Robot-Assisted Colorectal Cancer Surgery Mitigates Early Postoperative Immunosuppression and Angiogenesis
Abstract
1. Introduction
2. Results
2.1. Immune-Respone Mediators
2.1.1. Inducers and Mediators of Th1 Response
2.1.2. Inducers and Mediators of Th2 Response
2.2. Growth Factors
2.2.1. Angiogenic Growth Factors
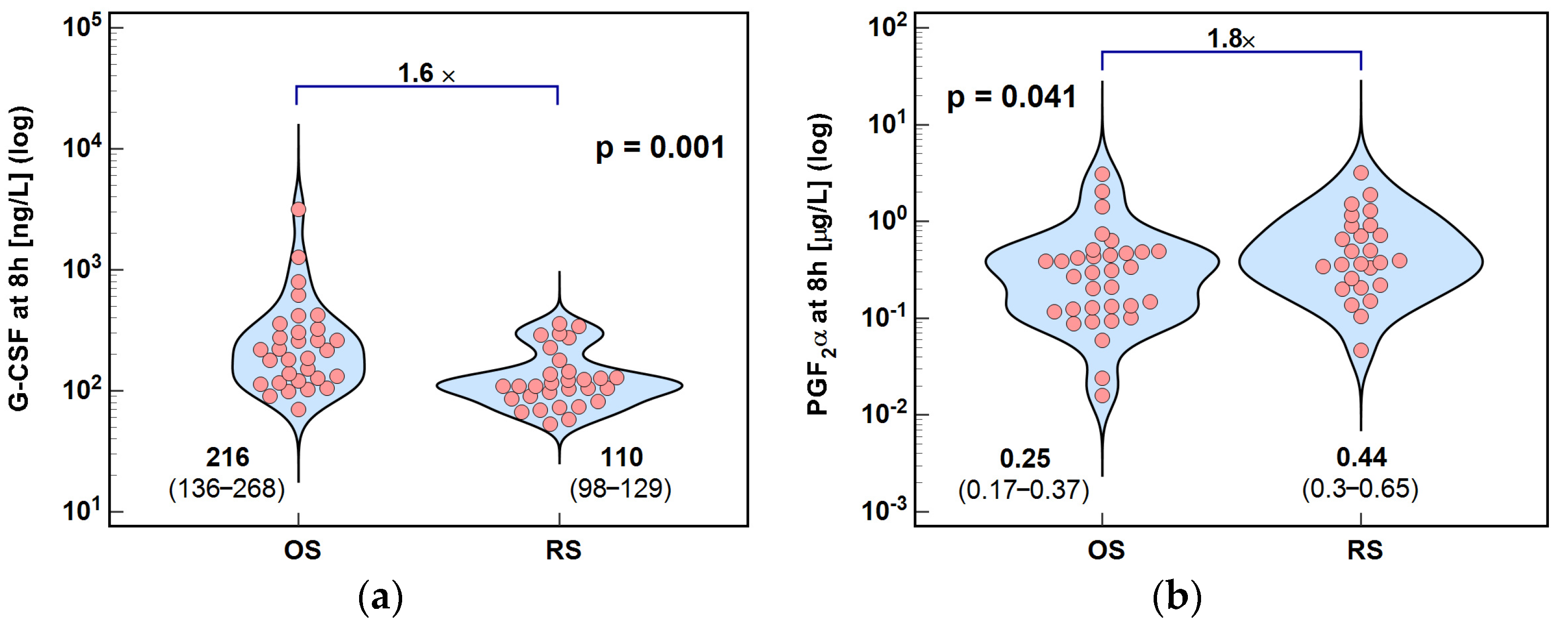
2.2.2. Colony-Stimulating Factors
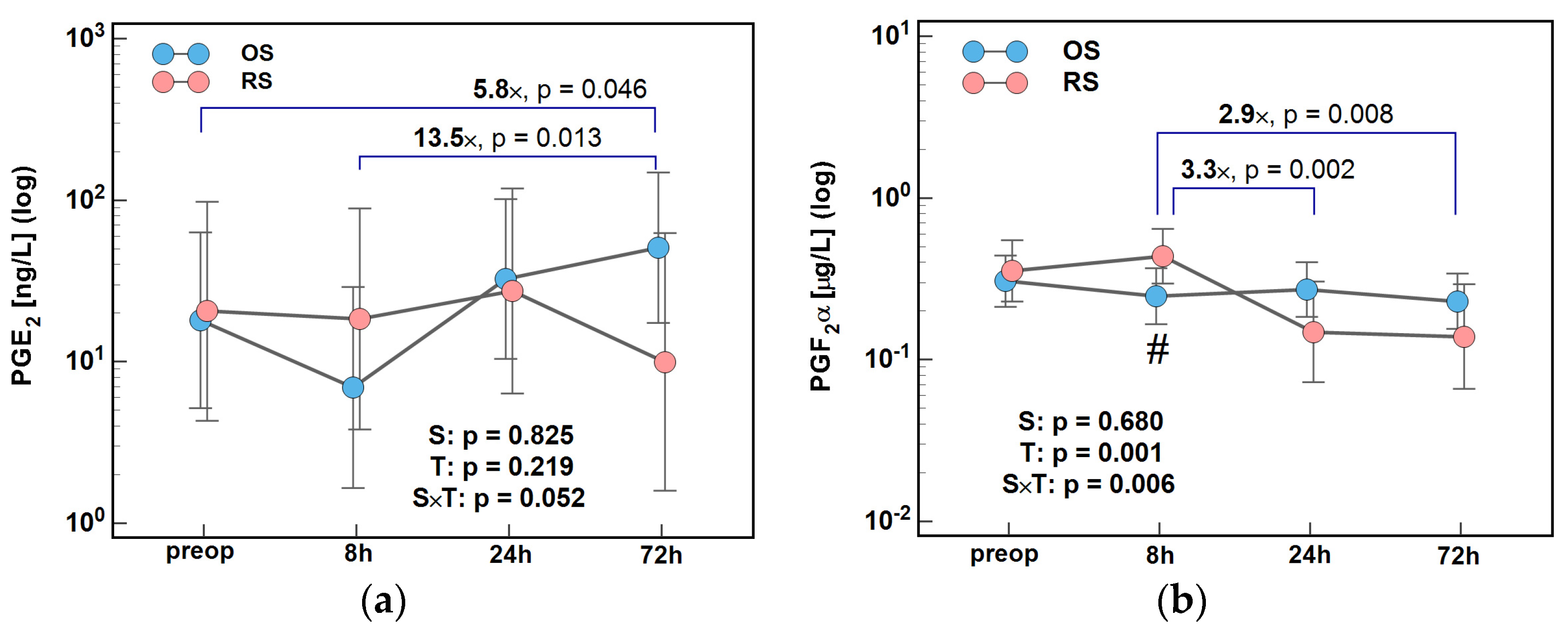
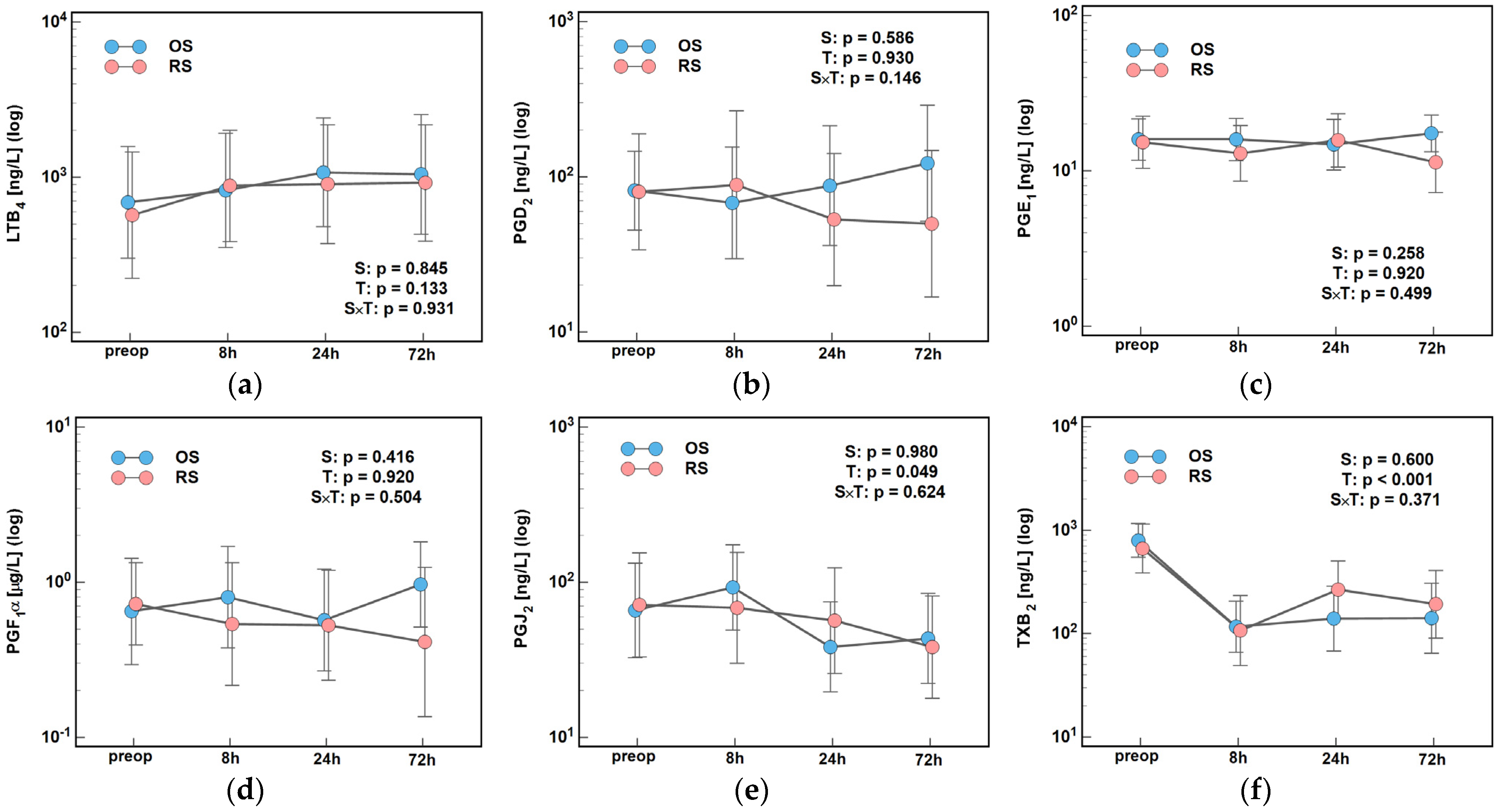
2.3. Lipid Mediators of Inflammation, Immunity, and Angiogenesis
3. Discussion
4. Limitations
5. Materials and Methods
5.1. Patients
5.1.1. Study Type and Design
5.1.2. Patients’ Characteristics
5.1.3. Treatment
5.2. Ethical Considerations
5.3. Preparative and Analytical Methods
5.3.1. Blood
5.3.2. Profiling Cytokines
5.3.3. Profiling Lipid Inflammatory Mediators
Materials
Targeted Metabolomic Analysis
5.4. Statistical Analysis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| ASA | Physical status classification system of the American Society of Anesthesiologists |
| BMI | Body mass index |
| CAR | Compensatory anti-inflammatory response |
| CARS | CAR syndrome |
| CCI | Chronic critical illness |
| CI | Confidence interval |
| COX-2 | Cyclooxygenase 2 |
| CRC | Colorectal cancer |
| F | Females |
| FA | Formic acid |
| FGF2 | Basic fibroblast growth factor |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte–macrophage colony-stimulating factor |
| HIF-1α | Hypoxia-inducible factor 1α |
| HLN | Harvested lymph nodes |
| IFNγ | Interferon γ |
| IL | Interleukin |
| IP-10 | Interferon-gamma-inducible protein 10 kD; CXCL10 |
| LC | Left colon |
| LMWH | Low-molecular-weight heparin |
| LoS | Length of surgery |
| LS | Conventional laparoscopy (laparoscopic surgery) |
| M | Males |
| MARS | Mixed antagonist response syndrome |
| MCP1 | Monocyte chemoattractant protein-1; CCL2 |
| MDSCs | Myeloid-derived suppressor cells |
| NK | Natural killer cells |
| NKT | Natural killer T cells |
| NLR | Neutrophil-to-lymphocyte ratio |
| OS | Open surgery |
| PDGF-BB | Platelet-derived growth factor BB |
| PG | Prostaglandin |
| PICS | “Persistent inflammation, immunosuppression, and catabolism syndrome” |
| RC | Right colon |
| RE | Rectum |
| RS | Robot-assisted surgery |
| SIR | Systemic inflammatory response |
| SIRS | SIR syndrome |
| TGFβ | Transforming Growth Factor β |
| TGFβRII | TGFβ receptor II |
| TNM | “Tumor-node-metastasis” cancer staging system |
| TX | Thromboxane |
| VEGF-A | Vascular endothelial growth factor A |
References
- American Cancer Society: Cancer Facts and Figures 2024. American Cancer Society, 2024. Available online: https://www.cancer.gov/types/colorectal (accessed on 10 November 2024).
- Global Cancer Observatory (GCO). Available online: https://gco.iarc.who.int/en (accessed on 10 November 2024).
- Zhou, J.; Yang, Q.; Zhao, S.; Sun, L.; Li, R.; Wang, J.; Wang, L.; Wang, D. Evolving landscape of colorectal cancer: Global and regional burden, risk factor dynamics, and future scenarios (the Global Burden of Disease 1990–2050). Ageing Res. Rev. 2025, 104, 102666. [Google Scholar] [CrossRef]
- Zhang, J.; Ou, D.; Xie, A.; Chen, D.; Li, X. Global burden and cross-country health inequalities of early-onset colorectal cancer and its risk factors from 1990 to 2021 and its projection until 2036. BMC Public Health 2024, 24, 3124. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Santucci, C.; Mignozzi, S.; Malvezzi, M.; Boffetta, P.; Collatuzzo, G.; Levi, F.; La Vecchia, C.; Negri, E. European cancer mortality predictions for the year 2024 with focus on colorectal cancer. Ann. Oncol. 2024, 35, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Didkowska, J.A.; Barańska, K.; Miklewska, M.J.; Wojciechowska, U. Cancer incidence and mortality in Poland in 2023. Nowotw. J. Oncol. 2024, 74, 75–93. [Google Scholar] [CrossRef]
