CpARF6 Controls Lobed Leaf Formation in Zucchini
Abstract
1. Introduction
2. Results
2.1. Lobed Leaf Trait in Zucchini Is Controlled by Two Recessive Genes
2.2. Gene Mapping of the Candidate Locus for the Lobed Leaf Trait
2.3. Fine Mapping of the Candidate Locus for the Lobed Leaf Trait
2.4. Expression Profiles of Genes in the Candidate Region
2.5. Non-Synonymous Variation of CpARF6 Confers the Lobed Leaf Trait
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. BSA-Seq Analysis
4.3. Kompetitive Allele-Specific PCR (KASP) Genotyping
4.4. qRT-PCR Experimental Method
4.5. Gene Cloning
5. Conlusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Hu, L.; Amas, J.C.; Thomas, W.J.W.; Wang, L.; Wang, X.; Wang, W.; Qu, G.; Shen, X.; Ji, R.; et al. BrRCO promotes leaf lobe formation by repressing BrACP5 expression in Brassica rapa. Hortic. Res. 2025, 12, uhaf084. [Google Scholar] [CrossRef]
- Tsukaya, H. Leaf development. Arab. Book 2013, 11, e0163. [Google Scholar] [CrossRef]
- Ledford, H. The lost art of looking at plants. Nature 2018, 553, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, J.; Liu, D.; Stiller, W.; Liu, D.; Zhang, Z.; Llewellyn, D.; Wilson, I. Integrated mapping and characterization of the gene underlying the okra leaf trait in Gossypium hirsutum L. J. Exp. Bot. 2016, 67, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Zhang, F.; Wu, H.; Xia, H.; Wu, J.; Tu, Z.; Yang, L.; Li, H. Comprehensive deciphering the alternative splicing patterns involved in leaf morphogenesis of Liriodendron chinense. BMC Plant Biol. 2024, 24, 250. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, A.; He, W.; Li, Q.; Zhao, B.; Zhao, H.; Ke, X.; Guo, Y.; Sun, P.; Yang, T.; et al. GRAS family member LATERAL SUPPRESSOR regulates the initiation and morphogenesis of watermelon lateral organs. Plant Physiol. 2023, 193, 2592–2604. [Google Scholar] [CrossRef]
