JP-14: A Trace Amine-Associated Receptor 1 Agonist with Anti-Metabolic Disorder Potential
Abstract
1. Introduction
2. Results and Discussion
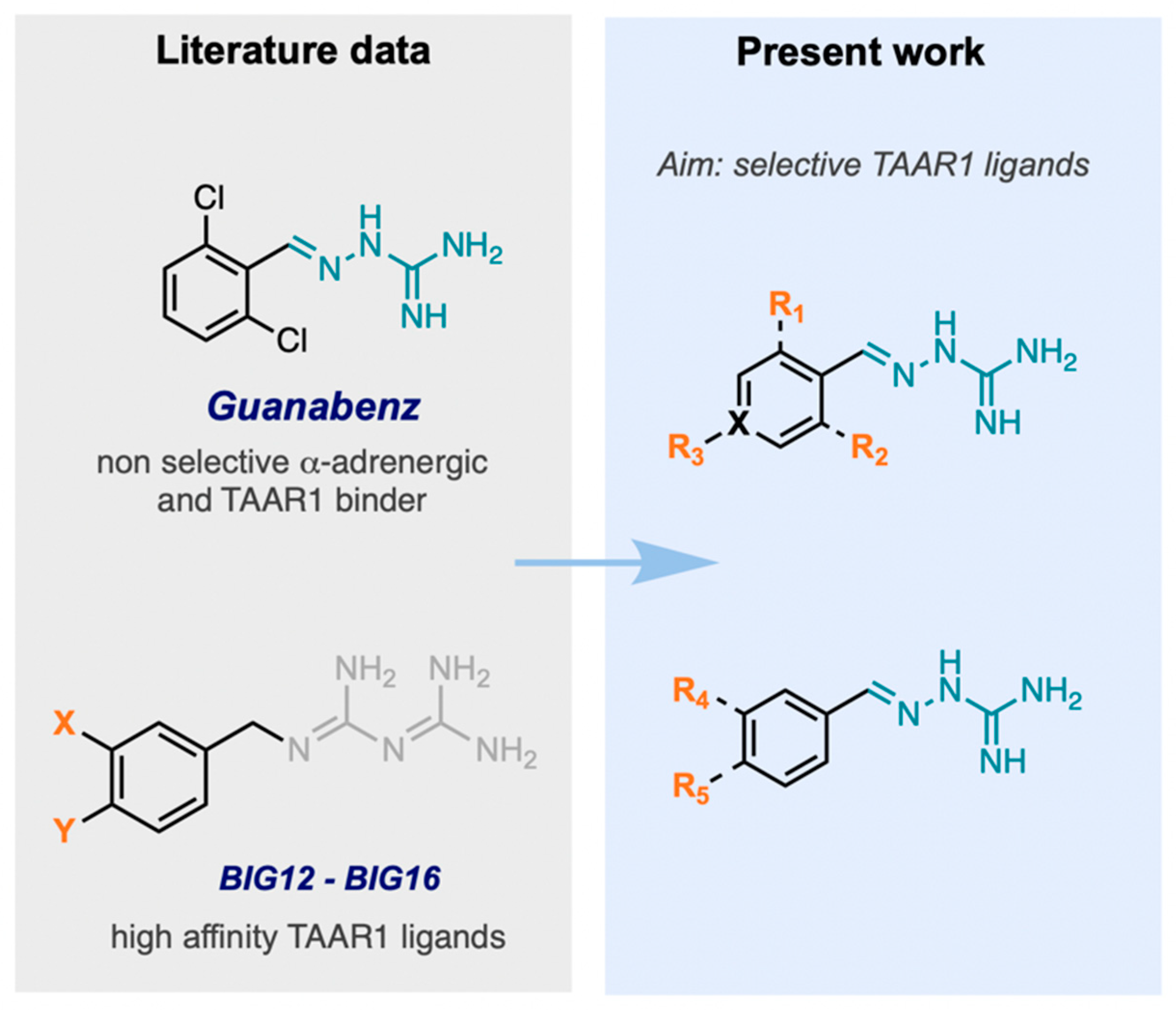
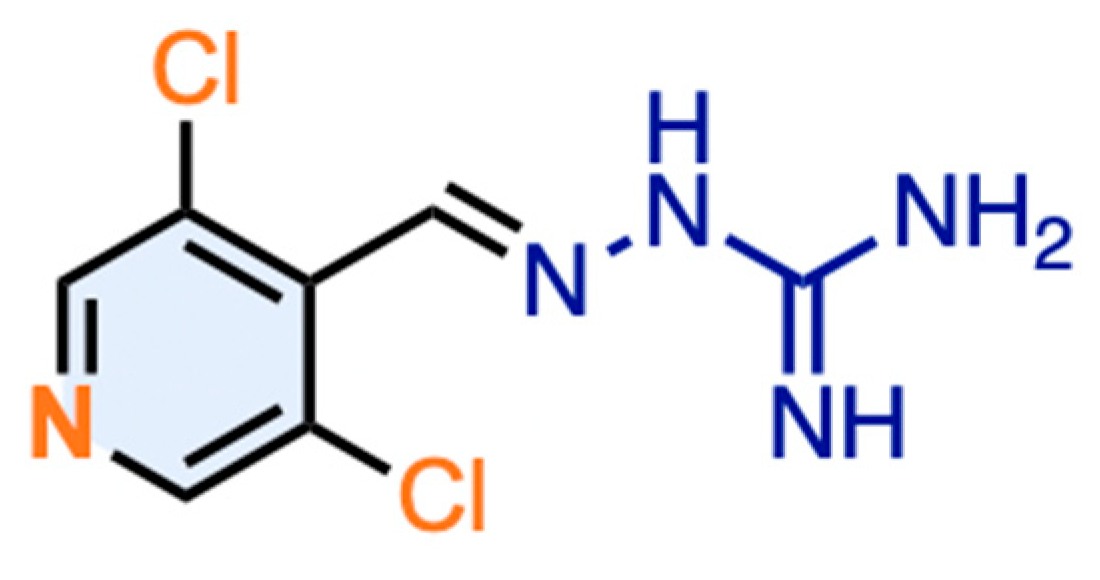
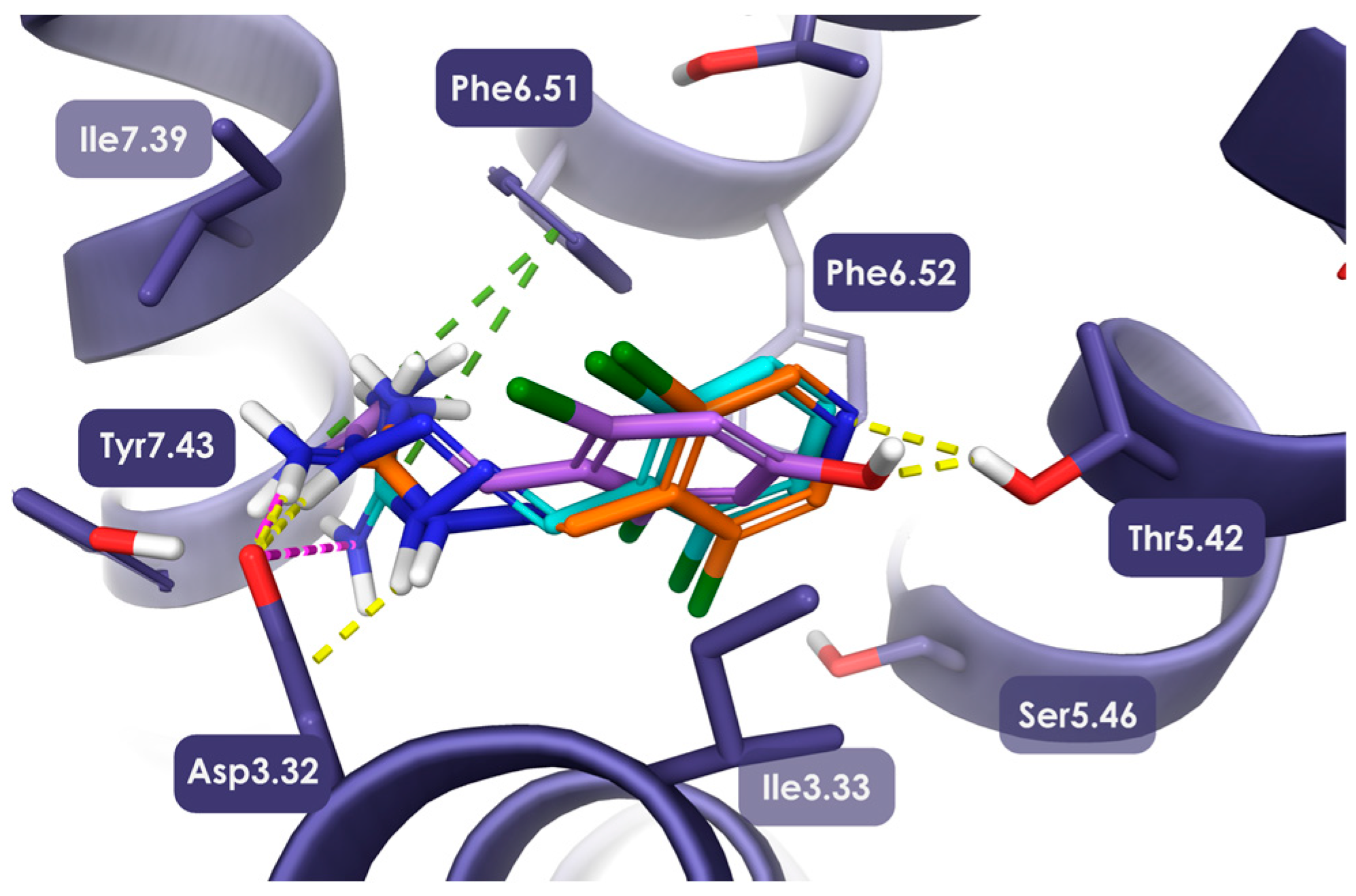
2.1. Design, Synthesis, and Identification of Compound JP-14 as Novel TAAR1 Ligand
2.2. Biological Activity in In Vitro and In Vivo Assays
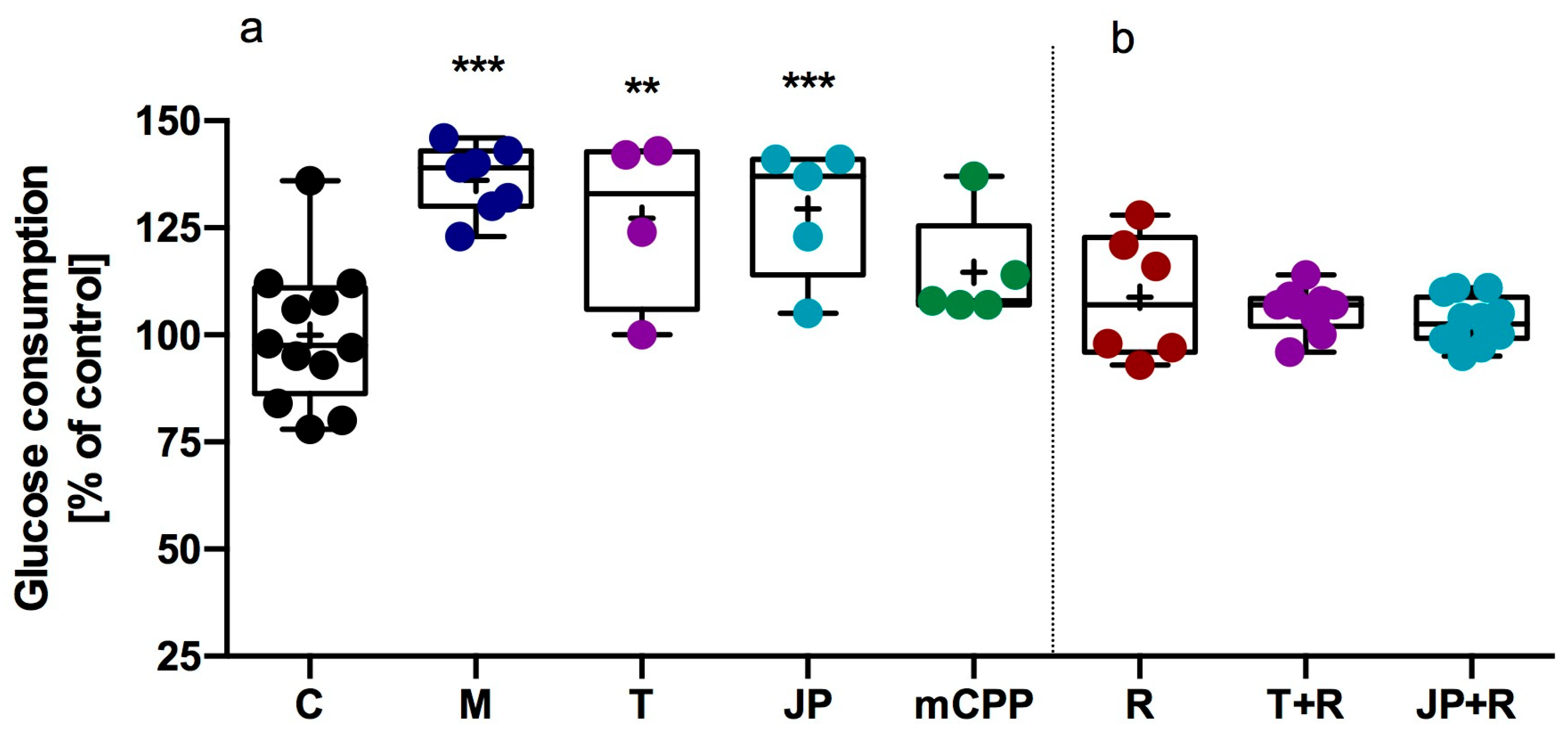
2.2.1. Influence of JP-14 on Glucose Metabolism in HepG2 Cells
2.2.2. Influence of JP-14 on Adipocyte Lipid Accumulation in 3T3-L1 Cells
2.2.3. Influence of Tested Compound on Steatosis and Phospholipidosis in 3T3-L1 Adipocytes
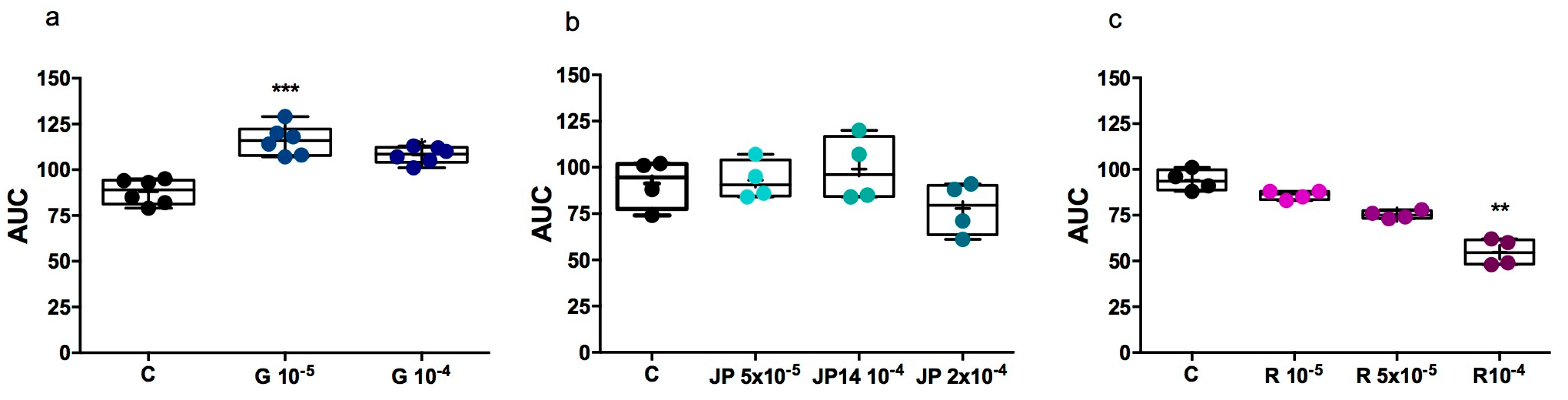
2.2.4. Influence of JP-14 on Lipid Utilization in Zebrafish Model
2.2.5. Influence of JP-14 on Fructose-Induction Lipid Disturbance in Zebrafish Model
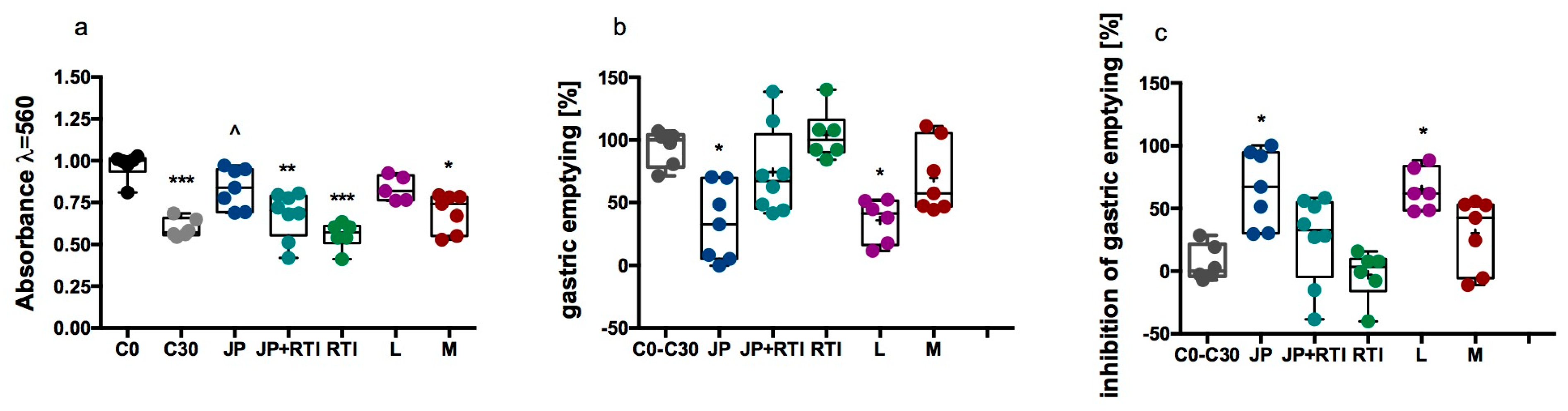
2.2.6. Influence of JP-14 on Gastric Emptying (Mouse Model)
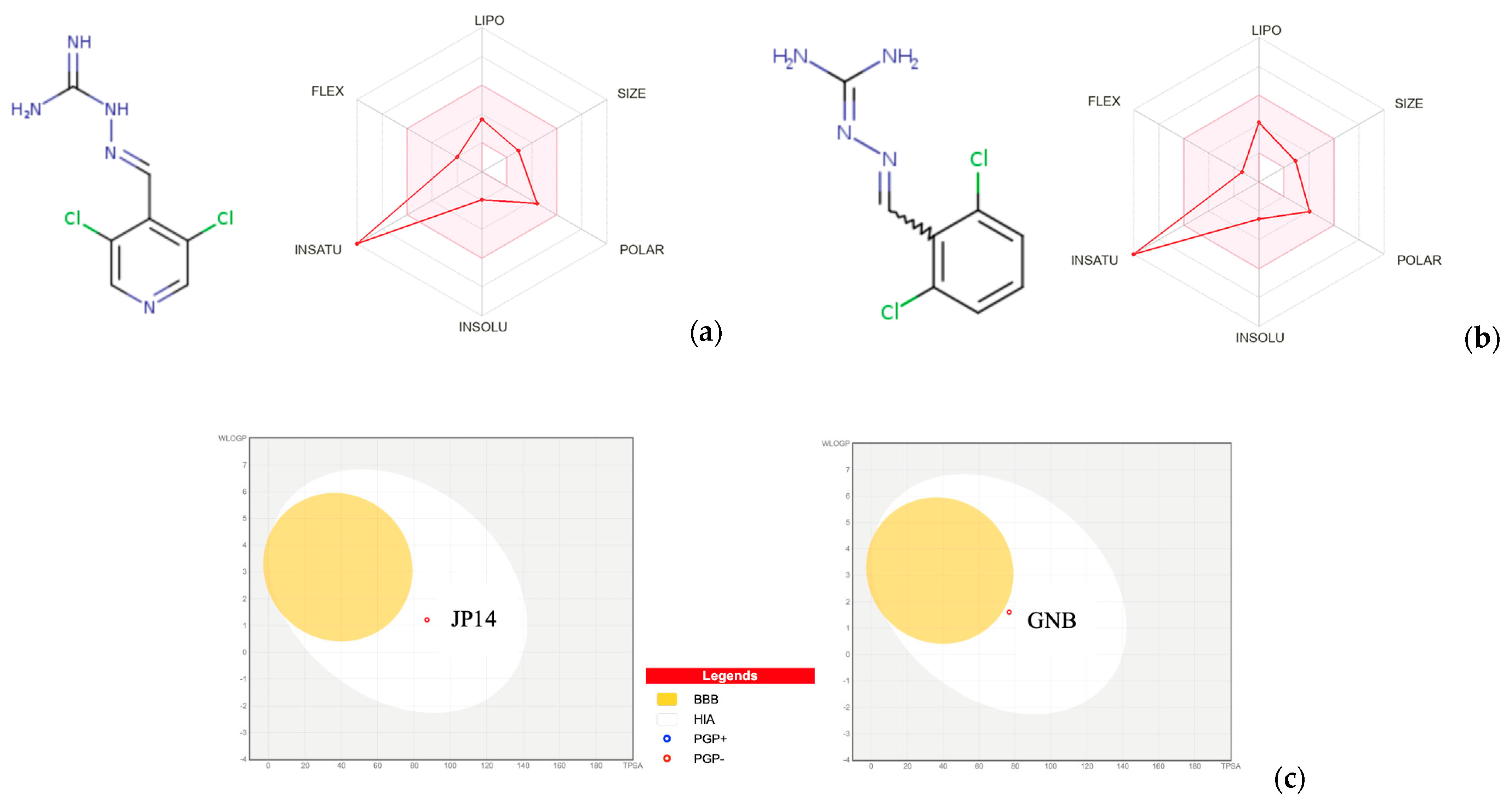
2.3. Chemical, Pharmacokinetic, and Drug-Likeness Properties
2.4. Toxicity Studies
2.4.1. Predicting the Most Frequently Assessed Toxicity Potentials with In Silico Models
2.4.2. Preliminary Cytotoxicity Assessment of Test Compounds
2.4.3. Influence of JP-14 on the Live Organism in the Danio rerio Model, Preliminary Toxicity Studies
2.4.4. Influence of JP-14 on Platelet Aggregation
3. Materials and Methods
3.1. Molecular Modeling
3.2. Chemistry
3.2.1. Chemical and Analytical Methods
3.2.2. Synthetic Procedures
(E)-2-((3,5-Dichloropyridin-4-yl)Methylene)Hydrazine-1-Carboximidamide (JP-14; 10)
3.3. In Vitro Assays
3.3.1. Cell Culture
T3-L1 Adipocytes
HepG2 Hepatocytes
Compound Preparation and Treatment
3.3.2. Cytotoxicity and Viability Assays
Membrane Integrity Assay
Cell Viability Assay
3.3.3. Lipid Accumulation and Glucose Consumption
AdipoRed Assay
Glucose Consumption (Amplex Red Assay)
High Content Analysis of Lipid Accumulation
3.3.4. Receptor-Binding Assay
3.3.5. Intrinsic Activity Assay
3.3.6. In Vitro Platelet Aggregation Tests
3.4. In Vivo Assays
3.4.1. Inhibition of Gastric Emptying (Mouse Model)
3.4.2. In Vivo Toxicity Assay
3.4.3. Nile Red Fluorescence Fat Metabolism Assay
3.4.4. Measurement of the Amount of Neutral Lipids in Zebrafish Larvae After Induction Metabolic Disorders with Fructose
3.5. In Silico Prediction of Chemical, Pharmacokinetic, and Drug-Likeness
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borowsky, B.; Adham, N.; Jones, K.A.; Raddatz, R.; Artymyshyn, R.; Ogozalek, K.L.; Durkin, M.M.; Lakhlani, P.P.; Bonini, J.A.; Pathirana, S.; et al. Trace Amines: Identification of a Family of Mammalian G Protein-Coupled Receptors. Proc. Natl. Acad. Sci. USA 2001, 98, 8966–8971. [Google Scholar] [CrossRef]
- Dedic, N.; Wang, L.; Hajos-Korcsok, E.; Hecksher-Sørensen, J.; Roostalu, U.; Vickers, S.P.; Wu, S.; Anacker, C.; Synan, C.; Jones, P.G.; et al. TAAR1 Agonists Improve Glycemic Control, Reduce Body Weight and Modulate Neurocircuits Governing Energy Balance and Feeding. Mol. Metab. 2024, 80, 101883. [Google Scholar] [CrossRef] [PubMed]
- Vaganova, A.N.; Shemyakova, T.S.; Lenskaia, K.V.; Rodionov, R.N.; Steenblock, C.; Gainetdinov, R.R. Trace Amine-Associated Receptors and Monoamine-Mediated Regulation of Insulin Secretion in Pancreatic Islets. Biomolecules 2023, 13, 1618. [Google Scholar] [CrossRef]
- Mühlhaus, J.; Dinter, J.; Jyrch, S.; Teumer, A.; Jacobi, S.F.; Homuth, G.; Kühnen, P.; Wiegand, S.; Grüters, A.; Völzke, H.; et al. Investigation of Naturally Occurring Single-Nucleotide Variants in Human TAAR1. Front. Pharmacol. 2017, 8, 807. [Google Scholar] [CrossRef]