- Luo, S.; Gong, J.; Zhu, Y.; Wang, L.; Zhang, K. Global, regional, and national burden of colorectal cancer in the elderly (aged > 60 years): A comprehensive analysis across 204 countries and territories (1990–2021). BMC Gastroenterol. 2025, 25, 570. [Google Scholar] [CrossRef]
- Li, X.; Xiao, X.; Wu, Z.; Li, A.; Wang, W.; Lin, R. Global, regional, and national burden of early-onset colorectal cancer and projection to 2050: An analysis based on the Global Burden of Disease Study 2021. Public Health 2025, 238, 245–253. [Google Scholar] [CrossRef]
- Sung, H.; Siegel, R.L.; Laversanne, M.; Jiang, C.; Morgan, E.; Zahwe, M.; Cao, Y.; Bray, F.; Jemal, A. Colorectal cancer incidence trends in younger versus older adults: An analysis of population-based cancer registry data. Lancet Oncol. 2025, 26, 51–63. [Google Scholar] [CrossRef]
- Chakedis, J.; Schmidt, C.R. Surgical Treatment of Metastatic Colorectal Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, M.; Krzystek-Korpacka, M.; Rząca, M.; Czarnecki, R.; Obuszko, Z.; Witkiewicz, W. Introduction of robotic surgery into a community hospital setting: A prospective comparison of robotic and open colorectal resection for cancer. Dig. Surg. 2017, 34, 489–494. [Google Scholar] [CrossRef]
- Lu, C.C.; Lu, C.T.; Chang, K.Y.; Chun-Li, W.; Wu, C.Y. Robot-assisted vs. laparoscopic right hemicolectomy in octogenarians and nonagenarians: An analysis of the US nationwide inpatient sample 2005–2018. Aging Clin. Exp. Res. 2024, 36, 193. [Google Scholar] [CrossRef]
- Hettiarachchi, T.S.; Askari, A.; Rudge, E.; Hao, L.T.; Sarwar, S.; Dowsett, D.; El Hadi, A.; Shaikh, I. Comparison of robotic vs laparoscopic left-sided colorectal cancer resections. J. Robot. Surg. 2023, 17, 205–213. [Google Scholar] [CrossRef]
- Dohrn, N.; Klein, M.F.; Gögenur, I. Robotic versus laparoscopic right colectomy for colon cancer: A nationwide cohort study. Int. J. Color. Dis. 2021, 36, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Baik, S.H.; Kwon, H.Y.; Kim, J.S.; Hur, H.; Sohn, S.K.; Cho, C.H.; Kim, H. Robotic versus laparoscopic low anterior resection of rectal cancer: Short-term outcome of a prospective comparative study. Ann. Surg. Oncol. 2009, 16, 1480–1487. [Google Scholar] [CrossRef]
- Chen, Y.T.; Huang, C.W.; Ma, C.J.; Tsai, H.L.; Yeh, Y.S.; Su, W.C.; Chai, C.Y.; Wang, J.Y. An observational study of patho-oncological outcomes of various surgical methods in total mesorectal excision for rectal cancer: A single center analysis. BMC Surg. 2020, 20, 23. [Google Scholar] [CrossRef]
- Sterk, M.F.M.; Crolla, R.M.P.H.; Verseveld, M.; Dekker, J.W.T.; van der Schelling, G.P.; Verhoef, C.; Olthof, P.B. Uptake of robot-assisted colon cancer surgery in the Netherlands. Surg. Endosc. 2023, 37, 8196–8203. [Google Scholar] [CrossRef]
