The Consequences of DNA Damage in the Early Embryo Are Important for Practical Procedures in Assisted Reproduction
Abstract
1. Introduction
2. First Mitosis and Cell Cycle Control
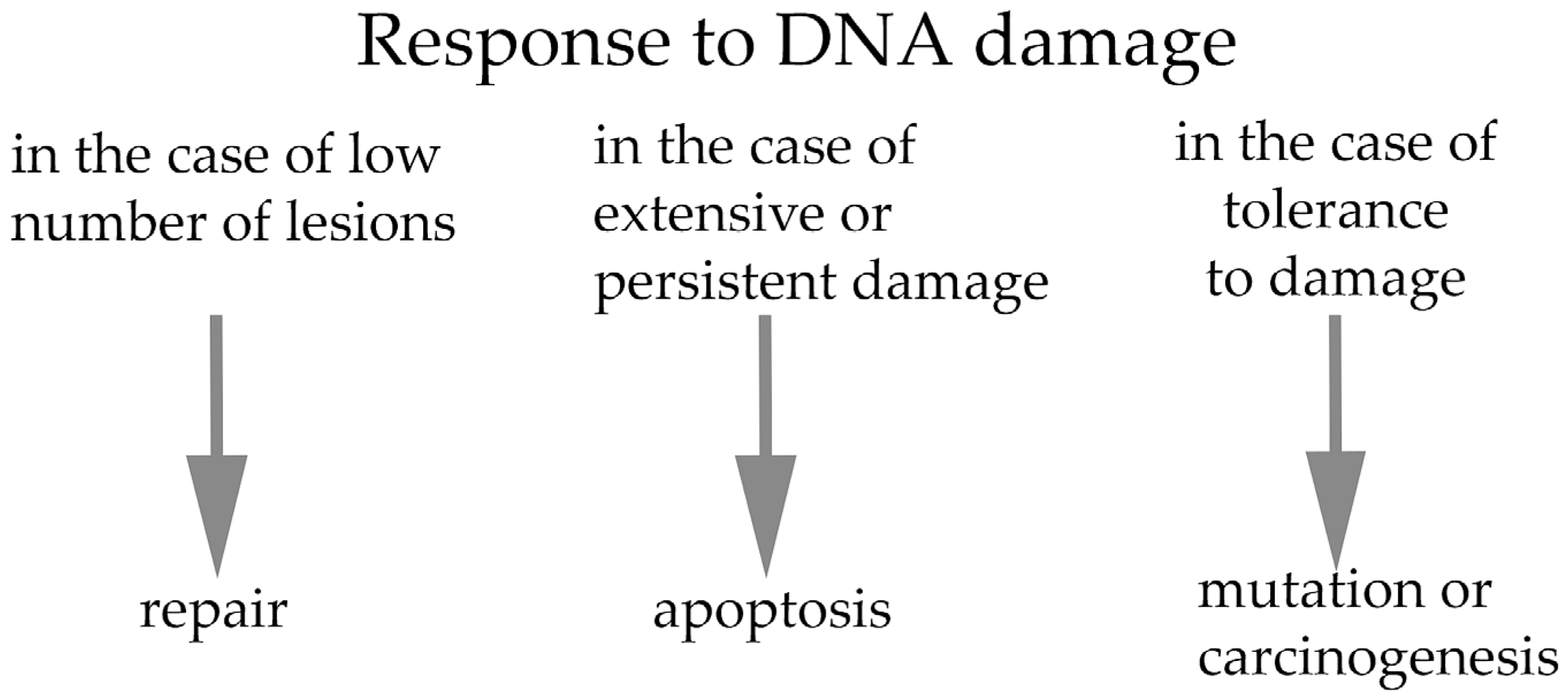
3. DNA Damage and Response
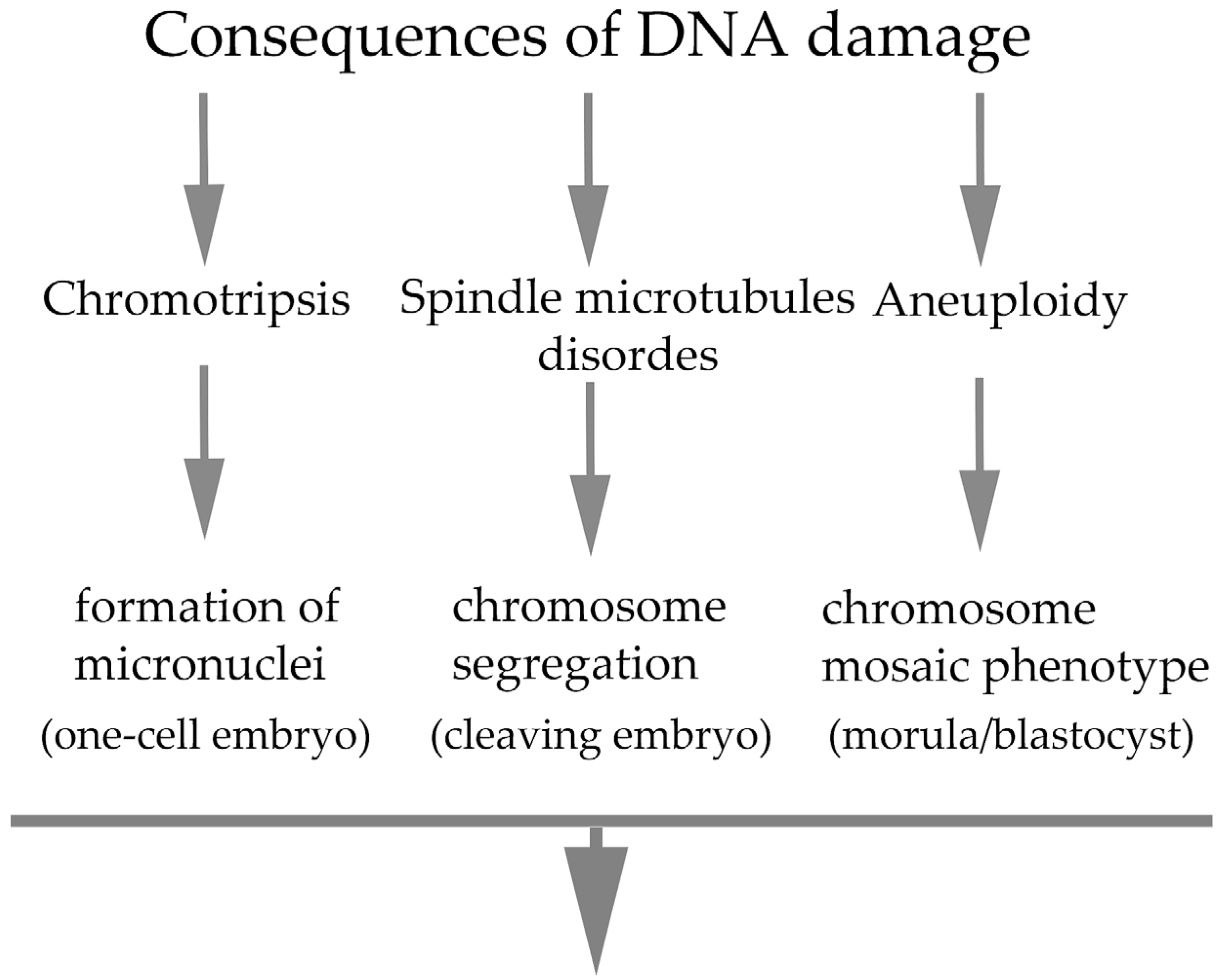
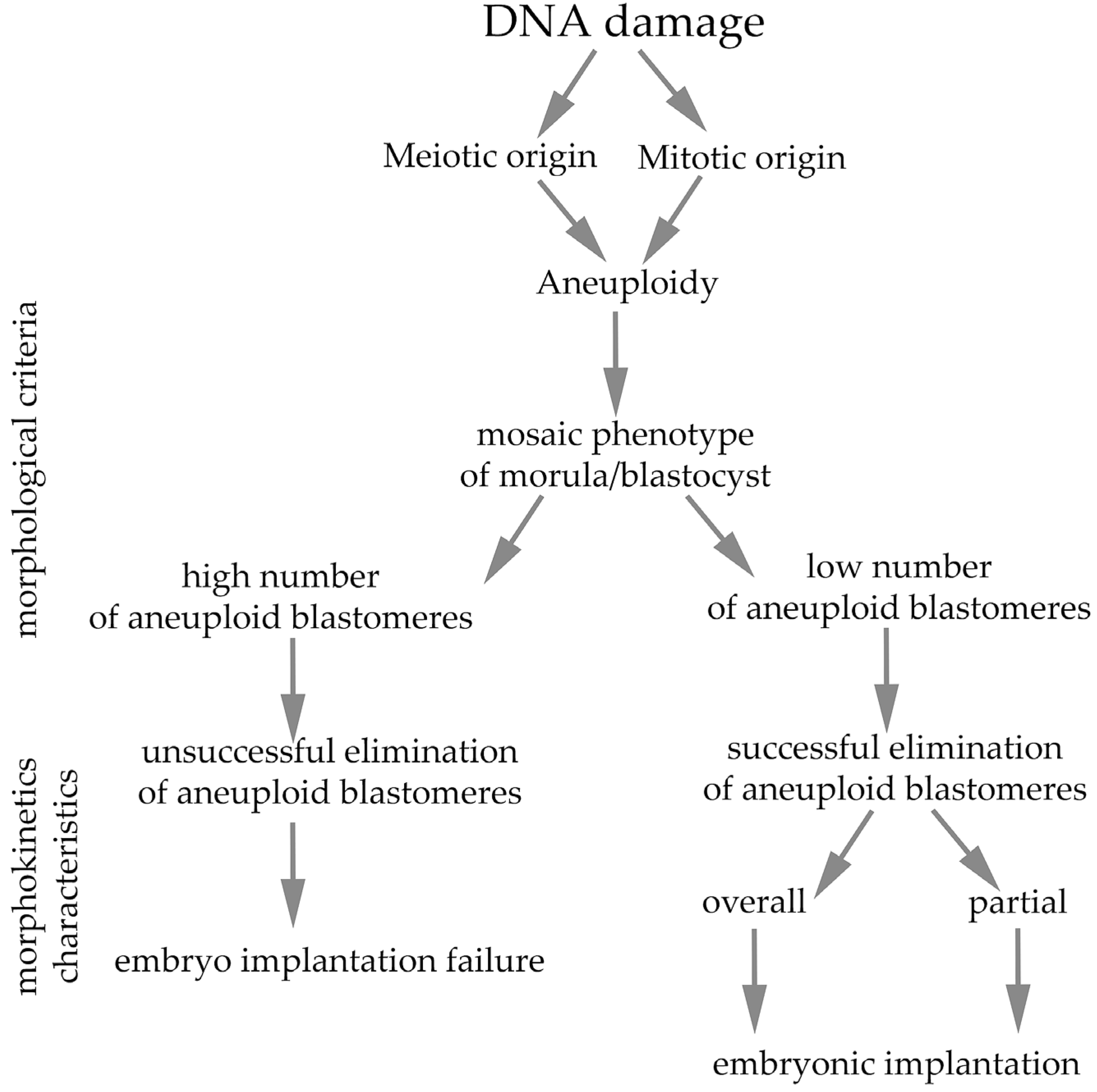
4. Consequences on Early Embryogenesis
Assisted Reproduction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mu, J.; Zhou, Z.; Sang, Q.; Wang, L. The physiological and pathological mechanisms of early embryonic development. Fundam. Res. 2022, 2, 859–872. [Google Scholar] [CrossRef]
- Bhakta, H.H.; Refai, F.H.; Avella, M.A. The molecular mechanisms mediating mammalian fertilization. Development 2019, 146, dev176966. [Google Scholar] [CrossRef]
- Gaspa-Toneu, L.; Peters, A.H. Nucleosomes in mammalian sperm: Conveying paternal epigenetic inheritance or subject to reprogramming between generations. Curr. Opin. Genet. Dev. 2023, 79, 102034. [Google Scholar] [CrossRef]
- Reichmann, J.; Nijmeijer, B.; Hossain, M.J.; Eguren, M.; Schneider, I.; Politi, A.Z.; Roberti, M.J.; Hufnagel, L.; Hiiragi, T.; Ellenberg, J. Dual-spindle formation in zygotes keeps parental genomes apart in early mammalian embryos. Science 2018, 361, 189–193. [Google Scholar] [CrossRef]
- Jord, A.A.; Verlhac, M.H. Spindle Assembly: Two Spindles for Two Genomes in a Mammalian zygote. Curr. Biol. 2018, 28, R931–R951. [Google Scholar] [CrossRef]
- Mullen, T.J.; Davis-Roca, A.C.; Wignall, S.M. Spindle assembly and chromosome dynamics during oocyte meiosis. Curr. Opin. Cell Biol. 2019, 60, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Dunkley, S.; Scheffler, K.; Mogessie, B. Cytoskeletal form and function in mammalian oocytes and zygotes. Curr. Opin. Cell Biol. 2022, 75, 102073. [Google Scholar] [CrossRef] [PubMed]
- Blengini, C.S.; Schindler, K. Acentriolar spindle assembly in mammalian female meiosis and the consequences of its perturbations on human reproduction. Biol. Reprod. 2022, 106, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Ciemerych, M.A.; Maro, B.; Kubiak, J.Z. Control of duration of the first two mitoses in a mouse embryo. Zygote 1999, 7, 293–300. [Google Scholar] [CrossRef]
- Bennabi, I.; Terret, M.E.; Verlhac, M.H. Meiotic spindle assembly and chromosome segregation in oocytes. J. Cell Biol. 2016, 215, 611–619. [Google Scholar] [CrossRef]
- Wyatt, C.D.R.; Pernaute, B.; Gohr, A.; Miret-Cuesta, M.; Goyeneche, L.; Rovira, Q.; Salzer, M.C.; Boke, E.; Bogdanovic, O.; Bonnal, S.; et al. A developmentally programmed splicing failure contributes to DNA damage response attenuation during mammalian zygotic genome activation. Sci. Adv. 2022, 8, eabn4935. [Google Scholar] [CrossRef]
- Anger, M.; Radonova, L.; Horakova, A.; Sekach, D.; Charousova, M. Impact of Global Transcriptional Silencing on Cell Cycle Regulation and Chromosome Segregation in Early Mammalian Embryos. Int. J. Mol. Sci. 2021, 22, 9073. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Hendrich, B.; Reik, W.; Dean, W. Dynamic reprogamming of DNA methylation in the early mouse embryo. Dev. Biol. 2002, 241, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Munisha, M.; Schimenti, J.C. Genome maintenance during embryogenesis. DNA Repair 2021, 106, 103195. [Google Scholar] [CrossRef]
- Musson, R.; Gasior, Ł.; Bisogno, S.; Ptak, G.E. DNA damage in preomplantation embryos and gametes: Specification, clinical relevance and repair strategies. Hum. Reprod. Update 2022, 28, dmab046. [Google Scholar] [CrossRef] [PubMed]
- Eckersley-Maslin, M.A.; Alda-Catalinas, C.; Reik, W. Dynamics of the epigenetic landscape during the maternal-to-zygotic transition. Nat. Rev. Mol. Cell Biol. 2018, 19, 436–450. [Google Scholar] [CrossRef]
- Pailas, A.; Niaka, K.; Zorzompokou, C.; Marangos, P. DNA damage response in fully grown mammalian oocytes. Cells 2022, 11, 798. [Google Scholar] [CrossRef]
- González-Marín, C.; Gosálvez, J.; Roy, R. Types, Causes, Detection and Repair of DNA Fragmentation in Animal and Human Sperm Cells. Int. J. Mol. Sci. 2012, 13, 14026–14052. [Google Scholar] [CrossRef]
