Selective Dehydration of Pentoses and Hexoses of Ulva rigida to Platform Chemicals Using Nb2O5 and ZrO2 Supported on Mesoporous Silicas as Heterogeneous Catalysts
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.1.1. N2 Sorption at −196 °C
2.1.2. X-Ray Diffraction
2.1.3. X-Ray Photoelectron Spectroscopy
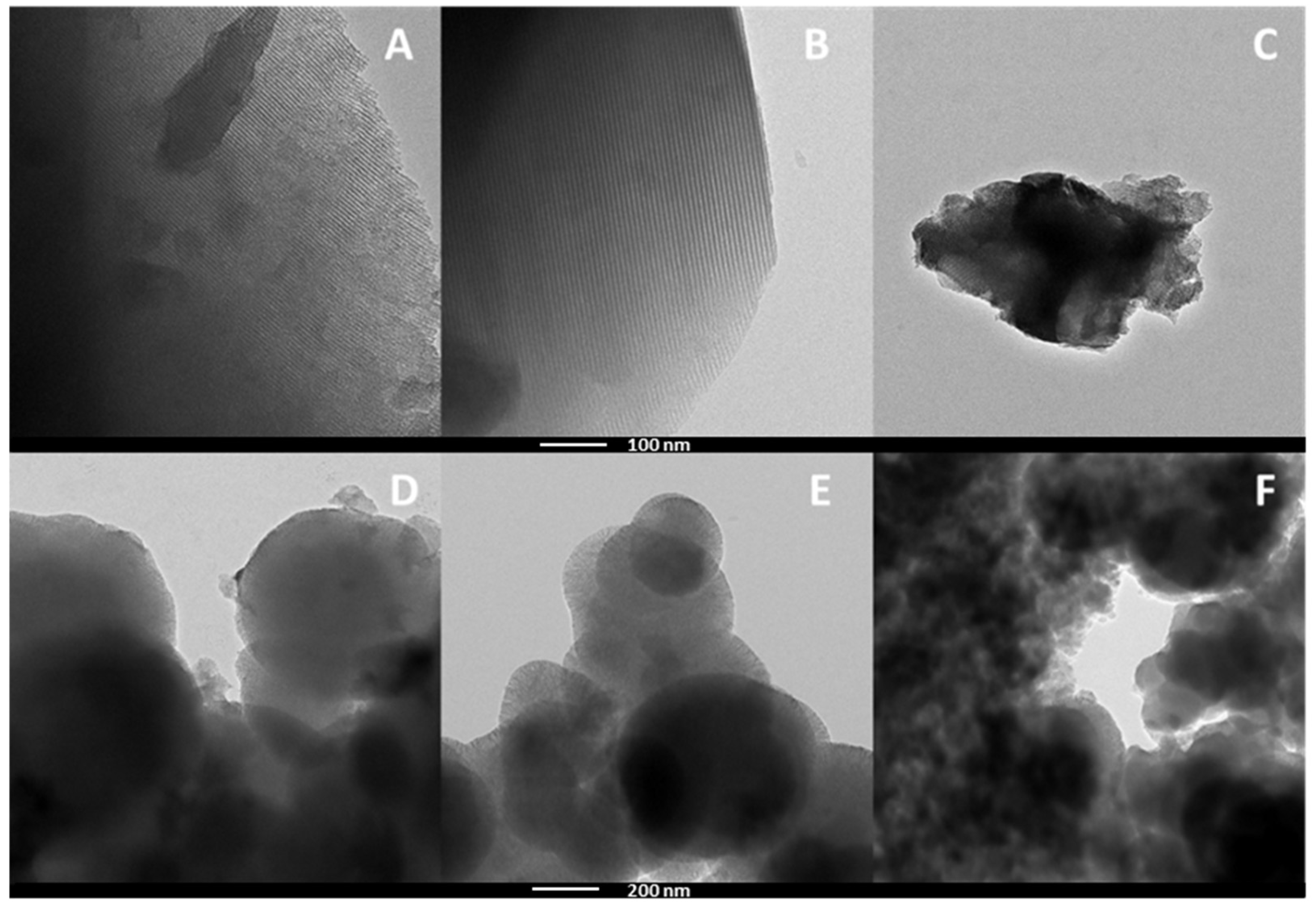
2.1.4. Transmission Electron Microscopy and Energy Dispersive X-Ray Spectroscopy
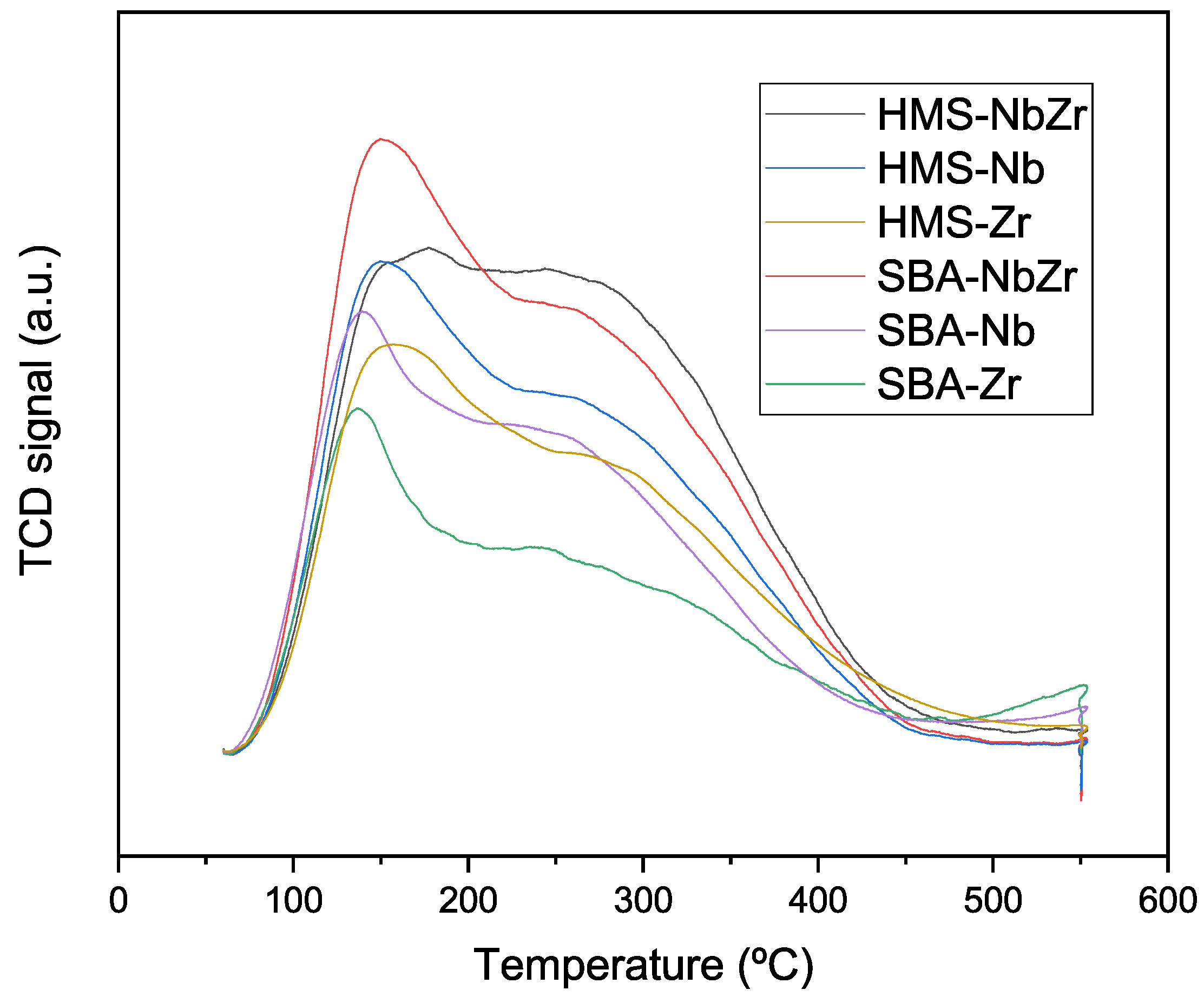
2.1.5. Ammonia Thermoprogrammed Desorption
2.1.6. Pyridine Adsorption Coupled to FTIR Spectroscopy
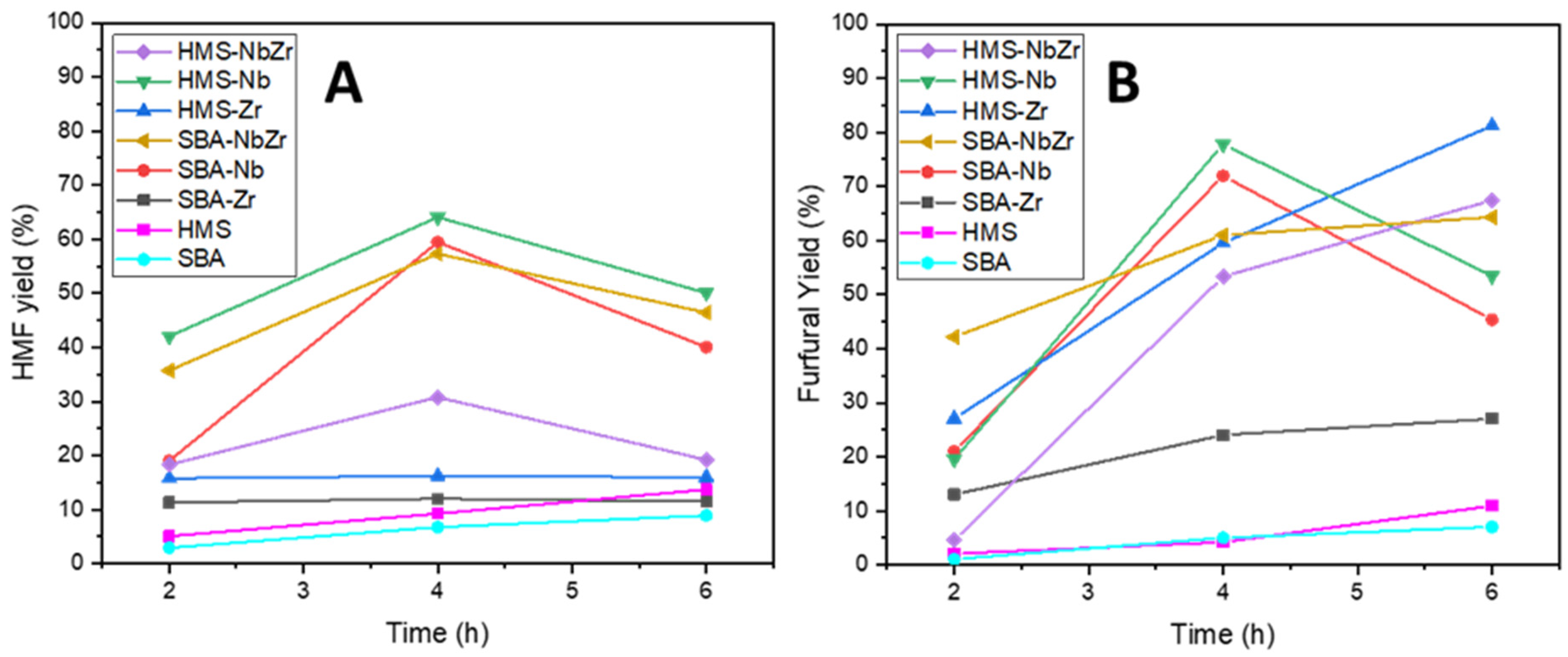
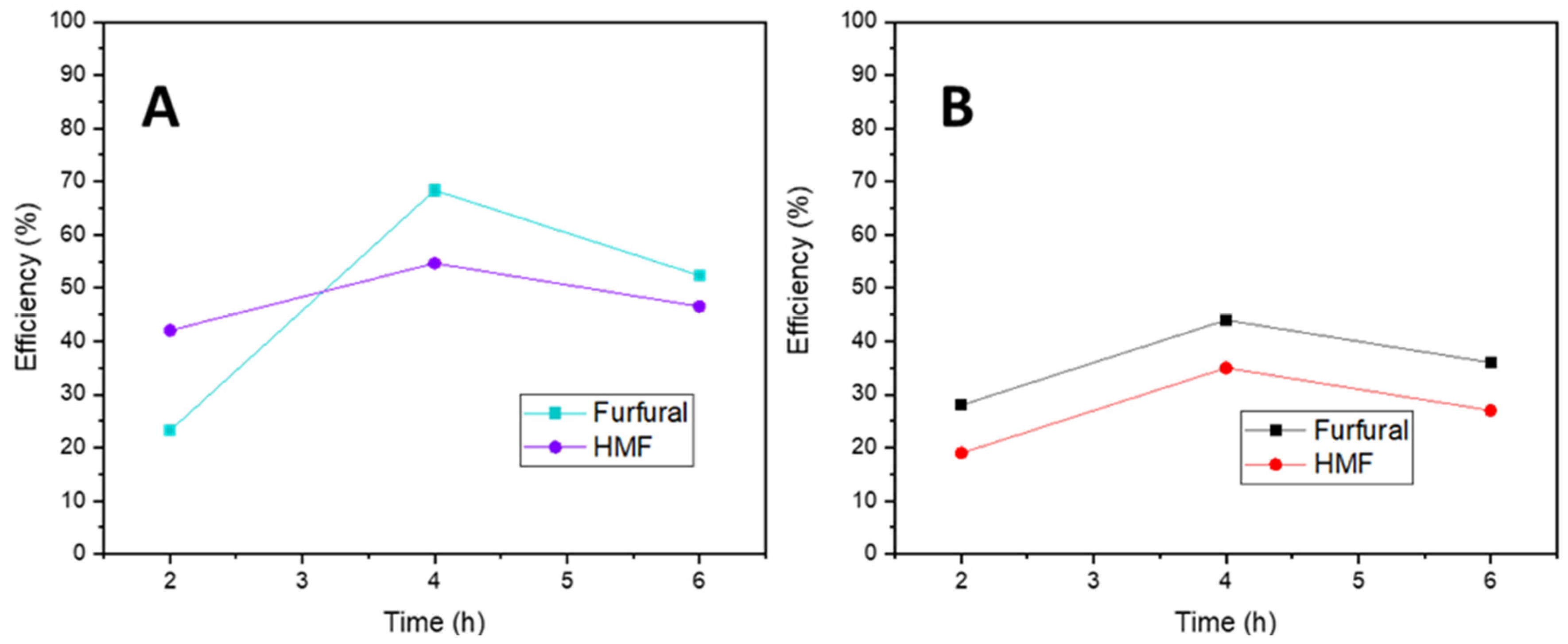
2.2. Catalytic Results
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization of Catalysts
3.3. Catalytic Tests
3.3.1. Dehydration of Monosaccharides
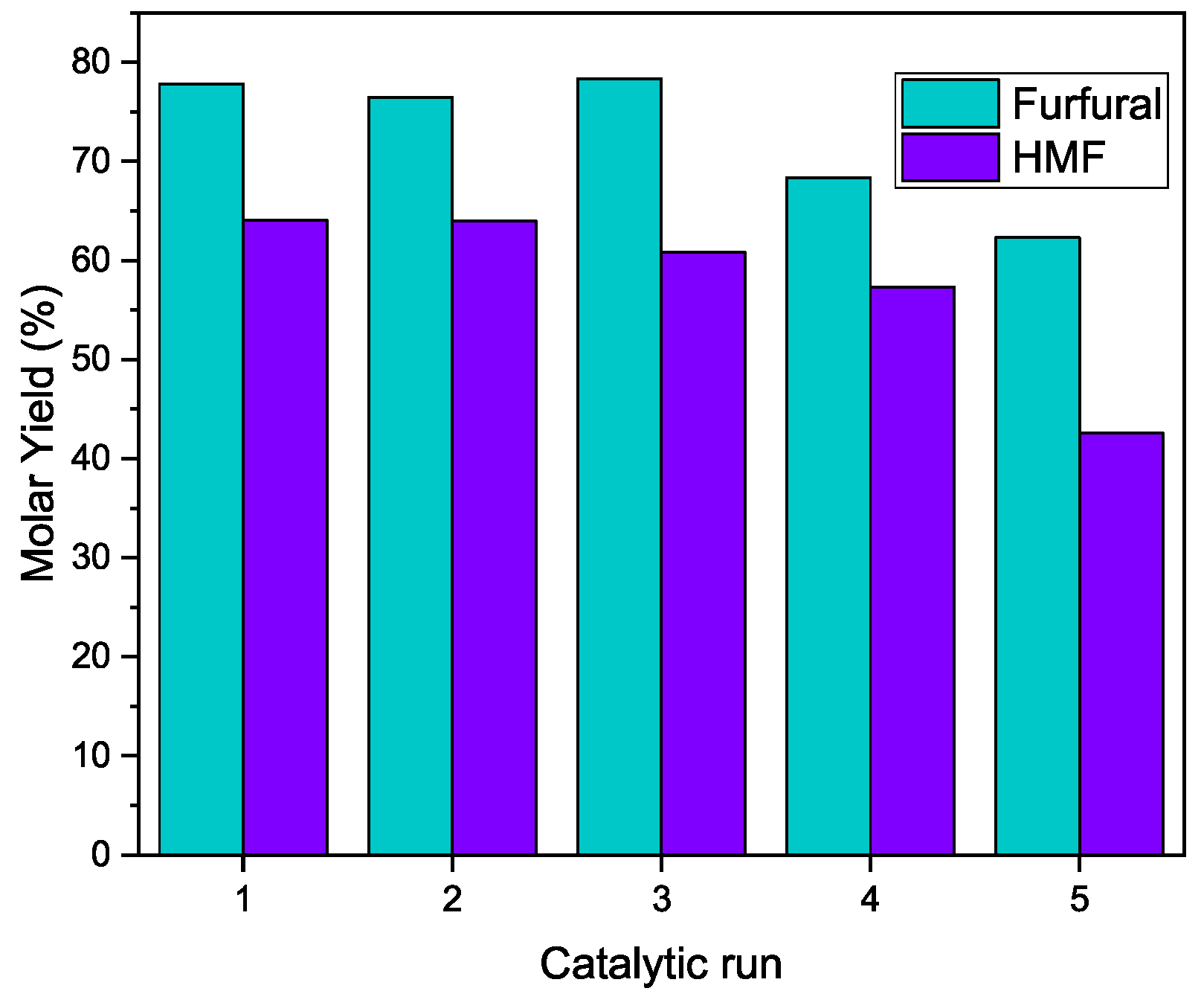
3.3.2. Catalyst Reutilization
3.3.3. Analysis Conditions of the Reaction Products
3.3.4. Furfural and HMF Quantification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sulis, D.B.; Lavoine, N.; Sederoff, H.; Jiang, X.; Marques, B.M.; Lan, K.; Cofre-Vega, C.; Barrangou, R.; Wang, J.P. Advances in Lignocellulosic Feedstocks for Bioenergy and Bioproducts. Nat. Commun. 2025, 16, 1244. [Google Scholar] [CrossRef] [PubMed]
- Fortuin, J.; Hoffmeester, L.J.; Minnaar, L.S.; den Haan, R. Advancing Cellulose Utilization and Engineering Consolidated Bioprocessing Yeasts: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2025, 109, 43. [Google Scholar] [CrossRef]
- Rao, T.S.S.B.; Gnanaprakasam, M.; Manimaran, R.; Balasubramanian, D.; Kale, U.; Kilikevičius, A. Sustainable Synthesis and Advanced Optimization of Prosopis Juliflora Biomass Catalyst for Efficient Biodiesel Production and Environmental Impact Assessment. Sci. Rep. 2025, 15, 4472. [Google Scholar] [CrossRef]
- Umesh; Moholkar, V.S. 2G Bioethanol for Sustainable Transport Sector: Review and Analysis of the Life Cycle Assessments. Curr. Pollut. Rep. 2025, 11, 11. [Google Scholar] [CrossRef]
- Cunha, J.T.; Romaní, A.; Domingues, L. Production of HMF-Derivatives from Wine Residues Using Saccharomyces Cerevisiae as Whole-Cell Biocatalyst. Bioresour. Bioprocess 2025, 12, 8. [Google Scholar] [CrossRef]
- Kant, G.; Hasan, A.; Yadav, P.; Pandey, A.; Srivastava, S. The Generational Shift in Biofuels: A Path toward Sustainable Energy Solutions. Biomass Bioenergy 2025, 196, 107757. [Google Scholar] [CrossRef]