- Bo, K.; Duan, Y.; Qiu, X.; Zhang, M.; Shu, Q.; Sun, Y.; He, Y.; Shi, Y.; Weng, Y.; Wang, C. Promoter variation in a homeobox gene, CpDll, is associated with deeply lobed leaf in Cucurbita pepo L. Theor. Appl. Genet. 2022, 135, 1223–1234. [Google Scholar] [CrossRef]
- Yan, X.; Yue, Z.; Li, S.; Chen, X.; Huang, X.; Feng, M.; Wang, Z.; Zhang, S.; Luan, F.; Liu, S.; et al. The HD-ZIP I transcription factor ClLL1 regulates lobed leaf development through the auxin pathway in watermelon. Plant J. 2025, 123, e70366. [Google Scholar]
- Bilsborough, G.D.; Runions, A.; Barkoulas, M.; Jenkins, H.W.; Hasson, A.; Galinha, C.; Laufs, P.; Hay, A.; Prusinkiewicz, P.; Tsiantis, M. Model for the regulation of Arabidopsis thaliana leaf margin development. Proc. Nat. Acad. Sci. USA 2011, 108, 3424–3429. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Han, R.; Liu, C.; Fang, G.; Yuan, Q.; Zheng, Z.; Yu, Q.; Jiang, J.; Liu, S.; Xie, L.; et al. Heritable epigenetic modification of BpPIN1 is associated with leaf shapes in Betula pendula. Tree Physiol. 2023, 43, 1811–1824. [Google Scholar] [CrossRef]
- Guilfoyle, T.J.; Hagen, G. Auxin response factors. Curr. Opin. Plant Biol. 2007, 10, 453–460. [Google Scholar] [CrossRef]
- Guan, C.; Wu, B.; Yu, T.; Wang, Q.; Krogan, N.T.; Liu, X.; Jiao, Y. Spatial auxin signaling controls leaf flattening in Arabidopsis. Curr. Biol. 2017, 27, 2940–2950. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lavelle, D.; Yu, C.; Zhang, W.; Chen, J.; Wang, X.; Michelmore, R.W.; Kuang, H. The upregulated LsKN1 gene transforms pinnately to palmately lobed leaves through auxin, gibberellin, and leaf dorsiventrality pathways in lettuce. Plant Biotechnol. J. 2022, 20, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Fang, N.; Lu, T.; Tameshige, T.; Nakata, M.T.; Jiang, Y.; Tan, L.; He, H.; Zhang, X.; Huang, Y.; et al. WOX1 controls leaf serration development via temporally restricting BRASSINAZOLE RESISTANT 1 and CUP SHAPED COTYLEDON 3 expression in Arabidopsis. J. Exp. Bot. 2025, 76, 478–492. [Google Scholar] [PubMed]