- Butnari, V.; Sultana, M.; Mansuri, A.; Rao, C.; Kaul, S.; Boulton, R.; Huang, J.; Rajendran, N. Comparison of early surgical outcomes of robotic and laparoscopic colorectal cancer resection reported by a busy district general hospital in England. Sci. Rep. 2024, 14, 9227. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Liu, Y.-C.; Chen, C.-C.; Chen, M.-C.; Chiu, T.-Y.; Huang, Y.-L.; Chiang, S.-W.; Lin, C.-L.; Chen, Y.-J.; Lin, C.-Y.; et al. Robotic-Assisted Colon Cancer Surgery: Faster Recovery and Less Pain Compared to Laparoscopy in a Retrospective Propensity-Matched Study. Cancers 2025, 17, 243. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Meyer, E.; Meurette, G.; Liot, E.; Toso, C.; Ris, F. Robotic versus laparoscopic right hemicolectomy: A systematic review of the evidence. J. Robot. Surg. 2024, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Pan, S.; Yang, S.; Wei, J.; Rong, J.; Wu, D. Impact of robotic surgery on postoperative gastrointestinal dysfunction following minimally invasive colorectal surgery: Incidence, risk factors, and short-term outcomes. Int. J. Color. Dis. 2024, 39, 166. [Google Scholar] [CrossRef]
- Law, W.L.; Foo, D.C.C. Comparison of short-term and oncologic outcomes of robotic and laparoscopic resection for mid- and distal rectal cancer. Surg. Endosc. 2017, 31, 2798–2807. [Google Scholar] [CrossRef]
- Laks, S.; Goldenshluger, M.; Lebedeyev, A.; Anderson, Y.; Gruper, O.; Segev, L. Robotic Rectal Cancer Surgery: Perioperative and Long-Term Oncological Outcomes of a Single-Center Analysis Compared with Laparoscopic and Open Approach. Cancers 2025, 17, 859. [Google Scholar] [CrossRef]
- Ferrari, D.; Violante, T.; Novelli, M.; Sassun, R.; Sileo, A.; Larson, D.W. Robotic Surgery in Emergency Colorectal Procedures: Analysis of Outcomes and Future Trends. J. Am. Coll. Surg. 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Safiejko, K.; Tarkowski, R.; Koselak, M.; Juchimiuk, M.; Tarasik, A.; Pruc, M.; Smereka, J.; Szarpak, L. Robotic-Assisted vs. Standard Laparoscopic Surgery for Rectal Cancer Resection: A Systematic Review and Meta-Analysis of 19,731 Patients. Cancers 2022, 14, 180. [Google Scholar] [CrossRef]
- Ng, K.T.; Tsia, A.K.V.; Chong, V.Y.L. Robotic versus conventional laparoscopic surgery for colorectal cancer: A systematic review and meta-analysis with trial sequential analysis. World J. Surg. 2019, 43, 1146–1161. [Google Scholar] [CrossRef] [PubMed]
- Ravendran, K.; Abiola, E.; Balagumar, K.; Raja, A.Z.; Flaih, M.; Vaja, S.P.; Muhidin, A.O.; Madouros, N. A Review of robotic surgery in colorectal surgery. Cureus 2023, 15, e37337. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.F.; Westfall, K.M.; Erozkan, K.; Henke, L.; Costedio, M.; Teetor, T.; Selfridge, J.E.; Steinhagen, E.; Charles, R. The rise of robotics: Surgical approaches for rectal cancer over time. Surg. Endosc. 2025, 39, 6702–6718. [Google Scholar] [CrossRef]
- Wang, W.; Liu, J.; Wang, J.; Huang, J.; Wang, J. Comparing robot-assisted vs. laparoscopic proctectomy for rectal cancer surgical and oncological outcomes. Front. Surg. 2025, 12, 1628649. [Google Scholar] [CrossRef]
- Hopkins, M.B.; Hawkins, A.T.; Tiwari, V.; Soda, M.; Martin, B.J.; Muldoon, R.L.; Ford, M.M.; Beck, D.; Geiger, T.M. Is newer always better? Comparing cost and short-term outcomes between laparoscopic and robotic right hemicolectomy. Surg. Endosc. 2022, 36, 2879–2885. [Google Scholar] [CrossRef]
- Wei, P.L.; Huang, Y.J.; Wang, W.; Huang, Y.M. Comparison of robotic reduced-port and laparoscopic approaches for left-sided colorectal cancer surgery. Asian J. Surg. 2023, 46, 698–704. [Google Scholar] [CrossRef]
- Dobson, G.P. Addressing the Global Burden of Trauma in Major Surgery. Front. Surg. 2015, 2, 43. [Google Scholar] [CrossRef]
- Bain, C.R.; Myles, P.S.; Corcoran, T.; Dieleman, J.M. Postoperative systemic inflammatory dysregulation and corticosteroids: A narrative review. Anaesthesia 2023, 78, 356–370. [Google Scholar] [CrossRef]
