Computational Modeling of PI3K/AKT Pathway in Bipolar Disorder and Type 2 Diabetes: Implications for Lithium Treatment and Curcumin as a Potential Alternative
Abstract
1. Introduction
2. Results
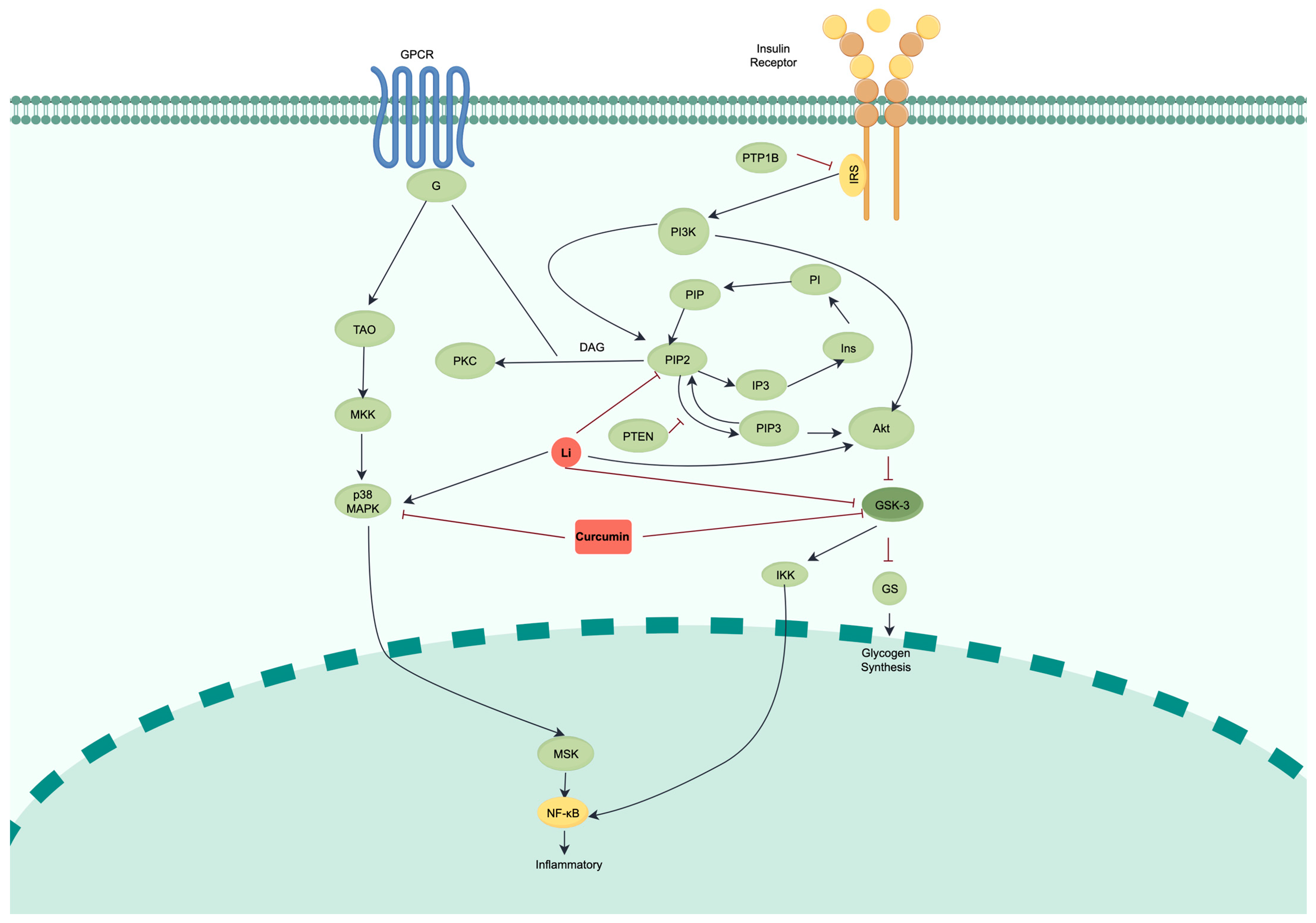
2.1. Constructing and Validating a Computational ODE Model of the BD-T2D Crosstalk Network
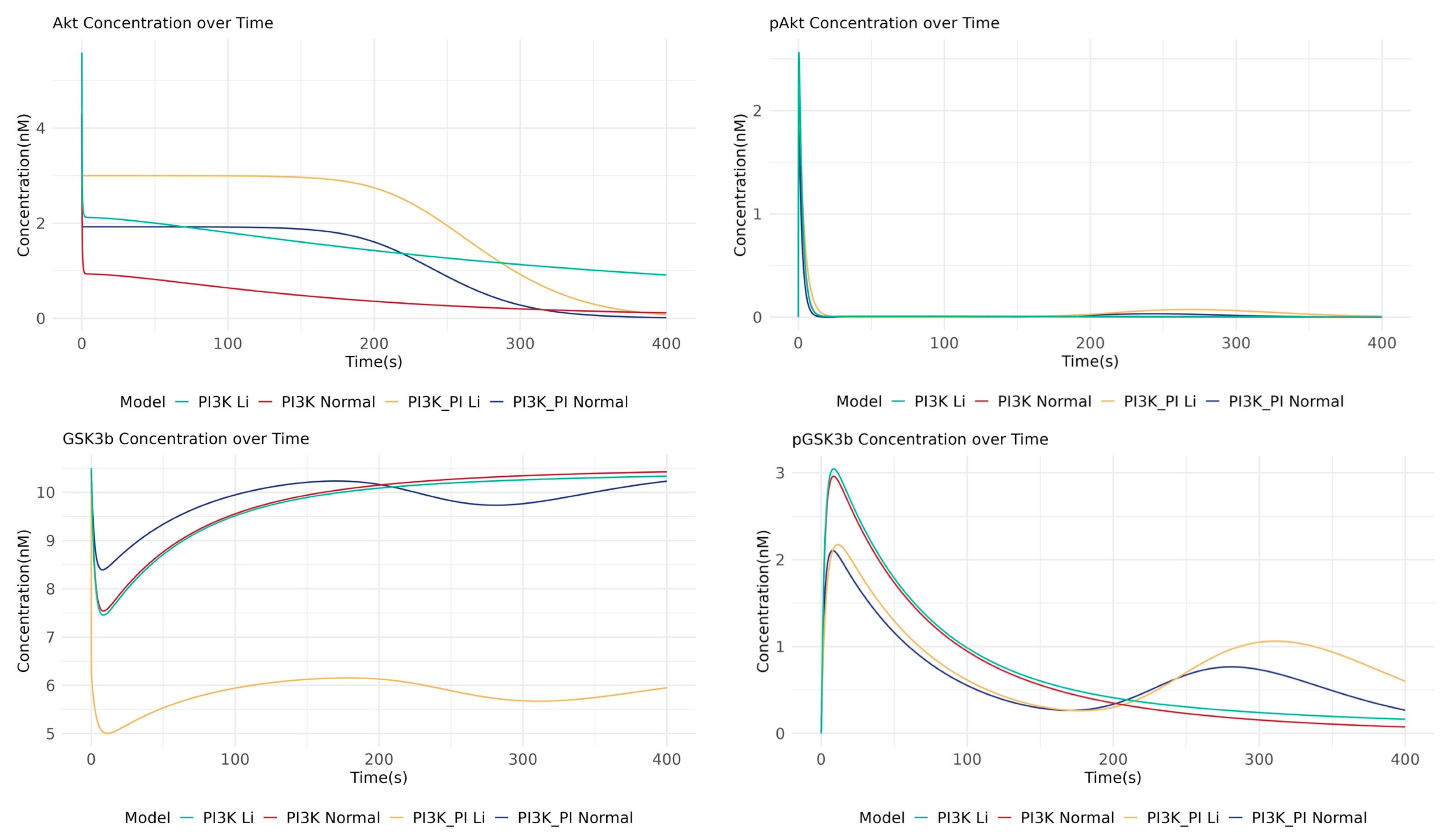
2.2. Crosstalk Simulation Reveals GSK3β as a Central Regulatory Node for Lithium’s Action
2.3. Deconvoluting Lithium’s Mechanism Suggests Direct GSK3β Inhibition as the Primary Mode of Action
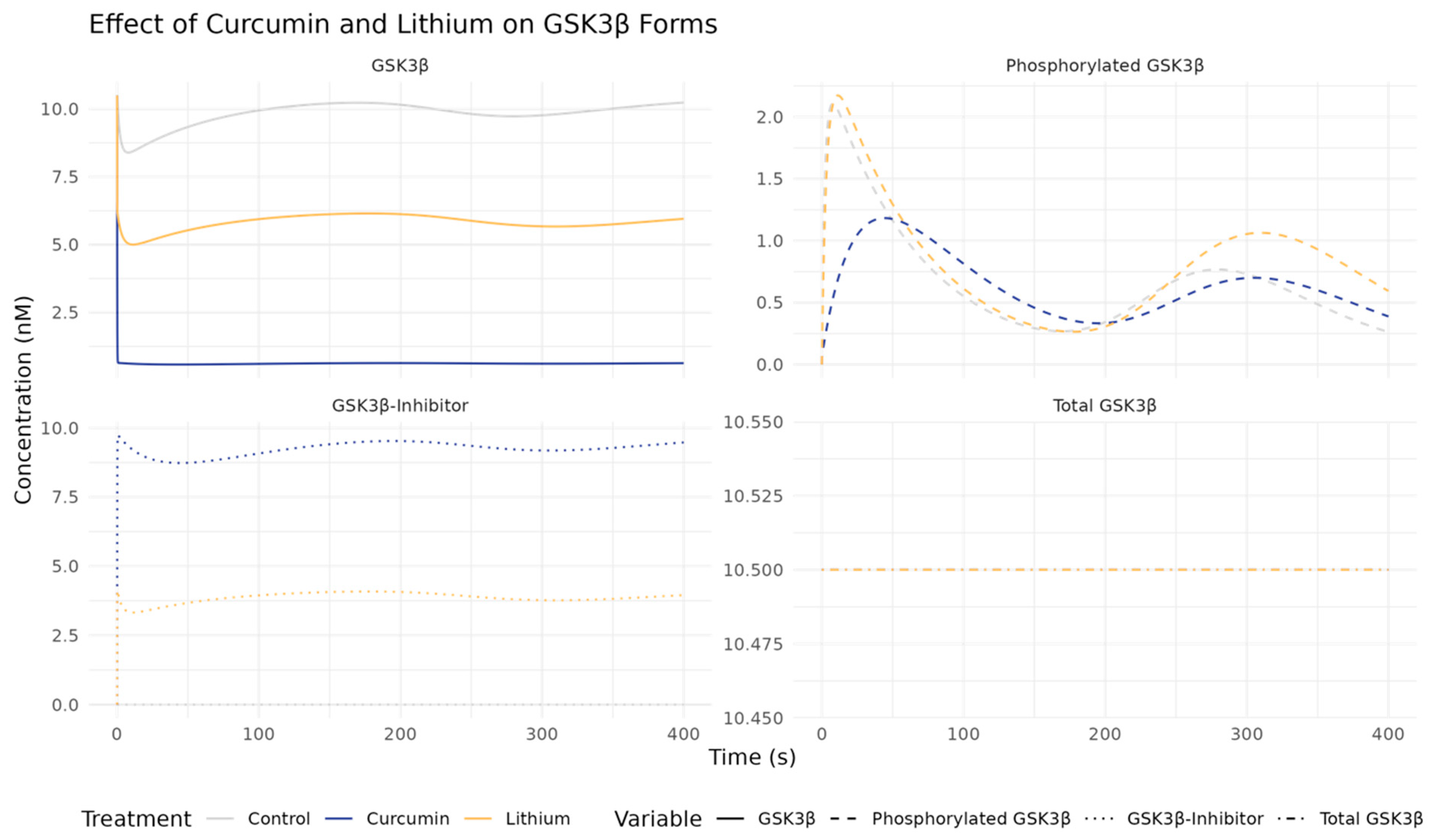
2.4. Curcumin vs. Lithium Effects on GSK3β and Related Pathways
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. Pathway Enrichment Analysis
4.3. ODE Simulation
4.4. Mass Action Kinetics
4.5. Enzyme-Catalyzed Reactions
4.6. Incorporating Inhibition in the ODE Model Using IC50
4.7. ODE Implementation in R
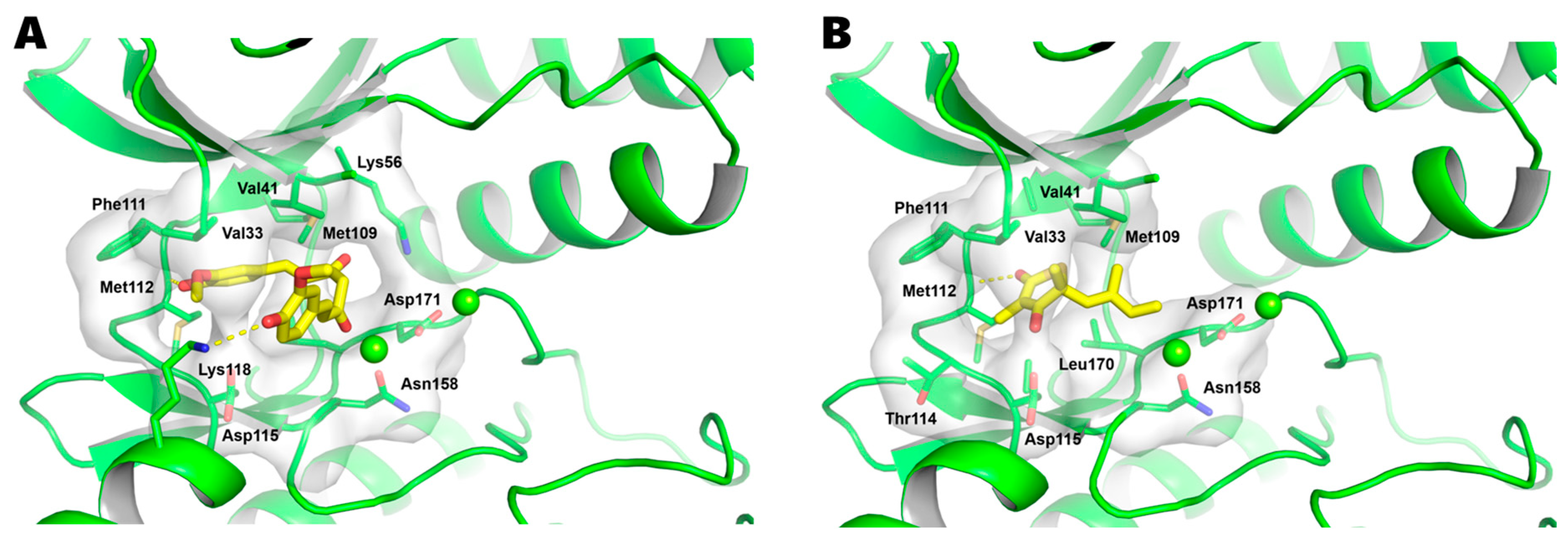
4.8. Molecular Docking
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosa, A.R.; Reinares, M.; Michalak, E.E.; Bonnin, C.M.; Sole, B.; Franco, C.; Comes, M.; Torrent, C.; Kapczinski, F.; Vieta, E. Functional Impairment and Disability across Mood States in Bipolar Disorder. Value Health J. Int. Soc. Pharmacoeconomics Outcomes Res. 2010, 13, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Grande, I.; Berk, M.; Birmaher, B.; Vieta, E. Bipolar Disorder. Lancet Lond. Engl. 2016, 387, 1561–1572. [Google Scholar] [CrossRef]