- Dedic, N.; Jones, P.G.; Hajos-Korcsok, E.; Synan, C.; Wu, S.; Anacker, C.; Vickers, S.P.; Hecksher-Sørensen, J.; Zeni, C.; Milanovic, S.; et al. TAAR1 Agonist Ulotaront Improves Glycemic Control and Reduces Body Weight in Rodent Models of Diabetes, Obesity, and Iatrogenic Weight Gain. CNS Spectr. 2023, 28, 259. [Google Scholar] [CrossRef]
- Hu, L.A.; Zhou, T.; Ahn, J.; Wang, S.; Zhou, J.; Hu, Y.; Liu, Q. Human and Mouse Trace Amine-Associated Receptor 1 Have Distinct Pharmacology towards Endogenous Monoamines and Imidazoline Receptor Ligands. Biochem. J. 2009, 424, 39–45. [Google Scholar] [CrossRef]
- Kotańska, M.; Marcinkowska, M.; Kuder, K.J.; Walczak, M.; Bednarski, M.; Siwek, A.; Kołaczkowski, M. Metabolic and Cardiovascular Benefits and Risks of 4-Hydroxy Guanabenz Hydrochloride: A2-Adrenoceptor and Trace Amine-Associated Receptor 1 Ligand. Pharmacol. Rep. 2023, 75, 1211–1229. [Google Scholar] [CrossRef] [PubMed]
- Glyakina, A.V.; Pavlov, C.D.; Sopova, J.V.; Gainetdinov, R.R.; Leonova, E.I.; Galzitskaya, O.V. Search for Structural Basis of Interactions of Biogenic Amines with Human TAAR1 and TAAR6 Receptors. Int. J. Mol. Sci. 2021, 23, 209. [Google Scholar] [CrossRef]
- Zagórska, A.; Marcinkowska, M.; Śniecikowska, J.; Bucki, A.; Siwek, A.; Kubacka, M.; Kazek, G.; Sapa, J.; Kotańska, M.; Kołaczkowski, M. Evaluation of Antiplatelet Activity of Novel Guanidine Derivatives in the Aspects of Their Adrenergic Receptor Activity. Acta Pol. Pharm. Drug Res. 2018, 75, 525–531. [Google Scholar]
- Marcinkowska, M.; Kotańska, M.; Zagórska, A.; Śniecikowska, J.; Kubacka, M.; Siwek, A.; Bucki, A.; Pawłowski, M.; Bednarski, M.; Sapa, J.; et al. Synthesis and Biological Evaluation of N-Arylpiperazine Derivatives of 4,4-Dimethylisoquinoline-1,3(2H,4H)-Dione as Potential Antiplatelet Agents. J. Enzym. Inhib. Med. Chem. 2018, 33, 536–545. [Google Scholar] [CrossRef]
- Tonelli, M.; Espinoza, S.; Gainetdinov, R.R.; Cichero, E. Novel Biguanide-Based Derivatives Scouted as TAAR1 Agonists: Synthesis, Biological Evaluation, ADME Prediction and Molecular Docking Studies. Eur. J. Med. Chem. 2017, 127, 781–792. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Q. TAAR Agonists. Cell. Mol. Neurobiol. 2020, 40, 257–272. [Google Scholar] [CrossRef]
- Tan, E.S.; Naylor, J.C.; Groban, E.S.; Bunzow, J.R.; Jacobson, M.P.; Grandy, D.K.; Scanlan, T.S. The Molecular Basis of Species-Specific Ligand Activation of Trace Amine-Associated Receptor 1 (TAAR1). ACS Chem. Biol. 2009, 4, 209–220. [Google Scholar] [CrossRef]
- Simmler, L.D.; Rickli, A.; Schramm, Y.; Hoener, M.C.; Liechti, M.E. Pharmacological Profiles of Aminoindanes, Piperazines, and Pipradrol Derivatives. Biochem. Pharmacol. 2014, 88, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Voigt, J.-P.; Fink, H. Serotonin Controlling Feeding and Satiety. Behav. Brain Res. 2015, 277, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Adefurin, A.; Vanderbilt, C.; Okafor, C.; Kawai, V.; Li, C.; Shah, A.; Wei, W.-Q.; Kurnik, D.; Stein, C.M. Alpha2A Adrenergic Receptor Genetic Variation Contributes to Hyperglycemia after Myocardial Infarction. Int. J. Cardiol. 2016, 215, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.H.; Braun, M.; Mahdi, T.; Andersson, S.A.; Travers, M.E.; Shigeto, M.; Zhang, E.; Almgren, P.; Ladenvall, C.; Axelsson, A.S.; et al. Reduced Insulin Exocytosis in Human Pancreatic β-Cells with Gene Variants Linked to Type 2 Diabetes. Diabetes 2012, 61, 1726–1733. [Google Scholar] [CrossRef] [PubMed]
