New Insights into Pathogenesis and Management of Keratoacanthoma: A Narrative Review
Abstract
1. Introduction
2. Epidemiology of Keratoacanthoma
3. Etiology
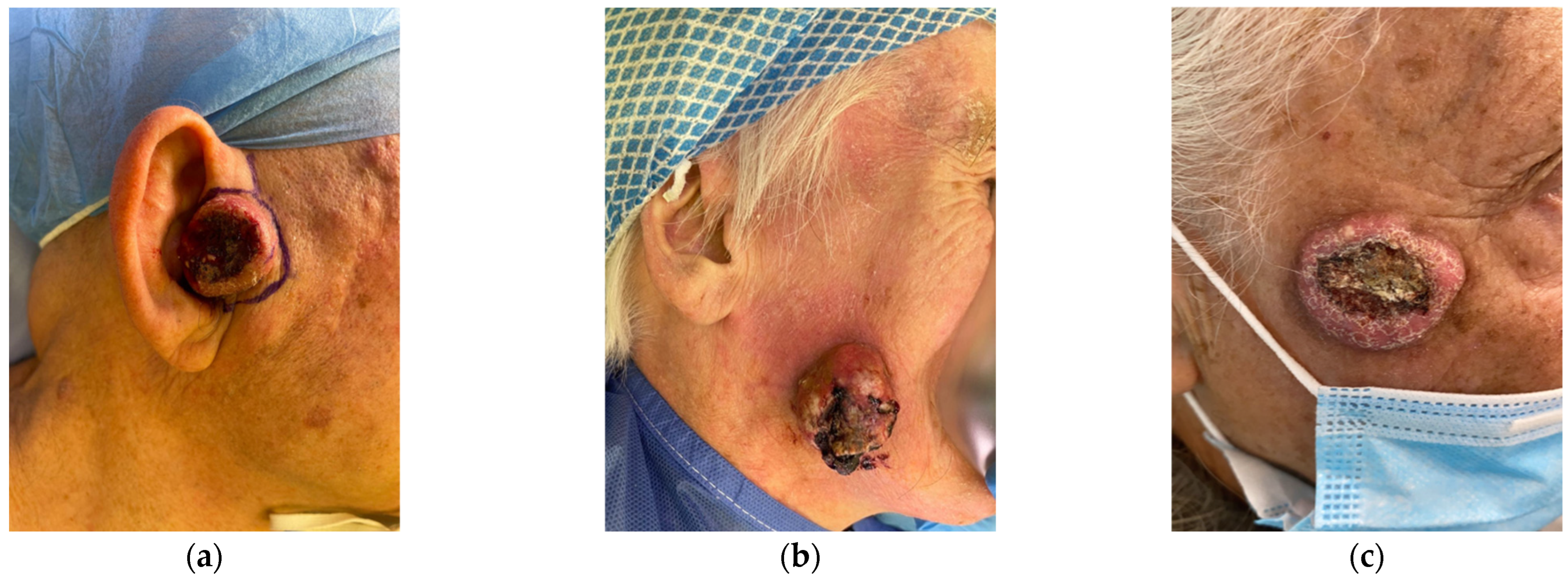
4. Clinical Manifestations
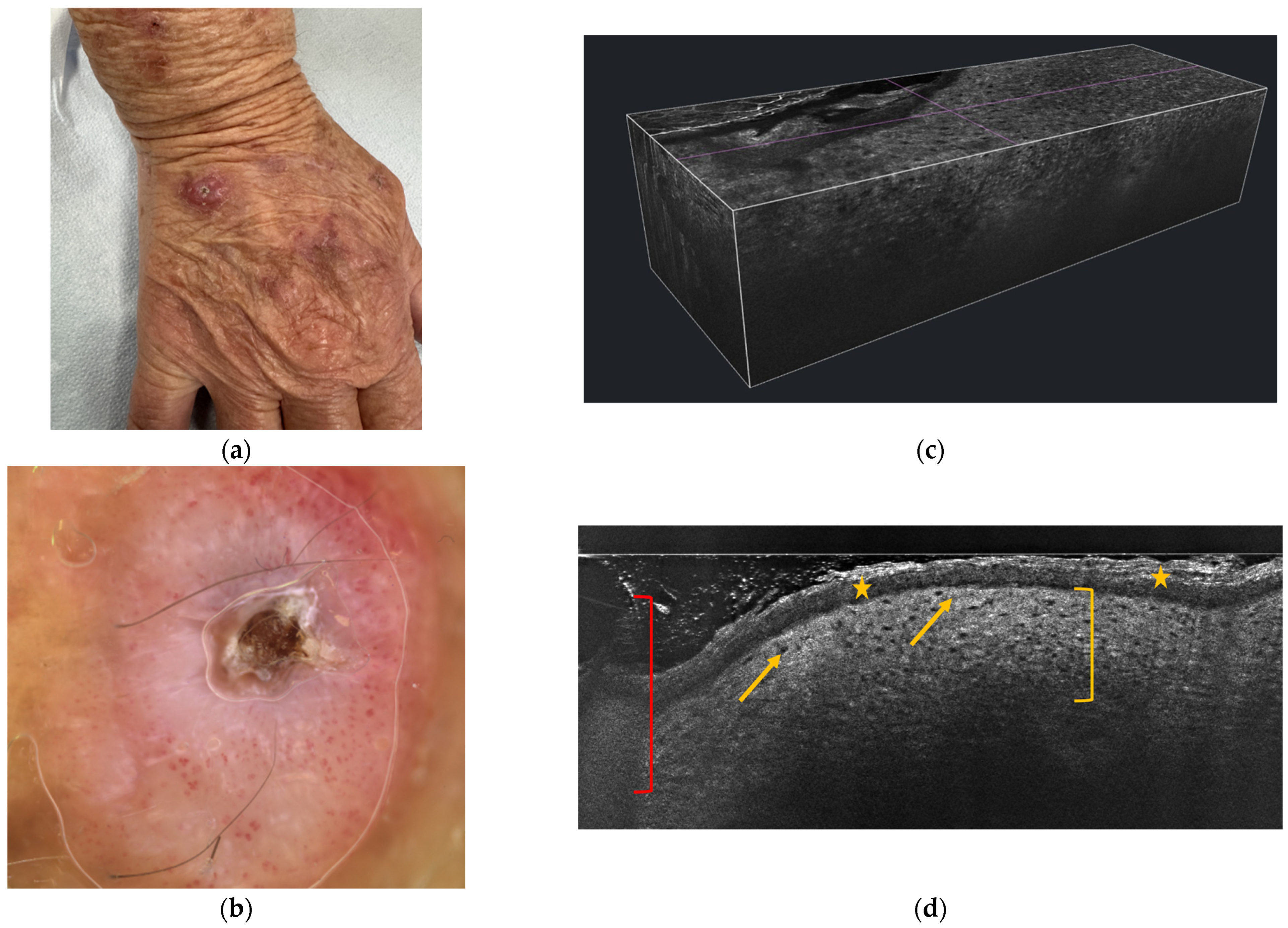
5. Dermoscopy
- Central Keratin Plug: One of the hallmark features of KA is the presence of a central, well-defined, yellowish or whitish keratin plug. This central crater is visible in most cases and is a key diagnostic feature.