- Russo, C.; Cirillo, V.; Pollaro, N.; Terribile, F.; Chiodini, A.; Maggio, A. The Global Energy Challenge: Second-Generation Feedstocks on Marginal Lands for a Sustainable Biofuel Production. Chem. Biol. Technol. Agric. 2025, 12, 10. [Google Scholar] [CrossRef]
- Ali, K.; Niaz, N.; Waseem, M.; Ashraf, W.; Hussain, M.; Khalid, M.U.; Tahir, A.B.; Raza, A.; Khan, I.M. Xylooligosaccharides: A Comprehensive Review of Production, Purification, Characterization, and Quantification. Food Res. Int. 2025, 201, 115631. [Google Scholar] [CrossRef] [PubMed]
- Bamidele, R.; Marszewski, M. Carbonization and Activation of Lignocellulosic Biomass in Liquid Phase at Sub-200 °C Temperatures. ACS Sustain. Chem. Eng. 2025, 13, 3010–3021. [Google Scholar] [CrossRef]
- Tang, Z.; Zhang, C.; Yin, J.; Fan, B.; He, Y.C.; Ma, C. Valorization of Rapeseed Straw through the Enhancement of Cellulose Accessibility, Lignin Removal and Xylan Elimination Using an n-Alkyltrimethylammonium Bromide-Based Deep Eutectic Solvent. Int. J. Biol. Macromol. 2025, 301, 140151. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Tang, Z.; He, Y.C. Valorization of Wheat Straw through Enhancement of Cellulose Accessibility, Xylan Elimination and Lignin Removal by Choline Chloride:P-Toluenesulfonic Acid Pretreatment. Int. J. Biol. Macromol. 2025, 301, 140335. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Borah, D.; Paul, P.; Rout, J. Integrated Biorefinery Approach for Sustainable Production of Biodiesel, Bioplastics and High Value Bioproducts from a Bloom Forming Alga, Botryococcus braunii. Sci. Total Environ. 2025, 964, 178599. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaran, M.; Rajan, K.G. Evaluating Value-Added Biochemical and Biodiesel Production from Chlorococcum humicolo Algal Biomass in Phycoremediation of Textile Dye Effluents. Bioresour. Technol. 2025, 419, 132009. [Google Scholar] [CrossRef]
- Hemavathy, R.V.; Ragini, Y.P.; Shruthi, S.; Ranjani, S.; Subhashini, S.; Thamarai, P. Biofuel Production from Marine Macroalgae: Pathways, Technologies, and Sustainable Energy Solutions. Ind. Crops. Prod. 2025, 224, 120282. [Google Scholar] [CrossRef]
- Smichi, N.; Messaoudi, Y.; Moujahed, N.; Messaoud, C.; Langar, H.; Bezzarga, M.; Gargouri, M. Bioethanol Production from Tunisian Macroalgal Biomass. EuroMediterr. J. Environ. Integr. 2024, 9, 1459–1469. [Google Scholar] [CrossRef]