- Stringer, J.M.; Winship, A.; Liew, S.H.; Hutt, K. The Capacity of Oocytes for DNA Repair. Cell. Mol. Life Sci. 2018, 75, 2777–2792. [Google Scholar] [CrossRef]
- Leem, J.; Bai, G.Y.; Oh, J.S. The Capacity to Repair Sperm DNA Damage in Zygotes is Enhanced by Inhibiting WIP1 Activity. Front. Cell Dev. Biol. 2022, 10, 841327. [Google Scholar] [CrossRef]
- Hamatani, T.; Carter, M.G.; Sharov, A.A.; Ko, M.S.G. Dynamics of global gene expression changes during mouse preimplantation development. Dev. Cell 2004, 6, 117–131. [Google Scholar] [CrossRef]
- Clift, D.; Schuh, M. Re-starting life: Fertilization and the transition from meiosis to mitosis. Nat. Rev. Mol. Cell Biol. 2013, 14, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Ladstatter, S.; Tachibana-Konwalski, K. A Surveillance mechanism ensure repair of DNA lesions during zygotic reprogramming. Cell 2016, 167, 1774–1787. [Google Scholar] [CrossRef]
- Jukam, D.; Shariati, S.A.M.; Skotheim, J.M. Zygotic genome activation in vertebrates. Dev. Cell 2017, 42, 316–332. [Google Scholar] [CrossRef]
- Zhang, M.; Kothari, P.; Mullins, M.; Lampson, M.A. Regulation of zygotic genome activation and DNA damage checkpoint acquisition at the mid-blastula transition. Cell Cycle 2014, 13, 3828–3838. [Google Scholar] [CrossRef]
- O’Farrell, P.H.; Stumpff, J.; Su, T.T. Embryonic cleavage cycles: How is a mouse like a fly? Curr. Biol. 2004, 14, R35–R45. [Google Scholar] [CrossRef]
- Farrell, J.A.; O’Farrell, P.H. From egg to gastrula: How the cell cycle is remodeled during the Drosophila mid-blastula transition. Annu. Rev. Genet. 2014, 48, 269–294. [Google Scholar] [CrossRef]
- Schindler-Johnson, M.; Petridou, N.I. Collective effects of cell cleavage Dynamics. Front. Cell Dev. Biol. 2024, 12, 1358971. [Google Scholar] [CrossRef]
- Mendieta-Serrano, M.A.; Schnabel, D.; Lomelí, H.; Salas-Vidal, E. Cell proliferation patterns in early zebrafish development. Anat. Rec. 2013, 296, 759–773. [Google Scholar] [CrossRef]
- Langley, A.R.; Smith, J.C.; Stemple, D.L.; Harvey, S.A. New insights into the maternal to zygotic transition. Development 2014, 141, 3834–3841. [Google Scholar] [CrossRef] [PubMed]
- Balder, P.; Jones, C.; Coward, K.; Yeste, M. Sperm chromatin: Evaluation, epigenetic signatures and relevance for embryo development and assisted reproductive technology outcomes. Eur. J. Cell Biol. 2024, 103, 151429. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Teklenburg, G.; Macklon, N.S.; Van Opstal, D.; Schuring-Blom, G.H.; Krijtenburg, P.J.; de Vreeden-Elbertse, J.; Fauser, B.C.; Baart, E.B. The fate of the mosaic embryo: Chromosomal constitution and development of day 4, 5 and 8 human embryos. Hum. Reprod. 2010, 25, 1916–1926. [Google Scholar] [CrossRef]
- Mertzanidou, A.; Wilton, L.; Cheng, J.; Spits, C.; Vanneste, E.; Moreau, Y.; Vermeesch, J.R.; Sermon, K. Microarray analysis reveals abnormal chromosomal complements in over 70% of 14 normally developing human embryos. Hum. Reprod. 2013, 28, 56–264. [Google Scholar] [CrossRef]
- Mu, X.F.; Jin, X.L.; Farnham, M.M.J.; Li, Y.; O’Neil, C. DNA damage-sensing kinases mediate the mouse 2-cell embryo’s response to genotoxic stress. Biol. Reprod. 2011, 85, 524–535. [Google Scholar] [CrossRef]
- Pacchierotti, F.; Ranaldi, R.; Derijck, A.A.; Heijden, G.V.D.; Boer, P.D. In vivo repair of DNA damage induced by X-rays in the early stages of mouse fertilization, and the influence of maternal PARP1 ablation. Mutat. Res. 2011, 714, 44–52. [Google Scholar] [CrossRef]
- Liu, Y.; Wanga, L.; Xua, X.; Yuana, Y.; Zhanga, B.; Lib, Z.; Xiea, Y.; Yana, Z.; Zhenga, Z.; Jib, J.; et al. The intra-S phase checkpoint directly regulates replication elongation to preserve the integrity of stalled replisomes. Proc. Natl. Acad. Sci. USA 2021, 118, e2019183118. [Google Scholar] [CrossRef]
- Tahahachi, S.; Kyogoku, H.; Hayakawa, T.; Miura, H.; Oji, A.; Kondo, Y.; Takebayashi, S.; Kitajima, T.S.; Hiratani, I. Embryonic genome instability upon DNA replication timing program emergence. Nature 2024, 633, 686–694. [Google Scholar] [CrossRef]
- Menezo, Y.; Dale, B.; Cohen, M. DNA damage and repair in human oocyte and embryos: A review. Zygote 2010, 18, 357–365. [Google Scholar] [CrossRef]