- Rolled Border: The lesion typically displays a smooth, well-demarcated, and elevated border that is slightly rolled. This appearance is characteristic of KA and helps in differentiating it from other tumors that may have irregular borders.
- Vascular Patterns: Dermoscopy often shows vessels that are linear, dotted, or hairpin-shaped in the peripheral areas of the lesion. These vessels may be more prominent around the edge of the keratin plug, contributing to the lesion’s raised appearance. Inflammatory and hyperplastic changes in the epidermis contribute to these vascular patterns.
- Homogeneous Pink or White Background: The background of the lesion may show a homogeneous pink or white color due to the underlying inflammatory changes and epithelial proliferation. This feature, when seen with the characteristic keratin plug, helps in the diagnosis.
- Yellowish Structures: Apart from the keratin plug, the lesion may exhibit yellowish areas or streaks within the lesion, which correspond to the presence of keratinized material beneath the surface.
- Regular Pigment Pattern: In most cases, the dermoscopic pattern of KA is regular, with no irregular pigment network or atypical vessels, which further helps to differentiate it from malignancies such as melanoma.
6. Reflectance Confocal Microscopy (RCM) and Line-Field Confocal Optical Coherence Tomography (LC-OCT)
7. Histology of Keratoacanthoma
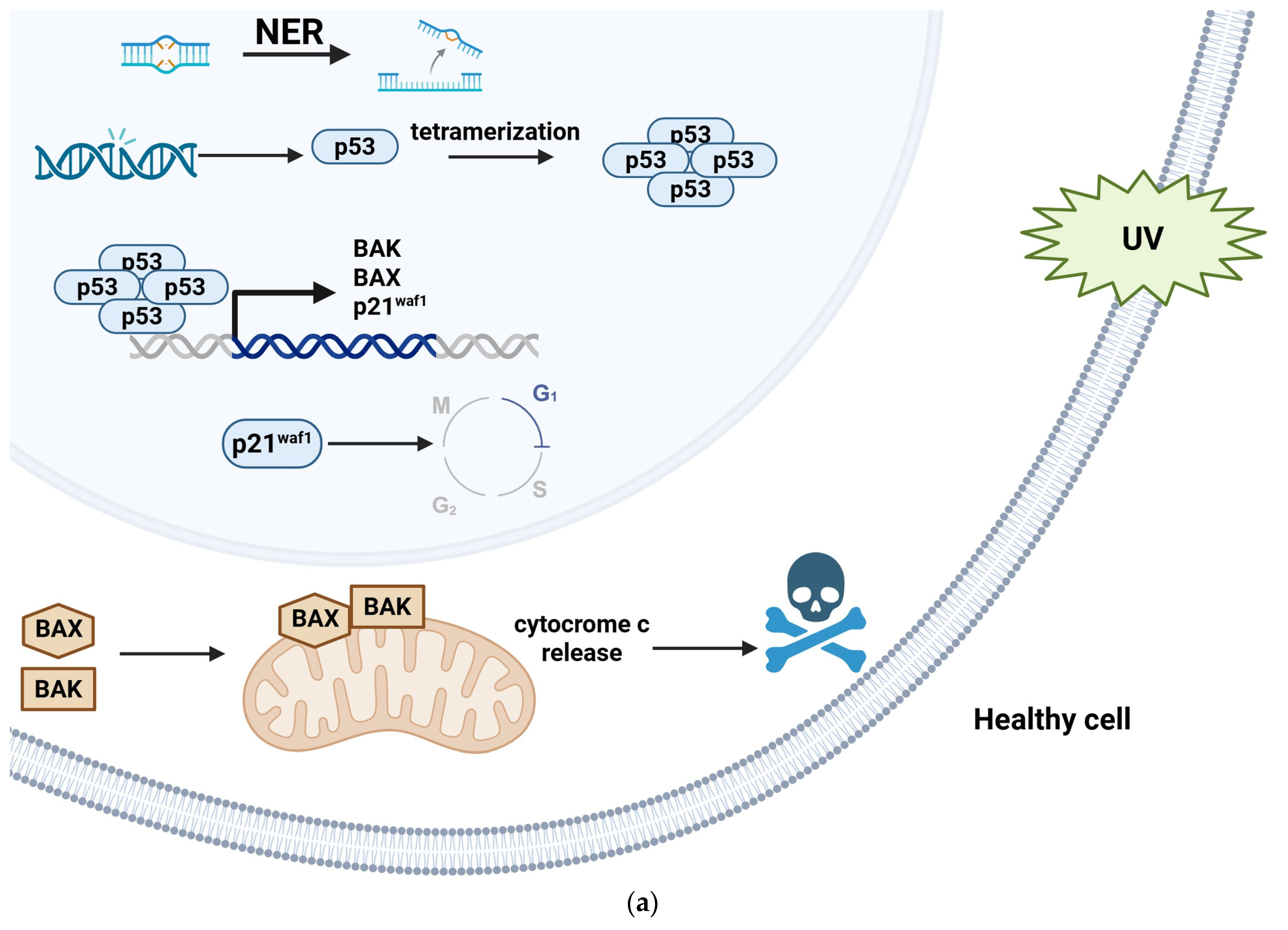
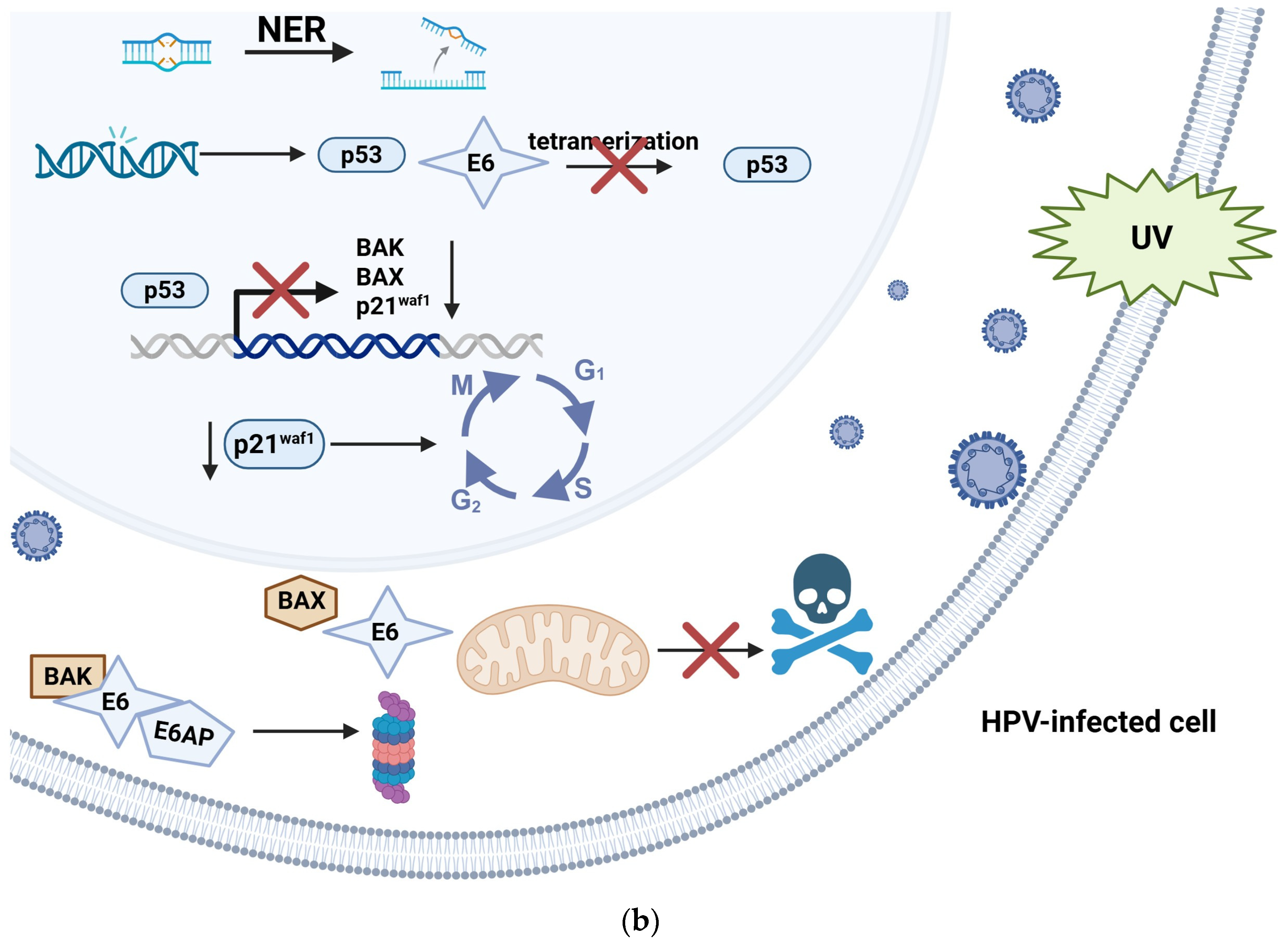
8. Human Papillomavirus and Carcinogenesis
9. Therapy
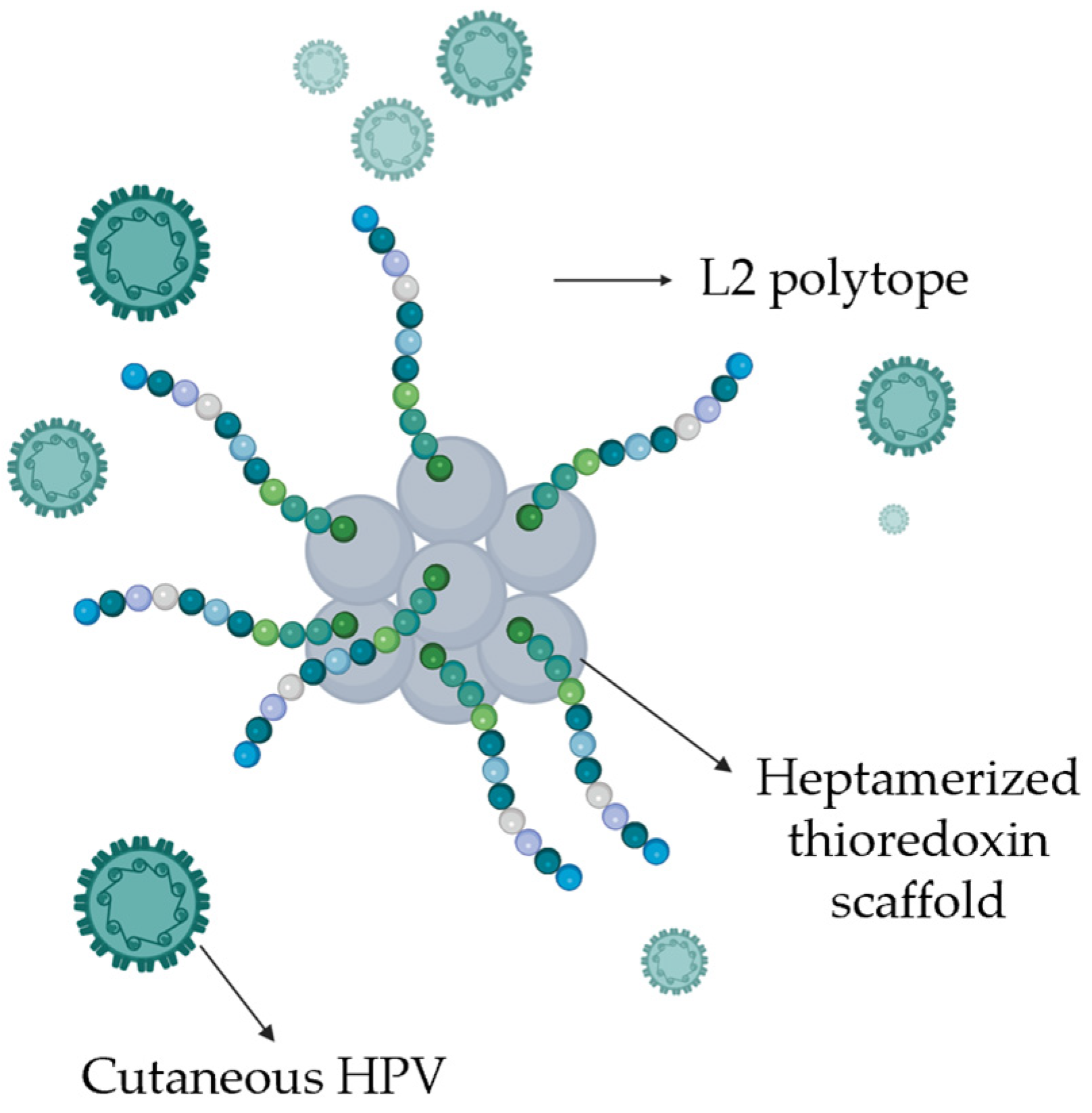
10. Anti-HPV Vaccines
11. Materials and Methods
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KA | Keratoacanthoma |
| SCC | Squamous Cell Carcinoma |
| MTX | Methotrexate |
| RCM | Reflectance Confocal Microscopy |
| LC-OCT | Line-Field Confocal Optical Coherence Tomography |
| HPV | Human Papillomavirus |
| UV | Ultraviolet |
| MSSE | Multiple Self-healing Squamous Epithelioma |
| EV | Epidermodysplasia Verruciformis |
| GEKA | Generalized Eruptive Keratoacanthoma |
| HR | High-Risk |
| LR | Low-Risk |
| NER | Nucleotide Excision Repair |
| E6AP | E6-Associated Protein |
| pRb | Retinoblastoma protein |
| RT | Radiotherapy |
| PDT | Photodynamic therapy |
| 5-FU | 5-Fluorouracil |
| EGFR | Epidermal Growth Factor Receptor |
References
- Robertson, S.J.; Bashir, S.J.; Pichert, G.; Robson, A.; Whittaker, S. Severe exacerbation of multiple self-healing squamous epithelioma (Ferguson–Smith disease) with radiotherapy, which was successfully treated with acitretin. Clin. Exp. Dermatol. 2010, 35, e100–e102. [Google Scholar] [CrossRef] [PubMed]
- Kwiek, B.; Schwartz, R.A. Keratoacanthoma (KA): An update and review. J. Am. Acad. Dermatol. 2016, 74, 1220–1233. [Google Scholar] [CrossRef] [PubMed]
- Miedzinski, F.; Kozakiewicz, J. Keratoacanthoma centrifugum—A special variety of keratoacanthoma. Hautarzt 1962, 13, 348–352. [Google Scholar]
- Ramselaar, C.G.; Ruitenberg, E.J.; Kruizinga, W. Regression of induced keratoacanthomas in anagen (hair growth phase) skin grafts in mice. Cancer Res. 1980, 40, 1668–1673. [Google Scholar]
- Zito, G.; Saotome, I.; Liu, Z.; Ferro, E.G.; Sun, T.Y.; Nguyen, D.X.; Bilguvar, K.; Ko, C.J.; Greco, V. Spontaneous tumour regression in keratoacanthomas is driven by Wnt/retinoic acid signalling cross-talk. Nat. Commun. 2014, 5, 3543. [Google Scholar] [CrossRef]
- Hu, W.; Cook, T.; Oh, C.W.; Penneys, N.S. Expression of the cyclin-dependent kinase inhibitor p27 in keratoacanthoma. J. Am. Acad. Dermatol. 2000, 42, 473–475. [Google Scholar] [CrossRef]
- Su, F.; Viros, A.; Milagre, C.; Trunzer, K.; Bollag, G.; Spleiss, O.; Reis-Filho, J.S.; Kong, X.; Koya, R.C.; Flaherty, K.T.; et al. RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N. Engl. J. Med. 2012, 366, 207–215. [Google Scholar] [CrossRef]
- Corominas, M.; Kamino, H.; Leon, J.; Pellicer, A. Oncogene activation in human benign tumors of the skin (keratoacanthomas): Is HRAS involved in differentiation as well as proliferation? Proc. Natl. Acad. Sci. USA 1989, 86, 6372–6376. [Google Scholar] [CrossRef]
- Sullivan, J.J. Keratoacanthoma: The Australian experience. Australas. J. Dermatol. 1997, 38, S36–S39. [Google Scholar] [CrossRef] [PubMed]
- Reizner, G.T.; Chuang, T.Y.; Elpern, D.J.; Stone, J.L.; Farmer, E.R. Basal cell carcinoma and keratoacanthoma in Hawaiians: An incidence report. J. Am. Acad. Dermatol. 1993, 29 5 Pt 1, 780–782. [Google Scholar] [CrossRef]
