Stimulatory Effect of Aluminum in Root Development of Pogostemon cablin: Integration of ROS Homeostasis and Gene Expression Networks
Abstract
1. Introduction
2. Results
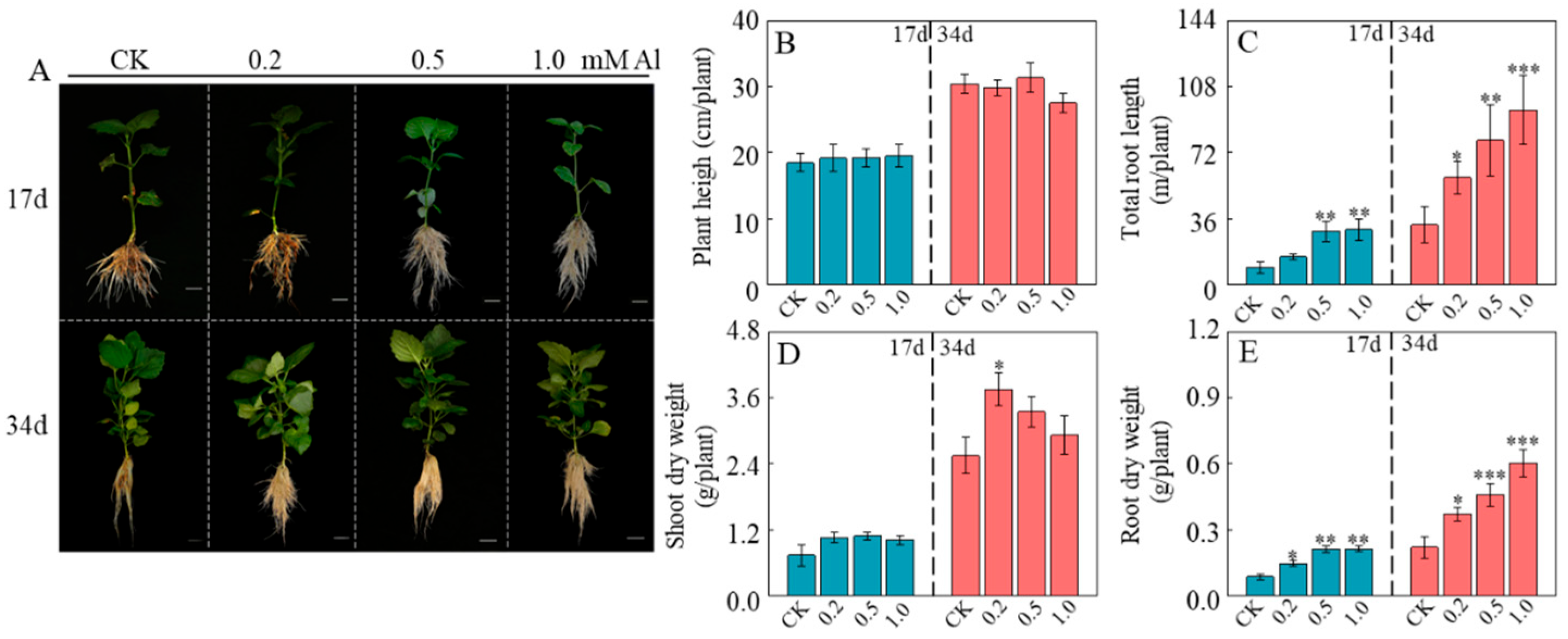
2.1. Al Promotes Root Growth in Patchouli Seedling
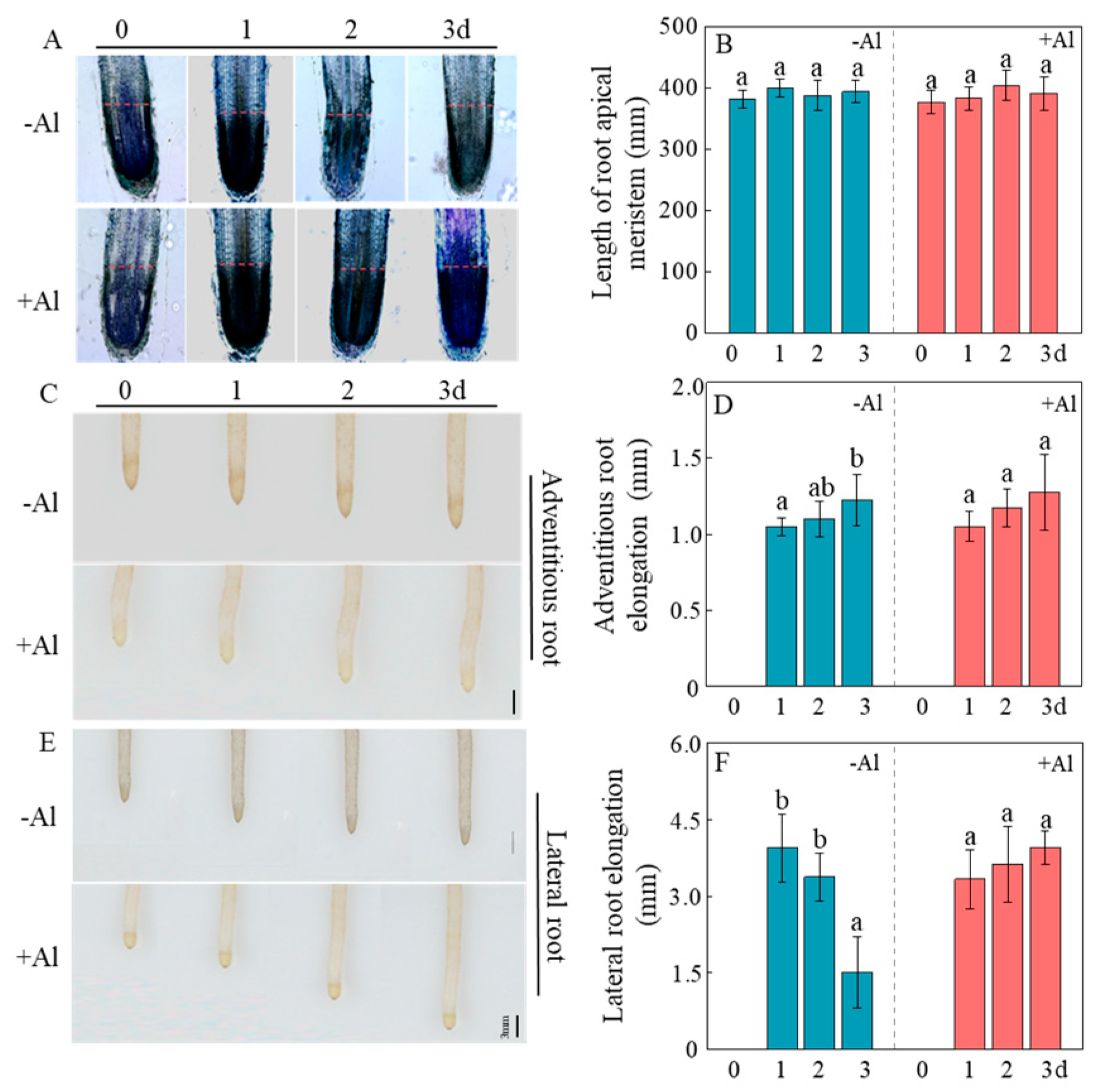
2.2. Al Enhances Cellular Activity in Root Meristematic Zones to Stimulate Root Growth
2.3. Al Enhances Cell Elongation in the Root Elongation Zone to Promote Root Growth
2.4. Al Treatment Reduces ROS Accumulation in Patchouli Roots
2.5. Al Remodels Patchouli Root Architecture by Boosting Root Initiation and Accelerating Lateral Root Development
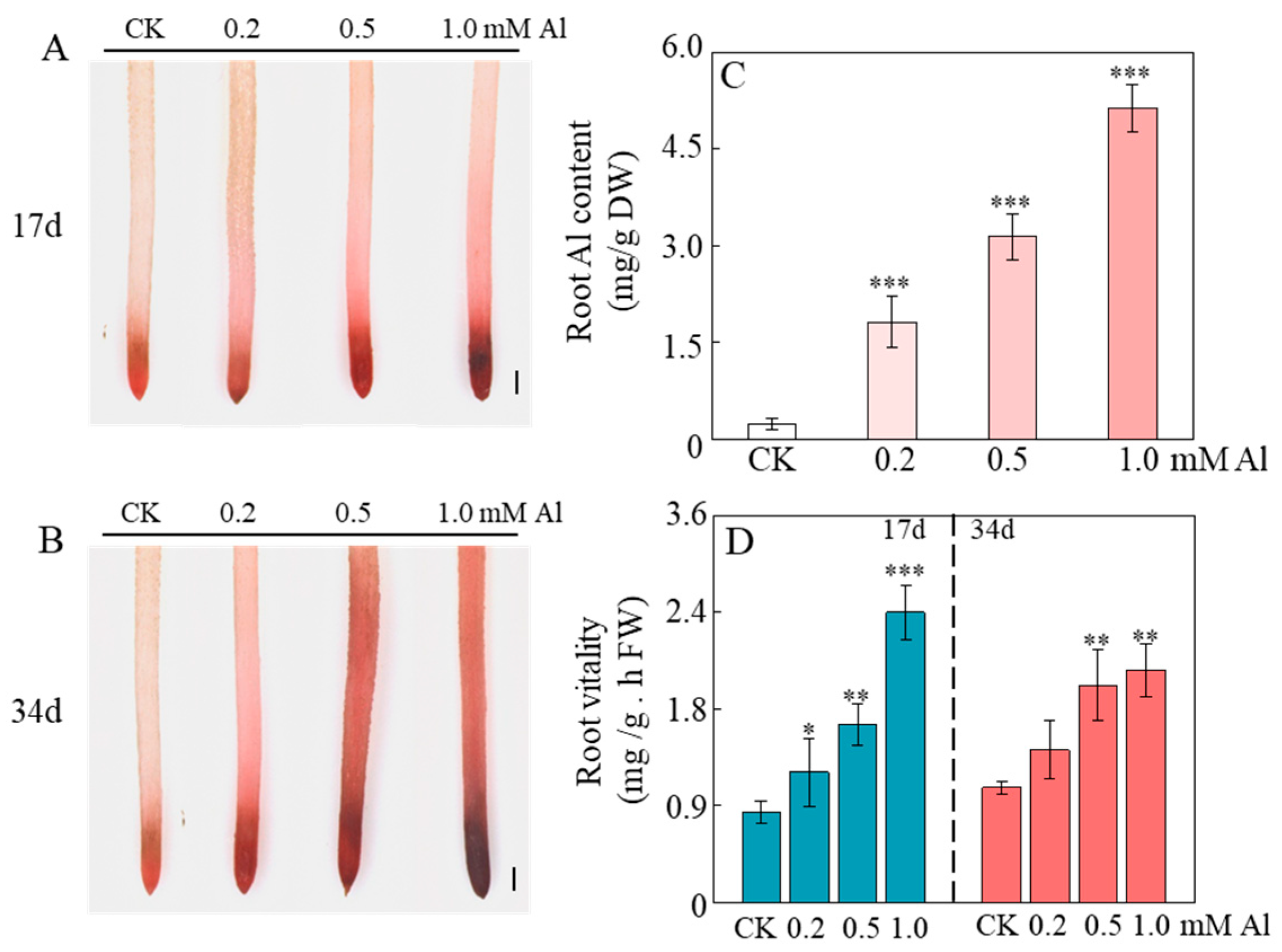
2.6. Al Exhibits Non-Essential but Beneficial Effects on Patchouli Root Growth
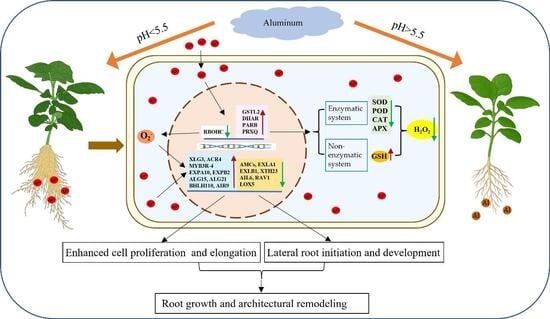
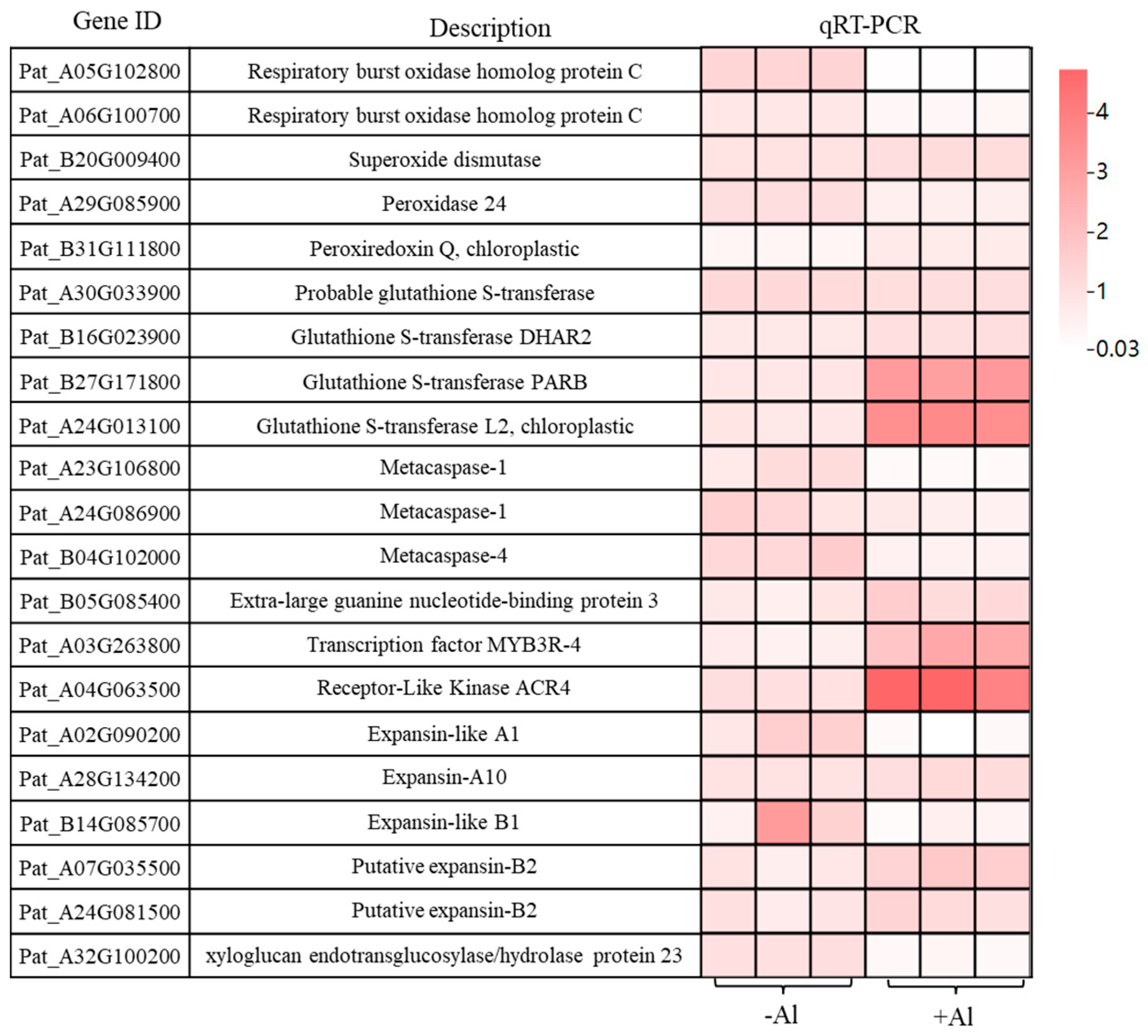
2.7. Al Modulates Gene Networks to Enhance Root Growth via ROS Regulation, Cell Proliferation, and Cell Wall Remodeling
2.8. Effect of Al on the Expression of Genes Related to Lateral Root Elongation in Patchoulit
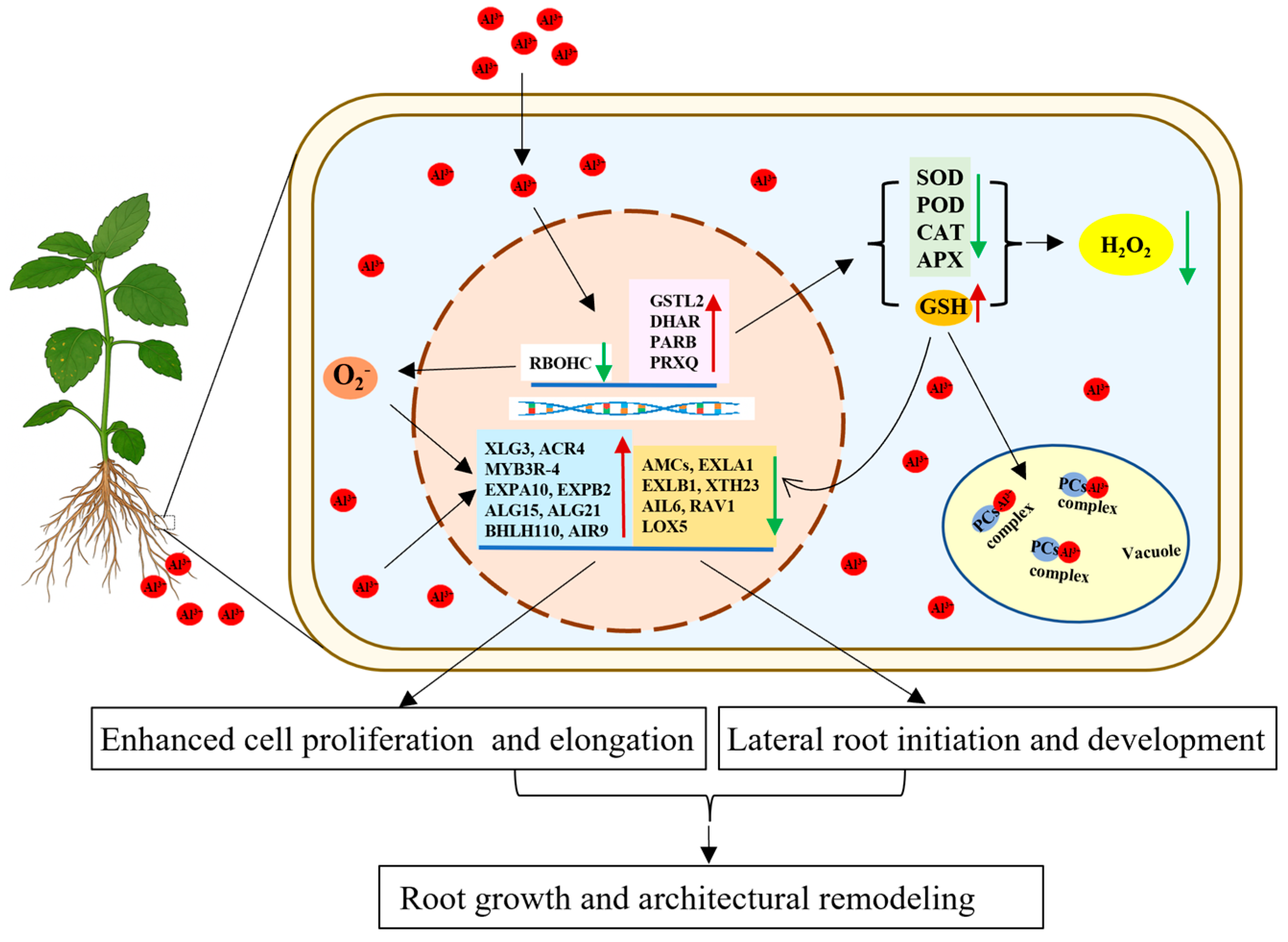
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Detection of ROS Level and Antioxidant Enzyme Activity
4.3. TTC Staining
4.4. Cell Elongation Measurements
4.5. Root Structure Analysis
4.6. Morin Staining
4.7. Determination of Al Content
4.8. Quantitative Real Time-PCR Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shetty, R.; Vidya, C.S.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: A review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef] [PubMed]
- Kinraide, T.B. Identity of the rhizotoxic aluminum species. Plant Soil 1991, 134, 167–178. [Google Scholar] [CrossRef]
- Horst, W.J.; Wang, Y.; Eticha, D. The role of the root apoplast in aluminum-induced inhibition of root elongation and in aluminum resistance of plants: A review. Ann. Bot. 2010, 106, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, N.; Huang, X.; Josy Karel, N.N.; He, Y.; Tang, H.; Li, Y.; Xu, J. Morphological, physiological, and transcriptomic analyses indicate that cell wall properties and antioxidant processes are potential targets for improving the aluminum tolerance of broad beans. Plant Physiol. Biochem. 2024, 216, 109164. [Google Scholar] [CrossRef]
- Li, Y.Z.; Lu, H.F.; Fan, X.W.; Sun, C.B.; Qing, D.J.; Dong, H.T.; Wang, L. Physiological responses and comparative transcriptional profiling of maize roots and leaves under imposition and removal of aluminum toxicity. Environ. Exp. Bot. 2021, 69, 158–166. [Google Scholar] [CrossRef]
- De Sousa, A.; Saleh, A.M.; Habeeb, T.H.; Hassan, Y.M.; Zrieq, R.; Wadaan, M.A.; Hozzein, W.N.; Selim, S.; Matos, M.; AbdElgawad, H. Silicon dioxide nanoparticles ameliorate the phytotoxic hazards of aluminum in maize grown on acidic soil. Sci. Total Environ. 2019, 693, 133636. [Google Scholar] [CrossRef]
