Class-Specific Effects of ARBs Versus ACE Inhibitors on Survival and Cardiovascular Outcomes in MASLD
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of the Study Population
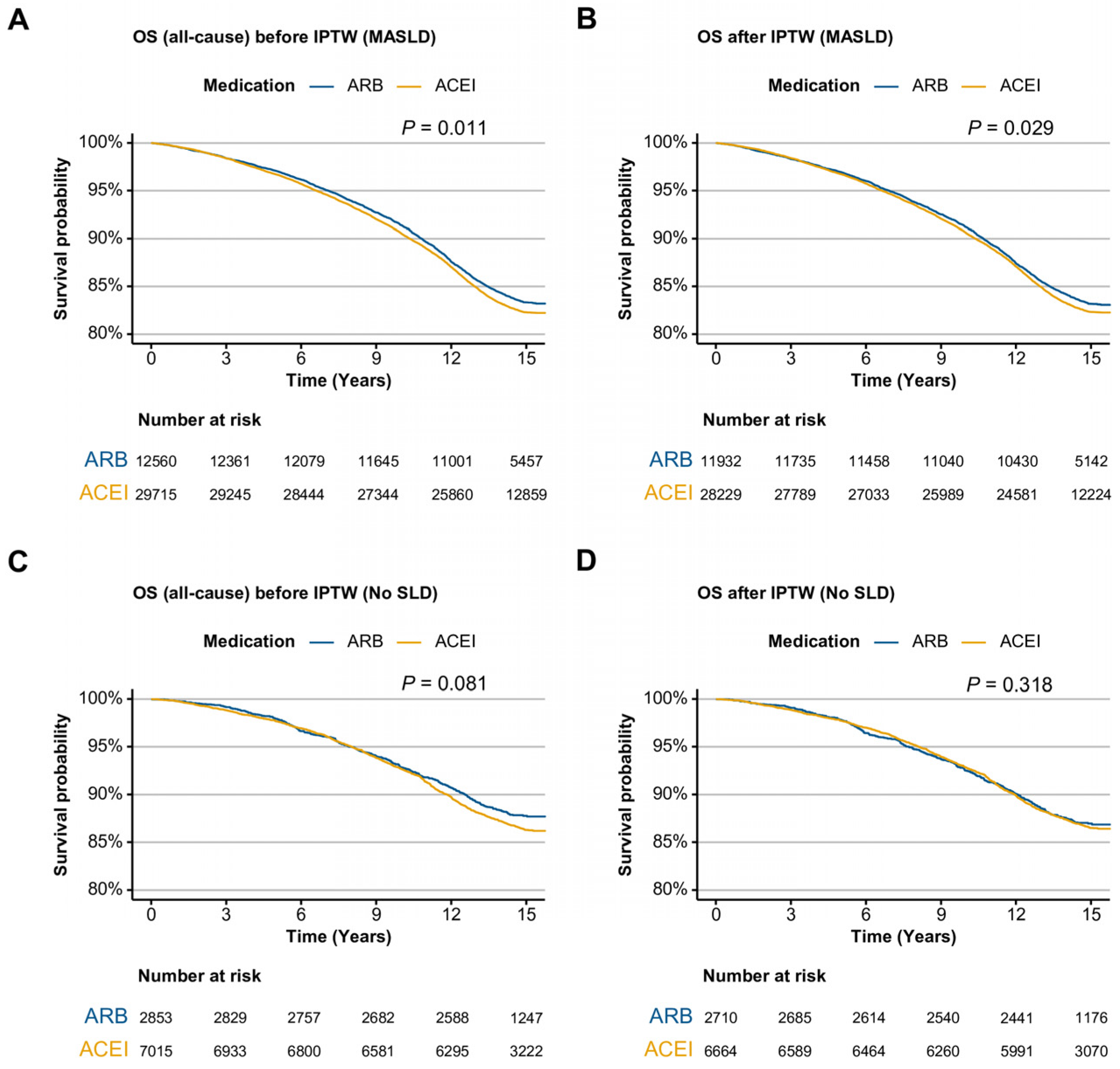
2.2. Overall Survival in ARB Users Versus ACEI Users in SLD
2.3. Cardiovascular Outcomes and Liver Related Events
2.4. Subgroup Analysis for Sex, Age, BMI and the Presence of Diabetes Mellitus
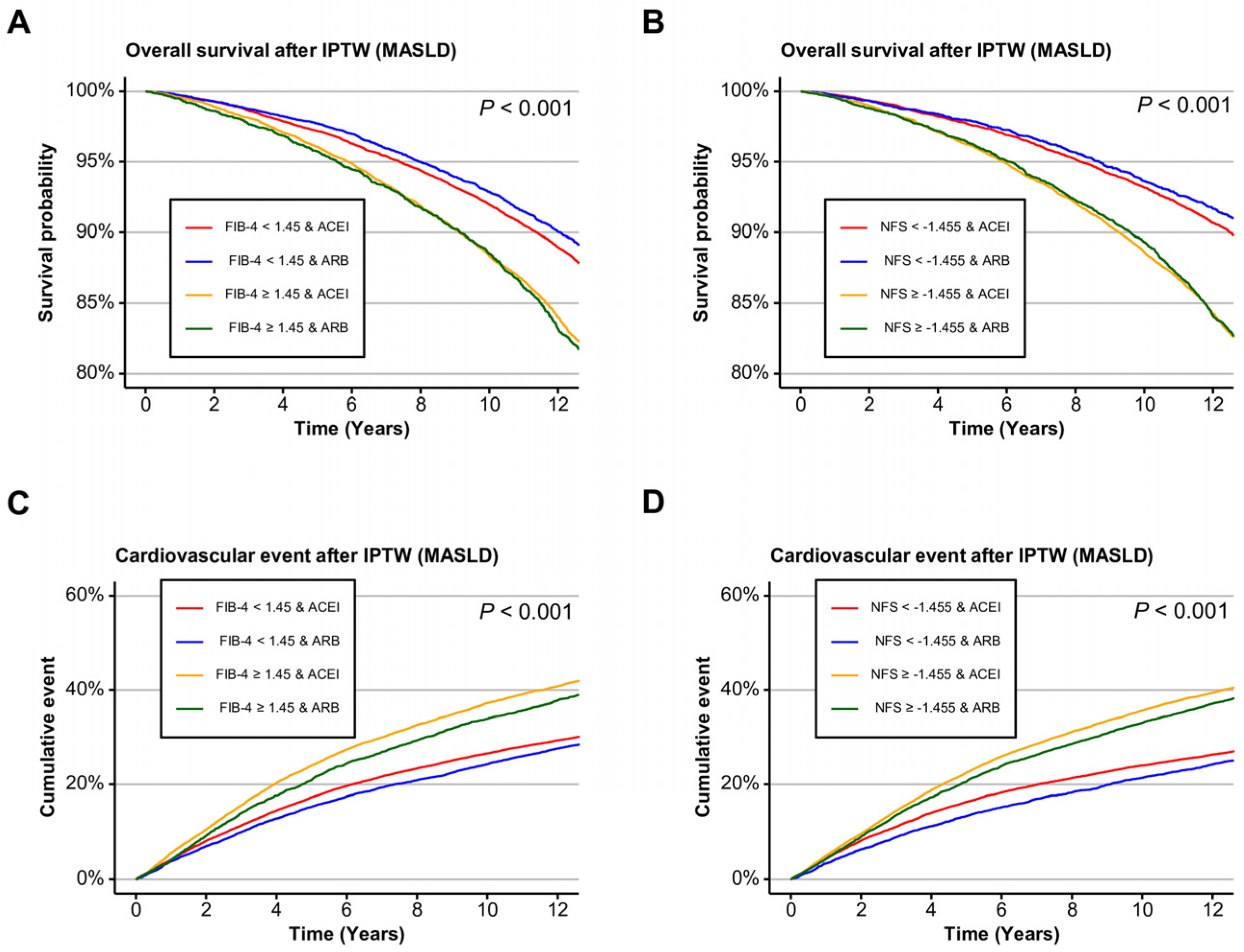
2.5. Liver Fibrosis Stratified Outcomes
2.6. Differential Spatial Expression of ACEI- and ARB-Target Genes in Mouse Liver
3. Discussion
4. Materials and Methods
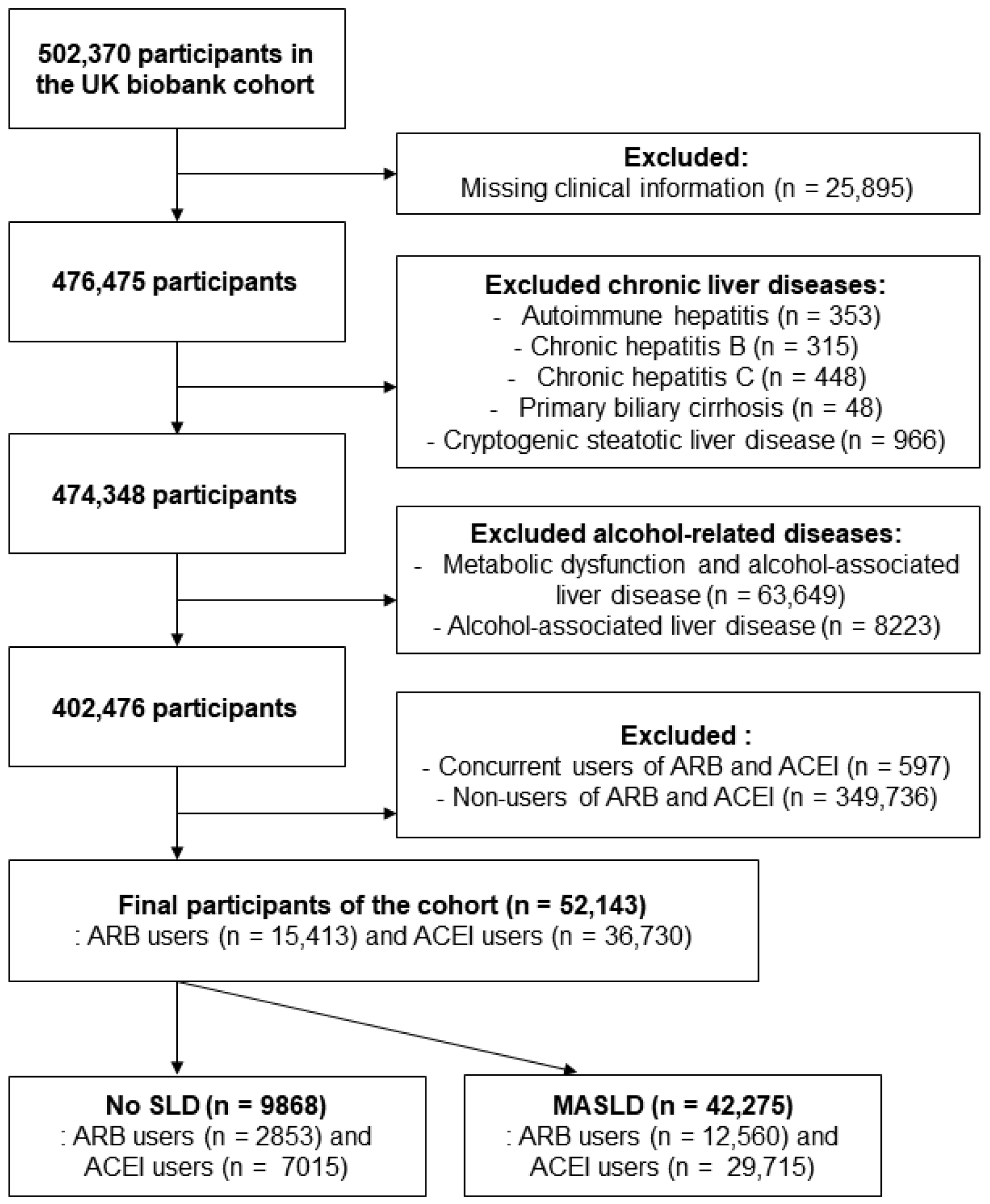
4.1. Study Design and Population
4.2. Noninvasive Assessment of Liver Fibrosis
4.3. Medication Exposure Definition
4.4. Spatial Transcriptomic Data from the Liver Cell Atlas
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACEI | angiotensin-converting enzyme inhibitor |
| ALT | alanine aminotransferase |
| ARB | angiotensin II receptor blocker |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| CI | confidence interval |
| FIB-4 | fibrosis-4 index |
| FLI | fatty liver index |
| GGT | gamma-glutamyl transpeptidase |
| HCC | hepatocellular carcinoma |
| HR | hazard ratio |
| IPTW | inverse probability of treatment weighting |
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| NAFLD | non-alcoholic fatty liver disease |
| NFS | NAFLD fibrosis score |
| RAS | Renin-angiotensin system |
| SLD | steatotic liver disease |
References
- Stefan, N.; Yki-Jarvinen, H.; Neuschwander-Tetri, B.A. Metabolic dysfunction-associated steatotic liver disease: Heterogeneous pathomechanisms and effectiveness of metabolism-based treatment. Lancet Diabetes Endocrinol. 2025, 13, 134–148. [Google Scholar] [CrossRef]
- Kim, D.; Danpanichkul, P.; Wijarnpreecha, K.; Cholankeril, G.; Loomba, R.; Ahmed, A. Current burden of steatotic liver disease and fibrosis among adults in the United States, 2017–2023. Clin Mol Hepatol. 2025, 31, 382–393. [Google Scholar] [CrossRef]