- Xu, S.; Egli, D. Genome organization and stability in mammalian pre-implantation development. DNA Repair 2024, 144, 103780. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N.; Kaldis, P. Regulation of the embryonic cell cycle during mammalian preimplantation development. Curr. Top. Dev. Biol. 2016, 120, 2–53. [Google Scholar] [CrossRef]
- ShaltielI, A.; Krenning, L.; Bruinsma, W.; Medema, R.H. The same, only differentDNA damage checkpoints and their reversal throughout the cell cycle. J. Cell Sci. 2015, 128, 607–620. [Google Scholar] [CrossRef]
- Wang, W.H.; Sun, Q.Y. Meiotic spindle, spindle checkpoint and embryonic aneuploidy. Front. Biosci. 2016, 11, 620–636. [Google Scholar] [CrossRef]
- Byrne, A.T.; Southgate, J.; Brison, D.R.; Leese, H.J. Analysis of apoptosis in the preimplantation bovine embryo using TUNEL. J. Reprod. Fertil. 1999, 117, 97–105. [Google Scholar] [CrossRef]
- Marangos, P.; Carrol, J. Oocytes progress beyond prophase in the presence DNA damage. Curr. Biol. 2012, 22, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Ou-Yang, Y.C.; Wang, Z.W.; Wang, Z.B.; Jing, Z.Z.; Luo, S.H.; Hou, Y.; Liu, Y.H.; Schatten, H.; Sun, Q.Y. The effect of DNA double-strand breaks on mouse oocyte meiotic maturation. Cell Cycle 2013, 12, 1233–1241. [Google Scholar] [CrossRef]
- Baran, V.; Duricek, T.; Pisko, J.; Drutovic, D.; Solc, P. Cleavage of Early Mouse Embryo with Damaged DNA. Int. J. Mol. Sci. 2022, 23, 3516, Erratum in Int. J. Mol. Sci. 2025, 26, 2171. [Google Scholar] [CrossRef] [PubMed]
- Bazrgar, M.; Gourabi, H.; Yazdi, P.E.; Vazirinasab, H.; Fakhri, M.; Hassani, F.; Valojerdi, M.R. DNA repair signalling pathway genes are overexpressed in poor-quality pre-implantation human embryos with complex aneuploidy. Eur. J. Obs. Gynecol. Reprod. Biol. 2014, 175, 152–156. [Google Scholar] [CrossRef]
- Fatehi, A.N.; Bevers, M.M.; Schoevers, E.; Roelen, B.A.J.; Colenbrander, B.; Gadella, B.M. DNA damage in bovine sperm does not block fertilization and early embryonic development but induces apoptosis after the first cleavages. J. Androl. 2006, 27, 176–188. [Google Scholar] [CrossRef]
- Ford, E.; Currie, C.E.; Taylor, D.M.; Erent, M.; Marston, A.L.; Hartshorne, G.M.; McAinsh, A.D. The First Mitotic Division of the Human Embryo Is Highly Error-Prone. bioRxiv 2020, 1–13. [Google Scholar] [CrossRef]
- Cavazza, T.; Takeda, Y.; Politi, A.Z.; Aushev, M.; Aldag, P.; Baker, C.; Choudhary, M.; Bucevicius, J.; Lukinavicius, G.; Elder, K.; et al. Parental Genome Unification Is Highly Error-Prone in Mammalian Embryos. Cell 2021, 184, 2860–2877.e22. [Google Scholar] [CrossRef]
- Palmerola, K.L.; Amrane, S.; Angeles, A.D.L.; Xu, S.; Wang, N.; Pinho, J.; Zuccaro, M.V.; Taglialatela, A.; Massey, D.J.; Turocy, J.; et al. Replication Stress Impairs Chromosome Segregation and Preimplantation Development in Human Embryos. Cell 2022, 185, 2988–3007.e20. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, P. Mammalian zygotic genome activation. Semin. Cell Dev. Biol. 2018, 84, 18–126. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, J.C.; Coonen, E.; Bras, M.; van Wissen, L.C.; Ignoul-Vanvuchelen, R.; Bergers-Jansen, J.M.; Derhaag, J.; Geraedts, J.P.; Evers, J. Comparison of in-vitro development of embryos originating from either conventional in-vitro fertilization or intracytoplasmic sperm injection. Hum. Reprod. 2000, 15, 402–409. [Google Scholar] [CrossRef]
- Pisko, J.; Spirkova, A.; Cikos, S.; Olexikova, L.; Kovarikova, V.; Sefcikova, Z.; Fabian, D. Apoptotic cells in mouse blastocysts are eliminated by neighbouring blastomeres. Sci. Rep. 2021, 11, 9228. [Google Scholar] [CrossRef]
- Shukla, V.; Høffding, M.K.; Hoffmann, E.R. Genome diversity and instability in human germ cells and preimplantation embryos. Semin. Cell Dev. Biol. 2021, 113, 132–147. [Google Scholar] [CrossRef]
- Girardi, L.; Serdarogullari, M.; Patassini, C.; Poli, M.; Fabiani, M.; Caroselli, S.; Coban, O.; Findikli, N.; Boynukalin, F.K.; Bahceci, M.; et al. Incidence, origin, and predictive model for the detection and clinical management of segmental aneuploidies in human embryos. Am. J. Hum. Genet. 2020, 106, 525–534. [Google Scholar] [CrossRef]
