From Protein Misfolding to Extracellular Matrix Disorganisation: Understanding Disease Pathology in Rare Skeletal Dysplasias
Abstract
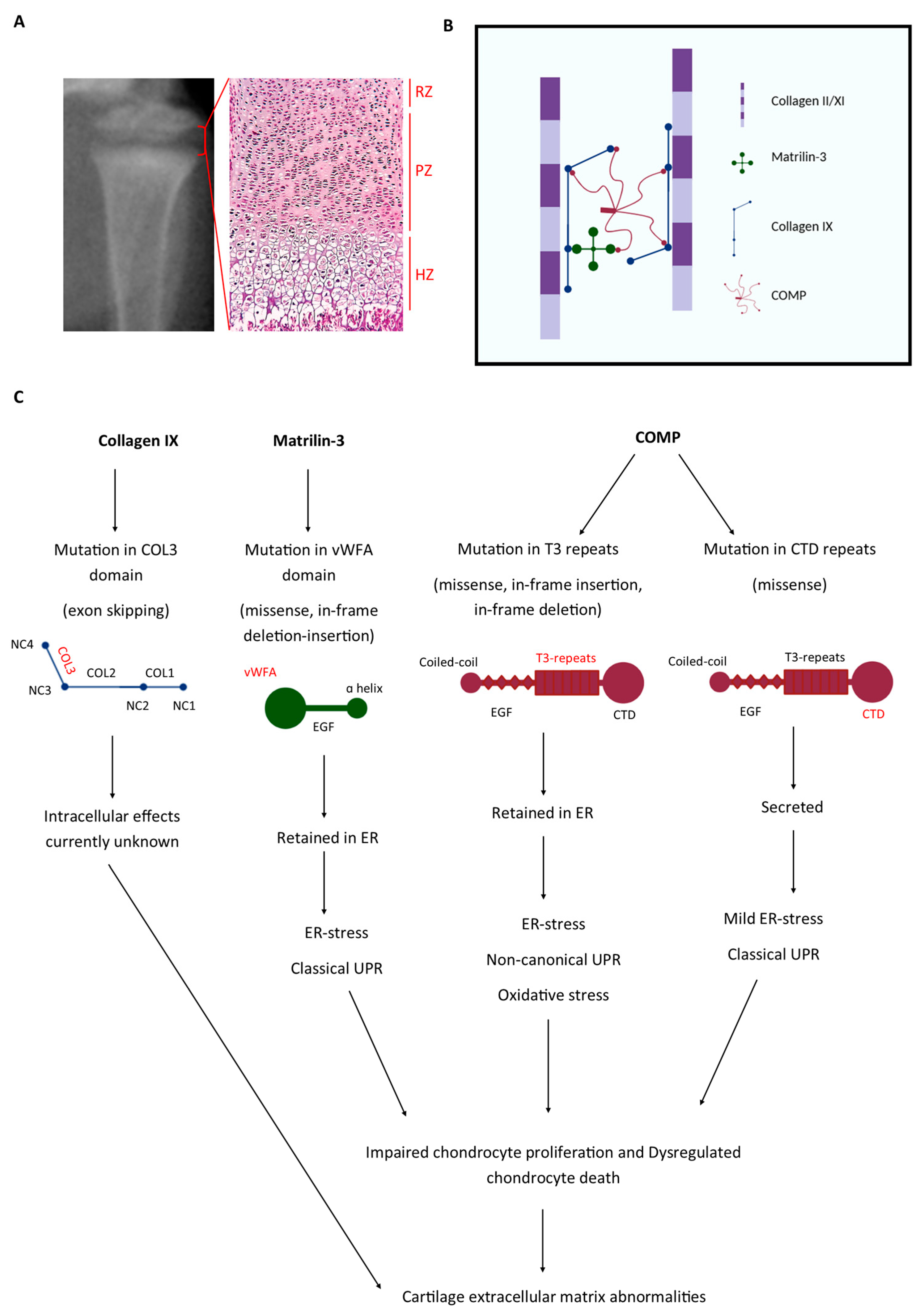
1. Introduction
2. Pseudoachondroplasia and Multiple Epiphyseal Dysplasia
2.1. Type IX Collagen
| Gene | Gene Location | Protein Change | Reference |
|---|---|---|---|
| COL9A1 | IVS8 as + 3, ins A | Exon 8 and/or 10 skipping resulting in the in-frame deletion of 25/21/49 amino acids from the COL3 domain | [5] |
| COL9A2 | IVS3 ds − 1, G > A | Exon 3 skipping resulting in the in-frame deletion of 12 amino acids from the COL3 domain | [25,28] |
| COL9A2 | IVS3 ds − 1, G > C | [29] | |
| COL9A2 | IVS3 ds, G > A | [30] | |
| COL9A2 | IVS3 ds, G > C | [30] | |
| COL9A2 | IVS3 ds + 2, T > C | [25,26] | |
| COL9A2 | IVS3 ds + 4, A > C | [30] | |
| COL9A2 | IVS3 ds + 5, G > C | [28] | |
| COL9A2 | IVS3 ds + 6, T > G | [21] | |
| COL9A3 | IVS2 as − 1, G > A | [22,31] | |
| COL9A3 | IVS2 as − 2, A > T | [7] | |
| COL9A3 | IVS2 as − 2, A > G | [30] | |
| COL9A3 | IVS3 ds + 5, G > A | [27] | |
| COL9A3 | Exon 2, c.G104 > A | p.Gly35Asp missense mutation in the COL3 domain | [23] |