- Ramadan, S.; Tay, L.; Kaur, H.; Parrish, T.; Abdelmoteleb, S.; Totlani, J.; Tadros, E.; Hirsch, D.; Murphy, N.; Meyer, A.; et al. Bipolar Disorder: Systematic Review of Approved Psychiatric Medications (2008-2024) and Pipeline Phase-3 Medications. J. Affect. Disord. 2025, 390, 119778. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 Diabetes: Principles of Pathogenesis and Therapy. Lancet Lond. Engl. 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Młynarska, E.; Czarnik, W.; Dzieża, N.; Jędraszak, W.; Majchrowicz, G.; Prusinowski, F.; Stabrawa, M.; Rysz, J.; Franczyk, B. Type 2 Diabetes Mellitus: New Pathogenetic Mechanisms, Treatment and the Most Important Complications. Int. J. Mol. Sci. 2025, 26, 1094. [Google Scholar] [CrossRef]
- Strain, W.D.; Paldánius, P.M. Diabetes, Cardiovascular Disease and the Microcirculation. Cardiovasc. Diabetol. 2018, 17, 57. [Google Scholar] [CrossRef]
- Miola, A.; De Filippis, E.; Veldic, M.; Ho, A.M.-C.; Winham, S.J.; Mendoza, M.; Romo-Nava, F.; Nunez, N.A.; Gardea Resendez, M.; Prieto, M.L.; et al. The Genetics of Bipolar Disorder with Obesity and Type 2 Diabetes. J. Affect. Disord. 2022, 313, 222–231. [Google Scholar] [CrossRef]
- Sharma, A.N.; Bauer, I.E.; Sanches, M.; Galvez, J.F.; Zunta-Soares, G.B.; Quevedo, J.; Kapczinski, F.; Soares, J.C. Common Biological Mechanisms between Bipolar Disorder and Type 2 Diabetes: Focus on Inflammation. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 54, 289–298. [Google Scholar] [CrossRef]
- Hajek, T.; McIntyre, R.; Alda, M. Bipolar Disorders, Type 2 Diabetes Mellitus, and the Brain. Curr. Opin. Psychiatry 2016, 29, 1–6. [Google Scholar] [CrossRef]
- Ringin, E.; Dunstan, D.W.; McIntyre, R.S.; Berk, M.; Owen, N.; Rossell, S.L.; Van Rheenen, T.E. Interactive Relationships of Type 2 Diabetes and Bipolar Disorder with Cognition: Evidence of Putative Premature Cognitive Ageing in the UK Biobank Cohort. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2023, 48, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Calkin, C.V.; Gardner, D.M.; Ransom, T.; Alda, M. The Relationship between Bipolar Disorder and Type 2 Diabetes: More than Just Co-Morbid Disorders. Ann. Med. 2013, 45, 171–181. [Google Scholar] [CrossRef]