- Vergilis-Kalner, I.J.; Kriseman, Y.; Goldberg, L.H. Keratoacanthomas: Overview and comparison between Houston and minneapolis experiences. J. Drugs Dermatol. 2010, 9, 117–121. [Google Scholar] [PubMed]
- Ambur, A.; Clark, A.; Nathoo, R. An Updated Review of the Therapeutic Management of Keratoacanthomas. J. Clin. Aesthet. Dermatol. 2022, 15, 30–36. [Google Scholar]
- Joshi, S.; De Angelis, P.M.; Zucknick, M.; Schjølberg, A.R.; Andersen, S.N.; Clausen, O.P.F. Role of the Wnt signaling pathway in keratoacanthoma. Cancer Rep. 2020, 3, e1219. [Google Scholar] [CrossRef]
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Zito, P.M.; Scharf, R. Keratoacanthoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK499931/ (accessed on 27 April 2025).
- Ilaria, P.; Nevena, S.; Ersilia, T.; Federica, T.; Felice, F.; Agnieszka Ewa, D.; Francesco, F.; Concetta, P. Generalized Eruptive Keratoacanthoma (GEKA) after Pfizer mRNABNT162b2 (Comirnaty®) COVID-19 Vaccination Successfully Treated with Cemiplimab. Viruses. 2024, 16, 1260. [Google Scholar] [CrossRef] [PubMed]
- Yumeen, S.; Robinson-Bostom, L.; Firoz, E.F. Solitary Eruptive Keratoacanthoma Developing at Site of COVID-19 Vaccine Injection. Rhode Isl. Med. J. 2023, 106, 49–51. [Google Scholar]
- Mitri, F.; Hartschuh, W.; Toberer, F. Multiple Keratoacanthomas after a Recent Tattoo: A Case Report. Case Rep. Dermatol. 2021, 13, 23–27. [Google Scholar] [CrossRef]
- Brongo, S.; Moccia, L.S.; Nunziata, V.; D’Andrea, F. Keratoacanthoma arising after site injection infection of cosmetic collagen filler. Int. J. Surg. Case Rep. 2013, 4, 429–431. [Google Scholar] [CrossRef][Green Version]
- Kim, J.H.; Lee, S.H.; Hong, S.P.; Kim, J.; Kim, S.W. Solitary Keratoacanthoma at the Recipient Site of a Full-Thickness Skin Graft: A Case Report and Review of the Literature. Arch. Plast. Surg. 2023, 50, 59–62. [Google Scholar] [CrossRef]
- Fradet, M.; Sibaud, V.; Tournier, E.; Lamant, L.; Boulinguez, S.; Brun, A.; Pages, C.; Meyer, N. Multiple Keratoacanthoma-like Lesions in a Patient Treated with Pembrolizumab. Acta Derm. Venereol. 2019, 99, 1301–1302. [Google Scholar] [CrossRef]
- Li, S.; Ye, X.; Li, X.; Yang, Y. Case Report: Multiple cutaneous keratoacanthoma-like lesions in a colorectal cancer patient treated with sintilimab. Front. Immunol. 2025, 16, 1535220. [Google Scholar] [CrossRef]
- Frances, L.; Guijarro, J.; Marin, I.; Leiva-Salinas, M.d.C.; Bouret, A.M. Multiple eruptive keratoacanthomas associated with leflunomide. Dermatol. Online J. 2013, 19, 18968. [Google Scholar] [CrossRef] [PubMed]
- Karampinis, E.; Kostopoulou, C.; Toli, O.; Marinos, L.; Papadimitriou, G.; Roussaki Schulze, A.V.; Zafiriou, E. Multiple Keratoacanthoma-like Syndromes: Case Report and Literature Review. Medicina 2024, 60, 371. [Google Scholar] [CrossRef]
- Neagu, N.; Dianzani, C.; Venuti, A.; Bonin, S.; Voidăzan, S.; Zalaudek, I.; Conforti, C. The role of HPV in keratinocyte skin cancer development: A systematic review. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 40–46. [Google Scholar] [CrossRef]
- Ljubojevic, S.; Skerlev, M. HPV-associated diseases. Clin. Dermatol. 2014, 32, 227–234. [Google Scholar] [CrossRef]
- Conforti, C.; Paolini, F.; Venuti, A.; Dianzani, C.; Zalaudek, I. The detection rate of human papillomavirus in well-differentiated squamous cell carcinoma and keratoacanthoma: Is there new evidence for a viral pathogenesis of keratoacanthoma? Br. J. Dermatol. 2019, 181, 1309–1311. [Google Scholar] [CrossRef]
- McLaughlin-Drubin, M.E. Human papillomaviruses and non-melanoma skin cancer. Semin. Oncol. 2015, 42, 284–290. [Google Scholar] [CrossRef]
- Arroyo Mühr, L.S.; Hultin, E.; Bzhalava, D.; Eklund, C.; Lagheden, C.; Ekström, J.; Johansson, H.; Forslund, O.; Dillner, J. Human papillomavirus type 197 is commonly present in skin tumors. Int. J. Cancer 2015, 136, 2546–2555. [Google Scholar] [CrossRef]
- Eversole, L.R.; Leider, A.S.; Alexander, G. Intraoral and labial keratoacanthoma. Oral Surg. Oral Med. Oral Pathol. 1982, 54, 663–667. [Google Scholar] [CrossRef]
- Cristalli, M.P.; Marini, R.; La Monaca, G.; Vitolo, D.; Pompa, G.; Annibali, S. Double localization of keratoacantho-ma on the cutaneous and mucosal sides of the lower lip: Report of a case. Oral Implantol. 2013, 6, 94–98. [Google Scholar]