2.2. Cartilage Oligomeric Matrix Protein
2.3. Matrilin-3
3. Disease Mechanisms Determined in Mouse Models of PSACH-MED: ER Stress as a Common Denominator
Targeting ER Stress Is an Attractive Therapeutic Target in Genetic Bone Diseases
4. Changes in ECM Structure/Function in Cartilage in PSACH-MED
4.1. Understanding the Effects of Mutant Protein Expression in the Cartilage ECM in PSACH-MED
4.1.1. Secretion of Mutant Matrilin-3 Disturbs the Collagen Networks in Growth Plate Cartilage
4.1.2. Secretion of Mutant p.T585M COMP Alters Its Localisation in Growth Plate Cartilage
4.1.3. Secretion of Mutant p.Asp469del COMP Alters Localisation of Other Components of the ECM
4.2. ER Stress or Matrix Integrity—Which Is the Driver of Osteoarthritis in PSACH-MED?
5. Changes in ECM Structure/Function in Muscle, Ligaments, and Tendons in PSACH-MED
5.1. PSACH and MED Patients Present with Mild Myopathy
5.1.1. Myopathy in EDM3
5.1.2. Myopathy in EDM1
5.1.3. Myopathy in EDM5
5.2. Understanding the Mechanisms of PSACH-MED-Associated Myopathy
5.2.1. Myopathy in Transgenic Knock-In Mouse Models of PSACH and MED
5.2.2. Changes in Musculoskeletal ECM Structure/Function Due to the Expression of Mutant Type IX Collagen
5.2.3. Changes in Musculoskeletal ECM Structure/Function Due to the Expression of Mutant COMP
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBZ | Carbamazepine |
| CK | Creatine kinase |
| COL | Collagenous |
| COMP | Cartilage oligomeric matrix protein |
| CTD | C-terminal domain |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| ER | Endoplasmic reticulum |
| ERAD | ER-assisted protein degradation |
| FACIT | Fibril-associated collagens with interrupted triple helices |
| GSD | Genetic skeletal disease |
| MCDS | Metaphyseal chondrodysplasia type Schmid |
| MED | Multiple epiphyseal dysplasia |
| NC | Non-collagenous |
| PSACH | Pseudoachondroplasia |
| T3 | Type III |
| Tg | Thyroglobulin |
| TSP5 | Thrombospondin 5 |
| UPR | Unfolded protein response |
| vWFA | Von Willebrand factor A |
References
- Mortier, G.R.; Cohn, D.H.; Cormier-Daire, V.; Hall, C.; Krakow, D.; Mundlos, S.; Nishimura, G.; Robertson, S.; Sangiorgi, L.; Savarirayan, R.; et al. Nosology and classification of genetic skeletal disorders: 2019 revision. Am. J. Med. Genet. A 2019, 179, 2393–2419. [Google Scholar] [CrossRef]
- Briggs, M.D.; Chapman, K.L. Pseudoachondroplasia and multiple epiphyseal dysplasia: Mutation review, molecular interactions, and genotype to phenotype correlations. Hum. Mutat. 2002, 19, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.D.; Hoffman, S.M.G.; King, L.M.; Olsen, A.S.; Mohrenweiser, H.; Leroy, J.G.; Mortier, G.R.; Rimoin, D.L.; Lachman, R.S.; Gaines, E.S.; et al. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat. Genet. 1995, 10, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.L.; Mortier, G.R.; Chapman, K.; Loughlin, J.; Grant, M.E.; Briggs, M.D. Mutations in the region encoding the von Willebrand factor A domain of matrilin-3 are associated with multiple epiphyseal dysplasia. Nat. Genet. 2001, 28, 393–396. [Google Scholar] [CrossRef]
- Czarny-Ratajczak, M.; Lohiniva, J.; Rogala, P.; Kozlowski, K.; Perälä, M.; Carter, L.; Spector, T.D.; Kolodziej, L.; Seppänen, U.; Glazar, R.; et al. A mutation in COL9A1 causes multiple epiphyseal dysplasia: Further evidence for locus heterogeneity. Am. J. Hum. Genet. 2001, 69, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Muragaki, Y.; Nishimura, I.; Henney, A.; Ninomiya, Y.; Olsen, B.R. The alpha 1 (IX) collagen gene gives rise to two different transcripts in both mouse embryonic and human fetal RNA. Proc. Natl. Acad. Sci. USA 1990, 87, 2400–2404. [Google Scholar] [CrossRef]
- Paassilta, P.; Lohiniva, J.; Annunen, S.; Bonaventure, J.; Le Merrer, M.; Pai, L.; Ala-Kokko, L. COL9A3: A Third Locus for Multiple Epiphyseal Dysplasia. Am. J. Human. Genet. 1999, 64, 1036–1044. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed]
- van der Rest, M.; Mayne, R. Type IX Collagen. In Structure and Function of Collagen Types; Mayne, R., Burgeson, R.E., Eds.; Academic Press: Cambridge, MA, USA, 1987; pp. 195–221. [Google Scholar]
- Nishimura, I.; Murgaki, Y.; Hayashi, M.; Ninomiya, Y.; Olsen, B.R. Tissue-Specific Expression of Type IX Collagen. Struct. Mol. Biol. Pathol. Collagen 1990, 580, 112–119. [Google Scholar] [CrossRef]
- Pihlajamaa, T.; Vuoristo, M.M.; Annunen, S.; Perälä, M.; Prockop, D.J.; Ala-Kokko, L. Human COL9A1 and COL9A2 genes. Two genes of 90 and 15 kb code for similar polypeptides of the same collagen molecule. Matrix Biol. 1998, 17, 237–241. [Google Scholar] [CrossRef]
- Savontaus, M.; Ihanamäki, T.; Perälä, M.; Metsäranta, M.; Sandberg-Lall, M.; Vuorio, E. Expression of type II and IX collagen isoforms during normal and pathological cartilage and eye development. Histochem. Cell Biol. 1998, 110, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Eyre, D.R.; Apon, S.; Wu, J.-J.; Ericsson, L.H.; Walsh, K.A. Collagen type IX: Evidence for covalent linkages to type II collagen in cartilage. FEBS Lett. 1987, 220, 337–341. [Google Scholar] [CrossRef]