- Onisiforou, A.; Zanos, P. One Path, Two Solutions: Network-Based Analysis Identifies Targetable Pathways for the Treatment of Comorbid Type II Diabetes and Neuropsychiatric Disorders. Comput. Struct. Biotechnol. J. 2024, 23, 3610–3624. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.; Campbell, H. Mechanisms of Insulin Resistance, Mitochondrial Dysfunction and the Action of the Ketogenic Diet in Bipolar Disorder. Focus on the PI3K/AKT/HIF1-a Pathway. Med. Hypotheses 2020, 145, 110299. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Bañuls, C.; Diaz-Morales, N.; Hernandez-Mijares, A.; Rocha, M.; Victor, V.M. Mitochondrial Dynamics in Type 2 Diabetes: Pathophysiological Implications. Redox Biol. 2017, 11, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Pinti, M.V.; Fink, G.K.; Hathaway, Q.A.; Durr, A.J.; Kunovac, A.; Hollander, J.M. Mitochondrial Dysfunction in Type 2 Diabetes Mellitus: An Organ-Based Analysis. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E268–E285. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Baldassari, F.; Bononi, A.; Bonora, M.; De Marchi, E.; Marchi, S.; Missiroli, S.; Patergnani, S.; Rimessi, A.; Suski, J.M.; et al. Mitochondrial Ca2+ and Apoptosis. Cell Calcium 2012, 52, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Starnawska, A.; Demontis, D.; Pen, A.; Hedemand, A.; Nielsen, A.L.; Staunstrup, N.H.; Grove, J.; Als, T.D.; Jarram, A.; O’Brien, N.L.; et al. CACNA1C Hypermethylation Is Associated with Bipolar Disorder. Transl. Psychiatry 2016, 6, e831. [Google Scholar] [CrossRef]