- Hagstrom, H.; Shang, Y.; Hegmar, H.; Nasr, P. Natural history and progression of metabolic dysfunction-associated steatotic liver disease. Lancet Gastroenterol. Hepatol. 2024, 9, 944–956. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Radosavljevic, T.; Brankovic, M.; Samardzic, J.; Djuretic, J.; Vukicevic, D.; Vucevic, D.; Jakovljevic, V. Altered Mitochondrial Function in MASLD: Key Features and Promising Therapeutic Approaches. Antioxidants 2024, 13, 906. [Google Scholar] [CrossRef]
- Eeda, V.; Patil, N.Y.; Joshi, A.D.; Awasthi, V. Advancements in metabolic-associated steatotic liver disease research: Diagnostics, small molecule developments, and future directions. Hepatol. Res. 2024, 54, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Borem, L.M.A.; Neto, J.F.R.; Brandi, I.V.; Lelis, D.F.; Santos, S.H.S. The role of the angiotensin II type I receptor blocker telmisartan in the treatment of non-alcoholic fatty liver disease: A brief review. Hypertens. Res. 2018, 41, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Roh, J.H.; Lee, S.; Yoon, J.H. Clinical implications of renin-angiotensin system inhibitors for development and progression of non-alcoholic fatty liver disease. Sci. Rep. 2021, 11, 2884. [Google Scholar] [CrossRef]
- McGrath, M.S.; Wentworth, B.J. The Renin-Angiotensin System in Liver Disease. Int. J. Mol. Sci. 2024, 25, 5807. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.; dos Santos, R.A.; da Costa Dias, F.L.; Teixeira, M.M.; Simoes e Silva, A.C. Renin-angiotensin system in the pathogenesis of liver fibrosis. World J. Gastroenterol. 2009, 15, 2579–2586. [Google Scholar] [CrossRef]
- Ning, Z.W.; Luo, X.Y.; Wang, G.Z.; Li, Y.; Pan, M.X.; Yang, R.Q.; Ling, X.G.; Huang, S.; Ma, X.X.; Jin, S.Y. MicroRNA-21 Mediates Angiotensin II-Induced Liver Fibrosis by Activating NLRP3 Inflammasome/IL-1beta Axis via Targeting Smad7 and Spry1. Antioxid. Redox Signal 2017, 27, 1–20. [Google Scholar] [CrossRef]
- Tandon, P.; Abraldes, J.G.; Berzigotti, A.; Garcia-Pagan, J.C.; Bosch, J. Renin-angiotensin-aldosterone inhibitors in the reduction of portal pressure: A systematic review and meta-analysis. J. Hepatol. 2010, 53, 273–282. [Google Scholar] [CrossRef]