- Basit, F.; Liu, J.; An, J.; Chen, M.; He, C.; Zhu, X.; Li, Z.; Hu, J.; Guan, Y. Seed priming with brassinosteroids alleviates aluminum toxicity in rice via improving antioxidant defense system and suppressing aluminum uptake. Environ. Sci. Pollut. Res. Int. 2022, 29, 10183–10197. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef]
- Sivaguru, M.; Baluska, F.; Volkmann, D.; Felle, H.H.; Horst, W.J. Impacts of aluminum on the cytoskeleton of the maize root apex. short-term effects on the distal part of the transition zone. Plant Physiol. 1999, 119, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Qifu, M.A.; Rengel, Z.; Kuo, J. Aluminum toxicity in rye (Secale cereale): Root growth and dynamics of cytoplasmic Ca2+ in intact root tips. Ann. Bot. 2002, 89, 241–244. [Google Scholar]
- Krtková, J.; Havelková, L.; Křepelová, A.; Fišer, R.; Vosolsobě, S.; Novotná, Z.; Martinec, J.; Schwarzerová, K. Loss of membrane fluidity and endocytosis inhibition are involved in rapid aluminum-induced root growth cessation in Arabidopsis thaliana. Plant Physiol. Biochem. 2012, 60, 88–97. [Google Scholar] [CrossRef]
- Ryan, P.R.; Kochian, L.V. Interaction between aluminum toxicity and calcium uptake at the root apex in near-isogenic lines of Wheat (Triticum aestivum L.) Differing in Aluminum Tolerance. Plant Physiol. 1993, 102, 975–982. [Google Scholar] [CrossRef]
- Doncheva, S.; Amenós, M.; Poschenrieder, C.; Barceló, J. Root cell patterning: A primary target for aluminum toxicity in maize. J. Exp. Bot. 2005, 56, 1213–1220. [Google Scholar] [CrossRef]
- Yang, Z.B.; Geng, X.; He, C.; Zhang, F.; Wang, R.; Horst, W.J.; Ding, Z. TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum-induced inhibition of root growth in Arabidopsis. Plant Cell 2014, 26, 2889–2904. [Google Scholar] [CrossRef]
- Wang, P.; Wan, N.; Horst, W.J.; Yang, Z.B. From stress to responses: Aluminum-induced signaling in the root apex. J. Exp. Bot. 2023, 74, 1358–1371. [Google Scholar] [CrossRef]
- Silva, I.R.; Smyth, T.J.; Moxley, D.F.; Carter, T.E.; Allen, N.S.; Rufty, T.W. Aluminum accumulation at nuclei of cells in the root tip. Fluorescence detection using lumogallion and confocal laser scanning microscopy. Plant Physiol. 2000, 123, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Kinraide, T.B. Ion fluxes considered in terms of membrane-surface electrical potentials. Funct. Plant Biol. 2001, 28, 607–618. [Google Scholar] [CrossRef]
- Ma, J.F. Syndrome of aluminum toxicity and diversity of aluminum resistance in higher plants. Int. Rev. Cytol. 2007, 264, 225–252. [Google Scholar]
- Rounds, M.A.; Larsen, P.B. Aluminum-dependent root-growth inhibition in Arabidopsis results from AtATR-regulated cell-cycle arrest. Curr. Biol. 2008, 18, 1495–1500. [Google Scholar] [CrossRef]