- Kotańska, M.; Knutelska, J.; Nicosia, N.; Mika, K.; Szafarz, M. Guanabenz—An Old Drug with a Potential to Decrease Obesity. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 963–974. [Google Scholar] [CrossRef]
- Yoshino, S.; Iwasaki, Y.; Matsumoto, S.; Satoh, T.; Ozawa, A.; Yamada, E.; Kakizaki, S.; Trejo, J.A.O.; Uchiyama, Y.; Yamada, M.; et al. Administration of Small-Molecule Guanabenz Acetate Attenuates Fatty Liver and Hyperglycemia Associated with Obesity. Sci. Rep. 2020, 10, 13671. [Google Scholar] [CrossRef]
- Ye, H.; Charpin-El Hamri, G.; Zwicky, K.; Christen, M.; Folcher, M.; Fussenegger, M. Pharmaceutically Controlled Designer Circuit for the Treatment of the Metabolic Syndrome. Proc. Natl. Acad. Sci. USA 2013, 110, 141–146. [Google Scholar] [CrossRef]
- Rutigliano, G.; Bandini, L.; Sestito, S.; Chiellini, G. 3-Iodothyronamine and Derivatives: New Allies Against Metabolic Syndrome? Int. J. Mol. Sci. 2020, 21, 2005. [Google Scholar] [CrossRef]
- Braulke, L.J.; Klingenspor, M.; DeBarber, A.; Tobias, S.C.; Grandy, D.K.; Scanlan, T.S.; Heldmaier, G. 3-Iodothyronamine: A Novel Hormone Controlling the Balance Between Glucose and Lipid Utilisation. J. Comp. Physiol. B 2008, 178, 167–177. [Google Scholar] [CrossRef]
- Haviland, J.A.; Reiland, H.; Butz, D.E.; Tonelli, M.; Porter, W.P.; Zucchi, R.; Scanlan, T.S.; Chiellini, G.; Assadi-Porter, F.M. NMR-based Metabolomics and Breath Studies Show Lipid and Protein Catabolism During Low Dose Chronic T1AM Treatment. Obesity 2013, 21, 2538–2544. [Google Scholar] [CrossRef] [PubMed]
- Assadi-Porter, F.M.; Reiland, H.; Sabatini, M.; Lorenzini, L.; Carnicelli, V.; Rogowski, M.; Selen Alpergin, E.S.; Tonelli, M.; Ghelardoni, S.; Saba, A.; et al. Metabolic Reprogramming by 3-Iodothyronamine (T1AM): A New Perspective to Reverse Obesity through Co-Regulation of Sirtuin 4 and 6 Expression. Int. J. Mol. Sci. 2018, 19, 1535. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V. Lipid Droplets and Liver Disease: From Basic Biology to Clinical Implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Breiden, B.; Sandhoff, K. Emerging Mechanisms of Drug-Induced Phospholipidosis. Biol. Chem. 2019, 401, 31–46, Erratum in Biol. Chem. 2022, 403, 251–251. [Google Scholar] [CrossRef]
- Buratta, S.; Urbanelli, L.; Ferrara, G.; Sagini, K.; Goracci, L.; Emiliani, C. A Role for the Autophagy Regulator Transcription Factor EB in Amiodarone-Induced Phospholipidosis. Biochem. Pharmacol. 2015, 95, 201–209. [Google Scholar] [CrossRef]
- Wiegand, M.D. Utilization of Yolk Fatty Acids by Goldfish Embryos and Larvae. Fish Physiol. Biochem. 1996, 15, 21–27. [Google Scholar] [CrossRef]
- Jones, K.S.; Alimov, A.P.; Rilo, H.L.; Jandacek, R.J.; Woollett, L.A.; Penberthy, W.T. A High Throughput Live Transparent Animal Bioassay to Identify Non-Toxic Small Molecules or Genes That Regulate Vertebrate Fat Metabolism for Obesity Drug Development. Nutr. Metab. 2008, 5, 23. [Google Scholar] [CrossRef]
- Regueiras, A.; Huguet, Á.; Conde, T.; Couto, D.; Domingues, P.; Domingues, M.R.; Costa, A.M.; Silva, J.L.D.; Vasconcelos, V.; Urbatzka, R. Potential Anti-Obesity, Anti-Steatosis, and Anti-Inflammatory Properties of Extracts from the Microalgae Chlorella Vulgaris and Chlorococcum Amblystomatis Under Different Growth Conditions. Mar. Drugs 2021, 20, 9. [Google Scholar] [CrossRef]
- He, L.; Babar, G.S.; Redel, J.M.; Young, S.L.; Chagas, C.E.; Moore, W.V.; Yan, Y. Fructose Intake: Metabolism and Role in Diseases. In Sugar Intake—Risks and Benefits and the Global Diabetes Epidemic; James Martins, I., Ed.; IntechOpen: London, UK, 2021; ISBN 978-1-83881-121-1. [Google Scholar]
