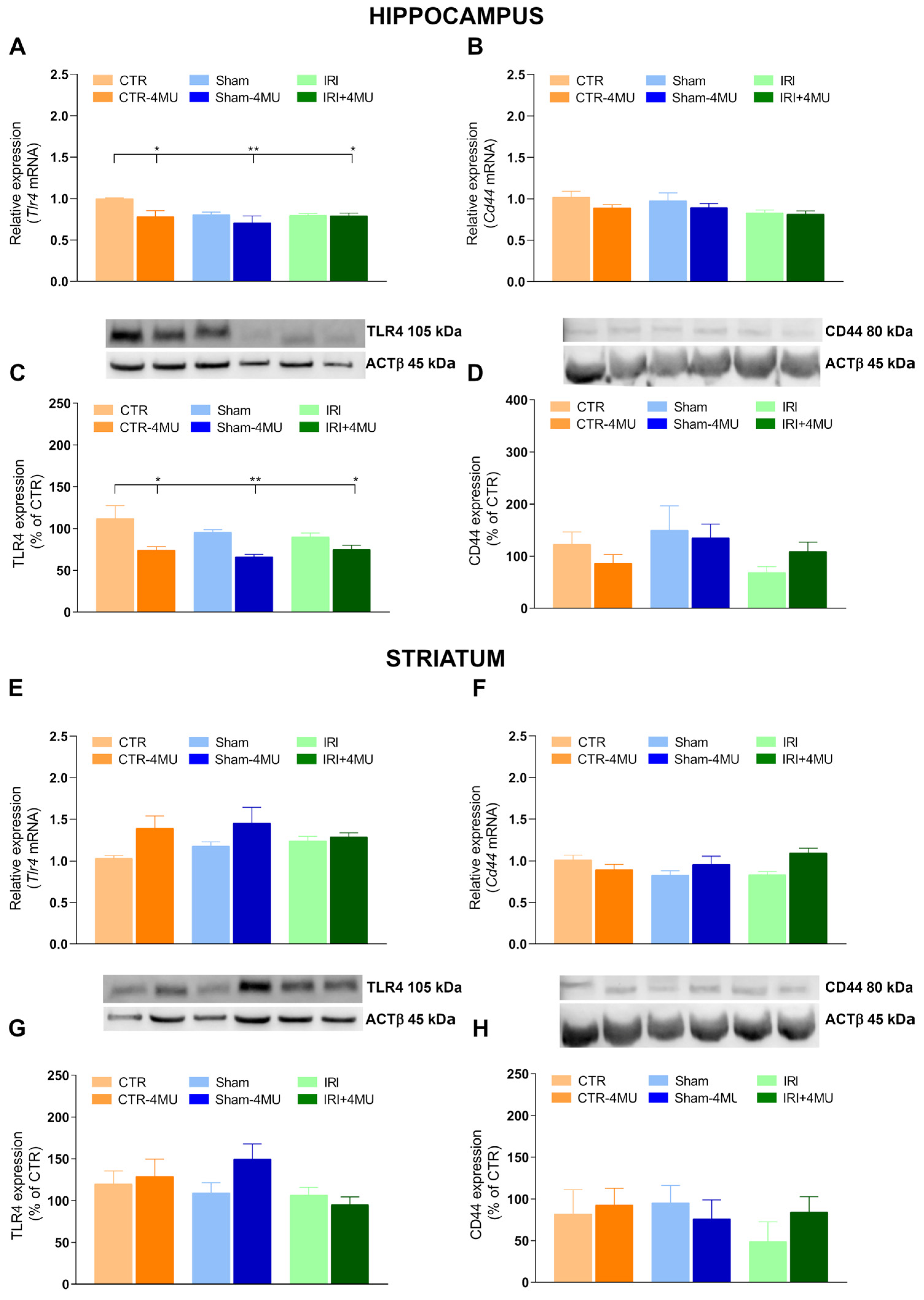
2.2. Effect of 4-MU on Hyaluronan Receptor Expression in the Rat Hippocampus and Striatum After Intestinal IRI
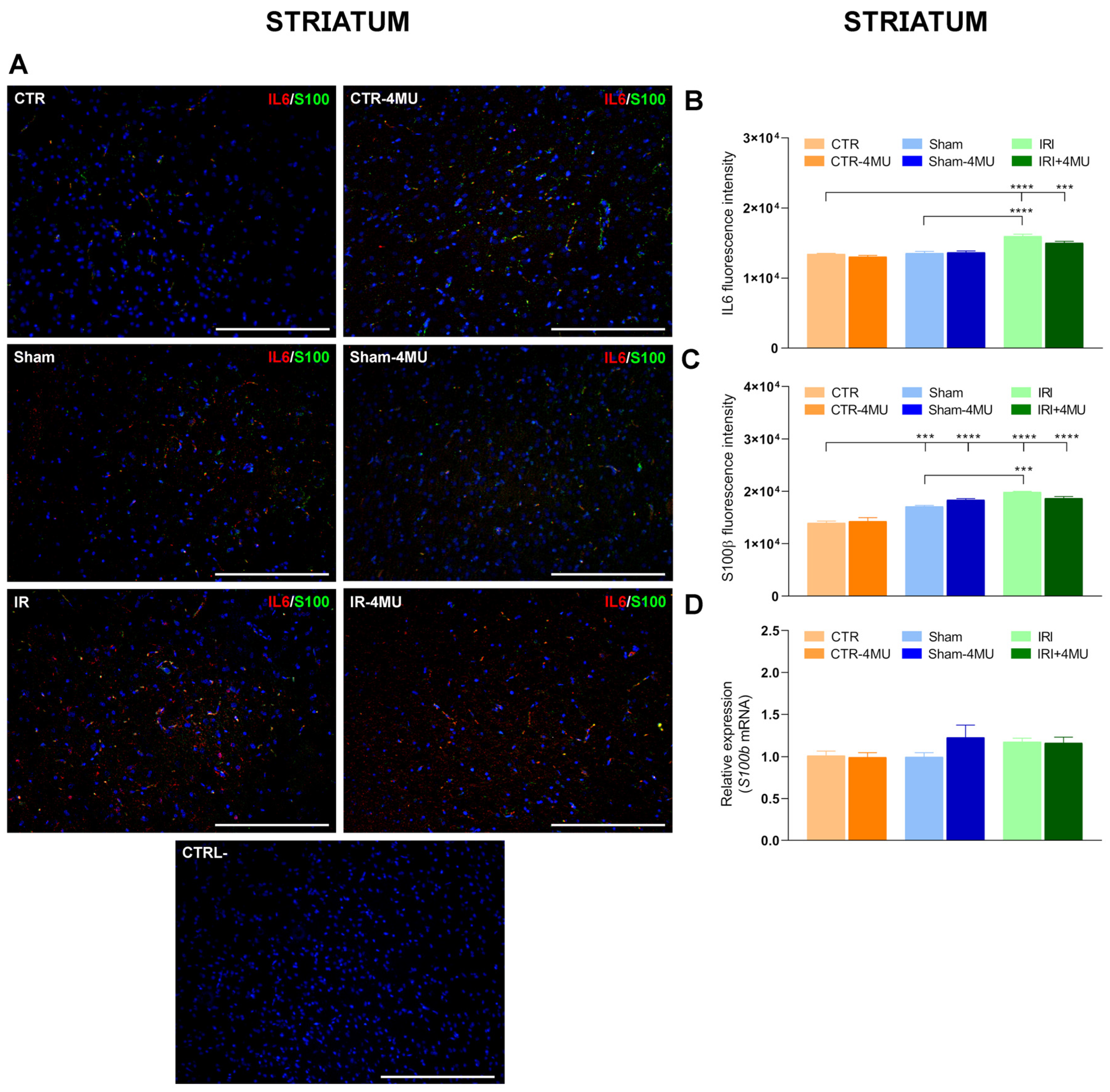
2.3. Effect of 4-MU Treatment on Intestinal IRI-Induced Changes in Proinflammatory Cytokines in the Rat Brain
2.4. 4-MU Modulation of Microglia in the Rat Brain After Intestinal IRI
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Intestinal IRI
4.3. Immunofluorescence Analysis
4.4. RNA Isolation and Quantitative RT PCR
4.5. Western Immunoblot Analysis
4.6. Chemicals
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IRI | Intestinal Ischemia and Reperfusion Injury |
| CNS | Central Nervous System |
| 4-MU | 4-Methylumbelliferone |
| HAS | Hyaluronan Synthase |
| ROS | Reactive Oxygen Species |
| NO | Nitric Oxide |
| TLR4 | Toll-Like Receptor 4 |
| MyD88 | Myeloid Differentiation Factor 88 |
| TNF-α | Tumor Necrosis Factor α |
| IL6 | Interleukin 6 |
| IL1β | Interleukin 1β |
| ACAN | Aggrecan |
| NCAN | Neurocan |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| NF-κB | Nuclear Factor κB |
| HAPLN1 | Hyaluronan and Proteoglycan Link Protein 1 |
| Bral | Brain-Specific Link Protein |
| ENS | Enteric Nervous System |
| CD44 | Cluster Differentiation 44 |
| RHAMM | Receptor for Hyaluronan-Mediated Motility |
| LYVE-1 | Lymphatic Vessel Endothelial Hyaluronan Receptor 1 |
| HARE | Hyaluronan Receptor for Endocytosis |
| HABP | Hyaluronan Binding Protein |
| HYAL | Hyaluronidase |
| pIκB | Phosphorylated Inhibitor of κB |
| IR | Immunoreactivity |
References
- Mallick, I.H.; Yang, W.; Winslet, M.C.; Seifalian, A.M. Ischemia-Reperfusion Injury of the Intestine and Protective Strategies against Injury. Dig. Dis. Sci. 2004, 49, 1359–1377. [Google Scholar] [CrossRef]
- Hierholzer, C.; Kalff, J.C.; Audolfsson, G.; Billiar, T.R.; Tweardy, D.J.; Bauer, A.J. Molecular and Functional Contractile Sequelae of Rat Intestinal Ischemia/Reperfusion Injury. Transplantation 1999, 68, 1244–1254. [Google Scholar] [CrossRef]
- Bielefeldt, K.; Whiteis, C.A.; Sharma, R.V.; Abboud, F.M.; Conklin, J.L. Reactive Oxygen Species and Calcium Homeostasis in Cultured Human Intestinal Smooth Muscle Cells. Am. J. Physiol. Gastrointest. Liver Physiol. 1997, 272, G1439–G1450. [Google Scholar] [CrossRef]
- Giaroni, C.; Zanetti, E.; Giuliani, D.; Oldrini, R.; Marchet, S.; Moro, E.; Borroni, P.; Trinchera, M.; Crema, F.; Lecchini, S.; et al. Protein Kinase c Modulates NMDA Receptors in the Myenteric Plexus of the Guinea Pig Ileum during in Vitro Ischemia and Reperfusion. Neurogastroenterol. Motil. 2011, 23, e91–e103. [Google Scholar] [CrossRef]