- Gilon, P.; Chae, H.-Y.; Rutter, G.A.; Ravier, M.A. Calcium Signaling in Pancreatic β-Cells in Health and in Type 2 Diabetes. Cell Calcium 2014, 56, 340–361. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Zhang, Y.; Wu, L.; Dong, S.; Wu, L.; Wu, L.; Du, S.; Zhang, Y.; Ma, D. PI3K/Akt Pathway-Mediated HO-1 Induction Regulates Mitochondrial Quality Control and Attenuates Endotoxin-Induced Acute Lung Injury. Lab. Investig. J. Tech. Methods Pathol. 2019, 99, 1795–1809. [Google Scholar] [CrossRef]
- Snitow, M.E.; Bhansali, R.S.; Klein, P.S. Lithium and Therapeutic Targeting of GSK-3. Cells 2021, 10, 255. [Google Scholar] [CrossRef]
- Sharma, M.D.; Garber, A.J.; Farmer, J.A. Role of Insulin Signaling in Maintaining Energy Homeostasis. Endocr. Pract. 2008, 14, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Cairns, K.; McCarvill, T.; Ruzickova, M.; Calkin, C.V. Course of Bipolar Illness Worsens after Onset of Insulin Resistance. J. Psychiatr. Res. 2018, 102, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Greenberg, M.L. Inositol Depletion, GSK3 Inhibition and Bipolar Disorder. Future Neurol. 2016, 11, 135–148. [Google Scholar] [CrossRef]
- Yildiz, A.; Sachs, G.S.; Dorer, D.J.; Renshaw, P.F. 31P Nuclear Magnetic Resonance Spectroscopy Findings in Bipolar Illness: A Meta-Analysis. Psychiatry Res. 2001, 106, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Shioiri, T.; Takahashi, S.; Inubushi, T. Measurement of Brain Phosphoinositide Metabolism in Bipolar Patients Using in Vivo 31P-MRS. J. Affect. Disord. 1991, 22, 185–190. [Google Scholar] [CrossRef]