- León-Marcos, L.; Fuente-Zapico, E.; Romero-Vargas, A.; Blandino, A.; Romero-García, L.I. Ultrasound Pretreatment of Third-Generation Biomass (Invasive Macroalga Rugulopteryx okamurae) to Obtain Platform Biocommodities. J. Appl. Phycol. 2024, 36, 2807–2821. [Google Scholar] [CrossRef]
- del Río, P.G.; Gullón, B.; Pérez-Pérez, A.; Romaní, A.; Garrote, G. Microwave Hydrothermal Processing of the Invasive Macroalgae Sargassum Muticum within a Green Biorefinery Scheme. Bioresour. Technol. 2021, 340, 125733. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and Harmful Algal Blooms: A Scientific Consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Bachot, X.; Riera, R. How the Invasive Algae Rugulopteryx Okamurae Affect Coastal Biodiversity? Insights from Coastal Fish Communities of Gran Canaria (NE Atlantic Ocean). J. Sea Res. 2025, 204, 102568. [Google Scholar] [CrossRef]
- Choudhary, P.; G., V.S.; Khade, M.; Savant, S.; Musale, A.; G., R.K.K.; Chelliah, M.S.; Dasgupta, S. Empowering Blue Economy: From Underrated Ecosystem to Sustainable Industry. J. Environ. Manag. 2021, 291, 112697. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Clare, A.S.; Rose, C.; Caldwell, G.S. Eutrophication and Warming-Driven Green Tides (Ulva Rigida) Are Predicted to Increase under Future Climate Change Scenarios. Mar. Pollut. Bull. 2017, 114, 439–447. [Google Scholar] [CrossRef]
- Liu, F.; Pang, S.; Chopin, T.; Xu, N.; Shan, T.; Gao, S.; Song, S. The Dominant Ulva Strain of the 2008 Green Algal Bloom in the Yellow Sea Was Not Detected in the Coastal Waters of Qingdao in the Following Winter. J. Appl. Phycol. 2010, 22, 531–540. [Google Scholar] [CrossRef]
- Burkholder, J.M.; Glibert, P.M. Eutrophication and Oligotrophication. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2013; pp. 347–371. ISBN 978-0-12-384720-1. [Google Scholar]
- Duarte, C.M. Seagrasses. In Encyclopedia of Biodiversity, 2nd ed.; Levin, S.A., Ed.; Academic Press: Waltham, MA, USA, 2001; pp. 540–550. ISBN 978-0-12-384720-1. [Google Scholar]
- Korzen, L.; Pulidindi, I.N.; Israel, A.; Abelson, A.; Gedanken, A. Single Step Production of Bioethanol from the Seaweed Ulva Rigida Using Sonication. RSC Adv. 2015, 5, 16223–16229. [Google Scholar] [CrossRef]