- Chen, H.W.; Lee, P.L.; Wang, C.N.; Hsu, H.J.; Chen, J.C. Silencing of PhLA, a CIN-TCP gene, causes defected petal conical epidermal cell formation and results in reflexed corolla lobes in petunia. Bot. Stud. 2020, 61, 24. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Y.; Wang, J.; Jin, X.; Zhang, Q.; Ackah, M.; Wang, Y.; Xu, D.; Zhao, W. ATAC-seq exposes differences in chromatin accessibility leading to distinct leaf shapes in mulberry. Plant Direct. 2022, 6, e464. [Google Scholar] [CrossRef]
- Nikovics, K.; Blein, T.; Peaucelle, A. The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. Plant Cell 2006, 18, 2929–2945. [Google Scholar] [CrossRef]
- Luo, M.; Yu, C.W.; Chen, F.F.; Zhao, L.; Tian, G.; Liu, X.; Cui, Y.; Yang, J.Y.; Wu, K. Histone deacetylase HDA6 is functionally associated with AS1 in repression of KNOX genes in Arabidopsis. PLoS Genet. 2012, 8, e1003114. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Zhang, C.; Gao, M.; Wang, Y.; Wang, J.; Chai, Q.; Zheng, Y.; Wang, X.; Li, Q.; et al. Genetic mapping and transcriptome profiling revealed leaf lobe formation and leaf size are regulated by GhRl4 in Gossypium hirsutum L. Theor. Appl. Genet. 2025, 138, 53. [Google Scholar] [CrossRef]
- Ni, X.; Huang, J.; Ali, B.; Zhou, W.; Zhao, J. Genetic analysis and fne mapping of the LOBED-LEAF 1 (BnLL1) gene in rapeseed (Brassica napus L.). Euphytica 2015, 204, 29–38. [Google Scholar]
- He, S.; Zhi, F.; Ge, A.; Liao, Y.; Li, K.; Min, Y.; Wei, S.; Peng, D.; Guo, Y.; Liu, Z.; et al. BnaC06.WIP2-BnaA09.STM transcriptional regulatory module promotes leaf lobe formation in Brassica napus. Int. J. Biol. Macromol. 2024, 271, 132544. [Google Scholar] [CrossRef]