- Kato, T.; Shioiri, T.; Murashita, J.; Hamakawa, H.; Inubushi, T.; Takahashi, S. Phosphorus-31 Magnetic Resonance Spectroscopy and Ventricular Enlargement in Bipolar Disorder. Psychiatry Res. 1994, 55, 41–50. [Google Scholar] [CrossRef]
- Davanzo, P.; Thomas, M.A.; Yue, K.; Oshiro, T.; Belin, T.; Strober, M.; McCracken, J. Decreased Anterior Cingulate Myo-Inositol/Creatine Spectroscopy Resonance with Lithium Treatment in Children with Bipolar Disorder. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2001, 24, 359–369. [Google Scholar] [CrossRef]
- Shimon, H.; Agam, G.; Belmaker, R.H.; Hyde, T.M.; Kleinman, J.E. Reduced Frontal Cortex Inositol Levels in Postmortem Brain of Suicide Victims and Patients with Bipolar Disorder. Am. J. Psychiatry 1997, 154, 1148–1150. [Google Scholar] [CrossRef]
- Chalecka-Franaszek, E.; Chuang, D.M. Lithium Activates the Serine/Threonine Kinase Akt-1 and Suppresses Glutamate-Induced Inhibition of Akt-1 Activity in Neurons. Proc. Natl. Acad. Sci. USA 1999, 96, 8745–8750. [Google Scholar] [CrossRef]
- Plum, L.; Schubert, M.; Brüning, J.C. The Role of Insulin Receptor Signaling in the Brain. Trends Endocrinol. Metab. 2005, 16, 59–65. [Google Scholar] [CrossRef]
- Chatterjee, D.; Beaulieu, J.M. Inhibition of Glycogen Synthase Kinase 3 by Lithium, a Mechanism in Search of Specificity. Front. Mol. Neurosci. 2022, 15, 1028963. [Google Scholar] [CrossRef]
- Klein, P.S.; Melton, D.A. A Molecular Mechanism for the Effect of Lithium on Development. Proc. Natl. Acad. Sci. USA 1996, 93, 8455–8459. [Google Scholar] [CrossRef]
- Stambolic, V.; Ruel, L.; Woodgett, J.R. Lithium Inhibits Glycogen Synthase Kinase-3 Activity and Mimics Wingless Signalling in Intact Cells. Curr. Biol. CB 1996, 6, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Németh, Z.H.; Deitch, E.A.; Szabó, C.; Fekete, Z.; Hauser, C.J.; Haskó, G. Lithium Induces NF-Kappa B Activation and Interleukin-8 Production in Human Intestinal Epithelial Cells. J. Biol. Chem. 2002, 277, 7713–7719. [Google Scholar] [CrossRef]