- Manquián-Cerda, K.; Cruces, E.; Escudey, M.; Zúñiga, G.; Calderón, R. Interactive effects of aluminum and cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets cultivated in vitro. Ecotoxicol. Environ. Saf. 2018, 150, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lv, T.; Huang, L.; Liu, X.; Jin, C.; Lin, X. Melatonin ameliorates aluminum toxicity through enhancing aluminum exclusion and reestablishing redox homeostasis in roots of wheat. J. Pineal Res. 2020, 68, e12642. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Wang, L.; Rao, I.M.; Wang, Z.; Chen, Z. AcEXPA1, an α-expansin gene, participates in the aluminum tolerance of carpetgrass (Axonopus compressus) through root growth regulation. Plant Cell Rep. 2024, 43, 159. [Google Scholar]
- Liu, G.; Li, D.; Mai, H.; Lin, X.; Lu, X.; Chen, K.; Wang, R.; Riaz, M.; Tian, J.; Liang, C. GmSTOP1-3 regulates flavonoid synthesis to reduce ROS accumulation and enhance aluminum tolerance in soybean. J. Hazard. Mater. 2024, 480, 136074. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Ryan, P.R.; Delhaize, E. Aluminum tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, J.V.; Piñeros, M.A.; Maciel, L.S.; Kochian, L.V. Emerging pleiotropic mechanisms underlying aluminum resistance and phosphorus acquisition on acidic soils. Front. Plant Sci. 2018, 9, 1420. [Google Scholar] [CrossRef]
- Fan, K.; Wang, M.; Gao, Y.; Ning, Q.; Shi, Y. Transcriptomic and bionomic analysis provides new insight into the beneficial effect of Al on tea roots’ growth and nutrient uptake. Plant Cell Rep. 2019, 38, 715–729. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a friend or foe of higher plants in acid soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Watanabe, T.; Osaki, M. Influence of aluminum and phosphorus on growth and xylem sap composition in Melastoma malabathricum L. Plant Soil 2001, 237, 63–70. Plant Soil 2001, 237, 63–70. [Google Scholar] [CrossRef]
- Tomioka, R.; Uchida, A.; Takenaka, C.; Tezuka, T. Effect of aluminum on nitrate reductase and photosynthetic activities in Quercus serrata seedlings. Environ. Sci. 2007, 14, 157–165. [Google Scholar] [PubMed]
- Matsumoto, H.; Motoda, H. Oxidative stress is associated with aluminum toxicity recovery in apex of pea root. Plant Soil 2013, 363, 399–410. [Google Scholar]
- Ranjan, A.; Sinha, R.; Sharma, T.R.; Pattanayak, A.; Singh, A.K. Alleviating aluminum toxicity in plants: Implications of reactive oxygen species signaling and crosstalk with other signaling pathways. Physiol. Plant 2021, 173, 1765–1784. [Google Scholar] [CrossRef] [PubMed]