- Jansen, G.; Gebert, D.; Kumar, T.R.; Simmons, E.; Murphy, S.; Teixeira, F.K. Tolerance thresholds underlie responses to DNA damage during germline development. Genes Dev. 2024, 38, 631–654. [Google Scholar] [CrossRef]
- Titus, S.; Li, F.; Stobezki, R.; Akula, K.; Unsal, E.; Jeong, K.; Dickler, M.; Robson, M.; Moy, F.; Goswami, S. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci. Transl. Med. 2013, 5, 172ra21. [Google Scholar] [CrossRef]
- Oktay, K.; Turan, V.; Titus, S.; Stobezki, R.; Liu, L.; Oktay, K.B. BRCA mutations, DNA repair deficiency, and ovarian aging. Biol. Reprod. 2015, 93, 67. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, A.; Gosalvez, J.; Agarwal, A.; Roy, R.; Johnston, S. DNA damage and repair in human reproductive cells. Int. J. Mol. Sci. 2018, 20, 31. [Google Scholar] [CrossRef]
- Turan, V.; Oktay, K. BRCA-related ATM-mediated DNA double strandbreak repair andovarianaging. Hum. Reprod. Update 2020, 26, 43–57. [Google Scholar] [CrossRef]
- Newman, H.; Horta, F.; Catt, S.; Vining, B.; Vollenhoven, B. DNA repair and response to sperm DNA damage in oocytes and embryos, and the potential consequences in ART: A systematic review. Mol. Hum. Reprod. 2022, 28, gaab071. [Google Scholar] [CrossRef]
- Zheng, W.W.; Song, G.; Wang, Q.L.; Liu, S.W.; Zhu, X.L.; Deng, S.M.; Zhong, A.; Tan, Y.M.; Tan, Y. Sperm DNA damage has a negative effect on early embryonic development following in vitro fertilization. Asian J. Androl. 2018, 20, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Middelkamp, S.; van Tol, H.T.A.; Spierings, D.C.J.; Boymans, S.; Guryev, V.; Roelen, B.A.J.; Lansdorp, P.M.; Cuppen, E.; Kuijk, E.W. Sperm DNA damage causes genomic instability in early embryonic development. Sci. Adv. 2020, 6, eaaz7602. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Baran, V.; Sakakibara, Y.; Brzakova, A.; Ferencova, I.; Motlik, J.; Kitajima, T.S.; Schultz, R.M.; Solc, P. DNA damage response during mouse oocyte maturation. Cell Cycle 2016, 15, 546–558. [Google Scholar] [CrossRef]
- Yukawa, M.; Oda, S.; Mitani, H.; Nagata, M.; Aoki, F. Deficiency in response to DNA double-strand breaks in mouse early preimplantation embryos. Biochem. Biophys. Res. Commun. 2007, 358, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.J.; Yang, L.X. Gamma-H2A.X–a novel biomarker for DNA double-strand breaks. In Vivo 2008, 22, 305–309. [Google Scholar] [PubMed]
- Xiao, J.; Liu, Y.; Li, Z.; Zhou, Y.; Lin, H.; Wu, X.; Chen, M.; Xioa, W. Effects of the insemination of hydrogen peroxide-treated epididymal mouse spermatozoa on H2AX repair and embryo development. PLoS ONE 2012, 7, e38742. [Google Scholar] [CrossRef]
- Grenier, L.; Robaire, B.; Hales, B.F. The activation of DNA damage detection and repair responses in cleavage-stage rat embryos by adameged paternal genome. Toxicol. Sci. 2012, 127, 555–566. [Google Scholar] [CrossRef]
- Wang, Z.W.; Ma, X.S.; Ma, J.Y.; Luo, Y.B.; Lin, F.; Wang, Z.B.; Fan, H.Y.; Schatten, H.; Sun, Q.Y. Laser microbeam-induced DNA damage inhibits cell division in early fertilized eggs and early embryos. Cell Cycle 2013, 12, 3336–3344. [Google Scholar] [CrossRef]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Gruhn, J.R.; Hoffman, E.R. Errors of the Egg: The Establishment and Progression of Human Aneuploidy Research in the Maternal Germline. Annu. Rev. Genet. 2022, 56, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Daughtry, B.L.; Chavez, S.L. Time-lapse imaging for the detection of chromosomal abnormalities in primate preimplantation embryos. Methods Mol. Biol. 2018, 1769, 293–317. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Probst, C.E.; Zayats, A.; Davidson, B.; Riedel, M.; Li, Y.; Venkatachalam, V. The in vitro micronucleus assay using imaging f low cytometry and deep learning. NPJ Syst. Biol. Appl. 2021, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, D.; Goldteyn, R.M. G2/M-phase checkpoint adaptation and micronuclei formation as mechanisms that contribute to genomic instability in human cells. Int. J. Mol. Sci. 2017, 18, 2344. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Spektor, A.; Cornils, H.; Francis, J.M.; Jackson, E.K.; Liu, S.; Meyerson, M.; Pellman, D. Chromothripsis from DNA damage in micronuclei. Nature 2015, 522, 179–184. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, M.; Liu, M.; Zhang, Y.; Mi, Z.; Mao, J.; Chen, H.; Huang, Y.; Huang, Y.; Liu, Z.; et al. Micronuclei in 2-cell embryos show higher blastocyst formation rates on human embryonic development. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 302, 26–32. [Google Scholar] [CrossRef]