- Trepiccione, F.; Pisitkun, T.; Hoffert, J.D.; Poulsen, S.B.; Capasso, G.; Nielsen, S.; Knepper, M.A.; Fenton, R.A.; Christensen, B.M. Early Targets of Lithium in Rat Kidney Inner Medullary Collecting Duct Include P38 and ERK1/2. Kidney Int. 2014, 86, 757–767. [Google Scholar] [CrossRef]
- Kubota, H.; Noguchi, R.; Toyoshima, Y.; Ozaki, Y.-I.; Uda, S.; Watanabe, K.; Ogawa, W.; Kuroda, S. Temporal Coding of Insulin Action through Multiplexing of the AKT Pathway. Mol. Cell 2012, 46, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.R.; Shanley, D.P. Computational Modelling of the Regulation of Insulin Signalling by Oxidative Stress. BMC Syst. Biol. 2013, 7, 41. [Google Scholar] [CrossRef]
- Kearney, A.L.; Norris, D.M.; Ghomlaghi, M.; Kin Lok Wong, M.; Humphrey, S.J.; Carroll, L.; Yang, G.; Cooke, K.C.; Yang, P.; Geddes, T.A.; et al. Akt Phosphorylates Insulin Receptor Substrate to Limit PI3K-Mediated PIP3 Synthesis. eLife 2021, 10, e66942. [Google Scholar] [CrossRef]
- Falkenburger, B.H.; Jensen, J.B.; Hille, B. Kinetics of PIP2 Metabolism and KCNQ2/3 Channel Regulation Studied with a Voltage-Sensitive Phosphatase in Living Cells. J. Gen. Physiol. 2010, 135, 99–114. [Google Scholar] [CrossRef]
- Borisov, N.; Aksamitiene, E.; Kiyatkin, A.; Legewie, S.; Berkhout, J.; Maiwald, T.; Kaimachnikov, N.P.; Timmer, J.; Hoek, J.B.; Kholodenko, B.N. Systems-Level Interactions between Insulin-EGF Networks Amplify Mitogenic Signaling. Mol. Syst. Biol. 2009, 5, 256. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, A.R.; Sherman, A.; Quon, M.J. A Mathematical Model of Metabolic Insulin Signaling Pathways. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1084–E1101. [Google Scholar] [CrossRef]