- Du, H.M.; Huang, Y.; Qu, M.; Li, Y.H.; Hu, X.Q.; Yang, W.; Li, H.J.; He, W.Z.; Ding, J.Z.; Liu, C.; et al. A maize ZmAT6 gene confers aluminum tolerance via reactive oxygen species scavenging. Front. Plant Sci. 2020, 9, 1016. [Google Scholar] [CrossRef] [PubMed]
- Ezaki, B.; Jayaram, K.; Higashi, A.; Takahashi, K. A combination of five mechanisms confers a high tolerance for aluminum to a wild species of Poaceae, Andropogon virginicus L. Environ. Exp. Bot. 2013, 93, 35–44. [Google Scholar] [CrossRef]
- Irany, R.P.; Anny, C.D.L.; Tatiane, L.P.; Raquel, A.A.; Maria, D.C.P.B. Gene expression and antioxidant enzymatic activity in passion fruit exposed to aluminum. Afr. J. Agric. Res. 2018, 13, 115–120. [Google Scholar] [CrossRef]
- Liu, W.; Xu, F.; Lv, T.; Zhou, W.; Chen, Y.; Jin, C.; Lu, L.; Lin, X. Spatial responses of antioxidative system to aluminum stress in roots of wheat (Triticum aestivum L.) plants. Sci. Total Environ. 2018, 15, 462–469. [Google Scholar] [CrossRef]
- Sivaguru, M.; Liu, J.; Kochian, L.V. Targeted expression of SbMATE in the root distal transition zone is responsible for sorghum aluminum resistance. Plant J. 2013, 76, 297–307. [Google Scholar] [CrossRef]
- Jiang, D.; Du, S.; Shi, J.; Xu, H.; Liu, S.; Han, H.; Xu, Y.; Wang, H.; Yan, M.; Huang, X.; et al. Glutathione mitigates aluminum toxicity in root-apex transition zone of rice through reducing aluminum absorption and maintaining redox balance. Plant Physiol. Biochem. 2025, 219, 109366. [Google Scholar] [CrossRef]
- Hajiboland, R.; Barceló, J.; Poschenrieder, C.; Tolrà, R. Amelioration of iron toxicity: A mechanism for aluminum-induced growth stimulation in tea plants. J. Inorg. Biochem. 2013, 128, 183–187. [Google Scholar] [CrossRef]
- Rehmus, A.; Bigalke, M.; Valarezo, C.; Mora-Castillo, J.; Wilcke, W. Aluminum toxicity to tropical montane forest tree seedlings in southern Ecuador: Response of biomass and plant morphology to elevated Al concentrations. Plant Soil 2015, 388, 87–97. [Google Scholar] [CrossRef]
- Schmitt, M.; Watanabe, T.; Jansen, S. The effects of aluminum on plant growth in a temperate and deciduous aluminum accumulating species. AoB Plants 2016, 8, plw065. [Google Scholar] [CrossRef]
- Liu, Y.J.; Tao, J.Y.; Cao, J.; Zeng, Y.P.; Li, X.; Ma, J.; Huang, Z.; Jiang, M.Y.; Sun, L.X. The beneficial effects of aluminum on the plant growth in Camellia japonica. J. Soil Sci. Plant Nutr. 2020, 20, 1799–1809. [Google Scholar] [CrossRef]
- Watanabe, T.; Jansen, S.; Osaki, M. The beneficial effect of aluminum and the role of citrate in Al accumulation in Melastoma malabathricum. New Phytol. 2005, 165, 773–780. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, M.; Liu, X.; Mao, Q.; Shi, C.; Kochian, L.V.; Liao, H. Aluminum is essential for root growth and development of tea plants (Camellia sinensis). J. Integr. Plant Biol. 2020, 62, 984–997. [Google Scholar] [CrossRef]