- van der Rest, M.; Mayne, R. Type IX collagen proteoglycan from cartilage is covalently cross-linked to type II collagen. J. Biol. Chem. 1988, 263, 1615–1618. [Google Scholar] [CrossRef]
- Eyre, D.R.; Wu, J.J.; Fernandes, R.J.; Pietka, T.A.; Weis, M.A. Recent developments in cartilage research: Matrix biology of the collagen II/IX/XI heterofibril network. Biochem. Soc. Trans. 2002, 30, 893–899. [Google Scholar] [CrossRef]
- Vaughan, L.; Mendler, M.; Huber, S.; Bruckner, P.; Winterhalter, K.H.; Irwin, M.I.; Mayne, R. D-periodic distribution of collagen type IX along cartilage fibrils. J. Cell Biol. 1988, 106, 991–997. [Google Scholar] [CrossRef]
- Blaschke, U.K.; Eikenberry, E.F.; Hulmes, D.J.; Galla, H.J.; Bruckner, P. Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J. Biol. Chem. 2000, 275, 10370–10378. [Google Scholar] [CrossRef] [PubMed]
- Blumbach, K.; Bastiaansen-Jenniskens, Y.M.; DeGroot, J.; Paulsson, M.; van Osch, G.J.; Zaucke, F. Combined role of type IX collagen and cartilage oligomeric matrix protein in cartilage matrix assembly: Cartilage oligomeric matrix protein counteracts type IX collagen-induced limitation of cartilage collagen fibril growth in mouse chondrocyte cultures. Arthritis Rheum. 2009, 60, 3676–3685. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.D.; Choi, H.; Warman, M.L.; Loughlin, J.A.; Wordsworth, P.; Sykes, B.C.; Irven, C.M.; Smith, M.; Wynne-Davies, R.; Lipson, M.H.; et al. Genetic mapping of a locus for multiple epiphyseal dysplasia (EDM2) to a region of chromosome 1 containing a type IX collagen gene. Am. J. Hum. Genet. 1994, 55, 678–684. [Google Scholar]
- Muragaki, Y.; Mariman, E.C.M.; Van Beersum, S.E.C.; Perala, M.; Van Mourik, J.B.A.; Warman, M.L.; Olsen, B.R.; Hamel, B.C.J. A mutation in the gene encoding the α2 chain of the fibril-associated collagen IX, COL9A2, causes multiple epiphyseal dysplasia (EDM2). Nat. Genet. 1996, 12, 103–105. [Google Scholar] [CrossRef]
- Spayde, E.C.; Joshi, A.P.; Wilcox, W.R.; Briggs, M.; Cohn, D.H.; Olsen, B.R. Exon skipping mutation in the COL9A2 gene in a family with multiple epiphyseal dysplasia. Matrix Biol. 2000, 19, 121–128. [Google Scholar] [CrossRef]
- Bönnemann, C.G.; Cox, G.F.; Shapiro, F.; Wu, J.J.; Feener, C.A.; Thompson, T.G.; Anthony, D.C.; Eyre, D.R.; Darras, B.T.; Kunkel, L.M. A mutation in the alpha 3 chain of type IX collagen causes autosomal dominant multiple epiphyseal dysplasia with mild myopathy. Proc. Natl. Acad. Sci. USA 2000, 97, 1212–1217. [Google Scholar] [CrossRef]
- Jeong, C.; Lee, J.Y.; Kim, J.; Chae, H.; Park, H.I.; Kim, M.; Kim, O.H.; Kim, P.; Lee, Y.K.; Jung, J. Novel COL9A3 mutation in a family diagnosed with multiple epiphyseal dysplasia: A case report. BMC Musculoskelet. Disord. 2014, 15, 371. [Google Scholar] [CrossRef]
- van Mourik, J.B.; Buma, P.; Wilcox, W.R. Electron microscopical study in multiple epiphyseal dysplasia type II. Ultrastruct. Pathol. 1998, 22, 249–251. [Google Scholar] [CrossRef]
- Jackson, G.C.; Marcus-Soekarman, D.; Stolte-Dijkstra, I.; Verrips, A.; Taylor, J.A.; Briggs, M.D. Type IX collagen gene mutations can result in multiple epiphyseal dysplasia that is associated with osteochondritis dissecans and a mild myopathy. Am. J. Med. Genet. A 2010, 152a, 863–869. [Google Scholar] [CrossRef] [PubMed]
- van Mourik, J.B.; Hamel, B.C.; Mariman, E.C. A large family with multiple epiphyseal dysplasia linked to COL9A2 gene. Am. J. Med. Genet. 1998, 77, 234–240. [Google Scholar] [CrossRef]
- Nakashima, E.; Kitoh, H.; Maeda, K.; Haga, N.; Kosaki, R.; Mabuchi, A.; Nishimura, G.; Ohashi, H.; Ikegawa, S. Novel COL9A3 mutation in a family with multiple epiphyseal dysplasia. Am. J. Med. Genet. A 2005, 132a, 181–184. [Google Scholar] [CrossRef]
- Holden, P.; Canty, E.G.; Mortier, G.R.; Zabel, B.; Spranger, J.; Carr, A.; Grant, M.E.; Loughlin, J.A.; Briggs, M.D. Identification of Novel pro-α2(IX) Collagen Gene Mutations in Two Families with Distinctive Oligo-Epiphyseal Forms of Multiple Epiphyseal Dysplasia. Am. J. Hum. Genet. 1999, 65, 31–38. [Google Scholar] [CrossRef]
- Fiedler, J.; Stöve, J.; Heber, F.; Brenner, R.E. Clinical phenotype and molecular diagnosis of multiple epiphyseal dysplasia with relative hip sparing during childhood (EDM2). Am. J. Med. Genet. 2002, 112, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.C.; Mittaz-Crettol, L.; Taylor, J.A.; Mortier, G.R.; Spranger, J.; Zabel, B.; Le Merrer, M.; Cormier-Daire, V.; Hall, C.M.; Offiah, A.; et al. Pseudoachondroplasia and multiple epiphyseal dysplasia: A 7-year comprehensive analysis of the known disease genes identify novel and recurrent mutations and provides an accurate assessment of their relative contribution. Hum. Mutat. 2012, 33, 144–157. [Google Scholar] [CrossRef]
- Lohiniva, J.; Paassilta, P.; Seppänen, U.; Vierimaa, O.; Kivirikko, S.; Ala-Kokko, L. Splicing mutations in the COL3 domain of collagen IX cause multiple epiphyseal dysplasia. Am. J. Med. Genet. 2000, 90, 216–222. [Google Scholar] [CrossRef]