- Mazet, F.; Tindall, M.J.; Gibbins, J.M.; Fry, M.J. A Model of the PI Cycle Reveals the Regulating Roles of Lipid-Binding Proteins and Pitfalls of Using Mosaic Biological Data. Sci. Rep. 2020, 10, 13244. [Google Scholar] [CrossRef]
- Wei, X.N.; Han, B.C.; Zhang, J.X.; Liu, X.H.; Tan, C.Y.; Jiang, Y.Y.; Low, B.C.; Tidor, B.; Chen, Y.Z. An Integrated Mathematical Model of Thrombin-, Histamine-and VEGF-Mediated Signalling in Endothelial Permeability. BMC Syst. Biol. 2011, 5, 112. [Google Scholar] [CrossRef]
- Suh, B.-C.; Horowitz, L.F.; Hirdes, W.; Mackie, K.; Hille, B. Regulation of KCNQ2/KCNQ3 Current by G Protein Cycling. J. Gen. Physiol. 2004, 123, 663–683. [Google Scholar] [CrossRef]
- Li, H.; Ung, C.Y.; Ma, X.H.; Li, B.W.; Low, B.C.; Cao, Z.W.; Chen, Y.Z. Simulation of Crosstalk between Small GTPase RhoA and EGFR-ERK Signaling Pathway via MEKK1. Bioinformatics 2009, 25, 358–364. [Google Scholar] [CrossRef]
- Ou, A.H.; Rosenthal, S.B.; Adli, M.; Akiyama, K.; Akula, N.; Alda, M.; Amare, A.T.; Ardau, R.; Arias, B.; Aubry, J.-M.; et al. Lithium Response in Bipolar Disorder Is Associated with Focal Adhesion and PI3K-Akt Networks: A Multi-Omics Replication Study. Transl. Psychiatry 2024, 14, 109. [Google Scholar] [CrossRef] [PubMed]
- De-Paula, V.J.; Forlenza, O.V. Lithium Modulates Multiple Tau Kinases with Distinct Effects in Cortical and Hippocampal Neurons According to Concentration Ranges. Naunyn. Schmiedebergs Arch. Pharmacol. 2022, 395, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.C.; Mallinger, A.G.; Dippold, C.S.; Forster Wells, K.; Frank, E.; Kupfer, D.J. Effects of Lithium on Platelet Membrane Phosphoinositides in Bipolar Disorder Patients: A Pilot Study. Psychopharmacology 2000, 149, 12–16. [Google Scholar] [CrossRef]