- Giaroni, C.; Marchet, S.; Carpanese, E.; Prandoni, V.; Oldrini, R.; Bartolini, B.; Moro, E.; Vigetti, D.; Crema, F.; Lecchini, S.; et al. Role of Neuronal and Inducible Nitric Oxide Synthases in the Guinea Pig Ileum Myenteric Plexus during in Vitro Ischemia and Reperfusion. Neurogastroenterol. Motil. 2013, 25, e114–e126. [Google Scholar] [CrossRef]
- Olanders, K.; Sun, Z.; Börjesson, A.; Dib, M.; Andersson, E.; Lasson, Å.; Ohlsson, T.; Andersson, R. The Effect of Intestinal Ischemia and Reperfusion Injury on ICAM-1 Expression, Endothelial Barrier Function, Neutrophil Tissue Influx, and Protease Inhibitor Levels in Rats. Shock 2002, 18, 86–92. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; McCartney, K.; Moore, T.A.; Thundyil, J.; Gelderblom, M.; Manzanero, S.; Arumugam, T.V. Intestinal Ischemia-Reperfusion Injury Leads to Inflammatory Changes in the Brain. Shock 2011, 36, 424–430. [Google Scholar] [CrossRef]
- Zhou, J.; Huang, W.Q.; Li, C.; Wu, G.Y.; Li, Y.S.; Wen, S.H.; Lei, W.L.; Liu, K.X. Intestinal Ischemia/Reperfusion Enhances Microglial Activation and Induces Cerebral Injury and Memory Dysfunction in Rats. Crit. Care Med. 2012, 40, 2438–2448. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zhang, L.Y.; Chen, Y.; Bai, Y.P.; Jia, J.; Feng, J.G.; Liu, K.X.; Zhou, J. Melatonin Alleviates Intestinal Injury, Neuroinflammation and Cognitive Dysfunction Caused by Intestinal Ischemia/Reperfusion. Int. Immunopharmacol. 2020, 85, 106596. [Google Scholar] [CrossRef] [PubMed]
- Sherman, L.S.; Matsumoto, S.; Su, W.; Srivastava, T.; Back, S.A. Hyaluronan Synthesis, Catabolism, and Signaling in Neurodegenerative Diseases. Int. J. Cell Biol. 2015, 2015, 368584. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.; Asher, R.; Dahl, D.; Labkovsky, B.; Perides, G.; Bignami, A. Permissive and Non-permissive Reactive Astrocytes: Immunofluorescence Study with Antibodies to the Glial Hyaluronate-binding Protein. J. Neurosci. Res. 1990, 25, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Ren, M.; Verma, A. Divergent Temporal Expression of Hyaluronan Metabolizing Enzymes and Receptors with Craniotomy vs. Controlled-Cortical Impact Injury in Rat Brain: A Pilot Study. Front. Neurol. 2014, 5, 173. [Google Scholar] [CrossRef]