- Li, P.; Su, T.; Li, H.; Wu, Y.; Wang, L.; Zhang, F.; Wang, Z.; Yu, S. Promoter variations in a homeobox gene, BrLMI1, contribute to leaf lobe formation in Brassica rapa ssp. chinensis Makino. Theor. Appl. Genet. 2023, 136, 188. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, X.; Yang, X.; Zhu, P. Fine mapping and identifcation of the leaf shape gene BoFL in ornamental kale. Theor. Appl. Genet. 2020, 133, 1303–1312. [Google Scholar]
- Andres, R.J.; Bowman, D.T.; Kaur, B.; Kuraparthy, V. Mapping and genomic targeting of the major leaf shape gene (L) in upland cotton (Gossypium hirsutum L.). Theor. Appl. Genet. 2014, 127, 167–177. [Google Scholar] [CrossRef]
- Jiao, K.; Li, X.; Guo, W.; Yuan, X.; Cui, X.; Chen, X. Genome resequencing of two accessions and fine mapping the locus of lobed leaflet margins in mungbean. Mol. Breed. 2016, 36, 128. [Google Scholar] [CrossRef]
- Wei, L.; Wen, S.; Ma, J.; Tu, Z.; Zhu, S.; Zhai, X.; Li, H. Overexpression of LtuHB6 from Liriodendron tulipifera causes lobed-leaf formation in Arabidopsis thaliana. Physiol. Mol. Biol. Plants 2022, 28, 1875–1887. [Google Scholar] [PubMed]
- Luo, X.; Guo, L.; Tagliere, E.; Yang, Z.; Liu, Z. Leaf dissection and margin serration are independently regulated by two regulators converging on the CUC2-auxin module in strawberry. Curr. Biol. 2024, 34, 769–780.e5. [Google Scholar] [CrossRef]
- Zhou, Y.M.; Chen, C.H.; Yi, S.Q.; Zhang, K.J.; Lyu, X.L.; Yang, J.H.; Hu, Z.Y.; Zhang, M.F. Homeodomain leucine zipper protein controls the lobed leaf formation by modulating auxin distribution in watermelon. Theor. Appl. Genet. 2025, 138, 156. [Google Scholar] [CrossRef]
- Gao, X.W.; Ning, X.F.; Wang, Y.M.; Wang, X.L.; Yan, W.L.; Zhang, Z.Q.; Li, G. Fine mapping of a gene that confers palmately lobed leaf (pll) in melon (Cucumis melo L.). Euphytica 2014, 200, 337–347. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, C.; Zhang, S.; Hu, B.; Wang, S.; Zhang, Z.; Huang, S. Mutation in a novel gene SMALL AND CORDATE LEAF 1 affects leaf morphology in cucumber. J. Integr. Plant Biol. 2017, 59, 736–741. [Google Scholar] [CrossRef]