- Hedbom, E.; Antonsson, P.; Hjerpe, A.; Aeschlimann, D.; Paulsson, M.; Rosa-Pimentel, E.; Sommarin, Y.; Wendel, M.; Oldberg, A.; Heinegård, D. Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J. Biol. Chem. 1992, 267, 6132–6136. [Google Scholar] [CrossRef]
- Murphy, J.M.; Heinegård, D.; McIntosh, A.; Sterchi, D.; Barry, F.P. Distribution of cartilage molecules in the developing mouse joint. Matrix Biol. 1999, 18, 487–497. [Google Scholar] [CrossRef]
- Carlson, C.B.; Lawler, J.; Mosher, D.F. Structures of thrombospondins. Cell Mol. Life Sci. 2008, 65, 672–686. [Google Scholar] [CrossRef]
- Halász, K.; Kassner, A.; Mörgelin, M.; Heinegård, D. COMP acts as a catalyst in collagen fibrillogenesis. J. Biol. Chem. 2007, 282, 31166–31173. [Google Scholar] [CrossRef]
- Schulz, J.N.; Nüchel, J.; Niehoff, A.; Bloch, W.; Schönborn, K.; Hayashi, S.; Kamper, M.; Brinckmann, J.; Plomann, M.; Paulsson, M.; et al. COMP-assisted collagen secretion--a novel intracellular function required for fibrosis. J. Cell Sci. 2016, 129, 706–716. [Google Scholar] [CrossRef]
- Rosenberg, K.; Olsson, H.; Mörgelin, M.; Heinegård, D. Cartilage Oligomeric Matrix Protein Shows High Affinity Zinc-dependent Interaction with Triple Helical Collagen. J. Biol. Chem. 1998, 273, 20397–20403. [Google Scholar] [CrossRef]
- Thur, J.; Rosenberg, K.; Nitsche, D.P.; Pihlajamaa, T.; Ala-Kokko, L.; Heinegård, D.; Paulsson, M.; Maurer, P. Mutations in Cartilage Oligomeric Matrix Protein Causing Pseudoachondroplasia and Multiple Epiphyseal Dysplasia Affect Binding of Calcium and Collagen I, II, and IX. J. Biol. Chem. 2001, 276, 6083–6092. [Google Scholar] [CrossRef] [PubMed]
- Holden, P.; Meadows, R.S.; Chapman, K.L.; Grant, M.E.; Kadler, K.E.; Briggs, M.D. Cartilage Oligomeric Matrix Protein Interacts with Type IX Collagen, and Disruptions to These Interactions Identify a Pathogenetic Mechanism in a Bone Dysplasia Family. J. Biol. Chem. 2001, 276, 6046–6055. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, P.E.; Chen, F.S.; Moergelin, M.; Carlson, C.S.; Leslie, M.P.; Perris, R.; Fang, C. Matrix-matrix interaction of cartilage oligomeric matrix protein and fibronectin. Matrix Biol. 2002, 21, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.H.; Herndon, M.E.; Patel, N.; Hecht, J.T.; Tuan, R.S.; Lawler, J. Interaction of cartilage oligomeric matrix protein/thrombospondin 5 with aggrecan. J. Biol. Chem. 2007, 282, 24591–24598. [Google Scholar] [CrossRef]
- Haudenschild, D.R.; Hong, E.; Yik, J.H.; Chromy, B.; Mörgelin, M.; Snow, K.D.; Acharya, C.; Takada, Y.; Di Cesare, P.E. Enhanced activity of transforming growth factor β1 (TGF-β1) bound to cartilage oligomeric matrix protein. J. Biol. Chem. 2011, 286, 43250–43258. [Google Scholar] [CrossRef]
- Ishida, K.; Acharya, C.; Christiansen, B.A.; Yik, J.H.; DiCesare, P.E.; Haudenschild, D.R. Cartilage oligomeric matrix protein enhances osteogenesis by directly binding and activating bone morphogenetic protein-2. Bone 2013, 55, 23–35. [Google Scholar] [CrossRef]
- Chen, F.H.; Thomas, A.O.; Hecht, J.T.; Goldring, M.B.; Lawler, J. Cartilage oligomeric matrix protein/thrombospondin 5 supports chondrocyte attachment through interaction with integrins. J. Biol. Chem. 2005, 280, 32655–32661. [Google Scholar] [CrossRef]
- Briggs, M.D.; Brock, J.; Ramsden, S.C.; Bell, P.A. Genotype to phenotype correlations in cartilage oligomeric matrix protein associated chondrodysplasias. Eur. J. Hum. Genet. 2014, 22, 1278–1282. [Google Scholar] [CrossRef]
- Svensson, L.; Aszódi, A.; Heinegård, D.; Hunziker, E.B.; Reinholt, F.P.; Fässler, R.; Oldberg, A. Cartilage oligomeric matrix protein-deficient mice have normal skeletal development. Mol. Cell Biol. 2002, 22, 4366–4371. [Google Scholar] [CrossRef]
- Délot, E.; King, L.M.; Briggs, M.D.; Wilcox, W.R.; Cohn, D.H. Trinucleotide expansion mutations in the cartilage oligomeric matrix protein (COMP) gene. Hum. Mol. Genet. 1999, 8, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Zankl, A.; Jackson, G.C.; Crettol, L.M.; Taylor, J.; Elles, R.; Mortier, G.R.; Spranger, J.; Zabel, B.; Unger, S.; Merrer, M.L.; et al. Preselection of cases through expert clinical and radiological review significantly increases mutation detection rate in multiple epiphyseal dysplasia. Eur. J. Hum. Genet. 2007, 15, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.; Jackson, G.C.; Barker, F.S.; Nundlall, S.; Bella, J.; Wright, M.J.; Mortier, G.R.; Neas, K.; Thompson, E.; Elles, R.; et al. Novel and recurrent mutations in the C-terminal domain of COMP cluster in two distinct regions and result in a spectrum of phenotypes within the pseudoachondroplasia—Multiple epiphyseal dysplasia disease group. Hum. Mutat. 2005, 25, 593–594. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.D.; Mortier, G.R.; Cole, W.G.; King, L.M.; Golik, S.S.; Bonaventure, J.; Nuytinck, L.; De Paepe, A.; Leroy, J.G.; Biesecker, L.; et al. Diverse mutations in the gene for cartilage oligomeric matrix protein in the pseudoachondroplasia-multiple epiphyseal dysplasia disease spectrum. Am. J. Hum. Genet. 1998, 62, 311–319. [Google Scholar] [CrossRef]