- Hirose, A.; Ono, M.; Saibara, T.; Nozaki, Y.; Masuda, K.; Yoshioka, A.; Takahashi, M.; Akisawa, N.; Iwasaki, S.; Oben, J.A.; et al. Angiotensin II type 1 receptor blocker inhibits fibrosis in rat nonalcoholic steatohepatitis. Hepatology 2007, 45, 1375–1381. [Google Scholar] [CrossRef]
- Namisaki, T.; Moriya, K.; Kitade, M.; Takeda, K.; Kaji, K.; Okura, Y.; Shimozato, N.; Sato, S.; Nishimura, N.; Seki, K.; et al. Effect of combined farnesoid X receptor agonist and angiotensin II type 1 receptor blocker on hepatic fibrosis. Hepatol. Commun. 2017, 1, 928–945. [Google Scholar] [CrossRef]
- Elhence, H.; Dodge, J.L.; Lee, B.P. Association of Renin-Angiotensin System Inhibition With Liver-Related Events and Mortality in Compensated Cirrhosis. Clin. Gastroenterol. Hepatol. 2024, 22, 315–323.e17. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.H.; Yeo, Y.H.; Kim, H.; Seki, E.; Rees, J.; Ma, K.S.; Moylan, C.A.; Rodriquez, L.M.; Abdelmalek, M.; Villanueva, A.; et al. Renin-angiotensin-aldosterone system inhibitor use improves clinical outcomes in patients with metabolic dysfunction-associated steatotic liver diseases: Target trial emulation using real-world data. Hepatology 2025. [Google Scholar] [CrossRef]
- Nehme, A.; Zibara, K. Efficiency and specificity of RAAS inhibitors in cardiovascular diseases: How to achieve better end-organ protection? Hypertens. Res. 2017, 40, 903–909. [Google Scholar] [CrossRef]
- Toth, P.P. Pleiotropic effects of angiotensin receptor blockers: Addressing comorbidities by optimizing hypertension therapy. J. Clin. Hypertens. 2011, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Givertz, M.M. Manipulation of the renin-angiotensin system. Circulation 2001, 104, e14–e18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iwanami, J.; Mogi, M.; Iwai, M.; Horiuchi, M. Inhibition of the renin-angiotensin system and target organ protection. Hypertens. Res. 2009, 32, 229–237. [Google Scholar] [CrossRef]