- Pereira, L. Chapter 3—Algal Polysaccharides. In Functional Ingredients from Algae for Foods and Nutraceuticals, 2nd ed.; Dominguez, H., Pereira, L., Kraan, S., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2023; pp. 151–212. ISBN 978-0-323-98819-3. [Google Scholar]
- Tziveleka, L.-A.; Ioannou, E.; Roussis, V. Ulvan, a Bioactive Marine Sulphated Polysaccharide as a Key Constituent of Hybrid Biomaterials: A Review. Carbohydr. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, G.; Yu, K.; Yu, H.; Wang, X. Furfural Production from Biomass–Derived Carbohydrates and Lignocellulosic Residues via Heterogeneous Acid Catalysts. Ind. Crops Prod. 2017, 98, 68–75. [Google Scholar] [CrossRef]
- Nikolla, E.; Román-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-Pot” Synthesis of 5-(Hydroxymethyl)Furfural from Carbohydrates Using Tin-Beta Zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef]
- García-López, E.I.; Pomilla, F.R.; Megna, B.; Testa, M.L.; Liotta, L.F.; Marcì, G. Catalytic Dehydration of Fructose to 5-Hydroxymethylfurfural in Aqueous Medium over Nb2O5-Based Catalysts. Nanomaterials 2021, 11, 1821. [Google Scholar] [CrossRef]
- Gromov, N.V.; Ogorodnikova, O.L.; Medvedeva, T.B.; Panchenko, V.N.; Yakovleva, I.S.; Isupova, L.A.; Timofeeva, M.N.; Taran, O.P.; Aymonier, C.; Parmon, V.N. Hydrolysis–Dehydration of Cellulose: Efficiency of NbZr Catalysts under Batch and Flow Conditions. Catalysts 2023, 13, 1298. [Google Scholar] [CrossRef]
- Alboofetileh, M.; Jeddi, S.; Subi, T.M.; Vasanthi, H.R. Sulfated Polysaccharides from Ulva Rigida as Antioxidant and Antibacterial Enhancers in Chicken Sausages. Algal Res. 2025, 86, 103940. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chang, S.S.; Clair, B.; Ruelle, J.; Beauchêne, J.; Di Renzo, F.; Quignard, F.; Zhao, G.J.; Yamamoto, H.; Gril, J. Mesoporosity as a New Parameter for Understanding Tension Stress Generation in Trees. J. Exp. Bot. 2009, 60, 3023–3030. [Google Scholar] [CrossRef]
- Janus, R.; Wądrzyk, M.; Lewandowski, M.; Natkański, P.; Łątka, P.; Kuśtrowski, P. Understanding Porous Structure of SBA-15 upon Pseudomorphic Transformation into MCM-41: Non-Direct Investigation by Carbon Replication. J. Ind. Eng. Chem. 2020, 92, 131–144. [Google Scholar] [CrossRef]
- Esperanza Adrover, M.; Pedernera, M.; Bonne, M.; Lebeau, B.; Bucalá, V.; Gallo, L. Synthesis and Characterization of Mesoporous SBA-15 and SBA-16 as Carriers to Improve Albendazole Dissolution Rate. Saudi Pharm. J. 2020, 28, 15–24. [Google Scholar] [CrossRef]