- Oohashi, T.; Edamatsu, M.; Bekku, Y.; Carulli, D. The Hyaluronan and Proteoglycan Link Proteins: Organizers of the Brain Extracellular Matrix and Key Molecules for Neuronal Function and Plasticity. Exp. Neurol. 2015, 274, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Vigetti, D.; Viola, M.; Karousou, E.; De Luca, G.; Passi, A. Metabolic Control of Hyaluronan Synthases. Matrix Biol. 2014, 35, 8–13. [Google Scholar] [CrossRef]
- Melero-Fernandez de Mera, R.M.; Arasu, U.T.; Kärnä, R.; Oikari, S.; Rilla, K.; Vigetti, D.; Passi, A.; Heldin, P.; Tammi, M.I.; Deen, A.J. Effects of Mutations in the Post-Translational Modification Sites on the Trafficking of Hyaluronan Synthase 2 (HAS2). Matrix Biol. 2019, 80, 85–103. [Google Scholar] [CrossRef]
- Vigetti, D.; Genasetti, A.; Karousou, E.; Viola, M.; Clerici, M.; Bartolini, B.; Moretto, P.; De Luca, G.; Hascall, V.C.; Passi, A. Modulation of Hyaluronan Synthase Activity in Cellular Membrane Fractions. J. Biol. Chem. 2009, 284, 30684–30694. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Sherman, L.S. Diverse Roles for Hyaluronan and Hyaluronan Receptors in the Developing and Adult Nervous System. Int. J. Mol. Sci. 2020, 21, 5988. [Google Scholar] [CrossRef]
- Van’t Spijker, H.M.; Kwok, J.C.F. A Sweet Talk: The Molecular Systems of Perineuronal Nets in Controlling Neuronal Communication. Front. Integr. Neurosci. 2017, 11, 33. [Google Scholar] [CrossRef]
- Galtrey, C.M.; Kwok, J.C.F.; Carulli, D.; Rhodes, K.E.; Fawcett, J.W. Distribution and Synthesis of Extracellular Matrix Proteoglycans, Hyaluronan, Link Proteins and Tenascin-R in the Rat Spinal Cord. Eur. J. Neurosci. 2008, 27, 1373–1390. [Google Scholar] [CrossRef]
- Costa, C.; Tortosa, R.; Domènech, A.; Vidal, E.; Pumarola, M.; Bassols, A. Mapping of Aggrecan, Hyaluronic Acid, Heparan Sulphate Proteoglycans and Aquaporin 4 in the Central Nervous System of the Mouse. J. Chem. Neuroanat. 2007, 33, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Bistoletti, M.; Bosi, A.; Caon, I.; Chiaravalli, A.M.; Moretto, P.; Genoni, A.; Moro, E.; Karousou, E.; Viola, M.; Crema, F.; et al. Involvement of Hyaluronan in the Adaptive Changes of the Rat Small Intestine Neuromuscular Function after Ischemia/Reperfusion Injury. Sci. Rep. 2020, 10, 11521. [Google Scholar] [CrossRef] [PubMed]
- Bosi, A.; Banfi, D.; Bistoletti, M.; Catizzone, L.M.; Chiaravalli, A.M.; Moretto, P.; Moro, E.; Karousou, E.; Viola, M.; Giron, M.C.; et al. Hyaluronan Regulates Neuronal and Immune Function in the Rat Small Intestine and Colonic Microbiota after Ischemic/Reperfusion Injury. Cells 2022, 11, 3370. [Google Scholar] [CrossRef] [PubMed]