- Bressan, A.C.G.; de Oliveira Carvalho Bittencourt, B.M.; Silva, G.S.; Habermann, G. Could the absence of aluminum (Al) impair the development of an Al-accumulating woody species from Brazilian savanna? Theor. Exp. Plant Physiol. 2021, 33, 281–292. [Google Scholar] [CrossRef]
- Maheshwari, M.L.; Vasantha, K.T.; Sharma, N.; Chandel, K.P.S. Patchouli-An Indian perspective. Indian Perfumer. 1993, 37, 9–11. [Google Scholar]
- Swamy, M.K.; Sinniah, U.R. A Comprehensive review on the phytochemical constituents and pharmacological activities of Pogostemon cablin Benth.: An aromatic medicinal plant of industrial importance. Molecules 2015, 20, 8521–8547. [Google Scholar] [CrossRef]
- Hu, G.; Peng, C.; Xie, X.; Zhang, S.; Cao, X. Availability, pharmaceutics, security, pharmacokinetics, and pharmacological activities of patchouli alcohol. Evid.-Based Complement. Altern. Med. 2017, 2017, 4850612. [Google Scholar] [CrossRef]
- Lin, R.F.; Feng, X.X.; Li, C.W.; Zhang, X.J.; Yu, X.T.; Zhou, J.Y.; Zhang, X.; Xie, Y.L.; Su, Z.R.; Zhan, J.Y. Prevention of UV radiation-induced cutaneous photoaging in mice by topical administration of patchouli oil. J. Ethnopharmacol. 2014, 154, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; He, L.; Wu, Y.; Ma, W.; Chen, H.; Ye, Z. Comparative proteomic analysis of Pogostemon cablin leaves after continuous cropping. Protein Expr. Purif. 2018, 152, 13–22. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Lai, Y.; Wang, S.; Li, P.; Xiao, J.; Fu, C.; Hu, H.; Wang, Y. Analysis of Pogostemon cablin from pharmaceutical research to market performances. Expert Opin. Investig. Drugs 2013, 22, 245–257. [Google Scholar] [CrossRef]
- Yi, J.X.; Lu, L.X.; Liu, G.D. Research on soil acidification and acidic soil’s melioration. J. South China Univ. Trop. Agric. 2006, 12, 23–28. [Google Scholar]
- Zou, J.Y.; Li, Y.F. Technical points of planting and management of Chinese herbal medicine Patchouli. South China Agric. 2015, 9, 51, 53. [Google Scholar]
- Luo, J.P.; Wu, Z. Analysis of macronutrients and trace elements in Pogostemon cablin from different origins. Chin. Tradit. Herb. Drugs 2001, 24, 2. [Google Scholar]
- Luo, J.P.; Feng, Y.F.; He, B.; Guo, X.L.; Che, X.X.; Cao, H.; Liu, Y.P.; Wu, Z.; Liu, H.H. Research on the authenticity of Pogostemon cablin. Chin. Tradit. Herb. Drugs 2005, 28, 5. [Google Scholar]
- Ur Rahman, S.; Han, J.C.; Ahmad, M.; Ashraf, M.N.; Khaliq, M.A.; Yousaf, M.; Wang, Y.; Yasin, G.; Nawaz, M.F.; Khan, K.A.; et al. Aluminum phytotoxicity in acidic environments: A comprehensive review of plant tolerance and adaptation strategies. Ecotoxicol. Environ. Saf. 2024, 269, 115791. [Google Scholar] [CrossRef] [PubMed]