- Chavez, S.L.; Loewke, K.E.; Han, J.; Moussavi, F.; Colls, P.; Munne, S.; Behr, B.; Pera, R.A.R. Dynamic blastomerebehaviour reflects human embryo ploidy by the four-cell stage. Nat. Commun. 2012, 3, 1251. [Google Scholar] [CrossRef]
- Daughtry, B.L.; Rosenkrantz, J.L.; Lazar, N.H.; Fei, S.S.; Redmayne, N.; Torkenczy, K.A.; Adey, A.; Yan, M.; Gao, L.; Park, B.; et al. Single-cell sequencing of primate preimplantation embryos reveals chromosome elimination via cellular fragmentation and blastomere exclusion. Genome Res. 2019, 29, 367–382. [Google Scholar] [CrossRef]
- Budrewicz, J.; Chavez, S.L. Insights into embryonic chromosomal instability: Mechanisms of DNA elimination during mammalian preimplantation development. Front. Cell Dev. Biol. 2024, 12, 1344092. [Google Scholar] [CrossRef]
- Shibasaki, I.; Sugiyama, H.; Kamada, Y.; Nagatomo, H.; Ito, D.; Wakayama, S.; Wakayama, M.O.T. Extracting and analyzing micronuclei from mousetwo-cell embryos fertilized with freeze-dried spermatozoa. Commun. Biol. 2025, 8, 6. [Google Scholar] [CrossRef]
- Mashiko, D.; Ikeda, Z.; Yao, T.; Tokoro, M.; Fukunaga, N.; Asada, Y.; Yamagata, K. Chromosome segregation error during early cleavage in mouse pre-implantation embryo does not necessarily cause developmental failure after blastocyst stage. Sci. Rep. 2020, 10, 854. [Google Scholar] [CrossRef]
- Hatano, Y.; Mashiko, D.; Tokoro, M.; Yao, T.; Yamagata, K. Chromosome counting in the mouse zygote using low-invasive super-resolution live-cell imaging. Genes Cells 2022, 27, 214–228. [Google Scholar] [CrossRef]
- Vázquez-Diez, C.; Yamagata, K.; Trivedi, S.; Haverfield, J.; FitzHarris, G. Micronucleus formation causes perpetual unilateral chromosome inheritance in mouse embryos. Proc. Natl. Acad. Sci. USA 2016, 113, 626–631. [Google Scholar] [CrossRef]
- Crasta, K.; Ganem, N.J.; Dagher, R.; Lentermenn, A.B.; Ivanova, E.V.; Pan, Y.; Nezi, L.; Protopopov, A.; Chowdhury, D.; Pellman, D. DNA breaks and chromosome pulverization from errors in mitosis. Nature 2012, 482, 53–60. [Google Scholar] [CrossRef]
- Lightfoot, D.A.; Kouznetsova, A.; Mahdy, E.; Wilbertz, J.; Höög, C. The fate of mosaic aneuploid embryos during mouse development. Dev. Biol. 2006, 289, 384–394. [Google Scholar] [CrossRef][Green Version]
- van Echten-Arends, J.; Mastenbroek, S.; Sikkema-Raddatz, B.; Korevaar, J.C.; Heineman, M.J.; van der Veen, F.; Repping, S. Chromosomal mosaicism in human preimplantation embryos: A systematic review. Hum. Reprod. Update 2011, 17, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Capalbo, A.; Poli, M.; Rienzi, L.; Girardi, L.; Patassini, C.; Fabiani, M.; Cimadomo, D.; Benini, F.; Farcomeni, A.; Cuzzi, J.; et al. Mosaic Human Preimplantation Embryos and Their Developmental Potential in a Prospective, Non-Selection Clinical Trial. Am. J. Hum. Genet. 2021, 108, 2238–2247. [Google Scholar] [CrossRef]
- Ribas-Maynou, J.; Novo, S.; Torres, T.; Salas-Huetos, A.; Rovira, S.; Antich, M.; Yeste, M. Sperm DNA integrity does play a crucial role for embryo development after ICSI, notably when good-quality oocytes from young donors are used. Biol. Res. 2022, 55, 41. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Cui, K.; Yi, Q.; Yu, L.; Xu, Y. DNA repair mechanisms in embryonic stem cells. Cell Mol. Life Sci. 2017, 74, 487–493. [Google Scholar] [CrossRef]
- Khokhlova, V.; Fesenko, Z.S.; Sopova, J.V.; Leonova, E.I. Features of DNA repair in thre early stages of mammalian embryonic development. Genes 2020, 11, 1138. [Google Scholar] [CrossRef]