- Yuzbashi, S.; Mousazadeh, M.; Ramezani, N.; Sid Kalal, H.; Sabour, B. Mesoporous Zirconium–Silica Nanocomposite Modified with Heteropoly Tungstophosphoric Acid Catalyst for Ultra-deep Oxidative Desulfurization. Appl. Organomet. Chem. 2019, 34, e5326. [Google Scholar] [CrossRef]
- Duan, G.; Zhang, C.; Li, A.; Yang, X.; Lu, L.; Wang, X. Preparation and Characterization of Mesoporous Zirconia Made by Using a Poly (Methyl Methacrylate) Template. Nanoscale Res. Lett. 2008, 3, 118. [Google Scholar] [CrossRef]
- García-Sancho, C.; Saboya, R.M.A.; Cecilia, J.A.; Sales, A.V.; Luna, F.M.T.; Rodríguez-Castellón, E.; Cavalcante, C.L. Influence of Pore Size and Loading for Nb2O5/SBA-15 Catalysts on Synthetic Ester Production from Free Fatty Acids of Castor Oil. Mol. Catal. 2017, 436, 267–275. [Google Scholar] [CrossRef]
- Özer, N.; Chen, D.G.; Lampert, C.M. Preparation and Properties of Spin-Coated Nb2O5 Films by the Sol-Gel Process for Electrochromic Applications. Thin Solid Film. 1996, 277, 162–168. [Google Scholar] [CrossRef]
- Garbassi, F.; Bart, J.C.J.; Petrini, G. XPS Study of Tellurium—Niobium and Tellurium—Tantalum Oxide Systems. J. Electron Spectrosc. Relat. Phenom. 1981, 22, 95–107. [Google Scholar] [CrossRef]
- Sinha, S.; Badrinarayanan, S.; Sinha, A.P.B. An XPS Study of Hydrogen Implanted Zirconium. J. Less Common Met. 1987, 134, 229–236. [Google Scholar] [CrossRef]
- Baba, Y.; Sasaki, T.A. Application of X-ray-induced Auger Electron Spectroscopy to State Analyses of Hydrogen Implanted in Y, Zr and Nb Metals. Surf. Interface Anal. 1984, 6, 171–173. [Google Scholar] [CrossRef]
- Tanev, P.T.; Pinnavaia, T.J. A Neutral Templating Route to Mesoporous Molecular Sieves. Science 1995, 267, 865–867. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, L.; Wang, X.; Song, Y.; Chen, B. Conversion of Sugars into Methyl Lactate over Poly (Ionic Liquid)s Functionalized Sn/Beta Zeolites. Catal. Today 2024, 442, 114937. [Google Scholar] [CrossRef]
- Busca, G. Spectroscopic Characterization of the Acid Properties of Metal Oxide Catalysts. Catal. Today 1998, 41, 191–206. [Google Scholar] [CrossRef]
- Datka, J. Acidic Properties of Supported Niobium Oxide Catalysts: An Infrared Spectroscopy Investigation. J. Catal. 1992, 135, 186–199. [Google Scholar] [CrossRef]