- Čiamporová, M. Morphological and Structural Responses of Plant Roots to Aluminum at Organ, Tissue, and Cellular Levels. Biol. Plant. 2002, 45, 161–171. [Google Scholar] [CrossRef]
- Scancar, J.; Milacic, R. Aluminum speciation in environmental samples: A review. Anal. Bioanal. Chem. 2006, 386, 999–1012. [Google Scholar] [CrossRef]
- Cao, Y.; Lou, Y.; Han, Y.; Shi, J.; Wang, Y.; Wang, W.; Ming, F. Al toxicity leads to enhanced cell division and changed photosynthesis in Oryza rufipogon L. Mol. Biol. Rep. 2011, 38, 4839–4846. [Google Scholar] [CrossRef]
- Jaskowiak, J.; Tkaczyk, O.; Slota, M.; Kwasniewska, J.; Szarejko, I. Analysis of aluminum toxicity in Hordeum vulgare roots with an emphasis on DNA integrity and cell cycle. PLoS ONE 2018, 13, e0193156. [Google Scholar] [CrossRef]
- Che, J.; Yamaji, N.; Shen, R.F.; Ma, J.F. An Al-inducible expansin gene, OsEXPA10 is involved in root cell elongation of rice. Plant J. 2016, 88, 132–142. [Google Scholar] [CrossRef]
- Xu, P.; Fang, S.; Chen, H.; Cai, W. The brassinosteroid-responsive xyloglucan endotransglucoseylase/hydrolase 19 (XTH19) and XTH23 genes are involved in lateral root development under salt stress in Arabidopsis. Plant J. 2020, 104, 59–75. [Google Scholar] [CrossRef]
- Wu, Z.; Cui, C.; Xing, A.; Xu, X.; Sun, Y.; Tian, Z.; Li, X.; Zhu, J.; Wang, G.; Wang, Y. Identification and response analysis of xyloglucan endotransglycosylase/hydrolases (XTH) family to fluoride and aluminum treatment in Camellia sinensis. BMC Genom. 2021, 22, 761. [Google Scholar] [CrossRef]
- Luo, S.; Pan, C.; Liu, S.; Liao, G.; Li, A.; Wang, Y.; Wang, A.; Xiao, D.; He, L.F.; Zhan, J. Identification and functional characterization of the xyloglucan endotransglucosylase/hydrolase 32 (AhXTH32) in peanut during aluminum-induced programmed cell death. Plant Physiol. Biochem. 2023, 194, 161–168. [Google Scholar] [CrossRef]
- Tatsumi, A.; Nagayama, T.; Teramoto, A.; Nakamura, A.; Yokoyama, R.; Furukawa, J.; Iwai, H. OsXTH19 overexpression improves aluminum tolerance via xyloglucan reduction in rice root cell wall. Plants 2025, 14, 1912. [Google Scholar] [CrossRef]
- Xu, F.J.; Jin, C.W.; Liu, W.J.; Zhang, Y.S.; Lin, X.Y. Pretreatment with H2O2 alleviates aluminum-induced oxidative stress in wheat seedlings. J. Integr. Plant Biol. 2011, 53, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Yu, Y.; Liu, W.; Lu, L.; Jin, C.; Lin, X. Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J. Integr. Plant Biol. 2015, 57, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Xu, H.; Zhang, Y.; Chen, G. Silicon mediated redox homeostasis in the root-apex transition zone of rice plays a key role in aluminum tolerance. Plant Physiol. Biochem. 2023, 201, 107871. [Google Scholar] [CrossRef]