- Song, M.F.; Cheng, F.; Wang, J.; Wei, Q.Z.; Fu, W.Y.; Yu, X.Q.; Li, J.; Chen, J.F.; Lou, Q.F. A leaf shape mutant provides insight into PINOID Serine/Threonine Kinase function in cucumber (Cucumis sativus L.). J. Integr. Plant Biol. 2019, 61, 1000–1014. [Google Scholar] [CrossRef]
- Rong, F.; Chen, F.; Huang, L.; Zhang, J.Y.; Zhang, C.W.; Hou, D.; Cheng, Z.H.; Weng, Y.Q.; Chen, P.; Li, Y.H. A mutation in class III homeodomain-leucine zipper (HD-ZIP III) transcription factor results in curly leaf (cul) in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2019, 132, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Narayan, J.A.; Manoj, V.M.; Nerkar, G.; Chakravarthi, M.; Dharshini, S.; Subramonian, N.; Premachandran, M.N.; Valarmathi, R.; Kumar, R.A.; Gomathi, R.; et al. Transgenic sugarcane with higher levels of BRK1 showed improved drought tolerance. Plant Cell Rep. 2023, 42, 1611–1628. [Google Scholar] [CrossRef]
- Dyutin, K.E. Spontaneous mutant of Cucurbita maxima Duch. squash with lobed leaves. Genetika 1980, 16, 176–178. [Google Scholar]
- Herrington, M.E.; Brown, P.J. Inheritance of leaf and fruit characteristics in Cucurbita maxima Duch. cv. Queensland Blue x C. ecuadorensis Cutler and Whitaker. Queensl. J. Agric. Anim. Sci. 1988, 45, 45–48. [Google Scholar]
- Montero-Pau, J.; Blanca, J.; Bombarely, A.; Ziarsolo, P.; Esteras, C.; Martí-Gómez, C.; Ferriol, M.; Gómez, P.; Jamilena, M.; Mueller, L.; et al. De novo assembly of the zucchini genome reveals a whole-genome duplication associated with the origin of the Cucurbita genus. Plant Biotechnol. J. 2018, 16, 1161–1171. [Google Scholar] [CrossRef]
- Cao, B.; Li, J.W.; Li, L.J.; Liu, Y.J.; Pan, Y.S.; Wang, Z.W. Genetic analysis of plant architecture and cob color traits in maize. Shandong Agric. Sci. 2007, 2, 8–9. [Google Scholar]