- Sigurdsson, S.T.; Paulson, O.B.; Hoj Nielsen, A.; Strandgaard, S. Bradykinin antagonist counteracts the acute effect of both angiotensin-converting enzyme inhibition and of angiotensin receptor blockade on the lower limit of autoregulation of cerebral blood flow. J. Cereb. Blood Flow Metab. 2014, 34, 467–471. [Google Scholar] [CrossRef]
- Benicky, J.; Sanchez-Lemus, E.; Pavel, J.; Saavedra, J.M. Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol. Neurobiol. 2009, 29, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Baek, C.H.; Lee, R.B.; Chang, J.W.; Yang, W.S.; Lee, S.K. Anti-Fibrotic Effect of Losartan, an Angiotensin II Receptor Blocker, Is Mediated through Inhibition of ER Stress via Up-Regulation of SIRT1, Followed by Induction of HO-1 and Thioredoxin. Int. J. Mol. Sci. 2017, 18, 305. [Google Scholar] [CrossRef]
- Ferreira, A.J.; Santos, R.A.; Bradford, C.N.; Mecca, A.P.; Sumners, C.; Katovich, M.J.; Raizada, M.K. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension 2010, 55, 207–213. [Google Scholar] [CrossRef]
- Kim, M.Y.; Baik, S.K.; Park, D.H.; Jang, Y.O.; Suk, K.T.; Yea, C.J.; Lee, I.Y.; Kim, J.W.; Kim, H.S.; Kwon, S.O.; et al. Angiotensin receptor blockers are superior to angiotensin-converting enzyme inhibitors in the suppression of hepatic fibrosis in a bile duct-ligated rat model. J. Gastroenterol. 2008, 43, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Bramel, E.E.; Bagirzadeh, R.; Saqib, M.; Creamer, T.J.; Espinoza Camejo, W.A.; Roker, L.A.; Pardo Habashi, J.; Dietz, H.C.; Gallo MacFarlane, E. Distinct Contribution of Global and Regional Angiotensin II Type 1a Receptor Inactivation to Amelioration of Aortopathy in Tgfbr1 (M318R/+) Mice. Front. Cardiovasc. Med. 2022, 9, 936142. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, T.; Sun, H.; Pinchuk, I.V.; Milewicz, D.M.; Tilton, R.G.; Brasier, A.R. Deletion of NF-kappaB/RelA in Angiotensin II-Sensitive Mesenchymal Cells Blocks Aortic Vascular Inflammation and Abdominal Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1881–1890. [Google Scholar] [CrossRef]