- Janette, A.; Pecaro, B.; Lonergan, M.; Lingen, M.W. Solitary intraoral keratoacanthoma: Report of a case. J. Oral Maxillofac. Surg. 1996, 54, 1026–1030. [Google Scholar] [CrossRef]
- Habel, G.; O’Regan, B.; Eissing, A.; Khoury, F.; Donath, K. Intra-oral keratoacanthoma: An eruptive variant and review of the literature. Br. Dent. J. 1991, 170, 336–339. [Google Scholar] [CrossRef]
- Whyte, A.M.; Hansen, L.S.; Lee, C. The intraoral keratoacanthoma: A diagnostic problem. Br. J. Oral Maxillofac. Surg. 1986, 24, 438–441. [Google Scholar] [CrossRef]
- Heidenreich, R.K.; Gongloff, R.K.; Wescott, W.B. A solitary, exophytic, crateriform lesion on the mandibular retromolar gingiva. J. Am. Dent. Assoc. 1986, 112, 377–379. [Google Scholar] [CrossRef]
- Svirsky, J.A.; Freedman, P.D.; Lumerman, H. Solitary intraoral keratoacanthoma. Oral Surg. Oral Med. Oral Pathol. 1977, 43, 116–122. [Google Scholar] [CrossRef]
- Zegarelli, D.J. Solitary intraoral keratoacanthoma. Oral Surg. Oral Med. Oral Pathol. 1975, 40, 785–788. [Google Scholar] [CrossRef]
- Divers, A.K.; Correale, D.; Lee, J.B. Keratoacanthoma centrifugum marginatum: A diagnostic and therapeutic challenge. Cutis 2004, 73, 257–262. [Google Scholar]
- Wu, T.P.; Miller, K.; Cohen, D.E.; Stein, J.A. Keratoacanthomas arising in association with prurigo nodules in pruritic, actinically damaged skin. J. Am. Acad. Dermatol. 2013, 69, 426–430. [Google Scholar] [CrossRef]
- Goudie, D.R.; D’Alessandro, M.; Merriman, B.; Lee, H.; Szeverényi, I.; Avery, S.; O’Connor, B.D.; Nelson, S.F.; Coats, S.E.; Stewart, A.; et al. Multiple self-healing squamous epithelioma is caused by a disease-specific spectrum of mutations in TGFBR1. Nat. Genet. 2011, 43, 365–369. [Google Scholar] [CrossRef]
- Grzybowski, M. A case of peculiar generalized epithelial tumours of the skin. Br. J. Dermatol. Syph. 1950, 62, 310–313. [Google Scholar] [CrossRef]
- Anzalone, C.L.; Cohen, P.R. Generalized eruptive keratoacanthomas of Grzybowski. Int. J. Dermatol. 2014, 53, 131–136. [Google Scholar] [CrossRef]
- Agarwal, M.; Chander, R.; Karmakar, S.; Walia, R. Multiple familial keratoacanthoma of Witten and Zak—A report of three siblings. Dermatology 1999, 198, 396–399. [Google Scholar] [CrossRef]
- Boateng, B.; Hornstein, O.P.; von den Driesch, P.; Kiesewetter, F. Multiple keratoacanthomas (Witten-Zak type) in prurigo simplex subacuta. Hautarzt 1995, 46, 114–117. [Google Scholar] [CrossRef]
- Rosendahl, C.; Cameron, A.; Argenziano, G.; Zalaudek, I.; Tschandl, P.; Kittler, H. Dermoscopy of squamous cell carcinoma and keratoacanthoma. Arch. Dermatol. 2012, 148, 1386–1392. [Google Scholar] [CrossRef]
- Zalaudek, I.; Giacomel, J.; Schmid, K.; Bondino, S.; Rosendahl, C.; Cavicchini, S.; Tourlaki, A.; Gasparini, S.; Bourne, P.; Keir, J.; et al. Dermatoscopy of facial actinic keratosis, intraepidermal carcinoma, and invasive squamous cell carcinoma: A progression model. J. Am. Acad. Dermatol. 2012, 66, 589–597. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Wang, H.; Zheng, L.; Guo, Z.; Fan, X.; Gao, M. The first report of diagnosing of keratoacanthoma in Chinese Han patients using dermoscopy and reflectance confocal microscopy. Skin. Res. Technol. 2021, 27, 422–427. [Google Scholar] [CrossRef]
- Nguyen, K.P.; Peppelman, M.; Hoogedoorn, L.; Van Erp, P.E.J.; Gerritsen, M.J.P. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: A systematic review. Eur. J. Dermatol. 2016, 26, 549–565. [Google Scholar] [CrossRef]
- Latriglia, F.; Ogien, J.; Tavernier, C.; Fischman, S.; Suppa, M.; Perrot, J.L.; Dubois, A. Line-Field Confocal Optical Coherence Tomography (LC-OCT) for Skin Imaging in Dermatology. Life 2023, 13, 2268. [Google Scholar]
- Cinotti, E.; Brunetti, T.; Cartocci, A.; Tognetti, L.; Suppa, M.; Malvehy, J.; Perez-Anker, J.; Puig, S.; Perrot, J.L.; Rubegni, P. Diagnostic Accuracy of Line-Field Confocal Optical Coherence Tomography for the Diagnosis of Skin Carcinomas. Diagnostics 2023, 13, 361. [Google Scholar] [CrossRef]
- Schwartz, R.A. Keratoacanthoma: A clinico-pathologic enigma. Dermatol. Surg. 2004, 30 2 Pt 2, 326–333. [Google Scholar] [CrossRef]
- Ogita, A.; Ansai, S.I.; Misago, N.; Anan, T.; Fukumoto, T.; Saeki, H. Histopathological diagnosis of epithelial crateriform tumors: Keratoacanthoma and other epithelial crateriform tumors. J. Dermatol. 2016, 43, 1321–1331. [Google Scholar] [CrossRef]
- Takai, T. Advances in histopathological diagnosis of keratoacanthoma. J. Dermatol. 2017, 44, 304–314. [Google Scholar] [CrossRef]