- Filpa, V.; Bistoletti, M.; Caon, I.; Moro, E.; Grimaldi, A.; Moretto, P.; Baj, A.; Giron, M.C.; Karousou, E.; Viola, M.; et al. Changes in Hyaluronan Deposition in the Rat Myenteric Plexus after Experimentally-Induced Colitis. Sci. Rep. 2017, 7, 4021–4044. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, I.; Kojima, K.; Takagaki, K.; Endo, M.; Kannagi, R.; Ito, M.; Maruo, Y.; Sato, H.; Yasuda, T.; Mita, S.; et al. A Novel Mechanism for the Inhibition of Hyaluronan Biosynthesis by 4-Methylumbelliferone. J. Biol. Chem. 2004, 279, 33281–33289. [Google Scholar] [CrossRef]
- Vigetti, D.; Rizzi, M.; Viola, M.; Karousou, E.; Genasetti, A.; Clerici, M.; Bartolini, B.; Hascall, V.C.; De Luca, G.; Passi, A. The Effects of 4-Methylumbelliferone on Hyaluronan Synthesis, MMP2 Activity, Proliferation, and Motility of Human Aortic Smooth Muscle Cells. Glycobiology 2009, 19, 537–546. [Google Scholar] [CrossRef]
- Girardello, R.; Baranzini, N.; Molteni, M.; Rossetti, C.; Tettamanti, G.; de Eguileor, M.; Grimaldi, A. The Medicinal Leech as a Valuable Model for Better Understanding the Role of a TLR4-like Receptor in the Inflammatory Process. Cell Tissue Res. 2019, 377, 245–257. [Google Scholar] [CrossRef]
- Hinz, M.; Scheidereit, C. The IκB Kinase Complex in NF-κB Regulation and Beyond. EMBO Rep. 2013, 15, 46–61. [Google Scholar] [CrossRef]
- Bourgognon, J.-M.; Cavanagh, J. The Role of Cytokines in Modulating Learning and Memory and Brain Plasticity. Brain Neurosci. Adv. 2020, 4, 239821282097980. [Google Scholar] [CrossRef]
- Adami, C.; Sorci, G.; Blasi, E.; Agneletti, A.L.; Bistoni, F.; Donato, R. S100b Expression in and Effects on Microglia. Glia 2001, 33, 131–142. [Google Scholar] [CrossRef]
- Arranz, A.M.; Perkins, K.L.; Irie, F.; Lewis, D.P.; Hrabe, J.; Xiao, F.; Itano, N.; Kimata, K.; Hrabetova, S.; Yamaguchi, Y. Hyaluronan Deficiency Due to Has3 Knock-out Causes Altered Neuronal Activity and Seizures via Reduction in Brain Extracellular Space. J. Neurosci. 2014, 34, 6164–6176. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Nikolskaya, A.I.; Goriainov, S.V.; Astakhova, A.A.; Sergeeva, M.G. Inhibitor of Hyaluronic Acid Synthesis 4-Methylumbelliferone as an Anti-Inflammatory Modulator of Lps-Mediated Astrocyte Responses. Int. J. Mol. Sci. 2020, 21, 8203. [Google Scholar] [CrossRef]
- Rilla, K.; Pasonen-Seppänen, S.; Deen, A.J.; Koistinen, V.V.T.; Wojciechowski, S.; Oikari, S.; Kärnä, R.; Bart, G.; Törrönen, K.; Tammi, R.H.; et al. Hyaluronan Production Enhances Shedding of Plasma Membrane-Derived Microvesicles. Exp. Cell Res. 2013, 319, 2006–2018. [Google Scholar] [CrossRef]