- Ferder, L.; Ferder, M.D.; Inserra, F. The Role of High-Fructose Corn Syrup in Metabolic Syndrome and Hypertension. Curr. Hypertens. Rep. 2010, 12, 105–112. [Google Scholar] [CrossRef]
- Basaranoglu, M.; Basaranoglu, G.; Bugianesi, E. Carbohydrate Intake and Nonalcoholic Fatty Liver Disease: Fructose as a Weapon of Mass Destruction. Hepatobiliary Surg. Nutr. 2015, 4, 109–116. [Google Scholar] [CrossRef]
- Sapp, V.; Gaffney, L.; EauClaire, S.F.; Matthews, R.P. Fructose Leads to Hepatic Steatosis in Zebrafish That Is Reversed by Mechanistic Target of Rapamycin (mTOR) Inhibition. Hepatology 2014, 60, 1581–1592. [Google Scholar] [CrossRef]
- He, J.-H.; Guo, S.-Y.; Zhu, F.; Zhu, J.-J.; Chen, Y.-X.; Huang, C.-J.; Gao, J.-M.; Dong, Q.-X.; Xuan, Y.-X.; Li, C.-Q. A Zebrafish Phenotypic Assay for Assessing Drug-Induced Hepatotoxicity. J. Pharmacol. Toxicol. Methods 2013, 67, 25–32. [Google Scholar] [CrossRef]
- Zhou, C.; Lai, Y.; Huang, P.; Xie, L.; Lin, H.; Zhou, Z.; Mo, C.; Deng, G.; Yan, W.; Gao, Z.; et al. Naringin Attenuates Alcoholic Liver Injury by Reducing Lipid Accumulation and Oxidative Stress. Life Sci. 2019, 216, 305–312. [Google Scholar] [CrossRef]
- Janssen, P.; Vanden Berghe, P.; Verschueren, S.; Lehmann, A.; Depoortere, I.; Tack, J. Review Article: The Role of Gastric Motility in the Control of Food Intake: Review: Regulation of Food Intake by Gastric Motility. Aliment. Pharmacol. Ther. 2011, 33, 880–894. [Google Scholar] [CrossRef]
- Vaganova, A.N.; Zhukov, I.S.; Shemiakova, T.S.; Rozhkov, K.A.; Alferova, L.S.; Karaseva, A.B.; Ermolenko, E.I.; Gainetdinov, R.R. Functional Analysis of TAAR1 Expression in the Intestine Wall and the Effect of Its Gene Knockout on the Gut Microbiota in Mice. Int. J. Mol. Sci. 2024, 25, 13216. [Google Scholar] [CrossRef]
- Raab, S.; Wang, H.; Uhles, S.; Cole, N.; Alvarez-Sanchez, R.; Künnecke, B.; Ullmer, C.; Matile, H.; Bedoucha, M.; Norcross, R.D.; et al. Incretin-like Effects of Small Molecule Trace Amine-Associated Receptor 1 Agonists. Mol. Metab. 2016, 5, 47–56. [Google Scholar] [CrossRef]
- Halawi, H.; Khemani, D.; Eckert, D.; O’Neill, J.; Kadouh, H.; Grothe, K.; Clark, M.M.; Burton, D.D.; Vella, A.; Acosta, A.; et al. Effects of Liraglutide on Weight, Satiation, and Gastric Functions in Obesity: A Randomised, Placebo-Controlled Pilot Trial. Lancet Gastroenterol. Hepatol. 2017, 2, 890–899. [Google Scholar] [CrossRef]
- Maselli, D.; Atieh, J.; Clark, M.M.; Eckert, D.; Taylor, A.; Carlson, P.; Burton, D.D.; Busciglio, I.; Harmsen, W.S.; Vella, A.; et al. Effects of Liraglutide on Gastrointestinal Functions and Weight in Obesity: A Randomized Clinical and Pharmacogenomic Trial. Obesity 2022, 30, 1608–1620. [Google Scholar] [CrossRef]
- Milanović, S.; Dedic, N.; Lew, R.; Burton, D.; Koblan, K.S.; Camilleri, M.; Hopkins, S.C. TAAR1 Agonist Ulotaront Delays Gastric Emptying of Solids in Patients with Schizophrenia and Concurrent Metabolic Syndrome with Prediabetes. Diabetes Obes. Metab. 2024, 26, 2466–2475. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A Simple, Robust, and Efficient Description of n-Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef]
- Wildman, S.A.; Crippen, G.M. Prediction of Physicochemical Parameters by Atomic Contributions. J. Chem. Inf. Comput. Sci. 1999, 39, 868–873. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. In Silico Prediction of Aqueous Solubility Using Simple QSPR Models: The Importance of Phenol and Phenol-like Moieties. J. Chem. Inf. Model. 2012, 52, 2950–2957. [Google Scholar] [CrossRef]