- Rosenthal, M.D.; Moore, F.A. Persistent Inflammation, Immunosuppression, and Catabolism: Evolution of Multiple Organ Dysfunction. Surg. Infect. 2016, 17, 167–172. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Decker, D.; Schondorf, M.; Bidlingmaier, F.; Hirner, A.; von Ruecker, A.A. Surgical stress induces a shift in the type-1/type-2 T-helper cell balance, suggesting down-regulation of cell-mediated and up-regulation of antibody-mediated immunity commensurate to the trauma. Surgery 1996, 119, 316–325. [Google Scholar] [CrossRef]
- Baier, P.K.; Wolff-Vorbeck, G.; Eggstein, S.; Baumgartner, U.; Hopt, U.T. Cytokine expression in colon carcinoma. Anticancer Res. 2005, 25, 2135–2139. [Google Scholar] [PubMed]
- Cusack, B.; Buggy, D.J. Anaesthesia, analgesia, and the surgical stress response. BJA Educ. 2020, 20, 321–328. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.P.; Wang, J.H.; Cotter, T.G.; Redmond, H.P. Less stress, more success? Oncological implications of surgery-induced oxidative stress. Gut 2013, 62, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.L.; Feelisch, M.; Martin, D.S. Perioperative oxidative stress: The unseen enemy. Anesth. Analg. 2019, 129, 1749–1760. [Google Scholar] [CrossRef]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef]
- Shibata, J.; Ishihara, S.; Tada, N.; Kawai, K.; Tsuno, N.H.; Yamaguchi, H.; Sunami, E.; Kitayama, J.; Watanabe, T. Surgical stress response after colorectal resection: A comparison of robotic, laparoscopic, and open surgery. Tech. Coloproctol. 2015, 19, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Zawadzki, M.; Szufnarowski, K.; Bednarz-Misa, I.; Gorska, S.; Witkiewicz, W.; Gamian, A. The perioperative dynamics of IL-7 following robot-assisted and open colorectal surgery. Sci. Rep. 2018, 8, 9126. [Google Scholar] [CrossRef]
- Fleszar, M.G.; Fortuna, P.; Zawadzki, M.; Hodurek, P.; Bednarz-Misa, I.; Witkiewicz, W.; Krzystek-Korpacka, M. Sex, Type of surgery, and surgical site infections are associated with perioperative cortisol in colorectal cancer patients. J. Clin. Med. 2021, 10, 589. [Google Scholar] [CrossRef]
- Zawadzki, M.; Krzystek-Korpacka, M.; Gamian, A.; Witkiewicz, W. Comparison of inflammatory responses following robotic and open colorectal surgery: A prospective study. Int. J. Color. Dis. 2017, 32, 399–407. [Google Scholar] [CrossRef]
- Krzystek-Korpacka, M.; Zawadzki, M.; Lewandowska, P.; Szufnarowski, K.; Bednarz-Misa, I.; Jacyna, K.; Witkiewicz, W.; Gamian, A. Distinct chemokine dynamics in early postoperative period after open and robotic colorectal surgery. J. Clin. Med. 2019, 8, 879. [Google Scholar] [CrossRef]
- Fleszar, M.G.; Fortuna, P.; Zawadzki, M.; Kosak, B.; Krzystek-Korpacka, M. Simultaneous LC-MS/MS-based quantification of free 3-nitro-L-tyrosine, 3-chloro-L-tyrosine, and 3-bromo-L-tyrosine in plasma of colorectal cancer patients during early postoperative period. Molecules 2020, 25, 5158. [Google Scholar] [CrossRef]
- Bednarz-Misa, I.; Fleszar, M.G.; Zawadzki, M.; Kapturkiewicz, B.; Kubiak, A.; Neubauer, K.; Witkiewicz, W.; Krzystek-Korpacka, M. L-Arginine/NO pathway metabolites in colorectal cancer: Relevance as disease biomarkers and predictors of adverse clinical outcomes following surgery. J. Clin. Med. 2020, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Kim, R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J. Transl. Med. 2018, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xiao, Q.; Ma, Q.; Han, L. Clinical treatment for persistent inflammation, immunosuppression and catabolism syndrome in patients with severe acute pancreatitis (Review). Exp. Ther. Med. 2023, 26, 495. [Google Scholar] [CrossRef]