- Connell, G. The Generation of Brønsted and Lewis Acid Sites on the Surface of Silica by Addition of Dopant Cations. J. Catal. 1987, 105, 285–298. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Jehng, J.-M.; Wachs, I.E. Molecular Structures of Supported Niobium Oxide Catalysts under Ambient Conditions. J. Mol. Catal. 1991, 67, 369–387. [Google Scholar] [CrossRef]
- Burcham, L.J.; Datka, J.; Wachs, I.E. In Situ Vibrational Spectroscopy Studies of Supported Niobium Oxide Catalysts. J. Phys. Chem. B 1999, 103, 6015–6024. [Google Scholar] [CrossRef]
- Burke, P. Acidic Properties of Oxides Containing Niobia on Silica and Niobia in Silica. J. Catal. 1991, 129, 38–46. [Google Scholar] [CrossRef]
- Zhou, Q.; Ding, A.; Zhang, L.; Wang, J.; Gu, J.; Wu, T.Y.; Gu, X.; Zhang, L. Furfural Production from the Lignocellulosic Agro-Forestry Waste by Solvolysis Method—A Technical Review. Fuel Process. Technol. 2024, 255, 108063. [Google Scholar] [CrossRef]
- Xu, J.; Luo, S.; Qiao, Y.; Li, C.; Zheng, Y.; Alam, M.A.; Zhang, S. Regulating Lewis Acid-Based Sites on Carbon Nitride for Selectivity Conversion of Glucose to Mannose and Fructose in Water. Biomass Bioenergy 2025, 194, 107631. [Google Scholar] [CrossRef]
- Gautam, R.; Saravanamurugan, S. Bifunctional Solid Acid Catalysed Conversion of Glucose to 5-Hydroxymethylfurfural with Sn-Doped Desilicated ZSM-5. Biomass Convers. Biorefin. 2025, 15, 21407–21418. [Google Scholar] [CrossRef]
- Choudhary, V.; Sandler, S.I.; Vlachos, D.G. Conversion of Xylose to Furfural Using Lewis and Brønsted Acid Catalysts in Aqueous Media. ACS Catal. 2012, 2, 2022–2028. [Google Scholar] [CrossRef]
- García Sancho, C.; Rubio, J.; Mérida Robles, J.; Moreno-Tost, R.; Santamaría–Gonzalez, J.; Maireles-Torres, P. Mesoporous Nb2O5 as Solid Acid Catalyst for Dehydration of D-Xylose into Furfural. Catal. Today 2014, 234, 119–124. [Google Scholar] [CrossRef]
- Carniti, P.; Gervasini, A.; Bossola, F.; Dal Santo, V. Cooperative Action of Brønsted and Lewis Acid Sites of Niobium Phosphate Catalysts for Cellobiose Conversion in Water. Appl. Catal. B 2016, 193, 93–102. [Google Scholar] [CrossRef]
- Gao, J.-X.; Tian, W.-J.; Zhang, H.-Y. Progress of Nb-Containing Catalysts for Carbon Dioxide Reduction: A Minireview. Tungsten 2022, 4, 284–295. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Vilarrasa-García, E.; García-Sancho, C.; Saboya, R.M.A.; Azevedo, D.C.S.; Cavalcante, C.L.; Rodríguez-Castellón, E. Functionalization of Hollow Silica Microspheres by Impregnation or Grafted of Amine Groups for the CO2 Capture. Int. J. Greenh. Gas Control 2016, 52, 344–356. [Google Scholar] [CrossRef]