- Habibi, J.; Aroor, A.R.; Das, N.A.; Manrique-Acevedo, C.M.; Johnson, M.S.; Hayden, M.R.; Nistala, R.; Wiedmeyer, C.; Chandrasekar, B.; DeMarco, V.G. The combination of a neprilysin inhibitor (sacubitril) and angiotensin-II receptor blocker (valsartan) attenuates glomerular and tubular injury in the Zucker Obese rat. Cardiovasc. Diabetol. 2019, 18, 40. [Google Scholar] [CrossRef]
- Tanaka, M.; Kaji, K.; Nishimura, N.; Asada, S.; Koizumi, A.; Matsuda, T.; Yorioka, N.; Tsuji, Y.; Fujinaga, Y.; Sato, S.; et al. Blockade of angiotensin II modulates insulin-like growth factor 1-mediated skeletal muscle homeostasis in experimental steatohepatitis. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119649. [Google Scholar] [CrossRef]
- Guilliams, M.; Bonnardel, J.; Haest, B.; Vanderborght, B.; Wagner, C.; Remmerie, A.; Bujko, A.; Martens, L.; Thoné, T.; Browaeys, R.; et al. Spatial proteogenomics reveals distinct and evolutionarily conserved hepatic macrophage niches. Cell 2022, 185, 379–396.e38. [Google Scholar] [CrossRef]
- Mathey, C.M.; Maj, C.; Scheer, A.B.; Fazaal, J.; Wedi, B.; Wieczorek, D.; Amann, P.M.; Löffler, H.; Koch, L.; Schöffl, C.; et al. Molecular Genetic Screening in Patients With ACE Inhibitor/Angiotensin Receptor Blocker-Induced Angioedema to Explore the Role of Hereditary Angioedema Genes. Front. Genet. 2022, 13, 914376. [Google Scholar] [CrossRef]
- Sotoodehnia, N.; Li, G.; Johnson, C.O.; Lemaitre, R.N.; Rice, K.M.; Rea, T.D.; Siscovick, D.S. Genetic variation in angiotensin-converting enzyme-related pathways associated with sudden cardiac arrest risk. Heart Rhythm 2009, 6, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Li, Y.; Wei, J.; Li, T.; Liao, B. Targeting Fibrosis: From Molecular Mechanisms to Advanced Therapies. Adv. Sci. 2025, 12, e2410416. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
| Clinical Characteristics | No SLD | MASLD | |||||
|---|---|---|---|---|---|---|---|
| Medication | SMD | Medication | SMD | ||||
| ARB, N = 2710 | ACEI, N = 6664 | ARB, N = 11,932 | ACEI, N = 28,229 | ||||