- Yin, J.; Chen, Y.; Huang, J.L.; Yan, L.; Kuang, Z.S.; Xue, M.M.; Sun, S.; Xiang, H.; Hu, Y.Y.; Dong, Z.M.; et al. Prognosis-related classification and dynamic monitoring of immune status in patients with sepsis: A prospective observational study. World J. Emerg. Med. 2021, 12, 185–191. [Google Scholar] [CrossRef]
- Horiguchi, H.; Loftus, T.J.; Hawkins, R.B.; Raymond, S.L.; Stortz, J.A.; Hollen, M.K.; Weiss, B.P.; Miller, E.S.; Bihorac, A.; Larson, S.D.; et al. Sepsis and Critical Illness Research Center Investigators. Innate Immunity in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome and Its Implications for Therapy. Front. Immunol. 2018, 9, 595. [Google Scholar] [CrossRef]
- Mira, J.C.; Brakenridge, S.C.; Moldawer, L.L.; Moore, F.A. Persistent Inflammation, Immunosuppression and Catabolism Syndrome. Crit. Care Clin. 2017, 33, 245–258. [Google Scholar] [CrossRef]
- Chadda, K.R.; Puthucheary, Z. Persistent inflammation, immunosuppression, and catabolism syndrome (PICS): A review of definitions, potential therapies, and research priorities. Br. J. Anaesth. 2024, 132, 507–518. [Google Scholar] [CrossRef]
- Forget, P.; Simonet, O.; De Kock, M. Cancer surgery induces inflammation, immunosuppression and neo-angiogenesis, but is it influenced by analgesics? F1000Research 2013, 2, 102. [Google Scholar] [CrossRef] [PubMed]
- Efron, P.A.; Mohr, A.M.; Bihorac, A.; Horiguchi, H.; Hollen, M.K.; Segal, M.S.; Baker, H.V.; Leeuwenburgh, C.; Moldawer, L.L.; Moore, F.A.; et al. Persistent inflammation, immunosuppression, and catabolism and the development of chronic critical illness after surgery. Surgery 2018, 164, 178–184. [Google Scholar] [CrossRef]
- Li, Q. Pituitary-immune bidirectional crosstalk under systemic inflammation. PLoS Biol. 2023, 21, e3002440. [Google Scholar] [CrossRef] [PubMed]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar] [CrossRef] [PubMed]
- Quirant-Sánchez, B.; Plans-Galván, O.; Lucas, E.; Argudo, E.; Martinez-Cáceres, E.M.; Arméstar, F. HLA-DR expression on monocytes and sepsis index are useful in predicting sepsis. Biomedicines 2023, 11, 1836. [Google Scholar] [CrossRef]
- Tai, L.H.; de Souza, C.T.; Bélanger, S.; Ly, L.; Alkayyal, A.A.; Zhang, J.; Rintoul, J.L.; Ananth, A.A.; Lam, T.; Breitbach, C.J.; et al. Preventing postoperative metastatic disease by inhibiting surgery-induced dysfunction in natural killer cells. Cancer Res. 2013, 73, 97–107. [Google Scholar] [CrossRef]
- Konjević, G.M.; Vuletić, A.M.; Mirjačić Martinović, K.M.; Larsen, A.K.; Jurišić, V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 2019, 117, 30–40. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Liu, C.; Su, L.; Zhang, D.; Fan, J.; Yang, Y.; Xiao, M.; Xie, J.; Xu, Y.; et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol. Med. 2020, 26, 97. [Google Scholar] [CrossRef]
- Dufour, J.H.; Dziejman, M.; Liu, M.T.; Leung, J.H.; Lane, T.E.; Luster, A.D. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 2002, 168, 3195–3204. [Google Scholar] [CrossRef]
- Madhurantakam, S.; Lee, Z.J.; Naqvi, A.; Prasad, S. Importance of IP-10 as a biomarker of host immune response: Critical perspective as a target for biosensing. Curr. Res. Biotechnol. 2023, 5, 100130. [Google Scholar] [CrossRef]