- Uehara, R.; Cerritelli, S.M.; Hasin, N.; Sakhuja, K.; London, M.; Iranzo, J.; Chon, H.; Grinberg, A.; Crouch, R.J. Two RNase H2 mutants with differential RNMP processing activity reveal a threshold of ribonucleotide tolerance for embryonic development. Cell Rep. 2018, 25, 1135–1145.e5. [Google Scholar] [CrossRef]
- Bolton, H.; Graham, S.J.L.; Van der Aa, N.; Kumar, P.; Theunis, K.; Fernandez Gallardo, E.; Voet, T.; Zernicka-Goetz, M. Mouse model of chromosome mosaicism reveals lineage-specific depletion of aneuploid cells and normal developmental potential. Nat. Commun. 2016, 7, 11165. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Gimeno, I.; Cañón-Beltrán, K.; Gutiérrez-Adán, A.; Rizos, D.; Gómez, E. Senescence and apoptosis during in vitro embryo develpmen in abovine model. Front. Cell Dev. Biol. 2020, 8, 619902. [Google Scholar] [CrossRef]
- Yu, B.; van Tol, H.T.A.; Stout, T.A.E.; Roelen, B.A.J. Cellular Fragments in the Perivitelline Space Are Not a Predictor of Expanded Blastocyst Quality. Front. Cell Dev. Biol. 2021, 8, 616801. [Google Scholar] [CrossRef]
- Harzif, A.K.; Andyra, A.F.; Sayogo, A.; Ummah, N.; Puspawardani, A.P.; Nurbaeti, P.; Wiweko, B. Embryo response to aneuploidy through self-correction mechanism: A literature review. Middle East Fertil. Soc. J. 2024, 29, 16. [Google Scholar] [CrossRef]
- Viotti, M.; Greco, E.; Grifo, J.A.; Madjunkov, M.; Librach, C.; Cetinkaya, M.; Kahraman, S.; Yakovlev, P.; Kornilov, N.; Corti, L.; et al. Chromosomal, gestational, and neonatal outcomes of embryos classified as a mosaic by preimplantation genetic testing for aneuploidy. Fertil. Steril. 2023, 120, 957–966. [Google Scholar] [CrossRef]
- Cimadomo, D.; Innocenti, F.; Taggi, M.; Saturno, G.; Campitiello, M.R.; Guido, M.; Vaiarelli, A.; Ubaldi, F.M.; Rienzi, L. How should the best human embryo in vitro be? Current and future challenges for embryo selection. Minerva Obstet. Gynecol. 2024, 76, 59–173. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Calonge, R.; Santamaria, N.; Rubio, T.; Moreno, J.M. Making and Selecting the Best Embryo in In vitro Fertilization. Arch. Med. Res. 2024, 55, 03068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zheng, P.S. Mechanism of chromosomal mosaicism in preimplantation embryos and its effect on embryo development. J. Assist. Reprod. Genet. 2024, 41, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Zhai, F.; Kong, S.; Song, S.; Guo, Q.; Ding, L.; Zhang, J.; Wang, N.; Kuo, Y.; Guan, S.; Yuan, P.; et al. Human embryos harbor complex mosaicism with broad presence of aneuploid cells during early development. Cell Discov. 2024, 10, 98. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Z.; Li, M.; Sun, L. Biopsy vs comprehensive embryo/blastocyst analysis: A closer look at embryonic chromosome evaluation. Hum. Reprod. Open. 2025, 2025, hoaf013. [Google Scholar] [CrossRef]
- Zhou, Y.; Yeb, F.; Zhang, L.; Kang, Q.; Luod, Y.; Jiang, N.; Lou, L.; Mao, Y.; Wang, L.; Jina, F. The role of DNA damage response in human embryonic stem cells exposed to atmospheric oxygen tension: Implications for embryo development and differentiation. Reprod. Toxicol. 2024, 128, 108648. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, H.; Zou, S.; Yu, X.; Li, J. Perspective in the Mechanisms for Repairing Sperm DNA Damage. Reprod. Sci. 2025, 32, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Casanovas, A.; Ribas-Maynou, J.; Lara-Cerrillo, S.; Jimenez-Macedo, A.R.; Hortal, O.; Benet, J.; Carrera, J.; García-Peiro, A. Double-stranded spermDNA damage is a cause of delayin embryo development and can impair implantation rates. Fertil. Steril. 2019, 111, 699–707. [Google Scholar] [CrossRef]
- Tsuiko, O.; Jatsenko, T.; Gracec, L.K.P.; Kurgd, A.; Vermeesche, J.R.; Lannerf, F.; Altmäeb, S.; Salumets, A. A speculative outlook on embryonic aneuploidy: Can molecular pathways be involved? Dev. Biol. 2019, 447, 3–13. [Google Scholar] [CrossRef]
- Fouks, Y.; Vaughan, D.; Sripada, V.; Penzias, A.S.; Bortoletto, P.; Sakkas, D. Do sperm factors influence embryonic aneuploidy? Long live the oocyte. Hum. Reprod. 2024, 39, 2442–2452. [Google Scholar] [CrossRef]