- Lim, B.; Pasternak, M.; Meyer, A.J.; Cobbett, C.S. Restricting glutamylcysteine synthetase activity to the cytosol or glutathione biosynthesis to the plastid is sufficient for normal plant development and stress tolerance. Plant Biol. 2014, 16, 58–67. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, J.J.; Kim, H.S.; Kim, Y.S.; Yoon, H.S. Enhanced glutathione content improves lateral root development and grain yield in rice plants. Plant Mol. Biol. 2021, 105, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.H.; Miao, Z.Q.; Qi, G.F.; Wu, J.; Cai, X.T.; Mao, J.L.; Xiang, C.B. MADS-box transcription factor AGL21 regulates lateral root development and responds to multiple external and physiological signals. Mol. Plant 2014, 7, 1653–1669. [Google Scholar] [CrossRef] [PubMed]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell 2007, 19, 118–130. [Google Scholar] [CrossRef]
- Buschmann, H.; Dols, J.; Kopischke, S.; Peña, E.J.; Andrade-Navarro, M.A.; Heinlein, M.; Szymanski, D.B.; Zachgo, S.; Doonan, J.H.; Lloyd, C.W. Arabidopsis KCBP interacts with AIR9 but stays in the cortical division zone throughout mitosis via its MyTH4-FERM domain. J. Cell Sci. 2015, 128, 2033–2046. [Google Scholar] [CrossRef]
- Zhang, Y.; Mitsuda, N.; Yoshizumi, T.; Horii, Y.; Oshima, Y.; Ohme-Takagi, M.; Matsui, M.; Kakimoto, T. Two types of bHLH transcription factor determine the competence of the pericycle for lateral root initiation. Nat. Plants 2021, 7, 633–643. [Google Scholar] [CrossRef]
- Vellosillo, T.; Martínez, M.; López, M.A.; Vicente, J.; Cascón, T.; Dolan, L.; Hamberg, M.; Castresana, C. Oxylipins produced by the 9-lipoxygenase pathway in Arabidopsis regulate lateral root development and defense responses through a specific signaling cascade. Plant Cell 2007, 19, 831–846. [Google Scholar] [CrossRef]
- Santuari, L.; Sanchez-Perez, G.F.; Luijten, M.; Rutjens, B.; Terpstra, I.; Berke, L.; Gorte, M.; Prasad, K.; Bao, D.; Timmermans Hereijgers, J.L.; et al. The PLETHORA gene regulatory network guides growth and cell differentiation in Arabidopsis roots. Plant Cell 2016, 28, 2937–2951. [Google Scholar] [CrossRef]
- Du, Y.; Scheres, B. PLETHORA transcription factors orchestrate de novo organ patterning during Arabidopsis lateral root outgrowth. Proc. Natl. Acad. Sci. USA 2017, 114, 11709–11714. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Ray, A.; Mandal, D.; Nag Chaudhuri, R. ABI3 mediated repression of RAV1 gene expression promotes efficient dehydration stress response in Arabidopsis thaliana. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194582. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Shin, H.Y.; Kang, H.K.; Shang, Y.; Park, S.Y.; Jeong, D.H.; Nam, K.H. Reciprocal inhibition of expression between RAV1 and BES1 modulates plant growth and development in Arabidopsis. J. Integr. Plant Biol. 2023, 65, 1226–1240. [Google Scholar] [CrossRef]