- Yu, J.; Wei, M.; Becknell, B.; Trotta, R.; Liu, S.; Boyd, Z.; Jaung, M.S.; Blaser, B.W.; Sun, J.; Benson, D.M., Jr.; et al. Pro- and antiinflammatory cytokine signaling: Reciprocal antagonism regulates interferon-gamma production by human natural killer cells. Immunity 2006, 24, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Mierzchała-Pasierb, M.; Zawadzki, M.; Diakowska, D.; Witkiewicz, W. Serum and erythrocyte antioxidant defense in colorectal cancer patients during early postoperative period: Potential modifiers and impact on clinical outcomes. Antioxidants 2021, 10, 999. [Google Scholar] [CrossRef] [PubMed]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 Signaling: The Yin and the Yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef]
- Mu, X.; Gu, R.; Tang, M.; Wu, X.; He, W.; Nie, X. IL-17 in wound repair: Bridging acute and chronic responses. Cell Commun. Signal. 2024, 22, 288. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, X.; Herjan, T.; Li, X. The role of interleukin-17 in tumor development and progression. J. Exp. Med. 2020, 217, e20190297. [Google Scholar] [CrossRef]
- Amicarella, F.; Muraro, M.G.; Hirt, C.; Cremonesi, E.; Padovan, E.; Mele, V.; Governa, V.; Han, J.; Huber, X.; Droeser, R.A.; et al. Dual role of tumour-infiltrating T helper 17 cells in human colorectal cancer. Gut 2017, 66, 692–704. [Google Scholar] [CrossRef]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Meier, C.; Brieger, A. The role of IL-8 in cancer development and its impact on immunotherapy resistance. Eur. J. Cancer 2025, 218, 115267. [Google Scholar] [CrossRef]
- Bazzichetto, C.; Milella, M.; Zampiva, I.; Simionato, F.; Amoreo, C.A.; Buglioni, S.; Pacelli, C.; Le Pera, L.; Colombo, T.; Bria, E.; et al. Interleukin-8 in Colorectal Cancer: A Systematic Review and Meta-Analysis of Its Potential Role as a Prognostic Biomarker. Biomedicines. 2022, 10, 2631. [Google Scholar] [CrossRef]
- Wang, L.; Lan, J.; Tang, J.; Luo, N. MCP-1 targeting: Shutting off an engine for tumor development (Review). Oncol. Lett. 2022, 23, 26. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Kong, B.; Michalski, C.W.; Friess, H.; Kleeff, J. Surgical procedure as an inducer of tumor angiogenesis. Exp. Oncol. 2010, 32, 186–189. [Google Scholar]
- Shi, Z.; Yao, C.; Shui, Y.; Li, S.; Yan, H. Research progress on the mechanism of angiogenesis in wound repair and regeneration. Front. Physiol. 2023, 14, 1284981. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Sekaran, S.; Rajamani Sekar, S.K. Surgical intervention as a driver of new angiogenesis in tumors-time to consider minimally invasive surgeries? Int. J. Surg. 2023, 109, 3222–3223. [Google Scholar] [CrossRef]
- Chen, J.; Gong, C.; Mao, H.; Li, Z.; Fang, Z.; Chen, Q.; Lin, M.; Jiang, X.; Hu, Y.; Wang, W.; et al. E2F1/SP3/STAT6 axis is required for IL-4-induced epithelial-mesenchymal transition of colorectal cancer cells. Int. J. Oncol. 2018, 53, 567–578. [Google Scholar] [CrossRef]
- Bednarz-Misa, I.; Diakowska, D.; Szczuka, I.; Fortuna, P.; Kubiak, A.; Rosińczuk, J.; Krzystek-Korpacka, M. Interleukins 4 and 13 and their receptors are differently expressed in gastrointestinal tract cancers, depending on the anatomical site and disease advancement, and improve colon cancer cell viability and motility. Cancers 2020, 12, 1463. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Hung, P.Y.; Chen, C.H.; Yu, Y.J.; Syu, M.S.; Hu, M.C. HSD3B1 expression is upregulated by interleukin 4 in HT-29 colon cancer cells via multiple signaling pathways. Int. J. Mol. Sci. 2022, 23, 13572. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; Zhao, H.; Yan, Y.; Lu, J. The role of interleukins in colorectal cancer. Int. J. Biol. Sci. 2020, 16, 2323–2339. [Google Scholar] [CrossRef]