- Tavianatou, A.G.; Caon, I.; Franchi, M.; Piperigkou, Z.; Galesso, D.; Karamanos, N.K. Hyaluronan: Molecular Size-Dependent Signaling and Biological Functions in Inflammation and Cancer. FEBS J. 2019, 286, 2883–2908. [Google Scholar] [CrossRef] [PubMed]
- Dubisova, J.; Burianova, J.S.; Svobodova, L.; Makovicky, P.; Martinez-Varea, N.; Cimpean, A.; Fawcett, J.W.; Kwok, J.C.F.; Kubinova, S. Oral Treatment of 4-Methylumbelliferone Reduced Perineuronal Nets and Improved Recognition Memory in Mice. Brain Res. Bull. 2022, 181, 144–156. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, V.B.; Lopez, L.E.; Munoz, D.; Chi, A.; Shirodkar, S.P.; Lokeshwar, S.D.; Escudero, D.O.; Dhir, N.; Altman, N. Antitumor Activity of Hyaluronic Acid Synthesis Inhibitor 4-Methylumbelliferone in Prostate Cancer Cells. Cancer Res. 2010, 70, 2613–2623. [Google Scholar] [CrossRef]
- Dresselhaus, E.C.; Meffert, M.K. Cellular Specificity of NF-ΚB Function in the Nervous System. Front. Immunol. 2019, 10, 1043. [Google Scholar] [CrossRef]
- Zhang, G.; Guo, L.; Yang, C.; Liu, Y.; He, Y.; Du, Y.; Wang, W.; Gao, F. A Novel Role of Breast Cancer-Derived Hyaluronan on Inducement of M2-like Tumor-Associated Macrophages Formation. Oncoimmunology 2016, 5, e1172154. [Google Scholar] [CrossRef]
- Frank, M.G.; Baratta, M.V.; Sprunger, D.B.; Watkins, L.R.; Maier, S.F. Microglia Serve as a Neuroimmune Substrate for Stress-Induced Potentiation of CNS pro-Inflammatory Cytokine Responses. Brain. Behav. Immun. 2007, 21, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.W.; Gilchrist, C.; Fehlings, M.G. High Molecular Weight Hyaluronan Reduces Lipopolysaccharide Mediated Microglial Activation. J. Neurochem. 2012, 122, 344–355. [Google Scholar] [CrossRef]
- Teeling, J.L.; Perry, V.H. Systemic Infection and Inflammation in Acute CNS Injury and Chronic Neurodegeneration: Underlying Mechanisms. Neuroscience 2009, 158, 1062–1073. [Google Scholar] [CrossRef]
- Banfi, D.; Moro, E.; Bosi, A.; Bistoletti, M.; Cerantola, S.; Crema, F.; Maggi, F.; Giron, M.C.; Giaroni, C.; Baj, A. Impact of Microbial Metabolites on Microbiota–Gut–Brain Axis in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 1623. [Google Scholar] [CrossRef]
- Tewari, B.P.; Chaunsali, L.; Prim, C.E.; Sontheimer, H. A Glial Perspective on the Extracellular Matrix and Perineuronal Net Remodeling in the Central Nervous System. Front. Cell. Neurosci. 2022, 16, 1022754. [Google Scholar] [CrossRef]