| Sex (Male) | 863 (31.8%) | 2123 (31.9%) | 0.001 | 7053 (59.1%) | 16,713 (59.2%) | 0.002 | |
| Age at recruitment | 60.70 ± 6.61 | 60.71 ± 6.58 | 0.003 | 60.38 ± 6.59 | 60.38 ± 6.61 | <0.001 | |
| Physical activity | 0.002 | 0.004 | |||||
| More than 4 times | 2290 (84.5%) | 5624 (84.4%) | 9066 (76.0%) | 21,490 (76.1%) | |||
| Under 4 times | 421 (15.5%) | 1040 (15.6%) | 2866 (24.0%) | 6739 (23.9%) | |||
| Body mass index (kg/m2) | 23.92 ± 2.31 | 23.94 ± 2.25 | 0.008 | 31.39 ± 5.00 | 31.36 ± 5.24 | 0.005 | |
| Waist circumference (cm) | 79.78 ± 7.26 | 79.82 ± 7.08 | 0.006 | 101.86 ± 12.09 | 101.82 ± 12.31 | 0.003 | |
| Type 2 diabetes | 320 (11.8%) | 794 (11.9%) | 0.003 | 4195 (35.2%) | 9906 (35.1%) | 0.001 | |
| Dyslipidemia | 1476 (54.4%) | 3629 (54.4%) | <0.001 | 8289 (69.5%) | 19,607 (69.5%) | <0.001 | |
| Hypertension | 2622 (96.7%) | 6449 (96.8%) | 0.002 | 11,672 (97.8%) | 27,598 (97.8%) | 0.004 | |
| ALT (U/L) | 18.82 ± 7.47 | 18.87 ± 7.78 | 0.006 | 27.53 ± 14.86 | 27.52 ± 16.72 | <0.001 | |
| GGT (U/L) | 24.15 ± 15.10 | 24.17 ± 14.94 | 0.001 | 46.90 ± 46.72 | 46.89 ± 45.93 | <0.001 | |
| Platelet (109/L) | 253.35 ± 62.08 | 253.11 ± 63.31 | 0.004 | 251.57 ± 62.71 | 251.59 ± 62.62 | <0.001 | |
| Albumin (g/L) | 4.53 ± 0.27 | 4.53 ± 0.26 | 0.001 | 4.51 ± 0.26 | 4.51 ± 0.26 | 0.002 | |
| FIB-4 score ≥ 1.45 | 1311 (48.4%) | 3222 (48.4%) | <0.001 | 4545 (38.1%) | 10,727 (38.0%) | 0.002 | |
| NFS ≥ −1.455 | 884 (32.6%) | 2202 (33.0%) | 0.009 | 6752 (56.6%) | 15,918 (56.4%) | 0.004 | |
| ASCVD risk score | 6.88 ± 6.11 | 6.95 ± 6.18 | 0.007 | 16.24 ± 13.44 | 16.03 ± 13.28 | 0.015 | |
| Before IPTW | ||||||
|---|---|---|---|---|---|---|
| Clinical Characteristics | Univariate Analysis | Multivariate Analysis | ||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Sex (Male) | 1.54 | 1.46, 1.62 | <0.001 | 1.38 | 1.29, 1.47 | <0.001 |
| Age at recruitment | 1.09 | 1.08, 1.09 | <0.001 | 1.08 | 1.08, 1.09 | <0.001 |
| Physical activity (Under 4 times) | 1.21 | 1.15, 1.28 | <0.001 | 1.18 | 1.12, 1.24 | <0.001 |