- Potts, R.O.; Guy, R.H. Predicting Skin Permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Martin, Y.C. A Bioavailability Score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef]
- Huang, S.; Strong, J.M.; Zhang, L.; Reynolds, K.S.; Nallani, S.; Temple, R.; Abraham, S.; Habet, S.A.; Baweja, R.K.; Burckart, G.J.; et al. New Era in Drug Interaction Evaluation: US Food and Drug Administration Update on CYP Enzymes, Transporters, and the Guidance Process. J. Clin. Pharmacol. 2008, 48, 662–670. [Google Scholar] [CrossRef]
- Sharom, F.J. The P-Glycoprotein Multidrug Transporter. Essays Biochem. 2011, 50, 161–178. [Google Scholar] [CrossRef]
- Offermanns, S. Activation of Platelet Function Through G Protein–Coupled Receptors. Circ. Res. 2006, 99, 1293–1304. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Hoener, M.C.; Berry, M.D. Trace Amines and Their Receptors. Pharmacol. Rev. 2018, 70, 549–620. [Google Scholar] [CrossRef]
- Koltai, K.; Kesmarky, G.; Feher, G.; Tibold, A.; Toth, K. Platelet Aggregometry Testing: Molecular Mechanisms, Techniques and Clinical Implications. Int. J. Mol. Sci. 2017, 18, 1803. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, R.; Priora, R.; Alessandrello Liotta, A.; Abbate, R. Platelet Function Tests: A Comparative Review. VHRM 2015, 2015, 133–148. [Google Scholar] [CrossRef]
- Mangin, P.H.; Neeves, K.B.; Lam, W.A.; Cosemans, J.M.E.M.; Korin, N.; Kerrigan, S.W.; Panteleev, M.A. In Vitro Flow-based Assay: From Simple toward More Sophisticated Models for Mimicking Hemostasis and Thrombosis. J. Thromb. Haemost. 2021, 19, 582–587. [Google Scholar] [CrossRef]
- Borst, O.; Gawaz, M. Glycoprotein VI—Novel Target in Antiplatelet Medication. Pharmacol. Ther. 2021, 217, 107630. [Google Scholar] [CrossRef] [PubMed]
- Stegner, D.; Nieswandt, B. Platelet Receptor Signaling in Thrombus Formation. J. Mol. Med. 2011, 89, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Belcher, P.R.; Drake-Holland, A.J.; Noble, M.I.M. The Antiplatelet Drug Target in Atherosclerotic Diseases. Cardiovasc. Hematol. Disord. Drug Targets 2006, 6, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P. The Growing Complexity of Platelet Aggregation. Blood 2007, 109, 5087–5095. [Google Scholar] [CrossRef]
- Fälker, K.; Ljungberg, L.U.; Kardeby, C.; Lindkvist, M.; Sirsjö, A.; Grenegård, M. Adrenoceptor α2A Signalling Countervails the Taming Effects of Synchronous Cyclic Nucleotide-Elevation on Thrombin-Induced Human Platelet Activation and Aggregation. Cell. Signal. 2019, 59, 96–109. [Google Scholar] [CrossRef]
- Keularts, I.M.; van Gorp, R.M.; Feijge, M.A.; Vuist, W.M.; Heemskerk, J.W. Alpha(2A)-Adrenergic Receptor Stimulation Potentiates Calcium Release in Platelets by Modulating cAMP Levels. J. Biol. Chem. 2000, 275, 1763–1772. [Google Scholar] [CrossRef]
- Revel, F.G.; Moreau, J.-L.; Pouzet, B.; Mory, R.; Bradaia, A.; Buchy, D.; Metzler, V.; Chaboz, S.; Groebke Zbinden, K.; Galley, G.; et al. A New Perspective for Schizophrenia: TAAR1 Agonists Reveal Antipsychotic- and Antidepressant-like Activity, Improve Cognition and Control Body Weight. Mol. Psychiatry 2013, 18, 543–556. [Google Scholar] [CrossRef]
- Dinter, J.; Mühlhaus, J.; Wienchol, C.L.; Yi, C.-X.; Nürnberg, D.; Morin, S.; Grüters, A.; Köhrle, J.; Schöneberg, T.; Tschöp, M.; et al. Inverse Agonistic Action of 3-Iodothyronamine at the Human Trace Amine-Associated Receptor 5. PLoS ONE 2015, 10, e0117774. [Google Scholar] [CrossRef]
- Schrödinger Release 2025-1: LigPrep; Schrödinger LLC: New York, NY, USA, 2025.