- Cribier, B.; Asch, P.; Grosshans, E. Differentiating squamous cell carcinoma from keratoacanthoma using histopathological criteria. Is it possible? A study of 296 cases. Dermatology 1999, 199, 208–212. [Google Scholar] [CrossRef]
- Savage, J.A.; Maize, J.C. Keratoacanthoma clinical behavior: A systematic review. Am. J. Dermatopathol. 2014, 36, 422–429. [Google Scholar] [CrossRef]
- Kossard, S.; Tan, K.B.; Choy, C. Keratoacanthoma and infundibulocystic squamous cell carcinoma. Am. J. Dermatopathol. 2008, 30, 127–134. [Google Scholar] [CrossRef]
- Weedon, D.D.; Malo, J.; Brooks, D.; Williamson, R. Squamous cell carcinoma arising in keratoacanthoma: A neglected phenomenon in the elderly. Am. J. Dermatopathol. 2010, 32, 423–426. [Google Scholar] [CrossRef]
- Piscioli, F.; Boi, S.; Zumiani, G.; Cristofolini, M. A gigantic, metastasizing keratoacanthoma. Report of a case and discussion on classification. Am. J. Dermatopathol. 1984, 6, 123–129. [Google Scholar] [CrossRef]
- Nelson, C.W.; Mirabello, L. Human papillomavirus genomics: Understanding carcinogenicity. Tumour Virus Res. 2023, 15, 200258. [Google Scholar] [CrossRef]
- Skelin, J.; Tomaić, V. Comparative analysis of alpha and beta HPV E6 oncoproteins: Insights into functional distinctions and divergent mechanisms of pathogenesis. Viruses 2023, 15, 2253. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30 (Suppl. S5), F55–F70. [Google Scholar] [CrossRef]
- Buck, C.B.; Cheng, N.; Thompson, C.D.; Lowy, D.R.; Steven, A.C.; Schiller, J.T.; Trus, B.L. Arrangement of L2 within the papillomavirus capsid. J. Virol. 2008, 82, 5190–5197. [Google Scholar] [CrossRef]
- Gheit, T. Mucosal and Cutaneous Human Papillomavirus Infections and Cancer Biology. Front. Oncol. 2019, 9, 355. [Google Scholar] [CrossRef]
- Müller, M.; Prescott, E.L.; Wasson, C.W.; Macdonald, A. Human papillomavirus E5 oncoprotein: Function and potential target for antiviral therapeutics. Future Virol. 2015, 10, 27–39. [Google Scholar] [CrossRef]
- Pešut, E.; Đukić, A.; Lulić, L.; Skelin, J.; Šimić, I.; Milutin Gašperov, N.; Tomaić, V.; Sabol, I.; Grce, M. Human Papillomaviruses-Associated Cancers: An Update of Current Knowledge. Viruses 2021, 13, 2234. [Google Scholar] [CrossRef]
- Messa, L.; Loregian, A. HPV-induced cancers: Preclinical therapeutic advancements. Expert. Opin. Investig. Drugs 2022, 31, 79–93. [Google Scholar] [CrossRef]
- Bertagnin, C.; Messa, L.; Pavan, M.; Celegato, M.; Sturlese, M.; Mercorelli, B.; Moro, S.; Loregian, A. A small molecule targeting the interaction between human papillomavirus E7 oncoprotein and cellular phosphatase PTPN14 exerts antitumoral activity in cervical cancer cells. Cancer Lett. 2023, 571, 216331. [Google Scholar] [CrossRef]
- Celegato, M.; Messa, L.; Goracci, L.; Mercorelli, B.; Bertagnin, C.; Spyrakis, F.; Suarez, I.; Cousido-Siah, A.; Travé, G.; Banks, L.; et al. A novel small-molecule inhibitor of the human papillomavirus E6-p53 interaction that reactivates p53 function and blocks cancer cells growth. Cancer Lett. 2020, 470, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Egawa, K.; Griffin, H.; Doorbar, J. Human Papillomaviruses; Epithelial Tropisms, and the Development of Neoplasia. Viruses 2015, 7, 3863–3890. [Google Scholar] [CrossRef] [PubMed]
- Tampa, M.; Mitran, C.I.; Mitran, M.I.; Nicolae, I.; Dumitru, A.; Matei, C.; Manolescu, L.; Popa, G.L.; Caruntu, C.; Georgescu, S.R. The Role of Beta HPV Types and HPV-Associated Inflammatory Processes in Cutaneous Squamous Cell Carcinoma. J. Immunol. Res. 2020, 2020, 5701639. [Google Scholar] [CrossRef] [PubMed]
- Orth, G. Genetics of Epidermodysplasia Verruciformis: Insights into Host Defense against Papillomaviruses. Semin. Immunol. 2006, 18, 362–374. [Google Scholar] [CrossRef]
- Quint, K.D.; Genders, R.E.; de Koning, M.N.; Borgogna, C.; Gariglio, M.; Bouwes Bavinck, J.N.; Doorbar, J.; Feltkamp, M.C.W. Human Beta-Papillomavirus Infection and Keratinocyte Carcinomas. J. Pathol. 2015, 235, 342–354. [Google Scholar] [CrossRef]
- Hufbauer, M.; Akgül, B. Molecular Mechanisms of Human Papillomavirus Induced Skin Carcinogenesis. Viruses 2017, 9, 187. [Google Scholar] [CrossRef]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight Damage to Cellular DNA: Focus on Oxidatively Generated Lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef]