- Song, X.; Traub, B.; Shi, J.; Kornmann, M. Possible roles of interleukin-4 and -13 and their receptors in gastric and colon cancer. Int. J. Mol. Sci. 2021, 22, 727. [Google Scholar] [CrossRef]
- Ito, S.E.; Shirota, H.; Kasahara, Y.; Saijo, K.; Ishioka, C. IL-4 blockade alters the tumor microenvironment and augments the response to cancer immunotherapy in a mouse model. Cancer Immunol. Immunother. 2017, 66, 1485–1496. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Perez, D.; Prados-Lopez, B.; Galvez, J.; Leon, J.; Carazo, A. Eosinophils in colorectal cancer: Emerging insights into anti-tumoral mechanisms and clinical implications. Int. J. Mol. Sci. 2024, 25, 6098. [Google Scholar] [CrossRef]
- Li, B.; Wang, F.; Ma, C.; Hao, T.; Geng, L.; Jiang, H. Predictive value of IL-18 and IL-10 in the prognosis of patients with colorectal cancer. Oncol. Lett. 2019, 18, 713–719. [Google Scholar] [CrossRef]
- Kouro, T.; Takatsu, K. IL-5- and eosinophil-mediated inflammation: From discovery to therapy. Int. Immunol. 2009, 21, 1303–1309. [Google Scholar] [CrossRef]
- Saraiva, A.L.; Carneiro, F. New insights into the role of tissue eosinophils in the progression of colorectal cancer: A literature review. Acta Med. Port. 2018, 31, 329–337. [Google Scholar] [CrossRef]
- Karagiannidis, I.; Salataj, E.; Said Abu Egal, E.; Beswick, E.J. G-CSF in tumors: Aggressiveness, tumor microenvironment and immune cell regulation. Cytokine 2021, 142, 155479. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, K.; Huddart, S. Surgical stress response and cancer outcomes: A narrative review. Dig. Med. Res. 2020, 3, 65. [Google Scholar] [CrossRef]
- Jin, K.; Qian, C.; Lin, J.; Liu, B. Cyclooxygenase-2-prostaglandin E2 pathway: A key player in tumor-associated immune cells. Front. Oncol. 2023, 13, 1099811. [Google Scholar] [CrossRef]
- Sheng, J.; Sun, H.; Yu, F.-B.; Li, B.; Zhang, Y.; Zhu, Y.-T. The Role of Cyclooxygenase-2 in colorectal cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef]
- Tong, D.; Liu, Q.; Wang, L.A.; Xie, Q.; Pang, J.; Huang, Y.; Wang, L.; Liu, G.; Zhang, D.; Lan, W.; et al. The roles of the COX2/PGE2/EP axis in therapeutic resistance. Cancer Metastasis Rev. 2018, 37, 355–368. [Google Scholar] [CrossRef]
- Glasner, A.; Avraham, R.; Rosenne, E.; Benish, M.; Zmora, O.; Shemer, S.; Meiboom, H.; Ben-Eliyahu, S. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J. Immunol. 2010, 184, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, J.; Wang, D.; Cen, B.; Lang, J.D.; DuBois, R.N. The COX-2-PGE2 pathway promotes tumor evasion in colorectal adenomas. Cancer Prev. Res. 2022, 15, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kulesza, A.; Paczek, L.; Burdzinska, A. The role of COX-2 and PGE2 in the regulation of immunomodulation and other functions of mesenchymal stromal cells. Biomedicines 2023, 11, 445. [Google Scholar] [CrossRef]
- Wang, Y.J.; Xie, X.L.; Liu, H.Q.; Tian, H.; Jiang, X.Y.; Zhang, J.N.; Chen, S.X.; Liu, T.; Wang, S.L.; Zhou, X.; et al. Prostaglandin F2α synthase promotes oxaliplatin resistance in colorectal cancer through prostaglandin F2α-dependent and F2α-independent mechanism. World J. Gastroenterol. 2023, 29, 5452–5470. [Google Scholar] [CrossRef] [PubMed]
- Qualtrough, D.; Kaidi, A.; Chell, S.; Jabbour, H.N.; Williams, A.C.; Paraskeva, C. Prostaglandin F(2alpha) stimulates motility and invasion in colorectal tumor cells. Int. J. Cancer 2007, 121, 734–740. [Google Scholar] [CrossRef]