- Stolakis, V.; Bertero, M.C. Molecular aspects of aneuploidy in preimplantation human embryos: A mini-review. Ann. Res. Hosp. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Chavli, E.A.; Klaasen, S.J.; Van Opstal, D.; Laven, J.S.; Kops, G.J.; Baart, E.B. Single-cell DNA sequencing reveals a high incidence of chromosomal abnormalities in human blastocysts. J. Clin. Investig. 2024, 134, e174483. [Google Scholar] [CrossRef]
- Nagaoka, S.I.; Hassold, T.J.; Hunt, P.A. Human aneuploidy: Mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 2012, 13, 493–504. [Google Scholar] [CrossRef]
- Vázquez-Diez, C.; FitzHarris, G. Causes and consequences of chromosome segregation error in preimplantation embryos. Reproduction 2018, 155, R63–R76. [Google Scholar] [CrossRef]
- Wartosch, L.; Schindler, K.; Schuh, M.; Gruhn, J.R.; Hoffmann, E.R.; McCoy, R.C.; Xing, J. Origins and mechanisms leading to aneuploidy in human eggs. Prenat. Diagn. 2021, 41, 620–630. [Google Scholar] [CrossRef]
- Charalambous, C.; Webster, A.; Schuh, M. Aneuploidy in mammalian oocytes and the impact of maternal ageing. Nat. Rev. Mol. Cell Biol. 2022, 24, 27–44. [Google Scholar] [CrossRef]
- Mihajlovic, A.I.; Haverfield, J.; FitzHarris, G. Distinct classes of lagging chromosome underpin age-related oocyte aneuploidy in mouse. Dev. Cell 2021, 56, 2273–2283.e3. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, A.I.; Byers, C.; Reinholdt, L.; FitzHarris, G. Spindle assembly checkpoint insensitivity allows meiosis-II despite chromosomal defects in aged eggs. EMBO Rep. 2023, 24, e57227. [Google Scholar] [CrossRef]
- Yin, L.; Mihajlovic, A.I.; Yang, G.; FitzHarris, G. Kinetochore deterioration concommitant with centromere weakening during aging in mouse oocyte meiosis-I. FASEB J. 2023, 37, e22922. [Google Scholar] [CrossRef] [PubMed]
- Tsuiko, O.; Catteeuw, M.; ZamaniEsteki, M.; Destouni, A.; Pascottini, O.B.; Besenfelder, U.; Havlicek, V.; Smits, K.; Kurg, A.; Salumets, A.; et al. Genome stability of bovine in vivo-conceived cleavage-stage embryos is higher compared to in vitro-produced embryos. Hum. Reprod. 2017, 32, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Pauerova, T.; Radonova, L.; Kovacovicova, K.; Novakova, L.; Skultety, M.; Anger, M. Aneuploidy during the onset of mouse embryo development. Reproduction 2020, 160, 773–782. [Google Scholar] [CrossRef]
- Duncan, F.E.; Chiang, T.; Schultz, R.M.; Lampson, M.A. Evidence that a defective spindle assembly checkpoint is not the primary cause of maternal age-associated aneuploidy in mouse eggs. Biol. Reprod. 2009, 81, 768–776. [Google Scholar] [CrossRef]
- Danylevska, A.; Kovacovicova, K.; Awadova, T.; Anger, M. The frequency of precocious segregation of sister chromatids in mouse female meiosis I is affected by genetic background. Chromosome Res. 2014, 22, 365–373. [Google Scholar] [CrossRef]
- Carbone, L.; Chavez, S.L. Mammalian pre-implantation chromosomal instability: Species comparison, evolutionary considerations, and pathological correlations. Syst. Biol. Reprod. Med. 2015, 61, 321–335. [Google Scholar] [CrossRef]
- Destouni, A.; Esteki, M.Z.; Catteeuw, M.; Tšuiko, O.; Dimitriadou, E.; Smits, K.; Kurg, A.; Salumets, A.; Van Soom, A.; Voet, T.; et al. Zygotes segregate entire parental genomes in distinct blastomere lineages causing cleavage-stage chimerism and mixoploidy. Genome Res. 2016, 26, 567–578. [Google Scholar] [CrossRef]
- Hornak, M.; Oracova, E.; Hulinska, P.; Urbankova, L.; Rubes, J. Aneuploidy detection in pigs using comparative genomic hybridization: From the oocytes to blastocysts. PLoS ONE 2012, 7, e30335. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Rito, T.; Metzger, J.; Naftaly, J.; Soman, R.; Hu, J.; Albertini, D.F.; Barad, D.H.; Brivanlou, A.H.; Gleicher, N. Depletion of aneuploid cells in human embryos and gastruloids. Nat. Cell Biol. 2021, 23, 314–321. [Google Scholar] [CrossRef]
- Capalbo, A.; Poli, M.; Jalas, C.; Forman, E.J.; Treff, N.R. On the reproductive capabilities of aneuploid human preimplantation embryos. Am. J. Hum. Genet. 2022, 109, 1572–1581. [Google Scholar] [CrossRef]