- Douki, T.; von Koschembahr, A.; Cadet, J. Insight in DNA Repair of UV-Induced Pyrimidine Dimers by Chromatographic Methods. Photochem. Photobiol. 2017, 93, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Brash, D.E.; Ziegler, A.; Jonason, A.S.; Simon, J.A.; Kunala, S.; Leffell, D.J. Sunlight and Sunburn in Human Skin Cancer: p53, Apoptosis, and Tumor Promotion. J. Investig. Dermatol. Symp. Proc. 1996, 1, 136–142. [Google Scholar] [PubMed]
- el-Deiry, W.S.; Harper, J.W.; O’Connor, P.M.; Velculescu, V.E.; Canman, C.E.; Jackman, J.; Pietenpol, J.A.; Burrell, M.; Hill, D.E.; Wang, Y.; et al. WAF1/CIP1 Is Induced in p53-Mediated G1 Arrest and Apoptosis. Cancer Res. 1994, 54, 1169–1174. [Google Scholar]
- Scheffner, M.; Werness, B.A.; Huibregtse, J.M.; Levine, A.J.; Howley, P.M. The E6 Oncoprotein Encoded by Human Papillomavirus Types 16 and 18 Promotes the Degradation of p53. Cell 1990, 63, 1129–1136. [Google Scholar] [CrossRef]
- Hengstermann, A.; Linares, L.K.; Ciechanover, A.; Whitaker, N.J.; Scheffner, M. Complete Switch from Mdm2 to Human Papillomavirus E6-Mediated Degradation of p53 in Cervical Cancer Cells. Proc. Natl. Acad. Sci. USA 2001, 98, 1218–1223. [Google Scholar] [CrossRef]
- Li, S.; Hong, X.; Wei, Z.; Xie, M.; Li, W.; Liu, G.; Guo, H.; Yang, J.; Wei, W.; Zhang, S. Ubiquitination of the HPV Oncoprotein E6 Is Critical for E6/E6AP-Mediated p53 Degradation. Front. Microbiol. 2019, 10, 2483. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Vande Pol, S.; Podjarny, A.; et al. Structure of the E6/E6AP/p53 Complex Required for HPV-Mediated Degradation of p53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef]
- Muench, P.; Probst, S.; Schuetz, J.; Leiprecht, N.; Busch, M.; Wesselborg, S.; Stubenrauch, F.; Iftner, T. Cutaneous Papillomavirus E6 Proteins Must Interact with p300 and Block p53-Mediated Apoptosis for Cellular Immortalization and Tumorigenesis. Cancer Res. 2010, 70, 6913–6924. [Google Scholar] [CrossRef]
- Muschik, D.; Braspenning-Wesch, I.; Stockfleth, E.; Rösl, F.; Hofmann, T.G.; Nindl, I. Cutaneous HPV23 E6 Prevents p53 Phosphorylation through Interaction with HIPK2. PLoS ONE 2011, 6, e27655. [Google Scholar] [CrossRef]
- Minoni, L.; Romero-Medina, M.C.; Venuti, A.; Sirand, C.; Robitaille, A.; Altamura, G.; Tommasino, M.; Accardi, R. Transforming Properties of Beta-3 Human Papillomavirus E6 and E7 Proteins. mSphere 2020, 5, e00398. [Google Scholar] [CrossRef]
- Cornet, I.; Bouvard, V.; Campo, M.S.; Thomas, M.; Banks, L.; Gissmann, L.; Tommasino, M. Comparative Analysis of Transforming Properties of E6 and E7 from Different Beta Human Papillomavirus Types. J. Virol. 2012, 86, 2366–2370. [Google Scholar] [CrossRef] [PubMed]
- White, E.A.; Kramer, R.E.; Tan, M.J.A.; Hayes, S.D.; Harper, J.W.; Howley, P.M. Comprehensive Analysis of Host Cellular Interactions with Human Papillomavirus E6 Proteins Identifies New E6 Binding Partners and Reflects Viral Diversity. J. Virol. 2012, 86, 13174–13186. [Google Scholar] [CrossRef]
- Accardi, R.; Dong, W.; Smet, A.; Cui, R.; Hautefeuille, A.; Gabet, A.; Tommasino, M. Skin Human Papillomavirus Type 38 Alters p53 Functions by Accumulation of ΔNp73. EMBO Rep. 2006, 7, 334–340. [Google Scholar] [CrossRef]
- Di, C.; Yang, L.; Zhang, H.; Ma, X.; Zhang, X.; Sun, C.; Zhu, H. Mechanisms, Function and Clinical Applications of ΔNp73. Cell Cycle 2013, 12, 1861–1867. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and Beyond—Mitochondrial Performance in Apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [PubMed]
- Jackson, S.; Harwood, C.; Thomas, M.; Banks, L.; Storey, A. Role of Bak in UV-Induced Apoptosis in Skin Cancer and Abrogation by HPV E6 Proteins. Genes. Dev. 2000, 14, 3065–3073. [Google Scholar] [CrossRef]
- Thomas, M.; Banks, L. Inhibition of Bak-Induced Apoptosis by HPV-18 E6. Oncogene 1998, 17, 2943–2954. [Google Scholar] [CrossRef]
- Magal, S.S.; Jackman, A.; Ish-Shalom, S.; Botzer, L.E.; Gonen, P.; Schlegel, R.; Shai, A. Downregulation of Bax mRNA Expression and Protein Stability by the E6 Protein of Human Papillomavirus 16. J. Gen. Virol. 2005, 86, 611–621. [Google Scholar] [CrossRef]
- Lee, J.O.; Russo, A.A.; Pavletich, N.P. Structure of the Retinoblastoma Tumour-Suppressor Pocket Domain Bound to a Peptide from HPV E7. Nature 1998, 391, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, S.; Zehbe, I.; Accardi, R.; Malanchi, I.; Dong, W.; Giarrè, M.; Tommasino, M. The E6 and E7 Proteins of the Cutaneous Human Papillomavirus Type 38 Display Transforming Properties. J. Virol. 2003, 77, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Wallace, N.A.; Robinson, K.; Howie, H.L.; Galloway, D.A. HPV 5 and 8 E6 Abrogate ATR Activity Resulting in Increased Persistence of UVB Induced DNA Damage. PLoS Pathog. 2012, 8, e1002807. [Google Scholar] [CrossRef]