- Heesch, S.; Broom, J.E.; Neill, K.F.; Farr, T.J.; Dalen, J.L.; Nelson, W.A. Ulva, Umbraulva and Gemina: Genetic survey of New Zealand taxa reveals diversity and introduced species. Eur. J. Phycol. 2009, 44, 143–154. [Google Scholar] [CrossRef]
- Massocato, T.F.; Robles-Carnero, V.; Moreira, B.R.; Castro-Varela, P.; Pinheiro-Silva, L.; Oliveira, W.d.S.; Vega, J.; Avilés, A.; Bonomi-Barufi, J.; Rörig, L.R.; et al. Growth, biofiltration and photosynthetic performance of Ulva spp. cultivated in fishpond effluents: An outdoor study. Front. Mar. Sci. 2022, 9, 981468. [Google Scholar] [CrossRef]
- El Harchi, M.; Kachkach, F.F.; El Mtili, N. Optimization of thermal acid hydrolysis for bioethanol production from Ulva rigida with yeast Pachysolen tannophilus. S. Afr. J. Bot. 2018, 115, 161–169. [Google Scholar] [CrossRef]
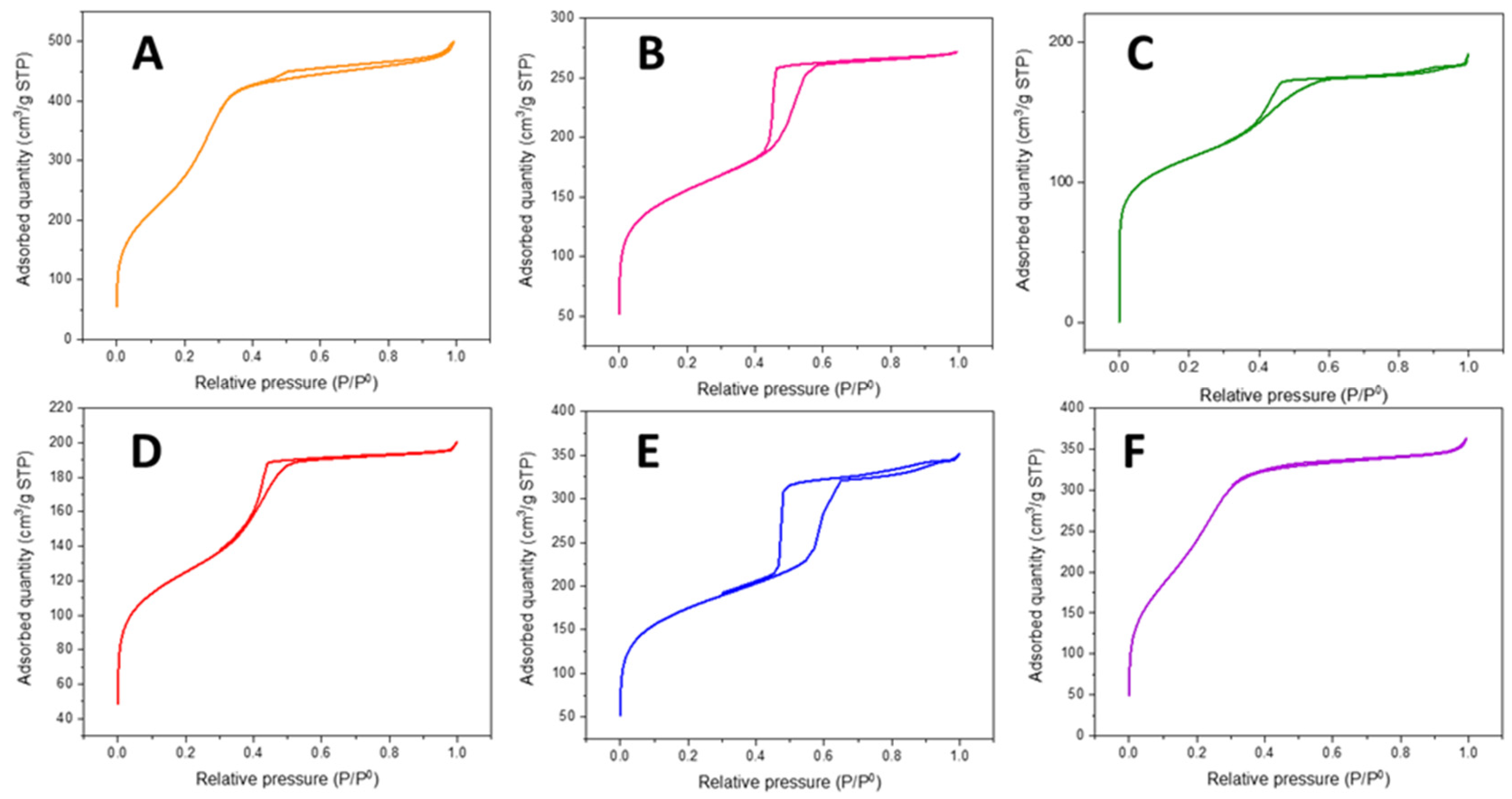
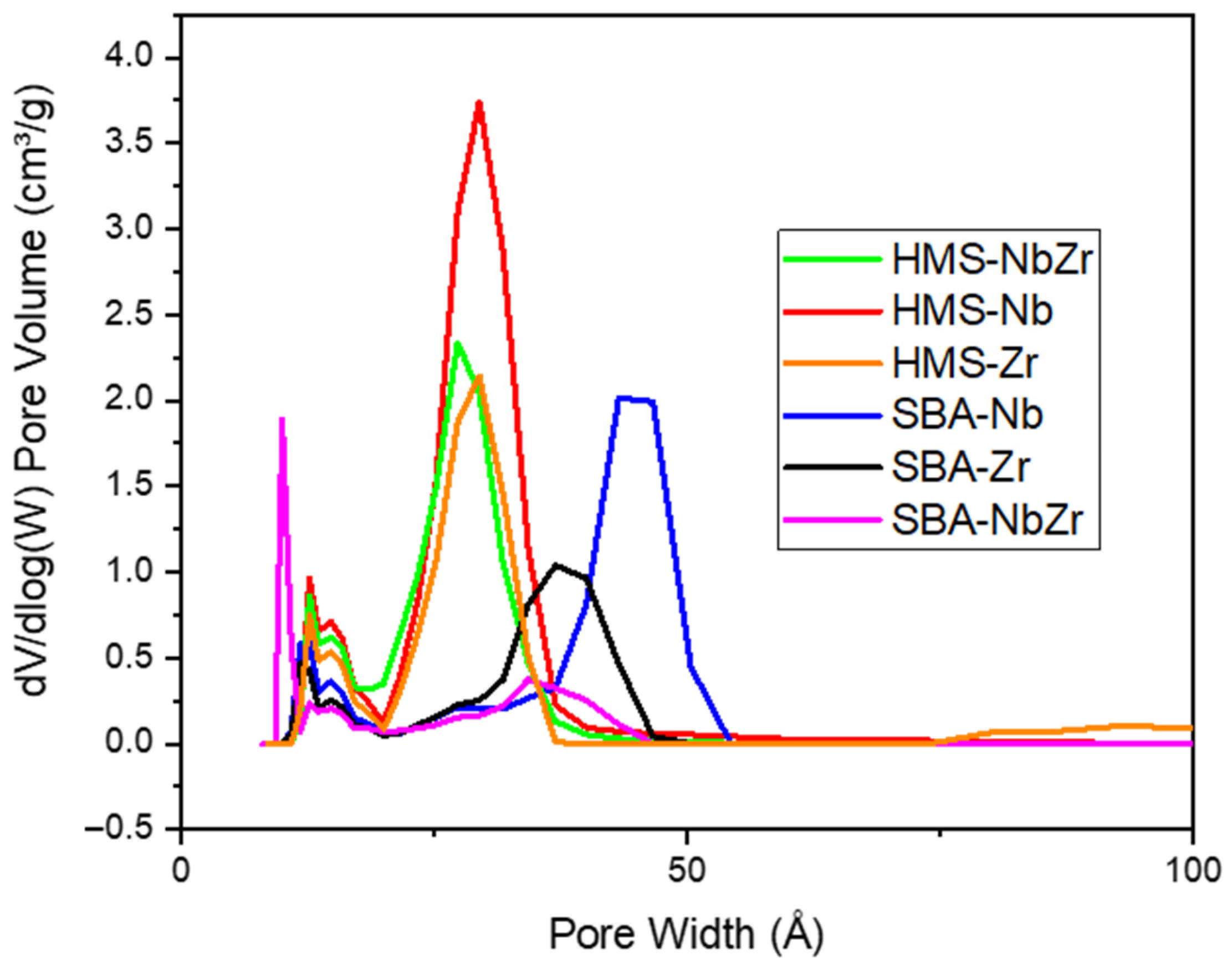
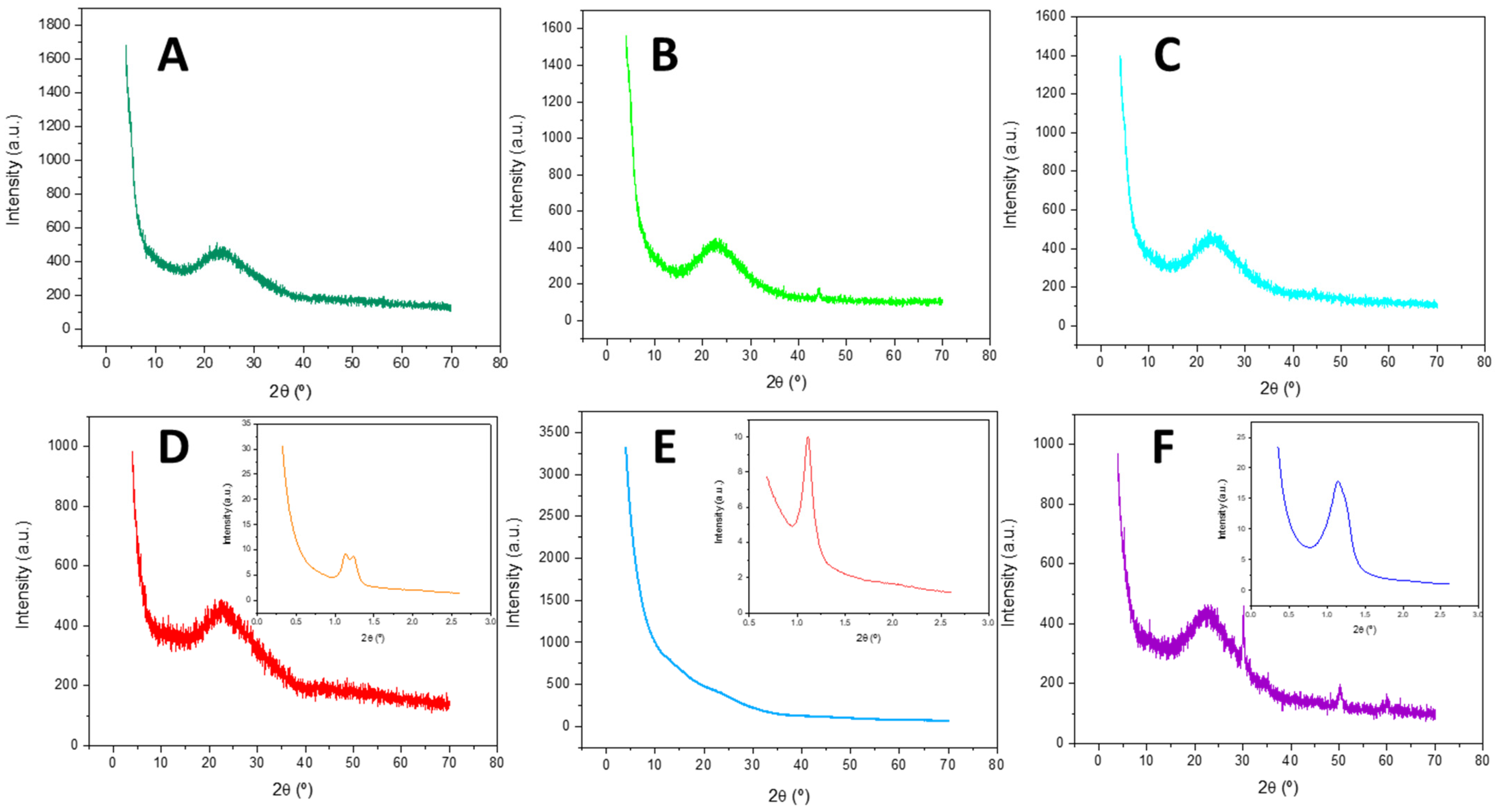
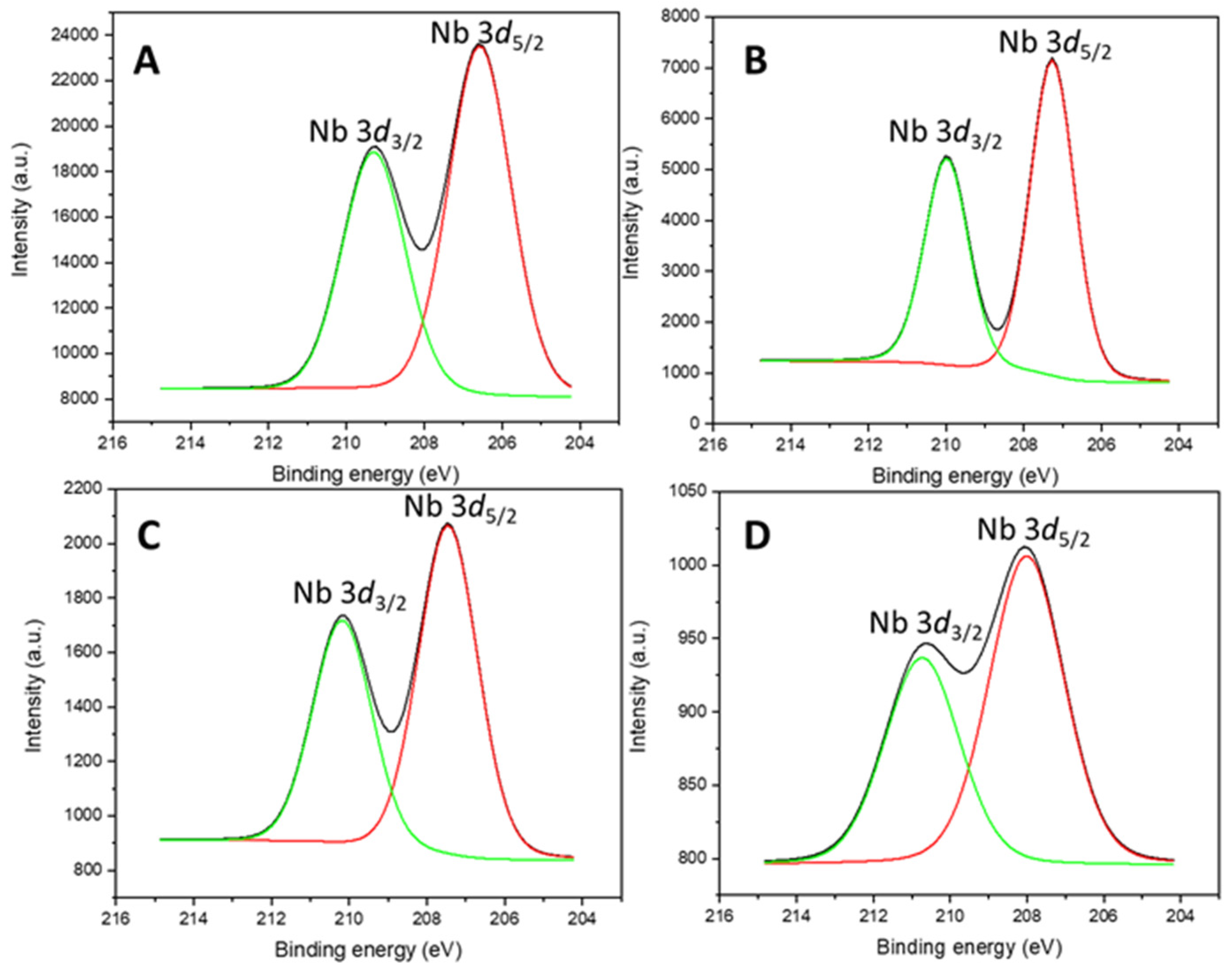


| Catalysts | SBET (m2/g) | t-Plot Micropore Area (m2/g) | External Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|---|
| HMS | 904 | 449 | 455 | 0.58 | 2.4 |
| SBA | 421 | 214 | 207 | 0.28 | 2.6 |
| HMS-NbZr | 988 | 855 | 132 | 0.54 | 3.7 |
| HMS-Nb | 1204 | 975 | 229 | 0.73 | 3.5 |
| HMS-Zr | 898 | 679 | 219 | 0.56 | 2.9 |
| SBA-NbZr | 424 | 113 | 310 | 0.30 | 3.6 |
| SBA-Nb | 555 | 150 | 405 | 0.42 | 3.6 |
| SBA-Zr | 445 | 241 | 204 | 0.23 | 3.4 |
| Catalysts | C 1s | O 1s | Si 2p | Nb 3d | Zr 3d | Si/Nb | Si/Zr | Si/(Nb + Zr) |
|---|---|---|---|---|---|---|---|---|
| HMS-NbZr | 9.49 | 63.39 | 25.49 | 7.24 | 5.32 | 3.52 | 4.79 | 2.03 |
| HMS-Nb | 8.40 | 64.09 | 27.23 | 4.80 | -- | 5.67 | -- | -- |
| HMS-Zr | 10.16 | 63.18 | 25.90 | -- | 3.77 | -- | 6.87 | -- |
| SBA-NbZr | 16.01 | 58.19 | 17.87 | 5.47 | 4.41 | 3.27 | 4.05 | 1.81 |
| SBA-Nb | 10.51 | 60.17 | 25.61 | 3.72 | -- | 6.88 | -- | -- |
| SBA-Zr | 11.54 | 60.88 | 23.20 | -- | 4.39 | -- | 5.28 | -- |
| Catalyst | Total Acidity (μmol/g) | Density of Acid Sites (μmol/m2) | Weak Sites a (μmol/g) | Moderate Sites b (μmol/g) | Strong Sites c (μmol/g) |
|---|---|---|---|---|---|
| HMS-NbZr | 430 | 0.43 | 210 | 160 | 60 |