- Song, H.R.; Lee, K.S.; Li, Q.W.; Koo, S.K.; Jung, S.C. Identification of cartilage oligomeric matrix protein (COMP) gene mutations in patients with pseudoachondroplasia and multiple epiphyseal dysplasia. J. Hum. Genet. 2003, 48, 222–225. [Google Scholar] [CrossRef]
- Deere, M.; Sanford, T.; Ferguson, H.L.; Daniels, K.; Hecht, J.T. Identification of twelve mutations in cartilage oligomeric matrix protein (COMP) in patients with pseudoachondroplasia. Am. J. Med. Genet. 1998, 80, 510–513. [Google Scholar] [CrossRef]
- Kennedy, J.; Jackson, G.; Ramsden, S.; Taylor, J.; Newman, W.; Wright, M.J.; Donnai, D.; Elles, R.; Briggs, M.D. COMP mutation screening as an aid for the clinical diagnosis and counselling of patients with a suspected diagnosis of pseudoachondroplasia or multiple epiphyseal dysplasia. Eur. J. Hum. Genet. 2005, 13, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Mabuchi, A.; Manabe, N.; Haga, N.; Kitoh, H.; Ikeda, T.; Kawaji, H.; Tamai, K.; Hamada, J.; Nakamura, S.; Brunetti-Pierri, N.; et al. Novel types of COMP mutations and genotype-phenotype association in pseudoachondroplasia and multiple epiphyseal dysplasia. Hum. Genet. 2003, 112, 84–90. [Google Scholar] [CrossRef]
- Mabuchi, A.; Haga, N.; Ikeda, T.; Manabe, N.; Ohashi, H.; Takatori, Y.; Nakamura, K.; Ikegawa, S. Novel mutation in exon 18 of the cartilage oligomeric matrix protein gene causes a severe pseudoachondroplasia. Am. J. Med. Genet. 2001, 104, 135–139. [Google Scholar] [CrossRef]
- Schmitz, M.; Becker, A.; Schmitz, A.; Weirich, C.; Paulsson, M.; Zaucke, F.; Dinser, R. Disruption of Extracellular Matrix Structure May Cause Pseudoachondroplasia Phenotypes in the Absence of Impaired Cartilage Oligomeric Matrix Protein Secretion. J. Biol. Chem. 2006, 281, 32587–32595. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.J.; Zhang, Z.; He, J.W.; Fu, W.Z.; Wang, C.; Zhang, Z.L. Identification of two novel mutations in the COMP gene in six families with pseudoachondroplasia. Mol. Med. Rep. 2016, 14, 2180–2186. [Google Scholar] [CrossRef]
- Susic, S.; McGrory, J.; Ahier, J.; Cole, W.G. Multiple epiphyseal dysplasia and pseudoachondroplasia due to novel mutations in the calmodulin-like repeats of cartilage oligomeric matrix protein. Clin. Genet. 1997, 51, 219–224. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; He, J.; Zhang, R.; Cao, Y.; Liu, X. A novel mutation in exon 11 of COMP gene in a Chinese family with pseudoachondroplasia. Genes. Dis. 2019, 6, 47–55. [Google Scholar] [CrossRef]
- Guo, B.-B.; Jin, J.-Y.; Yuan, Z.-Z.; Zeng, L.; Xiang, R. A Novel COMP Mutated Allele Identified in a Chinese Family with Pseudoachondroplasia. BioMed Res. Int. 2021, 2021, 6678531. [Google Scholar] [CrossRef]
- Newman, B.; Donnah, D.; Briggs, M.D. Molecular diagnosis is important to confirm suspected pseudoachondroplasia. J. Med. Genet. 2000, 37, 64–65. [Google Scholar] [CrossRef]
- Ballo, R.; Briggs, M.D.; Cohn, D.H.; Knowlton, R.G.; Beighton, P.H.; Ramesar, R.S. Multiple epiphyseal dysplasia, ribbing type: A novel point mutation in the COMP gene in a South African family. Am. J. Med. Genet. 1997, 68, 396–400. [Google Scholar] [CrossRef]
- Wagener, R.; Kobbe, B.; Paulsson, M. Primary structure of matrilin-3, a new member of a family of extracellular matrix proteins related to cartilage matrix protein (matrilin-1) and von Willebrand factor. FEBS Lett. 1997, 413, 129–134. [Google Scholar] [CrossRef]
- Whittaker, C.A.; Hynes, R.O. Distribution and evolution of von Willebrand/integrin A domains: Widely dispersed domains with roles in cell adhesion and elsewhere. Mol. Biol. Cell 2002, 13, 3369–3387. [Google Scholar] [CrossRef]
- Jackson, G.C.; Barker, F.S.; Jakkula, E.; Czarny-Ratajczak, M.; Mäkitie, O.; Cole, W.G.; Wright, M.J.; Smithson, S.F.; Suri, M.; Rogala, P.; et al. Missense mutations in the beta strands of the single A-domain of matrilin-3 result in multiple epiphyseal dysplasia. J. Med. Genet. 2004, 41, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, S.L.; Jackson, G.C.; Leighton, M.P.; Wagener, R.; Mäkitie, O.; Cole, W.G.; Briggs, M.D. Multiple epiphyseal dysplasia mutations in MATN3 cause misfolding of the A-domain and prevent secretion of mutant matrilin-3. Hum. Mutat. 2005, 26, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Nakashima, E.; Horikoshi, T.; Mabuchi, A.; Ikegawa, S. Mutation in the von Willebrand factor-A domain is not a prerequisite for the MATN3 mutation in multiple epiphyseal dysplasia. Am. J. Med. Genet. A 2005, 136, 285–286. [Google Scholar] [CrossRef]
- Mabuchi, A.; Haga, N.; Maeda, K.; Nakashima, E.; Manabe, N.; Hiraoka, H.; Kitoh, H.; Kosaki, R.; Nishimura, G.; Ohashi, H.; et al. Novel and recurrent mutations clustered in the von Willebrand factor A domain of MATN3 in multiple epiphyseal dysplasia. Hum. Mutat. 2004, 24, 439–440. [Google Scholar] [CrossRef]