- Forslund, O.; DeAngelis, P.M.; Beigi, M.; Schjølberg, A.R.; Clausen, O.P.F. Identification of human papillomavirus in keratoacanthomas. J. Cutan. Pathol. 2003, 30, 423–429. [Google Scholar] [CrossRef]
- Baek, Y.S.; Kim, Y.C.; Kim, K.E.; Jeon, J.; Kim, A.; Song, H.J.; Kim, C. Low detection rate of human papillomavirus in patients with cutaneous squamous cell carcinoma and keratoacanthoma. Eur. J. Dermatol. 2022, 32, 577–583. [Google Scholar] [CrossRef]
- Tran, D.C.; Li, S.; Henry, S.; Wood, D.J.; Chang, A.L.S. An 18-year retrospective study on the outcomes of keratoacanthomas with different treatment modalities at a single academic centre. Br. J. Dermatol. 2017, 177, 1749–1751. [Google Scholar] [CrossRef]
- Caccialanza, M.; Sopelana, N. Radiation therapy of keratoacanthomas: Results in 55 patients. Int. J. Radiat. Oncol. Biol. Phys. 1989, 16, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Donahue, B.; Cooper, J.S.; Rush, S. Treatment of aggressive keratoacanthomas by radiotherapy. J. Am. Acad. Dermatol. 1990, 23, 489–493. [Google Scholar] [CrossRef]
- Shimm, D.S.; Duttenhaver, J.R.; Doucette, J.; Wang, C.C. Radiation therapy of keratoacanthoma. Int. J. Radiat. Oncol. Biol. Phys. 1983, 9, 759–761. [Google Scholar] [CrossRef]
- Shaw, J.C.; Storrs, F.J.; Everts, E. Multiple keratoacanthomas after megavoltage radiation therapy. J. Am. Acad. Dermatol. 1990, 23 5 Pt 2, 1009–1011. [Google Scholar] [CrossRef]
- Kuflik, E.G. Cryosurgery for cutaneous malignancy: An update. Dermatol. Surg. 1997, 23, 1081–1087. [Google Scholar] [CrossRef]
- Gogia, R.; Grekin, R.C.; Shinkai, K. Eruptive self-resolving keratoacanthomas developing after treatment with photodynamic therapy and microdermabrasion. Dermatol. Surg. 2013, 39, 1717–1720. [Google Scholar] [CrossRef]
- Neumann, R.A.; Knobler, R.M. Argon laser treatment of small keratoacanthomas in difficult locations. Int. J. Dermatol. 1990, 29, 733–736. [Google Scholar] [CrossRef]
- Yoshioka, A.; Tanaka, S.; Hiraoka, O.; Koyama, Y.; Hirota, Y.; Ayusawa, D.; Nishimoto, I. Deoxyribonucleoside triphosphate imbalance. 5-Fluorodeoxyuridine-induced DNA double strand breaks in mouse FM3A cells and the mechanism of cell death. J. Biol. Chem. 1987, 262, 8235–8241. [Google Scholar] [CrossRef]
- Thompson, B.J.; Ravits, M.; Silvers, D.N. Clinical efficacy of short contact topical 5-Fluorouracil in the treatment of keratoacanthomas: A retrospective analysis. J. Clin. Aesthet. Dermatol. 2014, 7, 35–37. [Google Scholar]
- Goette, D.K.; Odom, R.B. Successful treatment of keratoacanthoma with intralesional fluorouracil. J. Am. Acad. Dermatol. 1980, 2, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Ziemer, M.; Fuchs, S.; Elsner, P. Combined 5-fluorouracil and Er:YAG laser treatment in a case of recurrent giant keratoacanthoma of the lower leg. Dermatol. Surg. 2004, 30 12 Pt 2, 1556–1560. [Google Scholar] [PubMed]
- Kiss, N.; Avci, P.; Bánvölgyi, A.; Lőrincz, K.; Szakonyi, J.; Gyöngyösi, N.; Fésűs, L.; Lee, G.; Wikonkál, N. Intralesional therapy for the treatment of keratoacanthoma. Dermatol. Ther. 2019, 32, e12872. [Google Scholar] [CrossRef]
- Jeon, H.C.; Choi, M.; Paik, S.H.; Ahn, C.H.; Park, H.S.; Cho, K.H. Treatment of keratoacanthoma with 5% imiquimod cream and review of the previous report. Ann. Dermatol. 2011, 23, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Dranchak, P.; Dahlin, J.L.; Lamy, L.; Li, W.; Oliphant, E.; Shrimp, J.H.; Rajacharya, G.H.; Tharakan, R.; Holland, D.O.; et al. Methotrexate-based PROTACs as DHFR-specific chemical probes. Cell Chem. Biol. 2024, 31, 221–233.e14. [Google Scholar] [CrossRef] [PubMed]
- Nofal, A.; Alakad, R.; Wahid, R.; Hoseiny, H.A.M. Intralesional methotrexate versus 5-flurouracil in the treatment of keratoacanthoma. Arch. Dermatol. Res. 2024, 316, 400. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.G.; Kim, I.H. Intralesional methotrexate for the treatment of keratoacanthoma: Retrospective study and review of the korean literature. Ann. Dermatol. 2014, 26, 172–176. [Google Scholar] [CrossRef]