- Schrödinger Release 2025-1: Protein Preparation Workflow; Epik Schrödinger LLC: New York, NY, USA, 2025.
- Schrödinger Release 2025-1: Glide; Schrödinger LLC: New York, NY, USA, 2025.
- Schrödinger Release 2025-1: Jaguar; Schrödinger LLC: New York, NY, USA, 2025.
- Kubacka, M.; Kazek, G.; Kotańska, M.; Filipek, B.; Waszkielewicz, A.M.; Mogilski, S. Anti-Aggregation Effect of Aroxyalkyl Derivatives of 2-Methoxyphenylpiperazine Is Due to Their 5-HT2A and A2-Adrenoceptor Antagonistic Properties. A Comparison with Ketanserin, Sarpogrelate, Prazosin, Yohimbine and ARC239. Eur. J. Pharmacol. 2018, 818, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, K.; Ohta, M.; Kanai, S.; Yoshida, Y.; Sato, N.; Nagata, A.; Matsui, T.; Noda, T.; Jimi, A.; Takiguchi, S.; et al. Enhanced Gastric Emptying of a Liquid Gastric Load in Mice Lacking Cholecystokinin-B Receptor: A Study of CCK-A,B, and AB Receptor Gene Knockout Mice. J. Gastroenterol. 2004, 39, 319–323. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]


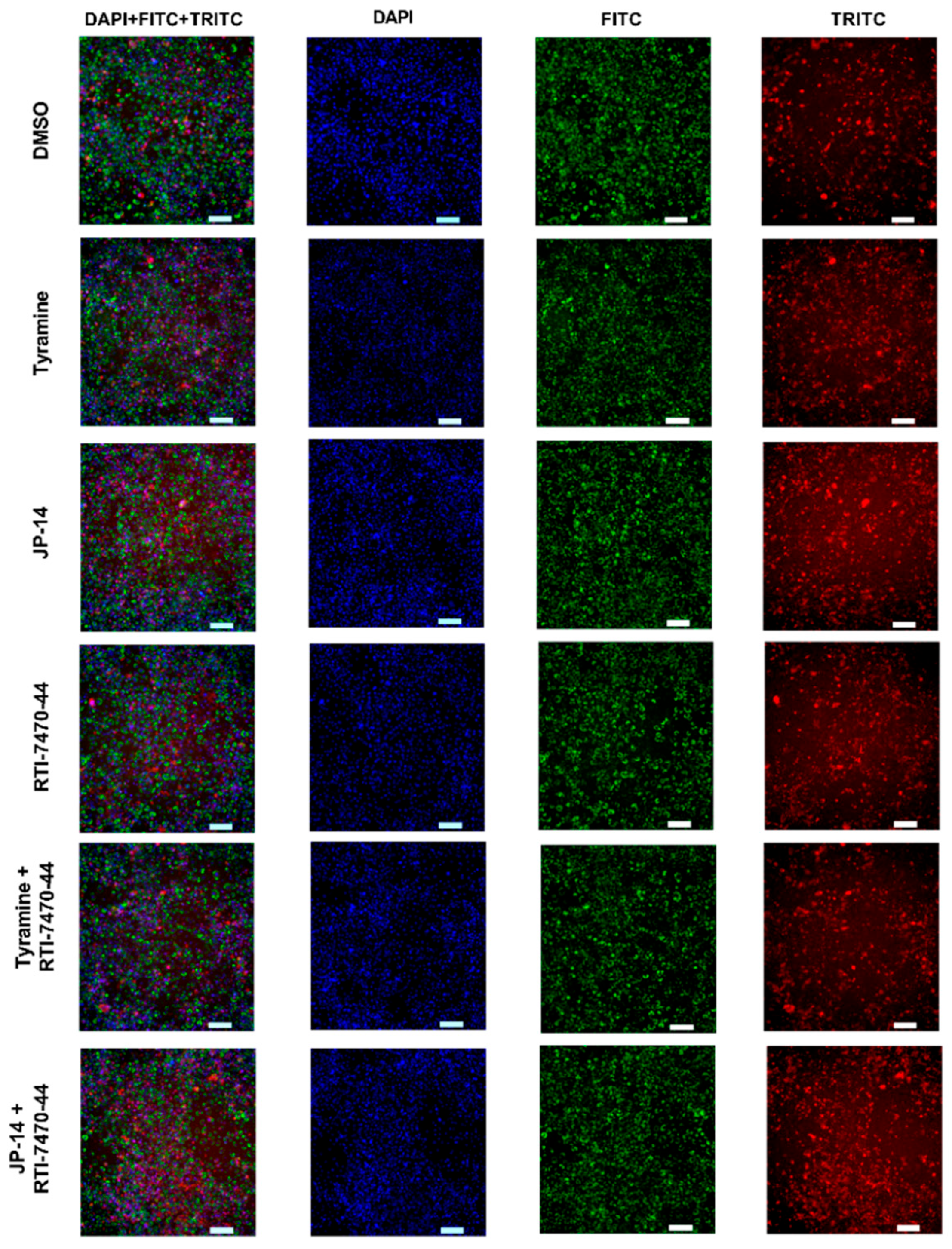
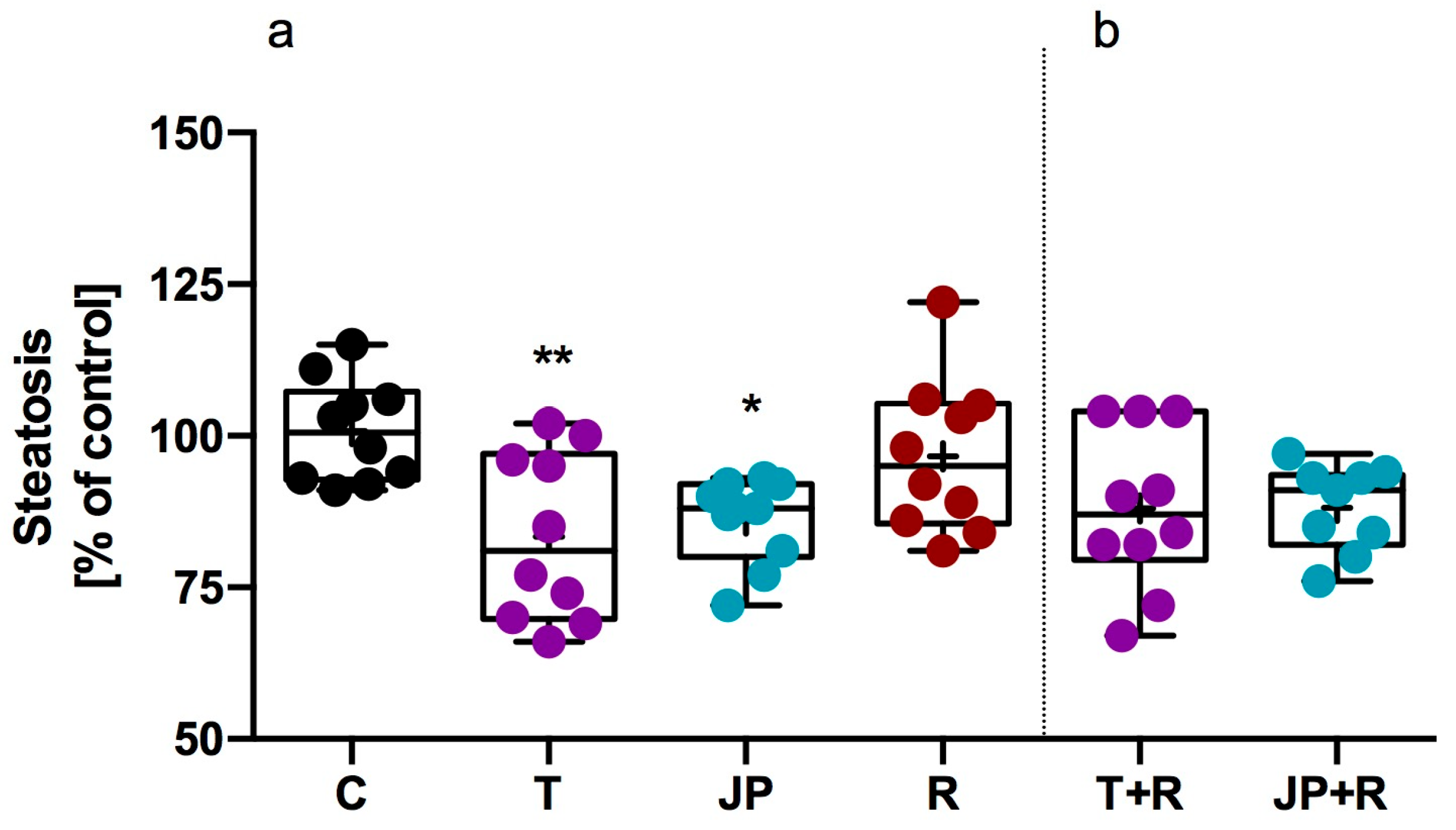
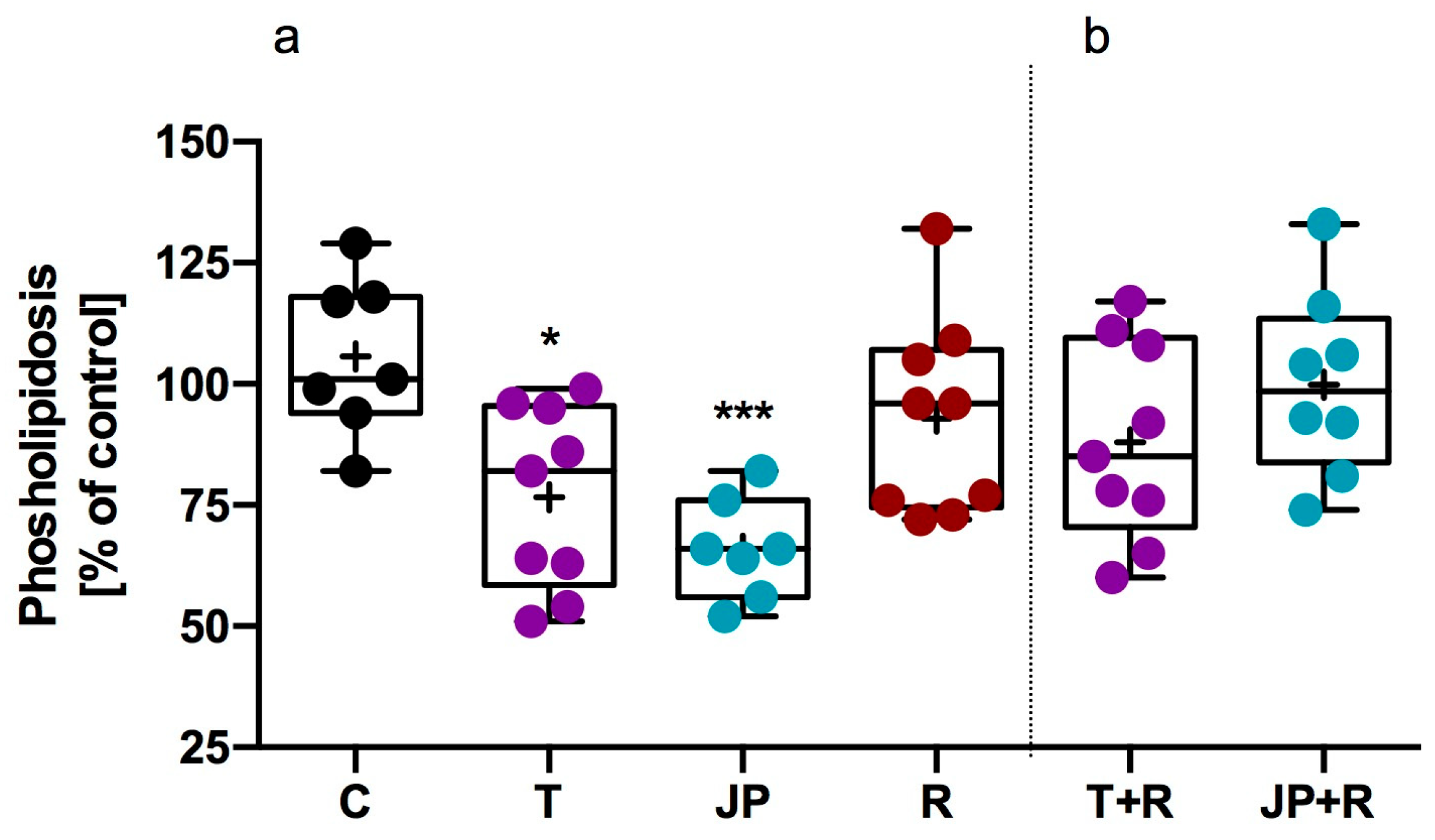
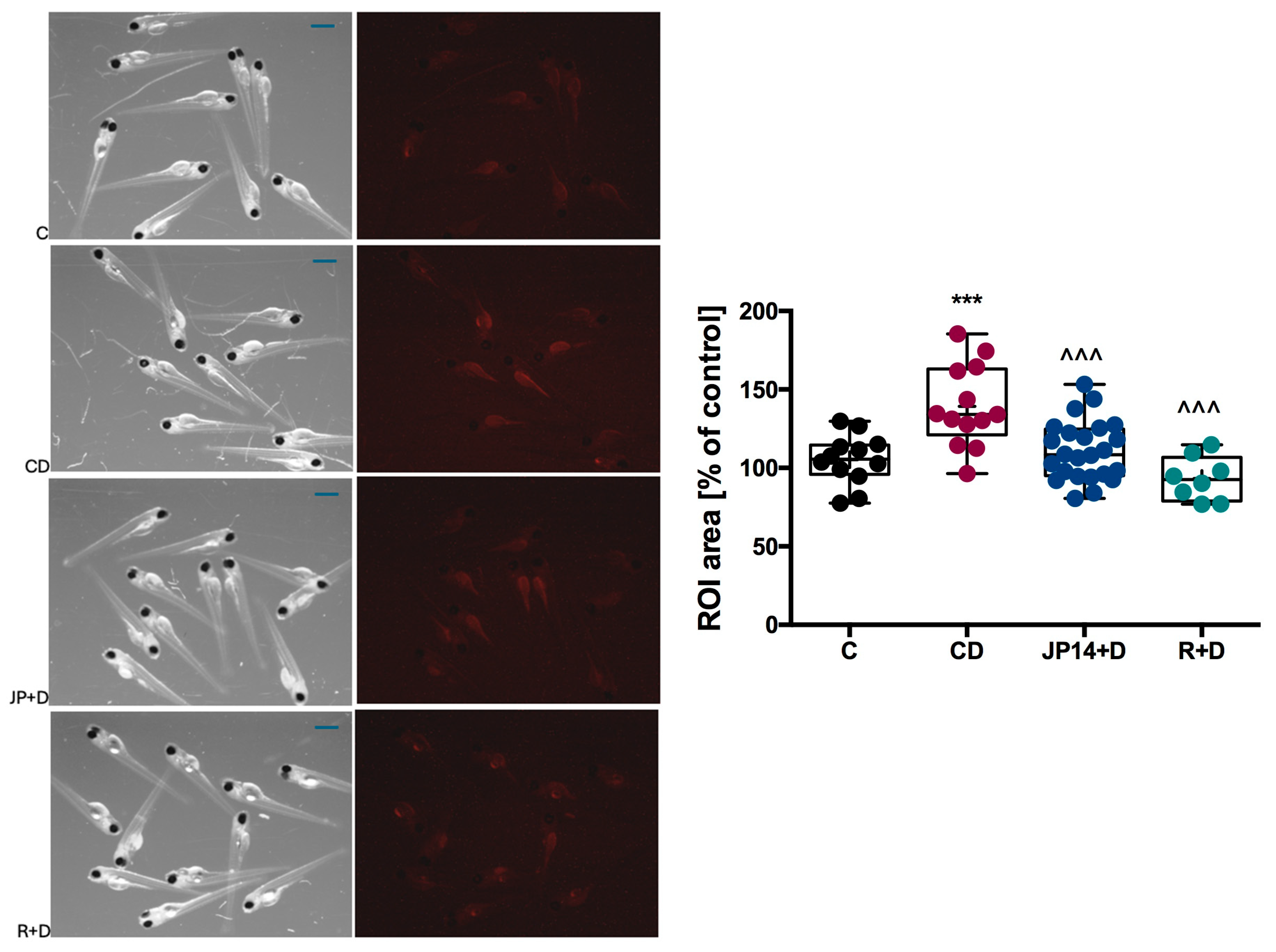


| Compd | TAAR1 Ago Emax/EC50 [µM] | TAAR1 Ant EC50 [nM] | 5-HT2C Ago EC50 [nM] | 5-HT2C Ant EC50 [nM] | α1 Ki [nM] | α2 Ki [nm.] | α2A Ago Emax/EC50 [nM] | α2B Ant IC50 [nM] |
|---|---|---|---|---|---|---|---|---|
| 10 (JP-14) | 11.29 ± 1.4 | n.a. | n.a. | n.a. | n.a. | 126.0 ± 27.0 | 15% | 622 |
| GNB |
60%/
0.301 ± 0.04 | n.a. | n.a. | n.a. | n.t. | 2.6 ± 0.6 | 16.32 | n.a. |
| 4-OH GNB |
34%/
(10−4M) a | n.t. | n.t. | n.t. | n.t. | 19.4 |
316.3
Ago |
330.2
Ant |
| TYR | 5.87 ± 0.47 | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. |
| RTI | n.a. | 167.6 ± 50.7 | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. |
| 5-HT | n.a. | n.a. | 0.8 ± 0.1 | n.t. | n.t. | n.t. | n.a. | n.t. |
| MSE | n.a. | n.a. | n.t. | 1.1 ± 0.1 | n.t. | n.t. | n.a. | n.t. |