- Mostert, A.K.; Dijkstra, P.F.; Jansen, B.R.; van Horn, J.R.; de Graaf, B.; Heutink, P.; Lindhout, D. Familial multiple epiphyseal dysplasia due to a matrilin-3 mutation: Further delineation of the phenotype including 40 years follow-up. Am. J. Med. Genet. A 2003, 120a, 490–497. [Google Scholar] [CrossRef]
- Fresquet, M.; Jowitt, T.A.; Ylöstalo, J.; Coffey, P.; Meadows, R.S.; Ala-Kokko, L.; Thornton, D.J.; Briggs, M.D. Structural and functional characterization of recombinant matrilin-3 A-domain and implications for human genetic bone diseases. J. Biol. Chem. 2007, 282, 34634–34643. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.T.; Tran, L.H.; Hoang, L.T.; Doan, P.K.T.; Nguyen, T.T.; Nguyen, T.H.; Tran, H.T.; Hoang, H.; Chu, H.H.; Luong, A.L.T. A novel p.A191D matrilin-3 variant in a Vietnamese family with multiple epiphyseal dysplasia: A case report. BMC Musculoskelet. Disord. 2020, 21, 216. [Google Scholar] [CrossRef]
- Leighton, M.P.; Nundlall, S.; Starborg, T.; Meadows, R.S.; Suleman, F.; Knowles, L.; Wagener, R.; Thornton, D.J.; Kadler, K.E.; Boot-Handford, R.P.; et al. Decreased chondrocyte proliferation and dysregulated apoptosis in the cartilage growth plate are key features of a murine model of epiphyseal dysplasia caused by a matn3 mutation. Hum. Mol. Genet. 2007, 16, 1728–1741. [Google Scholar] [CrossRef]
- Nundlall, S.; Rajpar, M.H.; Bell, P.A.; Clowes, C.; Zeeff, L.A.H.; Gardner, B.; Thornton, D.J.; Boot-Handford, R.P.; Briggs, M.D. An unfolded protein response is the initial cellular response to the expression of mutant matrilin-3 in a mouse model of multiple epiphyseal dysplasia. Cell Stress Chaperones 2010, 15, 835–849. [Google Scholar] [CrossRef]
- Suleman, F.; Gualeni, B.; Gregson, H.J.; Leighton, M.P.; Piróg, K.A.; Edwards, S.; Holden, P.; Boot-Handford, R.P.; Briggs, M.D. A novel form of chondrocyte stress is triggered by a COMP mutation causing pseudoachondroplasia. Hum. Mutat. 2012, 33, 218–231. [Google Scholar] [CrossRef]
- Schmitz, M.; Niehoff, A.; Miosge, N.; Smyth, N.; Paulsson, M.; Zaucke, F. Transgenic mice expressing D469Delta mutated cartilage oligomeric matrix protein (COMP) show growth plate abnormalities and sternal malformations. Matrix Biol. 2008, 27, 67–85. [Google Scholar] [CrossRef]
- Posey, K.L.; Yang, Y.; Veerisetty, A.C.; Sharan, S.K.; Hecht, J.T. Model systems for studying skeletal dysplasias caused by TSP-5/COMP mutations. Cell Mol. Life Sci. 2008, 65, 687–699. [Google Scholar] [CrossRef]
- Posey, K.L.; Veerisetty, A.C.; Liu, P.; Wang, H.R.; Poindexter, B.J.; Bick, R.; Alcorn, J.L.; Hecht, J.T. An inducible cartilage oligomeric matrix protein mouse model recapitulates human pseudoachondroplasia phenotype. Am. J. Pathol. 2009, 175, 1555–1563. [Google Scholar] [CrossRef]
- Piróg-Garcia, K.A.; Meadows, R.S.; Knowles, L.; Heinegård, D.; Thornton, D.J.; Kadler, K.E.; Boot-Handford, R.P.; Briggs, M.D. Reduced cell proliferation and increased apoptosis are significant pathological mechanisms in a murine model of mild pseudoachondroplasia resulting from a mutation in the C-terminal domain of COMP. Hum. Mol. Genet. 2007, 16, 2072–2088. [Google Scholar] [CrossRef] [PubMed]
- Gualeni, B.; Rajpar, M.H.; Kellogg, A.; Bell, P.A.; Arvan, P.; Boot-Handford, R.P.; Briggs, M.D. A novel transgenic mouse model of growth plate dysplasia reveals that decreased chondrocyte proliferation due to chronic ER stress is a key factor in reduced bone growth. Dis. Model. Mech. 2013, 6, 1414–1425. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.H.; Rajpar, M.H.; Preziosi, R.; Briggs, M.D.; Boot-Handford, R.P. Increased classical endoplasmic reticulum stress is sufficient to reduce chondrocyte proliferation rate in the growth plate and decrease bone growth. PLoS ONE 2015, 10, e0117016. [Google Scholar] [CrossRef]
- Boot-Handford, R.P.; Briggs, M.D. The unfolded protein response and its relevance to connective tissue diseases. Cell Tissue Res. 2010, 339, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Briggs, M.D.; Dennis, E.P.; Dietmar, H.F.; Pirog, K.A. New developments in chondrocyte ER stress and related diseases. F1000Res 2020, 9, F1000. [Google Scholar] [CrossRef]
- Mullan, L.A.; Mularczyk, E.J.; Kung, L.H.; Forouhan, M.; Wragg, J.M.; Goodacre, R.; Bateman, J.F.; Swanton, E.; Briggs, M.D.; Boot-Handford, R.P. Increased intracellular proteolysis reduces disease severity in an ER stress-associated dwarfism. J. Clin. Investig. 2017, 127, 3861–3865. [Google Scholar] [CrossRef]