| Body mass index (kg/m2) | 1.02 | 1.01, 1.02 | <0.001 | 0.98 | 0.97, 0.99 | <0.001 |
| Waist circumference (cm) | 1.02 | 1.02, 1.02 | <0.001 | 1.02 | 1.02, 1.02 | <0.001 |
| Type 2 diabetes | 1.75 | 1.67, 1.83 | <0.001 | 1.51 | 1.43, 1.59 | <0.001 |
| Dyslipidemia | 1.53 | 1.45, 1.62 | <0.001 | 1.08 | 1.02, 1.14 | 0.013 |
| ALT (U/L) | 0.99 | 0.99, 1.0 | <0.001 | 0.99 | 0.99, 0.99 | <0.001 |
| GGT (U/L) | 1.00 | 1.00, 1.00 | <0.001 | 1.00 | 1.00, 1.00 | <0.001 |
| Platelet (109/L) | 1.00 | 1.00, 1.00 | <0.001 | 1.00 | 1.00, 1.00 | <0.001 |
| Albumin (g/L) | 0.40 | 0.37, 0.43 | <0.001 | 0.51 | 0.47, 0.56 | <0.001 |
| FIB-4 score ≥ 1.45 | 1.56 | 1.49, 1.63 | <0.001 | 1.16 | 1.09, 1.23 | <0.001 |
| NFS ≥ −1.455 | 1.88 | 1.79, 1.98 | <0.001 | 1.03 | 0.96, 1.11 | 0.421 |
| ASCVD risk score | 1.03 | 1.03, 1.03 | <0.001 | 1.01 | 1.00, 1.01 | <0.001 |
| ARB user | 0.94 | 0.89, 0.98 | 0.011 | 0.94 | 0.89, 0.99 | 0.023 |
| After IPTW | ||||||
| Clinical Characteristics | Univariate Analysis | Multivariate Analysis | ||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Sex (Male) | 1.53 | 1.46, 1.61 | <0.001 | 1.39 | 1.30, 1.48 | <0.001 |
| Age at recruitment | 1.09 | 1.08, 1.10 | <0.001 | 1.08 | 1.08, 1.09 | <0.001 |
| Physical activity (Under 4 times) | 1.21 | 1.15, 1.28 | <0.001 | 1.18 | 1.12, 1.24 | <0.001 |
| Body mass index (kg/m2) | 1.02 | 1.01, 1.02 | <0.001 | 0.98 | 0.97, 0.99 | <0.001 |
| Waist circumference (cm) | 1.02 | 1.02, 1.02 | <0.001 | 1.02 | 1.02, 1.02 | <0.001 |
| Type 2 diabetes | 1.75 | 1.67, 1.83 | <0.001 | 1.50 | 1.42, 1.59 | <0.001 |
| Dyslipidemia | 1.54 | 1.45, 1.62 | <0.001 | 1.08 | 1.02, 1.14 | 0.009 |
| ALT (U/L) | 0.99 | 0.99, 1.00 | <0.001 | 0.99 | 0.99, 0.99 | <0.001 |
| GGT (U/L) | 1.00 | 1.00, 1.00 | <0.001 | 1.00 | 1.00, 1.00 | <0.001 |
| Platelet (109/L) | 1.00 | 1.00, 1.00 | <0.001 | 1.00 | 1.00, 1.00 | <0.001 |
| Albumin (g/L) | 0.4 | 0.36, 0.43 | <0.001 | 0.51 | 0.46, 0.56 | <0.001 |
| FIB-4 score ≥ 1.45 | 1.55 | 1.48, 1.63 | <0.001 | 1.16 | 1.09, 1.24 | <0.001 |