| PHT | n.t. | n.t. | n.t. | n.t. | 10.9 ± 0.8 | n.t. | n.t. | n.t. |
| CLO | n.t. | n.t. | n.t. | n.t. | n.t. | 3.1 ± 0.4 | n.t. | n.t. |
| BRIM | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | 7.25 | n.a. |
| YOH | n.t. | n.t. | n.t. | n.t. | n.t. | n.t. | n.a. | 5.43 ± 1.8 |
| Properties | JP-14 | Guanabenz | |
|---|---|---|---|
| Molecular weight | 232.07 g/mol | 231.08 g/mol | |
| Number heavy atoms | 14 | 14 | |
| Number aromatic heavy atoms | 6 | 6 | |
| Fraction Csp3 | 0.00 | 0.00 | |
| Number H-bond acceptors | 3 | 2 | |
| Number H-bond donors | 3 | 2 | |
| Number rotatable bonds | 3 | 2 | |
| Molar refractivity | 56.81 | 59.02 | |
| TPSA | 87.15 Å2 | 76.76 Å2 | |
| Lipophilicity | Log Po/w (iLOGP) | 0.65 | 1.57 |
| Log Po/w (WLOGP) | 1.21 | 1.60 | |
| Log Po/w (MLOGP) | 0.70 | 2.22 | |
| Log Po/w (XLOGP3) | 0.88 | 1.73 | |
| Water Solubility | Log S (ESOL) | −1.95 | −2.55 |
| Solubility | 2.59 × 100 mg/mL 1.12 × 10−2 mol/L | 6.55 × 10−1 mg/mL 2.83 × 10−3 mol/L | |
| Class | very soluble | soluble | |
| Log S (Ali) | −2.29 | −2.96 | |
| Solubility | 1.18 × 100 mg/mL 5.08 × 10−3 mol/L | 2.54 × 10−1 mg/mL 1.10 × 10−3 mol/L | |
| Class | Soluble | soluble | |
| Pharmacokinetics | GI absorption | High | High |
| Intestinal absorption (human) | 69.295% | 88.791% | |
| BBB permeant | No | No | |
| P-gp substrate | No | No | |
| Log Kp | −7.09 cm/s | −6.48 cm/s | |
| Bioavailability score | 0.55 | 0.55 | |
| Volume of distribution at steady state | 0.420 log L/kg | 0.928 log L/kg | |
| Fraction unbound (human) | 0.687 Fu | 0.416 Fu | |
| Inhibitor: CYP1A2, CYP2C19, CYP2C9, CYP2D6, CYP3A4 | No No | Yes No | |
| Total clearance | 0.376 log ml/min/kg | 0.209 log ml/min/kg | |
| Lipinski filter | Yes, 0 violation | Yes, 0 violation | |
| Synthetic accessibility score | 2.55 | 2.30 | |
| Toxicity Prediction | JP-14 | Guanabenz | Unit |
|---|---|---|---|
| Max. tolerated dose (human) | 0.625 | 0.357 | Numeric (log mg/kg/day) |