- Wang, F.B.; Fu, J.F.; Dong, L.F. Study on heredity laws of keratin membrane and sugar content in semi-leafless vegetable pea and their utilization in pea breeding. Yi Chuan 2004, 26, 907–910. [Google Scholar] [PubMed]
- Wu, L.; Tian, Z.; Zhang, J. Functional Dissection of Auxin Response Factors in Regulating Tomato Leaf Shape Development. Front. Plant Sci. 2018, 9, 957. [Google Scholar] [CrossRef]
- Hendelman, A.; Buxdorf, K.; Stav, R. Inhibition of lamina outgrowth following Solanum lycopersicum AUXIN RESPONSE FACTOR 10 (SlARF10) derepression. Plant Mol. Biolohy 2012, 78, 561–576. [Google Scholar]
- Ben-Gera, H.; Dafna, A.; Alvarez, J.P.; Bar, M.; Mauerer, M.; Ori, N. Auxin-mediated lamina growth in tomato leaves is restricted by two parallel mechanisms. Plant J. 2016, 86, 443–457. [Google Scholar] [CrossRef]
- Iwasaki, M.; Takahashi, H.; Iwakawa, H.; Nakagawa, A.; Ishikawa, T.; Tanaka, H.; Matsumura, Y.; Pekker, I.; Eshed, Y.; Vial-Pradel, S. Dual regulation of ETTIN (ARF3) gene expression by AS1-AS2, which maintains the DNA methylation level, is involved in stabilization of leaf adaxial-abaxial partitioning in Arabidopsis. Development 2013, 140, 1958–1969. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.; Li, H.; Fu, X.; Shang, H.; Zou, C.; Friml, J.; Xiao, G. GhARF16-1 modulates leaf development by transcriptionally regulating the GhKNOX2-1 gene in cotton. Plant Biotechnol. J. 2021, 19, 548–562. [Google Scholar] [PubMed]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysac- charide and polyphenol components. Plant Mol. Biol. Report. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Brown, J.; Pirrung, M.; McCue, L.A. FQC Dashboard: Integrates FastQC results into a web-based, interactive, and extensible FASTQ quality control tool. Bioinformatics 2017, 33, 3137–3139. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernysky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- Takagi, H.; Abe, A.; Yoshida, K.; Kosugi, S.; Natsume, S.; Mitsuoka, C.; Uemura, A.; Utsushi, H.; Tamiru, M.; Takuno, S.; et al. QTL-seq: Rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations. Plant J. 2013, 74, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Wang, Y.; Qi, C.; Luo, Y.; Wang, C.; Xu, W.; Qu, S. Fine mapping identifed the gibberellin 2-oxidase gene CpDw leading to a dwarf phenotype in squash (Cucurbita pepo L.). Plant Sci. 2021, 306, 110857. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
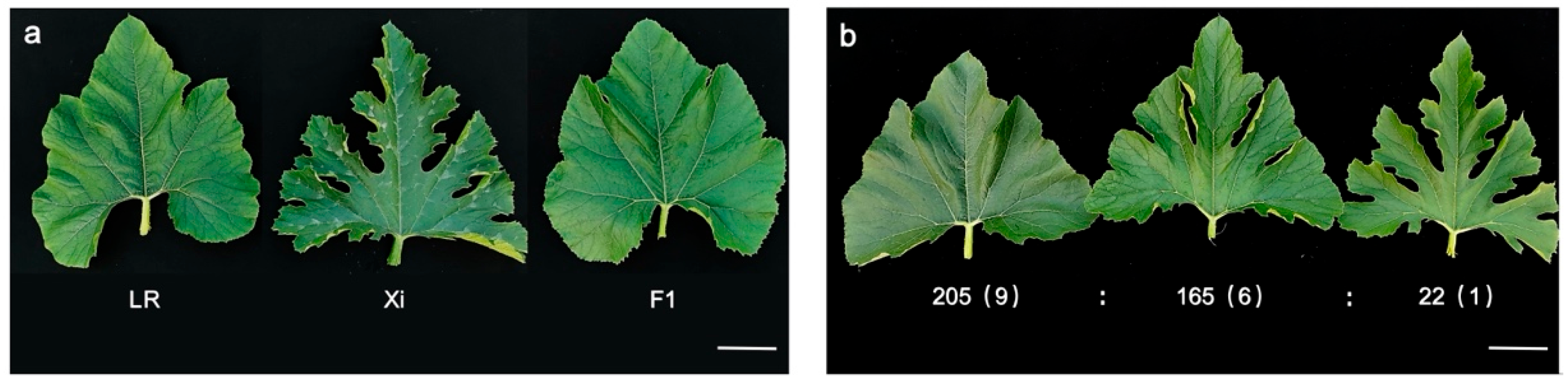

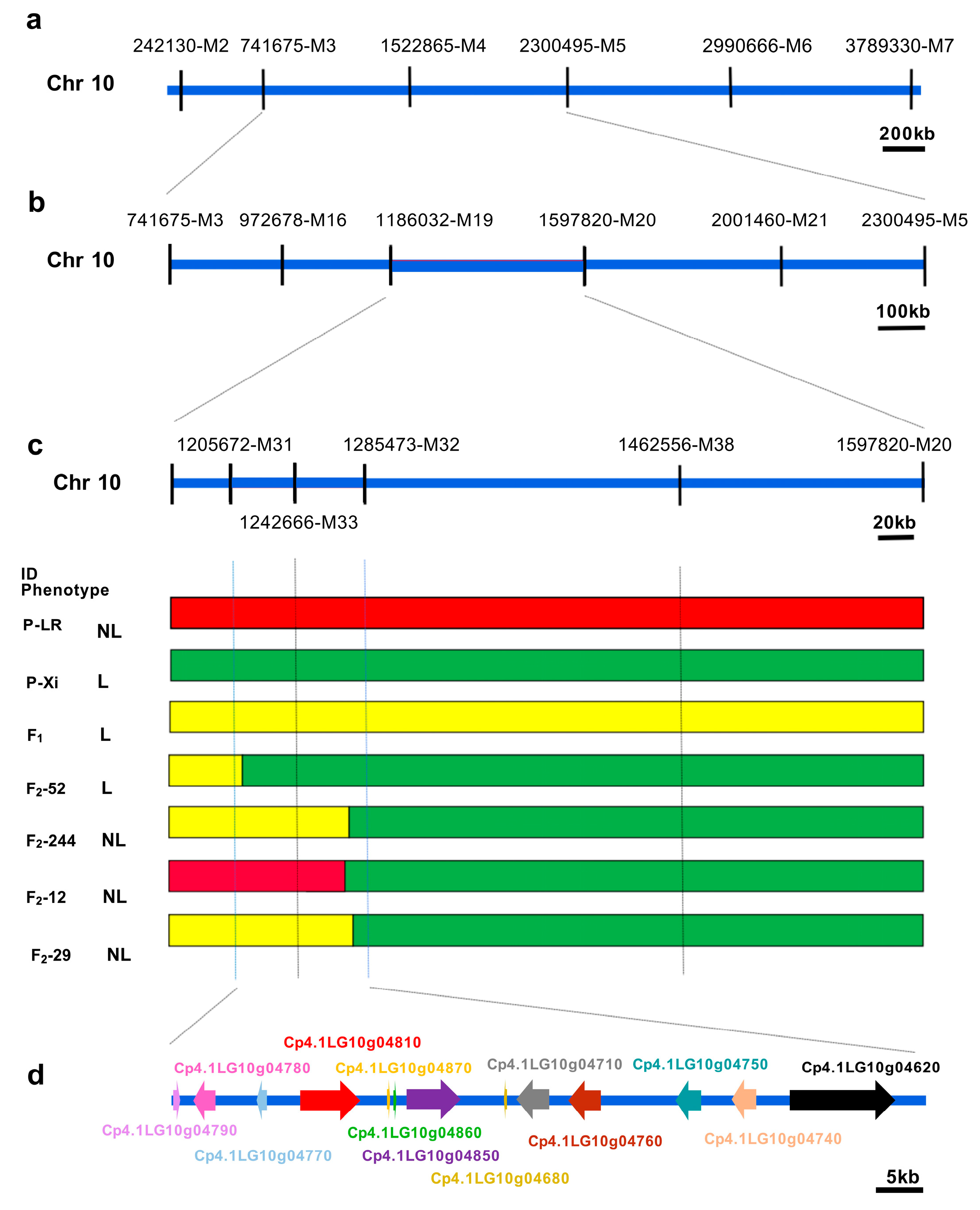
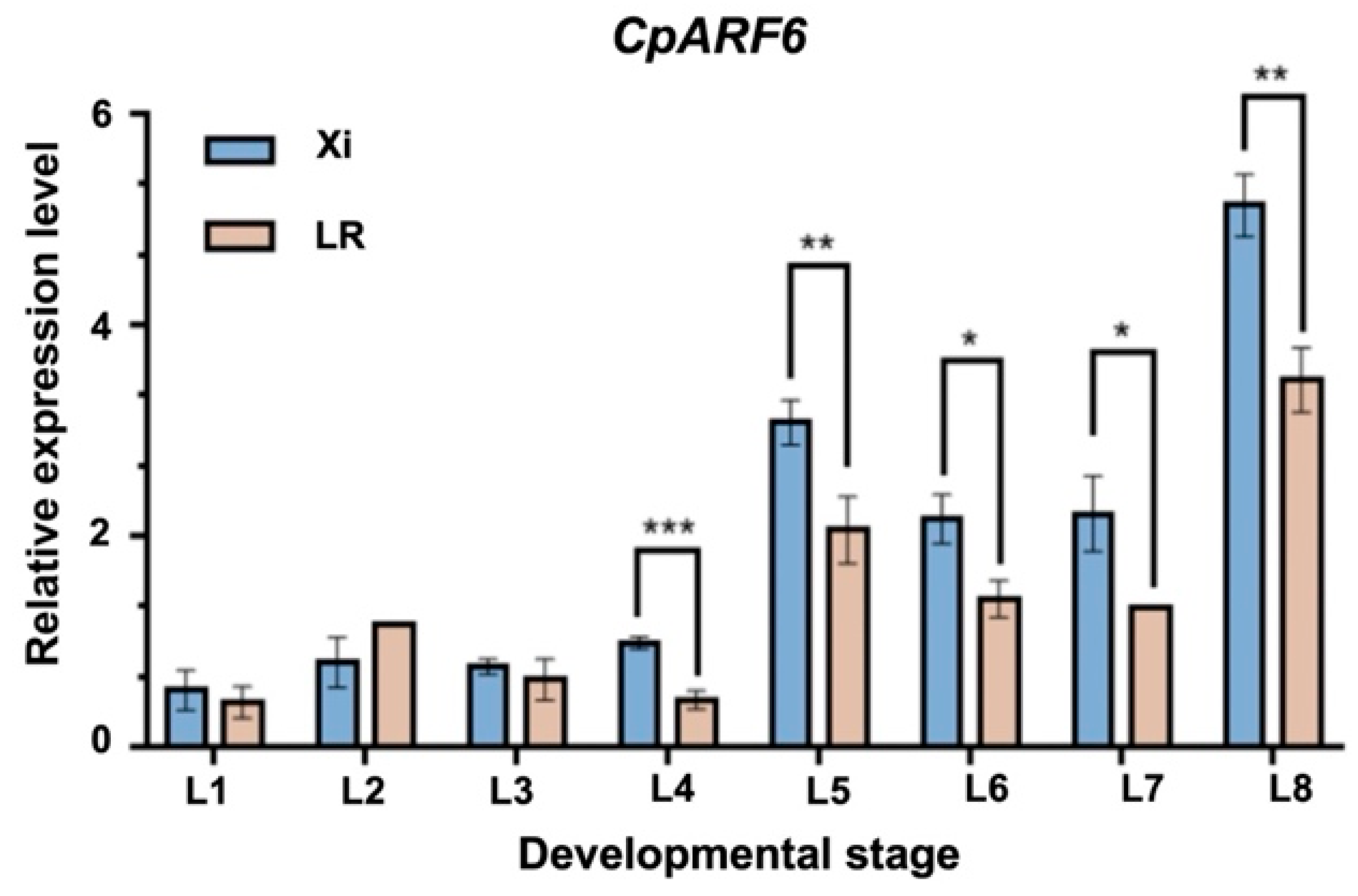
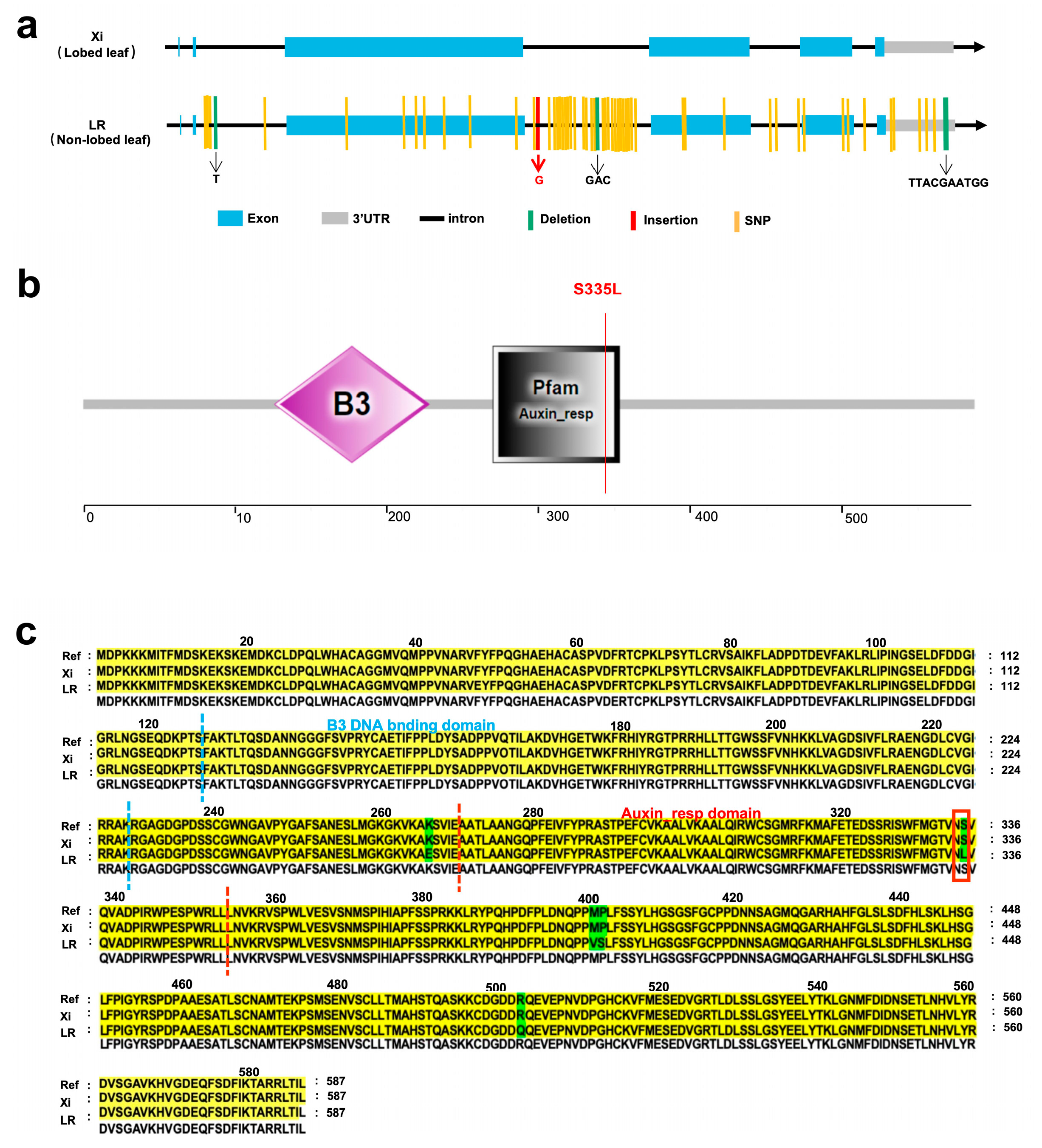
| Offspring | Population | Entire Leaf | Shallowly Lobed Leaf | Deeply Lobed Leaf | Expected Value | χ2 | χ20.05 |
|---|---|---|---|---|---|---|---|
| F2 | 392 | 205 | 165 | 22 | 9:6:1 | 3.549 | 5.991 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Shi, L.; Fei, S.; Zhang, M.; Zhou, Y.; Hu, Z.; Yang, J.; Zhang, M.; Lyu, X. CpARF6 Controls Lobed Leaf Formation in Zucchini. Int. J. Mol. Sci. 2025, 26, 10042. https://doi.org/10.3390/ijms262010042
Jiang S, Shi L, Fei S, Zhang M, Zhou Y, Hu Z, Yang J, Zhang M, Lyu X. CpARF6 Controls Lobed Leaf Formation in Zucchini. International Journal of Molecular Sciences. 2025; 26(20):10042. https://doi.org/10.3390/ijms262010042
Chicago/Turabian StyleJiang, Shufang, Lu Shi, Shuliang Fei, Mengyi Zhang, Yimei Zhou, Zhongyuan Hu, Jinghua Yang, Mingfang Zhang, and Xiaolong Lyu. 2025. "CpARF6 Controls Lobed Leaf Formation in Zucchini" International Journal of Molecular Sciences 26, no. 20: 10042. https://doi.org/10.3390/ijms262010042
APA StyleJiang, S., Shi, L., Fei, S., Zhang, M., Zhou, Y., Hu, Z., Yang, J., Zhang, M., & Lyu, X. (2025). CpARF6 Controls Lobed Leaf Formation in Zucchini. International Journal of Molecular Sciences, 26(20), 10042. https://doi.org/10.3390/ijms262010042