- Forouhan, M.; Sonntag, S.; Boot-Handford, R.P. Carbamazepine reduces disease severity in a mouse model of metaphyseal chondrodysplasia type Schmid caused by a premature stop codon (Y632X) in the Col10a1 gene. Hum. Mol. Genet. 2018, 27, 3840–3853. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.T.; Coustry, F.; Veerisetty, A.C.; Hossain, M.G.; Posey, K.L. Resveratrol Reduces COMPopathy in Mice Through Activation of Autophagy. JBMR Plus 2021, 5, e10456. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.P.; Watson, R.N.; McPate, F.; Briggs, M.D. Curcumin Reduces Pathological Endoplasmic Reticulum Stress through Increasing Proteolysis of Mutant Matrilin-3. Int. J. Mol. Sci. 2023, 24, 1496. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.T.; Veerisetty, A.C.; Hossain, M.G.; Chiu, F.; Posey, K.L. CurQ+, a Next-Generation Formulation of Curcumin, Ameliorates Growth Plate Chondrocyte Stress and Increases Limb Growth in a Mouse Model of Pseudoachondroplasia. Int. J. Mol. Sci. 2023, 24, 3845. [Google Scholar] [CrossRef]
- Piróg, K.A.; Briggs, M.D. Skeletal dysplasias associated with mild myopathy-a clinical and molecular review. J. Biomed. Biotechnol. 2010, 2010, 686457. [Google Scholar] [CrossRef]
- Jakkula, E.; Lohiniva, J.; Capone, A.; Bonafe, L.; Marti, M.; Schuster, V.; Giedion, A.; Eich, G.; Boltshauser, E.; Ala-Kokko, L.; et al. A recurrent R718W mutation in COMP results in multiple epiphyseal dysplasia with mild myopathy: Clinical and pathogenetic overlap with collagen IX mutations. J. Med. Genet. 2003, 40, 942–948. [Google Scholar] [CrossRef]
- Piróg, K.A.; Katakura, Y.; Mironov, A.; Briggs, M.D. Mild Myopathy Is Associated with COMP but Not MATN3 Mutations in Mouse Models of Genetic Skeletal Diseases. PLoS ONE 2013, 8, e82412. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Zunino, L.; Webbon, P.M.; Heinegård, D. The distribution of Cartilage Oligomeric Matrix Protein (COMP) in tendon and its variation with tendon site, age and load. Matrix Biol. 1997, 16, 255–271. [Google Scholar] [CrossRef]
- Fukuta, S.; Oyama, M.; Kavalkovich, K.; Fu, F.H.; Niyibizi, C. Identification of types II, IX and X collagens at the insertion site of the bovine achilles tendon. Matrix Biol. 1998, 17, 65–73. [Google Scholar] [CrossRef]
- DiCesare, P.; Hauser, N.; Lehman, D.; Pasumarti, S.; Paulsson, M. Cartilage oligomeric matrix protein (COMP) is an abundant component of tendon. FEBS Lett. 1994, 354, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Södersten, F.; Ekman, S.; Eloranta, M.L.; Heinegård, D.; Dudhia, J.; Hultenby, K. Ultrastructural immunolocalization of cartilage oligomeric matrix protein (COMP) in relation to collagen fibrils in the equine tendon. Matrix Biol. 2005, 24, 376–385. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Gerard, M.; Dowling, B.; Dart, A.J.; Birch, H.L.; Goodship, A.E. Correlation of cartilage oligomeric matrix protein (COMP) levels in equine tendon with mechanical properties: A proposed role for COMP in determining function-specific mechanical characteristics of locomotor tendons. Equine Vet. J. 2002, 34, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.T.; Deere, M.; Putnam, E.; Cole, W.; Vertel, B.; Chen, H.; Lawler, J. Characterization of cartilage oligomeric matrix protein (COMP) in human normal and pseudoachondroplasia musculoskeletal tissues. Matrix Biol. 1998, 17, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Weirich, C.; Keene, D.R.; Kirsch, K.; Heil, M.; Neumann, E.; Dinser, R. Expression of PSACH-associated mutant COMP in tendon fibroblasts leads to increased apoptotic cell death irrespective of the secretory characteristics of mutant COMP. Matrix Biol. 2007, 26, 314–323. [Google Scholar] [CrossRef]
| Disease | Gene | Protein | Type of Mutation |
|---|---|---|---|
| MED (EDM6, OMIM #614135) | COL9A1 | Type IX Collagen | Exon skipping and in-frame deletions |
| MED (EDM2, OMIM #600204) | COL9A2 | ||
| MED (EDM3, OMIM #600969) | COL9A3 | ||
| MED (EDM5, OMIM #607078) | MATN3 | Matrilin-3 | Antimorphic missense mutations and in-frame deletions |
| MED (EDM1, OMIM #134200) | COMP | Cartilage Oligomeric Matrix Protein | |
| PSACH (OMIM #177170) | COMP |
| Mutation | Location | Disease | Reference |
|---|---|---|---|
| p.Asn555Lys | CTD | EDM1 | [49] |
| p.Thr585Arg | CTD | PSACH/EDM1 | [30] |
| p.Thr585Met | CTD | PSACH/EDM1 | [5,50,51] |
| p.Thr585Lys | CTD | PSACH/EDM1 | [30] |
| p.Thr585Arg | CTD | PSACH/EDM1 | [30] |
| p.His587Arg | CTD | PSACH | [51,52] |
| p.Asp605Asn | CTD | EDM1 | [51] |
| p.Ser681Cys | CTD | EDM1 | [51] |
| p.Arg718Pro | CTD | EDM1 | [51,53] |
| p.Arg718Trp | CTD | EDM1 | [48,51,53,54] |
| p.Gly719Arg | CTD | PSACH | [55] |
| p.Gly719Ser | CTD | PSACH | [30,51] |
| p.Pro276Arg | T3 | EDM1 | [5] |
| p.Asp290Gly | T3 | PSACH | [30] |
| p.Ser298Leu | T3 | EDM1 | [48] |
| p.Asp299Arg | T3 | PSACH | [30] |
| p.Ser298Leu | T3 | PSACH | [53] |
| p.Ala311Asp | T3 | EDM1 | [30] |
| p.Asp316Tyr | T3 | EDM1 | [56] |
| p.Asp317Gly | T3 | EDM1 | [30] |
| p.Asp326Tyr | T3 | PSACH | [30,57] |
| p.Asp326Gly | T3 | EDM1 | [30] |
| p.Glu341_Asp342del | T3 | PSACH | [30] |
| p.Cys348Phe | T3 | EDM1 | [30] |
| p.Asn350_Asp372del | T3 | PSACH | [30] |
| p.Cys371Ser | T3 | EDM1 | [30,48] |
| p.Cys371Tyr | T3 | EDM1 | [58] |
| p.Asp374Asn | T3 | EDM1 | [48] |
| p.Asp376Asn | T3 | EDM1 | [48] |
| p.Asp378Val | T3 | PSACH | [30] |
| p.Asp385Asn | T3 | EDM1 | [48] |
| p.Asp385del | T3 | EDM1 | [53] |