- Annest, N.M.; VanBeek, M.J.; Arpey, C.J.; Whitaker, D.C. Intralesional methotrexate treatment for keratoacanthoma tumors: A retrospective study and review of the literature. J. Am. Acad. Dermatol. 2007, 56, 989–993. [Google Scholar] [CrossRef]
- Gavric, G.; Lekic, B.; Milinkovic Sreckovic, M.; Bosic, M.; Zivanovic, D. Keratoacanthoma centrifugum marginatum associated with mechanical trauma: Response to acitretin—A case report and review of the literature. Dermatol. Ther. 2020, 33, e13397. [Google Scholar] [CrossRef]
- Mizuta, H.; Takahashi, A.; Mori, T.; Namikawa, K.; Nakano, E.; Muto, Y.; Jinnai, S.; Nakama, K.; Tsutsui, K.; Yamazaki, N. Efficacy of oral retinoids for keratoacanthoma centrifugum marginatum. Dermatol. Ther. 2020, 33, e13291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Han, D.; Wang, T.; Liu, Y. Multiple keratoacanthoma and oral lichen planus successfully treated with systemic retinoids and review of multiple keratoacanthoma associated with lichen planus. Int. J. Dermatol. 2018, 57, 1125–1127. [Google Scholar] [CrossRef]
- Reid, D.C.; Guitart, J.; Agulnik, M.; Lacouture, M.E. Treatment of multiple keratoacanthomas with erlotinib. Int. J. Clin. Oncol. 2010, 15, 413–415. [Google Scholar] [CrossRef]
- Pogoda, C.S.; Roden, R.B.S.; Garcea, R.L. Immunizing against Anogenital Cancer: HPV Vaccines. PLoS Pathog. 2016, 12, e1005587. [Google Scholar] [CrossRef]
- Pouyanfard, S.; Spagnoli, G.; Bulli, L.; Balz, K.; Yang, F.; Odenwald, C.; Seitz, H.; Mariz, F.C.; Bolchi, A.; Ottonello, S.; et al. Minor Capsid Protein L2 Polytope Induces Broad Protection against Oncogenic and Mucosal Human Papillomaviruses. J. Virol. 2018, 92, e01930-17. [Google Scholar] [CrossRef]
- Mariz, F.C.; Balz, K.; Dittrich, M.; Zhang, Y.; Yang, F.; Zhao, X.; Bolchi, A.; Ottonello, S.; Müller, M. A broadly protective vaccine against cutaneous human papillomaviruses. NPJ Vaccines 2022, 7, 116. [Google Scholar] [CrossRef] [PubMed]

| Therapy Type | Description | Mechanism | Effectiveness | ||
|---|---|---|---|---|---|
 surgical | Complete surgical removal | Surgical removal with histopathological control | Gold-standard8% recurrence | ||
 physical | Radiotherapy | Targeted destruction of lesions via | Energy | Effective, but rarely used | |
| Cryotherapy | Cold | Effective (~87%), but not widely studied | |||
| PDT 1 | Locally activated drug | Limited available data | |||
| Argon laser | Laser | Effective for solitary KA 2 | |||
 topical | Local application of | 5-FU 3 | Inhibition of DNA synthesis | Very effective, first-line treatment | |
| Imiquimod | Local immune response activation | Effective | |||
 systemic | Retinoids | Control of proliferation, differentiation, and immune response | Valuable option for generalized eruptive KA 2 | ||
| Erlotinib | EGFR 4 inhibition | Still limited experience | |||
 active surveillance | Watch-and-wait approach | KA can regress spontaneously | Rarely appropriate, but can be considered as a strategy mainly for elderly patients | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyeraci, M.; Didona, D.; Abeni, D.; Magri, F.; Ricci, F.; Bertagnin, C.; Loregian, A.; Di Lella, G.; Di Guardo, A.; Panebianco, A.; et al. New Insights into Pathogenesis and Management of Keratoacanthoma: A Narrative Review. Int. J. Mol. Sci. 2025, 26, 10040. https://doi.org/10.3390/ijms262010040
Hyeraci M, Didona D, Abeni D, Magri F, Ricci F, Bertagnin C, Loregian A, Di Lella G, Di Guardo A, Panebianco A, et al. New Insights into Pathogenesis and Management of Keratoacanthoma: A Narrative Review. International Journal of Molecular Sciences. 2025; 26(20):10040. https://doi.org/10.3390/ijms262010040
Chicago/Turabian StyleHyeraci, Mariafrancesca, Dario Didona, Damiano Abeni, Francesca Magri, Francesco Ricci, Chiara Bertagnin, Arianna Loregian, Giovanni Di Lella, Antonio Di Guardo, Annarita Panebianco, and et al. 2025. "New Insights into Pathogenesis and Management of Keratoacanthoma: A Narrative Review" International Journal of Molecular Sciences 26, no. 20: 10040. https://doi.org/10.3390/ijms262010040
APA StyleHyeraci, M., Didona, D., Abeni, D., Magri, F., Ricci, F., Bertagnin, C., Loregian, A., Di Lella, G., Di Guardo, A., Panebianco, A., Chello, C., Conforti, C., Dellambra, E., & Fania, L. (2025). New Insights into Pathogenesis and Management of Keratoacanthoma: A Narrative Review. International Journal of Molecular Sciences, 26(20), 10040. https://doi.org/10.3390/ijms262010040