- Keschenau, P.R.; Ribbe, S.; Tamm, M.; Hanssen, S.J.; Tolba, R.; Jacobs, M.J.; Kalder, J. Extracorporeal Circulation Increases Proliferation in the Intestinal Mucosa in a Large Animal Model. J. Vasc. Surg. 2016, 64, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.S.; Mapstone, M. Does Extracorporeal Circulation Harm the Brain? Neurology 2005, 65, 978–979. [Google Scholar] [CrossRef]
- Tian, X.; Azpurua, J.; Hine, C.; Vaidya, A.; Myakishev-Rempel, M.; Ablaeva, J.; Mao, Z.; Nevo, E.; Gorbunova, V.; Seluanov, A. High-Molecular-Mass Hyaluronan Mediates the Cancer Resistance of the Naked Mole Rat. Nature 2013, 499, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Olivares, C.N.; Alaniz, L.D.; Menger, M.D.; Barañao, R.I.; Laschke, M.W.; Meresman, G.F. Inhibition of Hyaluronic Acid Synthesis Suppresses Angiogenesis in Developing Endometriotic Lesions. PLoS ONE 2016, 11, e0152302. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Noureddin, M.; Liu, C.; Ohashi, K.; Kim, S.Y.; Ramnath, D.; Powell, E.E.; Sweet, M.J.; Roh, Y.S.; Hsin, I.F.; et al. Hyaluronan Synthase 2-Mediated Hyaluronan Production Mediates Notch1 Activation and Liver Fibrosis. Sci. Transl. Med. 2019, 11, eaat9284. [Google Scholar] [CrossRef]
- Yates, T.J.; Lopez, L.E.; Lokeshwar, S.D.; Ortiz, N.; Kallifatidis, G.; Jordan, A.; Hoye, K.; Altman, N.; Lokeshwar, V.B. Dietary Supplement 4-Methylumbelliferone: An Effective Chemopreventive and Therapeutic Agent for Prostate Cancer. J. Natl. Cancer Inst. 2015, 107, djv085. [Google Scholar] [CrossRef]
- Filpa, V.; Carpanese, E.; Marchet, S.; Pirrone, C.; Conti, A.; Rainero, A.; Moro, E.; Chiaravalli, A.M.; Zucchi, I.; Moriondo, A.; et al. Nitric Oxide Regulates Homeoprotein OTX1 and OTX2 Expression in the Rat Myenteric Plexus after Intestinal Ischemia-Reperfusion Injury. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 312, G374–G389. [Google Scholar] [CrossRef] [PubMed]
- Bistoletti, M.; Caputi, V.; Baranzini, N.; Marchesi, N.; Filpa, V.; Marsilio, I.; Cerantola, S.; Terova, G.; Baj, A.; Grimaldi, A.; et al. Antibiotic Treatment-Induced Dysbiosis Differently Affects BDNF and TrkB Expression in the Brain and in the Gut of Juvenile Mice. PLoS ONE 2019, 14, e0212856. [Google Scholar] [CrossRef]
- Yoshida, H.; Nagaoka, A.; Kusaka-Kikushima, A.; Tobiishi, M.; Kawabata, K.; Sayo, T.; Sakai, S.; Sugiyama, Y.; Enomoto, H.; Okada, Y.; et al. KIAA1199, a Deafness Gene of Unknown Function, Is a New Hyaluronan Binding Protein Involved in Hyaluronan Depolymerization. Proc. Natl. Acad. Sci. USA 2013, 110, 5612–5617. [Google Scholar] [CrossRef]