| p.Asp385Tyr | T3 | EDM1 | [30] |
| p.Cys387Arg | T3 | PSACH | [30] |
| p.Asp397His | T3 | EDM1 | [30] |
| p.Asp401Asn | T3 | EDM1 | [48] |
| p.Gly404Arg | T3 | EDM1 | [30] |
| p.Cys407Arg | T3 | EDM1 | [59] |
| p.Cys410Tyr | T3 | PSACH/EDM1 | [48] |
| p.Asn415Lys | T3 | EDM1 | [48] |
| p.Gly427Glu | T3 | EDM1 | [48,52] |
| p.Asp439Glu | T3 | PSACH | [60] |
| p.Gly440Arg | T3 | PSACH | [30] |
| p.Asp446Asn | T3 | PSACH | [30] |
| p.Cys448Ser | T3 | PSACH | [30] |
| p.Glu457del | T3 | EDM1 | [54,61] |
| p.Asp469-Asp473del | T3 | PSACH | [51] |
| p.Asp473dup | T3 | EDM1 | [47] |
| p.Asp473del | T3 | PSACH | [30] |
| p.Asp473His | T3 | PSACH | [30] |
| p.Asp473Tyr | T3 | PSACH | [51] |
| p.Asp473_Asp474insAsp | T3 | EDM1 | [48] |
| p.Asp475Asn | T3 | PSACH | [30] |
| p.Asp482Gly | T3 | PSACH | [30] |
| p.Asp482His | T3 | PSACH | [51] |
| p.Gly501Asp | T3 | EDM1 | [30] |
| p.Gly501Asp_Asp502Tyr | T3 | EDM1 | [30] |
| p.Asp507Gly | T3 | PSACH | [30] |
| p.Asp511Gly | T3 | PSACH | [30] |
| p.Asp515Gly | T3 | PSACH | [30] |
| p.Asn523Lys | T3 | EDM1 | [48,62] |
| p.Thr529Ile | T3 | PSACH | [30] |
| p.Thr529Ala | T3 | PSACH | [57] |
| p.Thr529Ile | T3 | PSACH | [30] |
| p.Thr529Ala | T3 | PSACH | [57] |
| Mutation | Location | Reference |
|---|---|---|
| p.Arg70His | Linker region | [67] |
| p.Phe105Ser | A domain: α1 | [68] |
| p.Thr120Met | A domain: βB | [65,68] |
| p.Arg121Trp | A domain: βB | [4,65,66,68] |
| p.Ala123Lys | A domain: βB | [68] |
| p.Ala128Pro | A domain: βB | [69] |
| p.Glu134Lys | A domain: βC | [65] |
| p.Asp171_Glu177delinsGlu | A domain: α4 | [30] |
| p.Ala173Asp | A domain: α4 | [70] |
| p.Ala191Asp | A domain: βD | [71] |
| p.Ile192Asn | A domain: βD | [65] |
| p.Val194Asp | A domain: βD | [4] |
| p.Thr195Lys | A domain: βD | [66] |
| p.Arg209Pro | A domain: α5 | [30] |
| p.Tyr218Asn | A domain: βD | [66] |
| p.Ala219Asp | A domain: βE | [65] |
| p.Lys231Asn | A domain: α6 | [70] |
| p.Val245Met | A domain: βF | [30] |
| Disease | Gene | Mutation | Approach Taken | Promoter | Reference |
|---|---|---|---|---|---|
| PSACH | COMP | p.Asp469del | Transgenic (rat COMP cDNA) | Col2a1 | [75] |
| PSACH | COMP | p.Asp469del | Transgenic (human COMP gene) | Native | [76] |
| PSACH | COMP | p.Asp469del | Transgenic (human COMP cDNA) | Col2a1 | [76] |
| PSACH | COMP | p.Asp469del | Transgenic inducible overexpression (human COMP cDNA) | Col2a1 and tetracycline responsive element | [77] |
| PSACH | COMP | p.Asp469del | Knock-in | Native | [74] |
| PSACH-MED | COMP | p.Thr585Met | Knock-in | Native | [78] |
| MED | MATN3 | p.Val194Asp | Knock-in | Native | [72,73] |
| Chondrodysplasia | TG | Rdw (p.Gly2320Arg) | Transgenic ‘ER-stress phenocopy’ | Col2a1 | [79] |
| Chondrodysplasia | TG | Cog (p.Lys2293Pro) | Transgenic ‘ER-stress phenocopy’ | Col2a1 | [80] |
| Gene | Mutation | Phenotype | Retained | ER Stress | Response to ER Stress | Reference |
|---|---|---|---|---|---|---|
| COMP | p.Asp469del | PSACH | Yes | Yes | Novel stress pathways | [74] |
| COMP | p.Thr585Met | Mild PSACH/MED | Slightly | Mild | Mild UPR | [78] |
| MATN3 | p.Val194Asp | MED | Yes | Yes | UPR | [72,73] |
| Gene | Mutation | Clinical Feature | Diagnosis | Reference |
|---|---|---|---|---|
| COL9A2 | Exon 3 Skipping IVS3 ds, G > C |
| MED | ESDN-01013 [88] |
| COL9A2 | Exon 3 skipping IVS3 ds + 4, A > C |
| MED | ESDN-01003 [88] |
| COL9A2 | Exon 3 Skipping IVS3 ds + 2, T > C |
| MED | ESDN-00997 [25] |
| COL9A2 | Exon 3 skipping IVS2 ds − 1, G > A |
| MED | ESDN-01013 [25] |
| COL9A3 | Exon skipping IVS2 as − 1, G > A |
| MED | [22] |
| COMP | T3 domain mutation p.Asp326Tyr |
| PSACH | ESDN-00385 |
| COMP | T3 domain mutation p.Glu457del |
| MED | ESDN-00430 [54,61] |
| COMP | CTD mutation p.Asp605Asn |
| MED | [49] |
| COMP | CTD mutation p.Arg718Trp |
| MED | ESDN-00066 ESDN-00080 [49,89] |
| MATN3 | p.Asp176Val |
| MED | ESDN-01071 [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dennis, E.P.; Briggs, M.D. From Protein Misfolding to Extracellular Matrix Disorganisation: Understanding Disease Pathology in Rare Skeletal Dysplasias. Int. J. Mol. Sci. 2025, 26, 10057. https://doi.org/10.3390/ijms262010057
Dennis EP, Briggs MD. From Protein Misfolding to Extracellular Matrix Disorganisation: Understanding Disease Pathology in Rare Skeletal Dysplasias. International Journal of Molecular Sciences. 2025; 26(20):10057. https://doi.org/10.3390/ijms262010057
Chicago/Turabian StyleDennis, Ella Patricia, and Michael Darren Briggs. 2025. "From Protein Misfolding to Extracellular Matrix Disorganisation: Understanding Disease Pathology in Rare Skeletal Dysplasias" International Journal of Molecular Sciences 26, no. 20: 10057. https://doi.org/10.3390/ijms262010057
APA StyleDennis, E. P., & Briggs, M. D. (2025). From Protein Misfolding to Extracellular Matrix Disorganisation: Understanding Disease Pathology in Rare Skeletal Dysplasias. International Journal of Molecular Sciences, 26(20), 10057. https://doi.org/10.3390/ijms262010057