- Allais, A.; FitzHarris, G. Absence of a robust mitotic timer mechanism in early preimplantation mouse embryos leads to chromosome instability. Development 2022, 149, dev200391. [Google Scholar] [CrossRef]
- Kort, D.H.; Chia, G.; Treff, N.R.; Tanaka, A.J.; Xing, T.; Vensand, L.B.; Micucci, S.; Prosser, R.; Lobo, R.A.; Sauer, M.V.; et al. Human embryos commonly form abnormal nuclei during development: A mechanism of DNA damage, embryonic aneuploidy, and developmental arrest. Hum. Reprod. 2016, 31, 312–323. [Google Scholar] [CrossRef]
- Yao, T.; Ueda, A.; Khurchabilig, A.; Mashiko, D.; Tokoro, M.; Nagai, H.; Sho, T.; Matoba, S.; Yamagata, K.; Sugimura, S. Micronucleus formation during early cleavage division is a potential hallmark of preimplantation embryonic loss in cattle. Biochem. Biophys. Res. Commun. 2022, 617, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Fan, W.; Yan, L.; Li, R.; Lian, Y.; Huang, J.; Li, J.; Xu, L.; Tang, F.; Xie, X.S.; et al. Genome analyses of single human oocytes. Cell 2013, 155, 1492–1506. [Google Scholar] [CrossRef] [PubMed]
- Gruhn, J.R.; Zielinska, A.P.; Shukla, V.; Blanshard, R.; Capalbo, A.; Cimadomo, D.; Nikiforov, D.; Chan, A.C.-H.; Newnham, L.J.; Vogel, I.; et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science 2019, 365, 1466–1469. [Google Scholar] [CrossRef]
- Mikwar, M.; MacFarlane, A.J.; Marchetti, F. Mechanisms of oocyte aneuploidy associated with advanced maternal age. Mutat. Res. Rev. 2020, 785, 108320. [Google Scholar] [CrossRef]
- van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zong, C.; Fan, W.; Yang, M.; Li, J.; Chapman, A.R.; Zhu, P.; Hu, X.; Xu, L.; Yan, L.; et al. Probing meiotic recombination and aneuploidy of single sperm cells by whole-genome sequencing. Science 2012, 338, 1627–1630. [Google Scholar] [CrossRef]
- Bell, A.D.; Mello, C.J.; Nemesh, J.; Brumbaugh, S.A.; Wysoker, A.; McCarroll, S.A. Insights into variation in meiosis from 31,228 human sperm genomes. Nature 2020, 583, 259–264. [Google Scholar] [CrossRef]
- Khan, S.M.; Bennett, J.P., Jr. Development of mitochondrial gene replacement therapy. J. Bioenerg. Biomembr. 2004, 36, 387–393. [Google Scholar] [CrossRef]
- Fogleman, S.; Santana, C.; Bishop, C.; Miller, A.; Capco, D.G. CRISPR/Cas9 and mitochondrial gene replacement therapy: Promising techniques and ethical considerations. Am. J. Stem Cells 2016, 5, 39–52. [Google Scholar] [PubMed]
- Labarta, E.; de Los Santos, M.J.; Escriba, M.J.; Pellicer, A.; Herraiz, S. Mitochondria as a tool for oocyte rejuvenation. Fertil. Steril. 2019, 111, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.F.; Soares, M.; Reis, S.A.; Ramalho-Santos, J.; Sousa, A.P.; Almendla-Santos, T. Does supplementation with mitochondria improve oocyte competence? A systematic review. Reproduction 2021, 161, 269–287. [Google Scholar] [CrossRef]
- Brooks, K.E.; Daughtry, B.L.; Davis, B.; Yan, M.Y.; Fei, S.S.; Shepherd, S.; Carbone, L.; Chavez, S.L. Molecular contribution to embryonic aneuploidy and karyotypic complexity ininitial cleavage divisions of mammalian development. Development 2022, 149, dev198341. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, V.; Čikoš, Š.; Fabian, D. The Consequences of DNA Damage in the Early Embryo Are Important for Practical Procedures in Assisted Reproduction. Int. J. Mol. Sci. 2025, 26, 10031. https://doi.org/10.3390/ijms262010031
Baran V, Čikoš Š, Fabian D. The Consequences of DNA Damage in the Early Embryo Are Important for Practical Procedures in Assisted Reproduction. International Journal of Molecular Sciences. 2025; 26(20):10031. https://doi.org/10.3390/ijms262010031
Chicago/Turabian StyleBaran, Vladimír, Štefan Čikoš, and Dušan Fabian. 2025. "The Consequences of DNA Damage in the Early Embryo Are Important for Practical Procedures in Assisted Reproduction" International Journal of Molecular Sciences 26, no. 20: 10031. https://doi.org/10.3390/ijms262010031
APA StyleBaran, V., Čikoš, Š., & Fabian, D. (2025). The Consequences of DNA Damage in the Early Embryo Are Important for Practical Procedures in Assisted Reproduction. International Journal of Molecular Sciences, 26(20), 10031. https://doi.org/10.3390/ijms262010031