- Fukui, Y.; Morihara, R.; Hu, X.; Nakano, Y.; Yunoki, T.; Takemoto, M.; Abe, K.; Yamashita, T. Suppression of PTBP1 in Hippocampal Astrocytes Promotes Neurogenesis and Ameliorates Recognition Memory in Mice with Cerebral Ischemia. Sci. Rep. 2024, 14, 20521. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-S.; Lõhelaid, H.; Shih, C.-C.; Liew, H.-K.; Wang, V.; Hu, W.-F.; Chen, Y.-H.; Saarma, M.; Airavaara, M.; Tseng, K.-Y. Targeting Rap1b Signaling Cascades with CDNF: Modulating Platelet Activation, Regulating Plasma Oxylipins and Mitigating Reperfusion Injury in Stroke. Mol. Ther. 2024, 32, 4021–4044. [Google Scholar] [CrossRef]
- Prajapati, K.S.; Kumar, S. Loss of MiR-6844 Alters Stemness/Self-Renewal and Cancer Hallmark(s) Markers through CD44-JAK2-STAT3 Signaling Axis in Breast Cancer Stem-like Cells. J. Cell. Biochem. 2023, 124, 1186–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.D.; Li, H.X.; Gan, H.; Tang, Z.; Guo, Y.Y.; Yao, S.Q.; Liuyu, T.; Zhong, B.; Lin, D. RNF115 Inhibits the Post-ER Trafficking of TLRs and TLRs-Mediated Immune Responses by Catalyzing K11-Linked Ubiquitination of RAB1A and RAB13. Adv. Sci. 2022, 9, e2105391. [Google Scholar] [CrossRef]
- Wang, H.C.; Lee, W. Sen Progesterone-Induced Migration Inhibition in Male Rat Aortic Smooth Muscle Cells through the CSrc/AKT/ERK 2/P38 Pathway-Mediated Up-Regulation of P27. Endocrinology 2014, 155, 1428–1435. [Google Scholar] [CrossRef]
- Cha, M.; Lee, K.H.; Lee, B.H. Astroglial Changes in the Zona Incerta in Response to Motor Cortex Stimulation in a Rat Model of Chronic Neuropathy. Sci. Rep. 2020, 10, 943. [Google Scholar] [CrossRef]







| Primary Antibody | Host Species | Dilution (HC) | Dilution (WB) | Specificity | Source |
|---|---|---|---|---|---|
| HABP | - | 1:100 | - | Hyaluronan [53] | Hokudo (BC41) |
| S100β | Rabbit | 1:100 | - | S100β [54] | GeneTex (GTX129573) |
| IL6 | Mouse | - | 1:200 | IL6 [55] | Santa Cruz (sc-57315) |
| IL6 | Rabbit | 1:150 | - | IL6 [55] | Sigma Aldrich (I2143) |
| CD44 | Rabbit | 1:1000 | CD44 [56] | Invitrogen (PA5-21419) | |
| TLR4 | Rabbit | 1:1000 | TLR4 [57] | ABclonal (A5258) | |
| pIκBα | Mouse | - | 1:400 | pIκΒ−α [58] | Santa Cruz (sc-8404) |
| ACTβ | Mouse | - | 1:1000 | Housekeeping [59] | Cell Signaling (#3700) |
| Secondary Antibody & Streptavidin complex | |||||
| Anti-rabbit Cy3-conjugated | Goat | 1:200 | - | Jackson (AB_2338006) | |
| Anti-rabbit FITC-conjugated | Goat | 1:200 | - | Jackson (AB_2337972) | |
| Streptavidin-Cy3 | - | 1:500 | - | Amersham (PA43001) | |
| Anti-rabbit IgG,HRP-linked | Donkey | 1:2000 | Amersham (NA934) | ||
| Anti-mouse IgG,HRP-linked | Horse | - | 1:2000 | Cell Signaling (#7076) |