| NFS ≥ −1.455 | 1.88 | 1.78, 1.97 | <0.001 | 1.03 | 0.96, 1.12 | 0.406 |
| ASCVD risk score | 1.03 | 1.03, 1.03 | <0.001 | 1.01 | 1.00, 1.01 | <0.001 |
| ARB user | 0.95 | 0.90, 1.00 | 0.029 | 0.94 | 0.90, 1.0 | 0.031 |
| Medication | Entire Cohort | ||||
|---|---|---|---|---|---|
| Number of Patients | Number of Events | Number of Events/ 100 Patient-Years | HR (95% CI) | p Value | |
| ARB | 15,413 | 4665 | 2.584 | Reference | |
| ACEI | 36,730 | 12,682 | 3.088 | 1.08 (1.05–1.12) | <0.001 |
| Medication | No SLD | ||||
| Number of Patients | Number of Events | Number of Events/ 100 Patient-Years | HR (95% CI) | p Value | |
| ARB | 2853 | 639 | 1.802 | Reference | |
| ACEI | 7015 | 1837 | 2.199 | 1.07 (0.97–1.17) | 0.162 |
| Medication | MASLD | ||||
| Number of Patients | Number of Events | Number of Events/ 100 Patient-Years | HR (95% CI) | p Value | |
| ARB | 12,560 | 4026 | 2.775 | Reference | |
| ACEI | 29,715 | 10,845 | 3.315 | 1.09 (1.05–1.13) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, T.; Seo, Y.J.; Lee, J.; Han, J.W.; Yang, H.; Yang, K. Class-Specific Effects of ARBs Versus ACE Inhibitors on Survival and Cardiovascular Outcomes in MASLD. Int. J. Mol. Sci. 2025, 26, 10061. https://doi.org/10.3390/ijms262010061
Ryu T, Seo YJ, Lee J, Han JW, Yang H, Yang K. Class-Specific Effects of ARBs Versus ACE Inhibitors on Survival and Cardiovascular Outcomes in MASLD. International Journal of Molecular Sciences. 2025; 26(20):10061. https://doi.org/10.3390/ijms262010061
Chicago/Turabian StyleRyu, Tom, Yeon Joo Seo, Jaejun Lee, Ji Won Han, Hyun Yang, and Keungmo Yang. 2025. "Class-Specific Effects of ARBs Versus ACE Inhibitors on Survival and Cardiovascular Outcomes in MASLD" International Journal of Molecular Sciences 26, no. 20: 10061. https://doi.org/10.3390/ijms262010061
APA StyleRyu, T., Seo, Y. J., Lee, J., Han, J. W., Yang, H., & Yang, K. (2025). Class-Specific Effects of ARBs Versus ACE Inhibitors on Survival and Cardiovascular Outcomes in MASLD. International Journal of Molecular Sciences, 26(20), 10061. https://doi.org/10.3390/ijms262010061