| HMS-Nb | 390 | 0.32 | 185 | 155 | 50 |
| HMS-Zr | 350 | 0.39 | 207 | 173 | 70 |
| SBA-NbZr | 460 | 1.08 | 293 | 107 | 60 |
| SBA-Nb | 360 | 0.65 | 191 | 97 | 72 |
| SBA-Zr | 220 | 0.49 | 136 | 54 | 30 |
| Catalyst | Acid Nature | Weak Sites a (μmol/g) | Moderate Sites b (μmol/g) | Strong Sites c (μmol/g) |
|---|---|---|---|---|
| HMS-NbZr | Brønsted | 24 | 13 | 22 |
| Lewis | 82 | 14 | 7 | |
| HMS-Nb | Brønsted | 10 | 24 | 11 |
| Lewis | 147 | 31 | 20 | |
| HMS-Zr | Brønsted | 30 | 26 | 29 |
| Lewis | 64 | 6 | 0 | |
| SBA-NbZr | Brønsted | 73 | 25 | 10 |
| Lewis | 5 | 53 | 40 | |
| SBA-Nb | Brønsted | 26 | 8 | 9 |
| Lewis | 81 | 28 | 11 | |
| SBA-Zr | Brønsted | 2 | 17 | 1 |
| Lewis | 28 | 31 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Carballo, G.; Torres-Olea, B.; García-Sancho, C.; Vega, J.; Figueroa, F.L.; Cecilia, J.A.; Maireles-Torres, P.; Moreno-Tost, R. Selective Dehydration of Pentoses and Hexoses of Ulva rigida to Platform Chemicals Using Nb2O5 and ZrO2 Supported on Mesoporous Silicas as Heterogeneous Catalysts. Int. J. Mol. Sci. 2025, 26, 10054. https://doi.org/10.3390/ijms262010054
Rodríguez-Carballo G, Torres-Olea B, García-Sancho C, Vega J, Figueroa FL, Cecilia JA, Maireles-Torres P, Moreno-Tost R. Selective Dehydration of Pentoses and Hexoses of Ulva rigida to Platform Chemicals Using Nb2O5 and ZrO2 Supported on Mesoporous Silicas as Heterogeneous Catalysts. International Journal of Molecular Sciences. 2025; 26(20):10054. https://doi.org/10.3390/ijms262010054
Chicago/Turabian StyleRodríguez-Carballo, Gabriela, Benjamín Torres-Olea, Cristina García-Sancho, Julia Vega, Félix L. Figueroa, Juan Antonio Cecilia, Pedro Maireles-Torres, and Ramón Moreno-Tost. 2025. "Selective Dehydration of Pentoses and Hexoses of Ulva rigida to Platform Chemicals Using Nb2O5 and ZrO2 Supported on Mesoporous Silicas as Heterogeneous Catalysts" International Journal of Molecular Sciences 26, no. 20: 10054. https://doi.org/10.3390/ijms262010054
APA StyleRodríguez-Carballo, G., Torres-Olea, B., García-Sancho, C., Vega, J., Figueroa, F. L., Cecilia, J. A., Maireles-Torres, P., & Moreno-Tost, R. (2025). Selective Dehydration of Pentoses and Hexoses of Ulva rigida to Platform Chemicals Using Nb2O5 and ZrO2 Supported on Mesoporous Silicas as Heterogeneous Catalysts. International Journal of Molecular Sciences, 26(20), 10054. https://doi.org/10.3390/ijms262010054