| Oral Rat Acute Toxicity (LD50) | 2.968 | 2.801 | Numeric (mol/kg) |
| Oral Rat Chronic Toxicity (LOAEL) | 1.525 | 2.498 | Numeric (log mg/kg b.w./day) |
| Hepatotoxicity | No | No | Categorical (Yes/No) |
| Skin Sensitisation | Yes | Yes | Categorical (Yes/No) |
| hERG I inhibitor | No | No | Categorical (Yes/No) |
| hERG II inhibitor | No | No | Categorical (Yes/No) |
| AMES toxicity | Yes | Yes | Categorical (Yes/No) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcinkowska, M.; Sniecikowska, J.; Głuch-Lutwin, M.; Mordyl, B.; Bednarski, M.; Bucki, A.; Sapa, M.; Kubacka, M.; Siwek, A.; Zagórska, A.; et al. JP-14: A Trace Amine-Associated Receptor 1 Agonist with Anti-Metabolic Disorder Potential. Int. J. Mol. Sci. 2025, 26, 10033. https://doi.org/10.3390/ijms262010033
Marcinkowska M, Sniecikowska J, Głuch-Lutwin M, Mordyl B, Bednarski M, Bucki A, Sapa M, Kubacka M, Siwek A, Zagórska A, et al. JP-14: A Trace Amine-Associated Receptor 1 Agonist with Anti-Metabolic Disorder Potential. International Journal of Molecular Sciences. 2025; 26(20):10033. https://doi.org/10.3390/ijms262010033
Chicago/Turabian StyleMarcinkowska, Monika, Joanna Sniecikowska, Monika Głuch-Lutwin, Barbara Mordyl, Marek Bednarski, Adam Bucki, Michał Sapa, Monika Kubacka, Agata Siwek, Agnieszka Zagórska, and et al. 2025. "JP-14: A Trace Amine-Associated Receptor 1 Agonist with Anti-Metabolic Disorder Potential" International Journal of Molecular Sciences 26, no. 20: 10033. https://doi.org/10.3390/ijms262010033
APA StyleMarcinkowska, M., Sniecikowska, J., Głuch-Lutwin, M., Mordyl, B., Bednarski, M., Bucki, A., Sapa, M., Kubacka, M., Siwek, A., Zagórska, A., Sapa, J., Kołaczkowski, M., & Kotańska, M. (2025). JP-14: A Trace Amine-Associated Receptor 1 Agonist with Anti-Metabolic Disorder Potential. International Journal of Molecular Sciences, 26(20), 10033. https://doi.org/10.3390/ijms262010033