- Kidd, P. Th1/Th2 Balance: The Hypothesis, its Limitations, and Implications for Health and Disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar]
- Bezu, L.; Öksüz, A.D.; Bell, M.; Buggy, D.; Diaz-Cambronero, O.; Enlund, M.; Forget, P.; Gupta, A.; Hollmann, M.W.; Ionescu, D.; et al. Perioperative immunosuppressive factors during cancer surgery: An updated review. Cancers 2024, 16, 2304. [Google Scholar] [CrossRef] [PubMed]
- Krzystek-Korpacka, M.; Fleszar, M.G.; Fortuna, P.; Gostomska-Pampuch, K.; Lewandowski, Ł.; Piasecki, T.; Kosyk, B.; Szeląg, A.; Trocha, M. Modulation of prostanoids profile and counter-regulation of SDF-1α/CXCR4 and VIP/VPAC2 expression by sitagliptin in non-diabetic rat model of hepatic ischemia-reperfusion injury. Int. J. Mol. Sci. 2021, 22, 13155. [Google Scholar] [CrossRef] [PubMed]
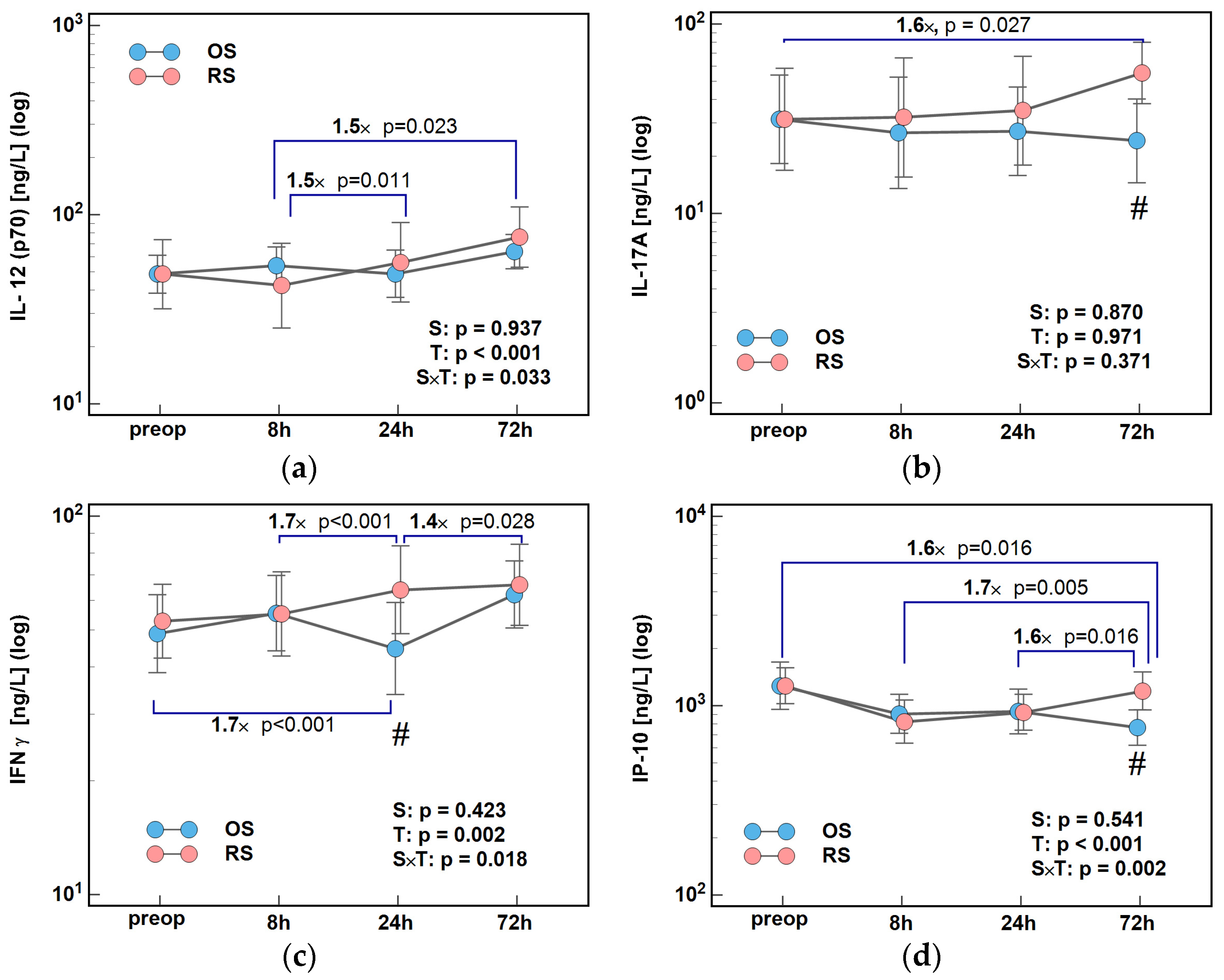
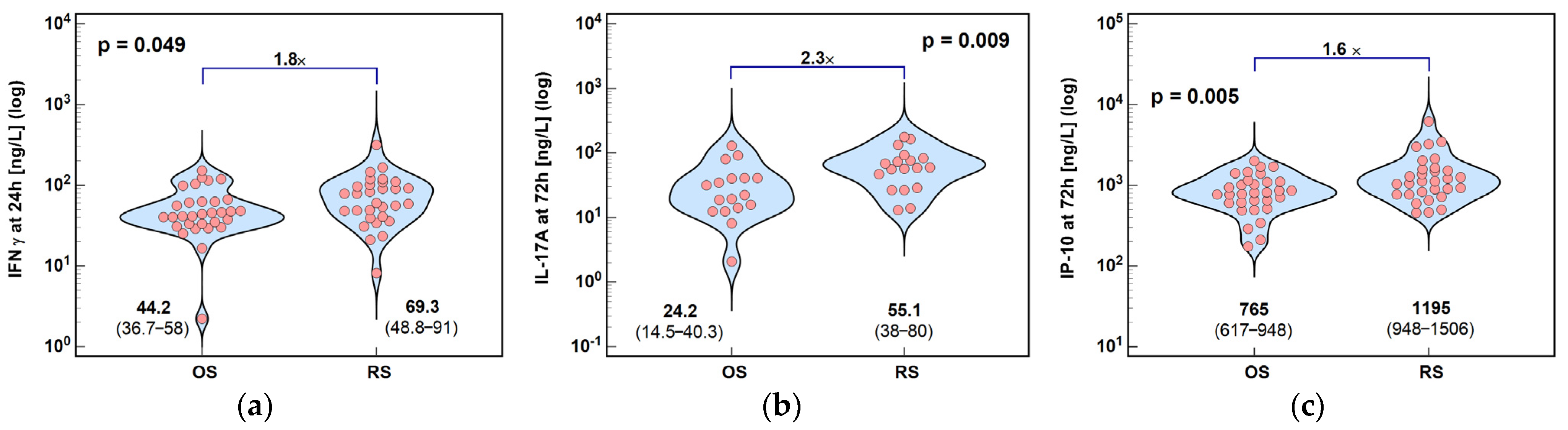
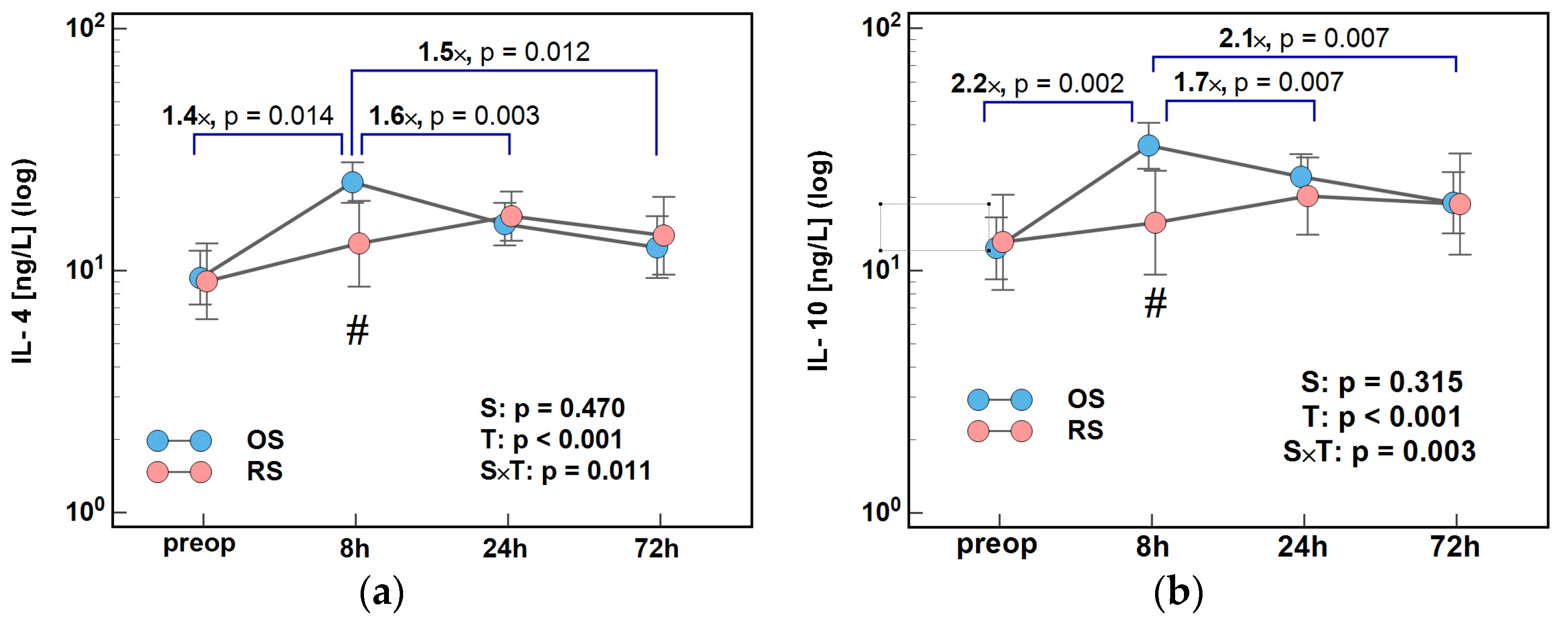
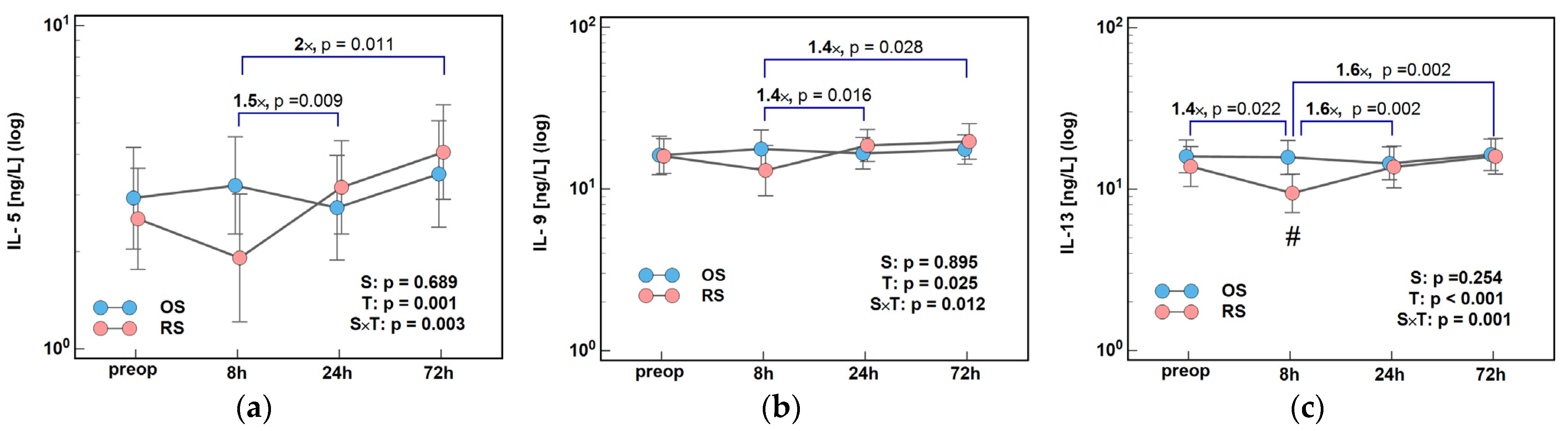
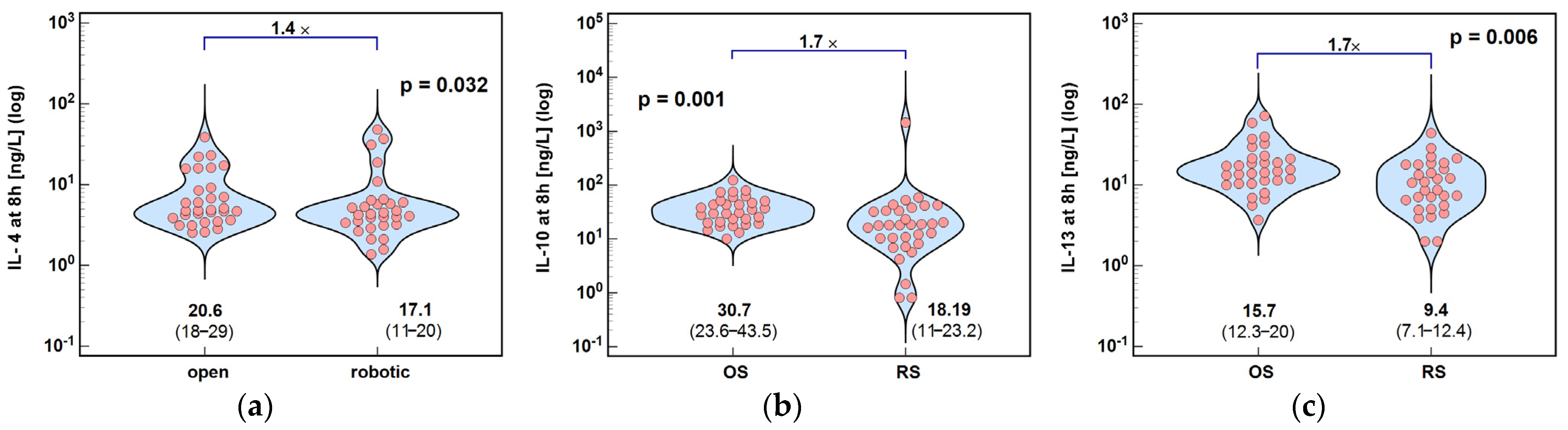
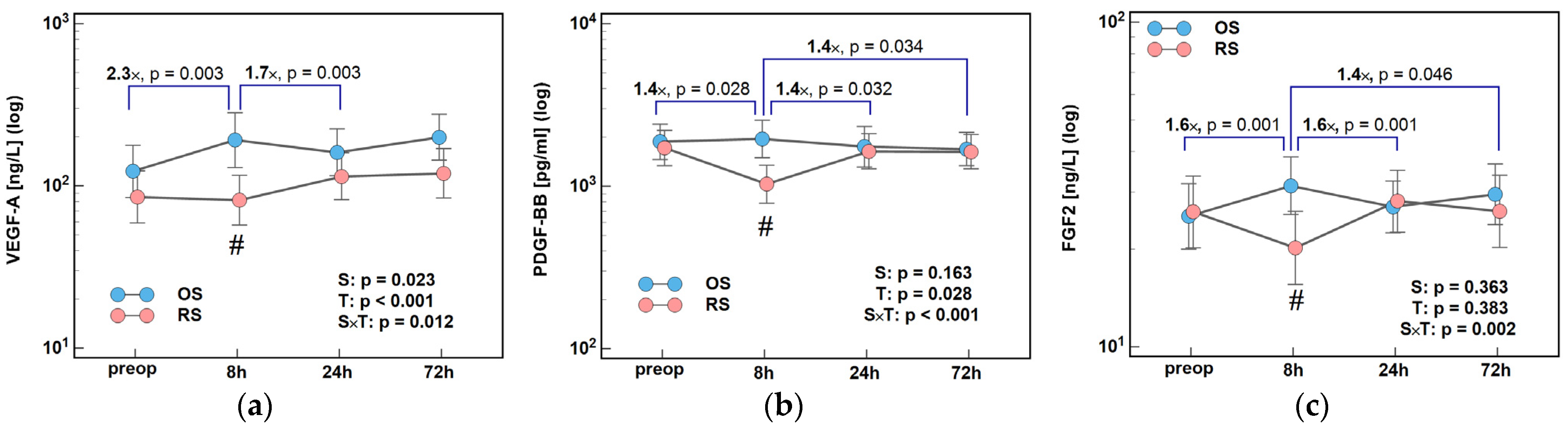
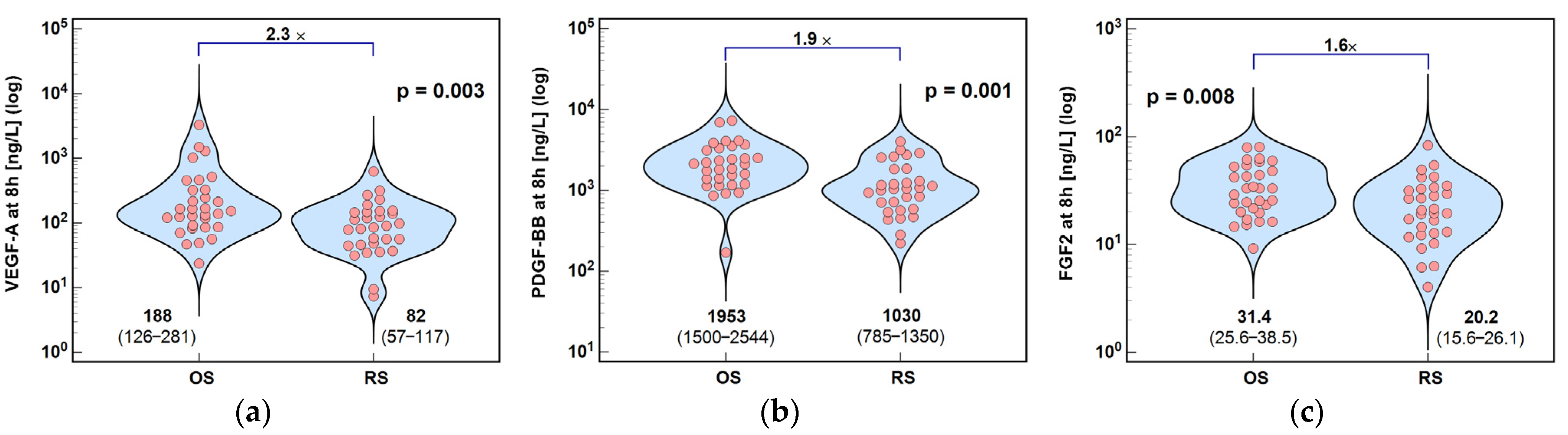
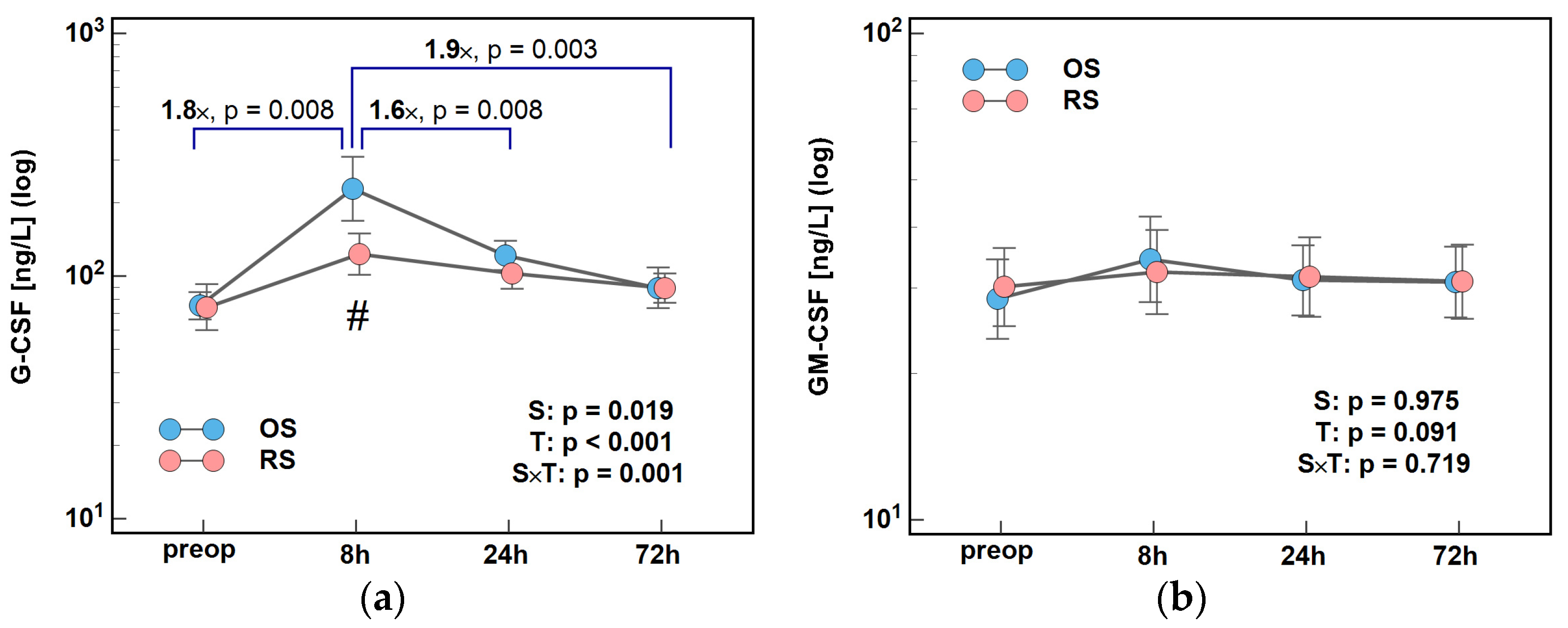
| Characteristics | Open Surgery (OS) | Robotic Surgery (RS) | p |
|---|---|---|---|
| N | 31 | 30 | - |
| Sex (F/M), n | 14/17 | 7/23 | 0.127 1 |
| Age [yrs.] | 68 (65–76) | 67 (62–72) | 0.302 2 |
| BMI [kg/m2] | 26 (25–28) | 25 (24–28) | 0.716 2 |
| ASA (1/2/3), n | 6/20/5 | 5/20/5 | 0.830 3 |
| NLR | 3.17 (2.59–3.49) | 3.58 (2.81–4.24) | 0.194 2 |
| pTNM (0/1/2/3/4) | 2/2/15/9/3 | 2/3/11/12/2 | 0.839 3 |
| T (tis/1/2/3/4) | 2/1/1/20/7 | 2/0/5/16/7 | 0.393 3 |
| N (0/1/2) | 19/4/8 | 16/8/6 | 0.395 3 |
| M (Y/N) | 28/3 | 28/2 | 0.970 1 |
| G (x/1/2/3/4) | 1/4/21/4/1 | 2/6/17/5/0 | 0.601 3 |
| Tumor (LC/RE/RC) | 11/14/6 | 7/11/12 | 0.199 3 |
| LoS [min] | 125 (115–150) | 205 (191–240) | <0.001 2 |
| HLN, n | 14 (12–17) | 13.5 (12–17) | 0.700 2 |
| Transfusion (Y/N), n | 26/5 | 28/2 | 0.425 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleszar, M.G.; Zawadzki, M.; Fortuna, P.; Bednarz-Misa, I.; Krauze, I.; Maciejewska, K.; Klekowski, J.; Chabowski, M.; Witkiewicz, W.; Krzystek-Korpacka, M. Robot-Assisted Colorectal Cancer Surgery Mitigates Early Postoperative Immunosuppression and Angiogenesis. Int. J. Mol. Sci. 2025, 26, 10041. https://doi.org/10.3390/ijms262010041
Fleszar MG, Zawadzki M, Fortuna P, Bednarz-Misa I, Krauze I, Maciejewska K, Klekowski J, Chabowski M, Witkiewicz W, Krzystek-Korpacka M. Robot-Assisted Colorectal Cancer Surgery Mitigates Early Postoperative Immunosuppression and Angiogenesis. International Journal of Molecular Sciences. 2025; 26(20):10041. https://doi.org/10.3390/ijms262010041
Chicago/Turabian StyleFleszar, Mariusz G., Marek Zawadzki, Paulina Fortuna, Iwona Bednarz-Misa, Izabela Krauze, Kamila Maciejewska, Jakub Klekowski, Mariusz Chabowski, Wojciech Witkiewicz, and Małgorzata Krzystek-Korpacka. 2025. "Robot-Assisted Colorectal Cancer Surgery Mitigates Early Postoperative Immunosuppression and Angiogenesis" International Journal of Molecular Sciences 26, no. 20: 10041. https://doi.org/10.3390/ijms262010041
APA StyleFleszar, M. G., Zawadzki, M., Fortuna, P., Bednarz-Misa, I., Krauze, I., Maciejewska, K., Klekowski, J., Chabowski, M., Witkiewicz, W., & Krzystek-Korpacka, M. (2025). Robot-Assisted Colorectal Cancer Surgery Mitigates Early Postoperative Immunosuppression and Angiogenesis. International Journal of Molecular Sciences, 26(20), 10041. https://doi.org/10.3390/ijms262010041