| Gene | Sequence |
|---|---|
| ACTβ | F 5′-CAGGGTGTGATGGTGGGTATGG-3′ R 5′-AGTTGGTGACAATGCCGTGTTC-3′ |
| Tnfa | F 5′-CCCAGACCCTCACACTCAGAT-3′ R 5′-TTGTCCCTTGAAGAGAACCTG-3′ |
| Il6 | F 5′-GTGCAATGGCAATTCTGATTGTA-3′ R 5′-CTAGGGTTTCAGTATTGCTCTGA-3′ |
| Nfkb | F 5′-CCTTTCTCTACTACCCCGAAATC-3′ R 5′-GAAGTTGGGCATGAGCTTCT-3′ |
| S100β | F 5′-GGGTGACAAGCACAAGCTGAA-3′ R 5′-AGCGTCTCCATCACTTTGTCCA-3′ |
| Cd44 | F 5′-AAGACATCGATGCCTCAAAC-3′ R 5′-CTCCAGTAGGCTGTGAAGTG-3′ |
| Tlr4 | F 5′-TGAGATTGCTCAAACATGGC-3′ R 5′-CGAGGCTTTTCCATCCAATA-3′ |
| Has1 | F 5′-AGTATACCTCGCGCTCCAGA-3′ R 5′-ACCACAGGGCGTTGTATAGC-3′ |
| Has2 | F 5′-TTGGCCGGTCGTCTCAA-3′ R 5′-CGTCCTCCGCCTGTCTGT-3′ |
| Has3 | F 5′-CCTCATCGCCACAGTCATACAA-3′ R 5′-CCACCAGCTGCACCGTTAGT-3′ |
| Hyal1 | F 5′-GCCCATAATGCCCTACGTCCA-3′ R 5′-TGGCTTGGCATGACTCCTTG-3′ |
| Hyal2 | F 5′-GGAGCGGGCTTAGCTGGTA-3′ R 5′-GGGCTACAGGAAGTGTCACC-3′ |
| Hyal3 | F 5′-CACCAGATCCTCCACAACCT-3′ R 5′-GAGGCTGCCTGGTAGACTTG-3′ |
| Acan | F 5′-TCCACATCAGAAGAGCCATAC-3′ R 5′-AGTCAAGGTCGCCAGAGG-3′ |
| Ncan | F 5′-TTTCAGTCCACAGCGATCAG-3′ R 5′-AGGAGAGGGATACAGCAGCA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosi, A.; Baranzini, N.; Ponti, A.; Moretto, P.; Moro, E.; Crema, F.; Cianci, R.; Karousou, E.; Viola, M.; Passi, A.; et al. Intestinal Ischemia/Reperfusion Injury Influences Hyaluronan Homeostasis in the Rat Brain. Int. J. Mol. Sci. 2025, 26, 10064. https://doi.org/10.3390/ijms262010064
Bosi A, Baranzini N, Ponti A, Moretto P, Moro E, Crema F, Cianci R, Karousou E, Viola M, Passi A, et al. Intestinal Ischemia/Reperfusion Injury Influences Hyaluronan Homeostasis in the Rat Brain. International Journal of Molecular Sciences. 2025; 26(20):10064. https://doi.org/10.3390/ijms262010064
Chicago/Turabian StyleBosi, Annalisa, Nicolò Baranzini, Alessandra Ponti, Paola Moretto, Elisabetta Moro, Francesca Crema, Rossella Cianci, Evgenia Karousou, Manuela Viola, Alberto Passi, and et al. 2025. "Intestinal Ischemia/Reperfusion Injury Influences Hyaluronan Homeostasis in the Rat Brain" International Journal of Molecular Sciences 26, no. 20: 10064. https://doi.org/10.3390/ijms262010064
APA StyleBosi, A., Baranzini, N., Ponti, A., Moretto, P., Moro, E., Crema, F., Cianci, R., Karousou, E., Viola, M., Passi, A., Vigetti, D., Baj, A., Grimaldi, A., Severgnini, P., & Giaroni, C. (2025). Intestinal Ischemia/Reperfusion Injury Influences Hyaluronan Homeostasis in the Rat Brain. International Journal of Molecular Sciences, 26(20), 10064. https://doi.org/10.3390/ijms262010064