- Bielach, A.; Duclercq, J.; Marhavý, P.; Benková, E. Genetic approach towards the identification of auxin-cytokinin crosstalk components involved in root development. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1469–1478. [Google Scholar] [CrossRef]
- Sahu, P.P.; Pandey, G.; Sharma, N.; Puranik, S.; Muthamilarasan, M.; Prasad, M. Epigenetic mechanisms of plant stress responses and adaptation. Plant Cell Rep. 2013, 32, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Kui, M.; He, K.; Yang, M.; Du, J.; Jiang, Y.; Hu, Y. Jasmonate-regulated Root Growth Inhibition and Root Hair Elongation. J. Exp. Bot. 2022, 74, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Fu, J.; Liao, H.; He, Y.; Nian, H.; Hu, Y.; Qiu, L.; Dong, Y.; Yan, X. Characterization of root architecture in an applied core collection for phosphorus efficiency of soybean germplasm. Chin. Sci. Bull. 2004, 49, 1611–1620. [Google Scholar] [CrossRef]
- De Smet, I.; Vassileva, V.; De Rybel, B.; Levesque, M.P.; Grunewald, W.; Van Damme, D.; Van Noorden, G.; Naudts, M.; Van Isterdael, G.; De Clercq, R.; et al. Receptor-Like Kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 2008, 322, 594–597. [Google Scholar] [CrossRef]
- Ding, L.; Pandey, S.; Assmann, S.M. Arabidopsis extra-large G proteins (XLGs) regulate root morphogenesis. Plant J. 2008, 53, 248–263. [Google Scholar] [CrossRef]
- Wan, B.S.; Chen, B.L.; Wu, K.L.; Zhou, L.Y.; Yan, H.J. Selection of Reference Genes in Real-time Quantitative PCR of Pogostemon cablin. Mol. Plant Breed. 2023, 1–20. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Lin, Z.; Yang, H.; Liang, C.; Jiang, W. Stimulatory Effect of Aluminum in Root Development of Pogostemon cablin: Integration of ROS Homeostasis and Gene Expression Networks. Int. J. Mol. Sci. 2025, 26, 10056. https://doi.org/10.3390/ijms262010056
Deng Z, Lin Z, Yang H, Liang C, Jiang W. Stimulatory Effect of Aluminum in Root Development of Pogostemon cablin: Integration of ROS Homeostasis and Gene Expression Networks. International Journal of Molecular Sciences. 2025; 26(20):10056. https://doi.org/10.3390/ijms262010056
Chicago/Turabian StyleDeng, Zongyu, Zhongqi Lin, Hulan Yang, Cuiyue Liang, and Weizhen Jiang. 2025. "Stimulatory Effect of Aluminum in Root Development of Pogostemon cablin: Integration of ROS Homeostasis and Gene Expression Networks" International Journal of Molecular Sciences 26, no. 20: 10056. https://doi.org/10.3390/ijms262010056
APA StyleDeng, Z., Lin, Z., Yang, H., Liang, C., & Jiang, W. (2025). Stimulatory Effect of Aluminum in Root Development of Pogostemon cablin: Integration of ROS Homeostasis and Gene Expression Networks. International Journal of Molecular Sciences, 26(20), 10056. https://doi.org/10.3390/ijms262010056