- Spanoudaki, M.; Papadopoulou, S.K.; Antasouras, G.; Papadopoulos, K.A.; Psara, E.; Vorvolakos, T.; Solovos, E.; Chrysafi, M.; Psallas, M.; Mentzelou, M.; et al. Curcumin as a Multifunctional Spice Ingredient against Mental Disorders in Humans: Current Clinical Studies and Bioavailability Concerns. Life Basel Switz. 2024, 14, 479. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ding, L.; Cao, S.; Feng, X.; Zhang, Q.; Chen, Y.; Zhang, N.; Qiu, F. Curcumin Metabolites Contribute to the Effect of Curcumin on Ameliorating Insulin Sensitivity in High-Glucose-Induced Insulin-Resistant HepG2 Cells. J. Ethnopharmacol. 2020, 259, 113015. [Google Scholar] [CrossRef]
- Bustanji, Y.; Taha, M.O.; Almasri, I.M.; Al-Ghussein, M.A.S.; Mohammad, M.K.; Alkhatib, H.S. Inhibition of Glycogen Synthase Kinase by Curcumin: Investigation by Simulated Molecular Docking and Subsequent in Vitro/in Vivo Evaluation. J. Enzyme Inhib. Med. Chem. 2009, 24, 771–778. [Google Scholar] [CrossRef]
- Li, B.; Ren, J.; Yang, L.; Li, X.; Sun, G.; Xia, M. Lithium Inhibits GSK3β Activity via Two Different Signaling Pathways in Neurons After Spinal Cord Injury. Neurochem. Res. 2018, 43, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.; Du, J. Potential Role of Akt Signaling in Chronic Kidney Disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2015, 30, 385–394. [Google Scholar] [CrossRef]
- Rej, S.; Pira, S.; Marshe, V.; Do, A.; Elie, D.; Looper, K.J.; Herrmann, N.; Müller, D.J. Molecular Mechanisms in Lithium-Associated Renal Disease: A Systematic Review. Int. Urol. Nephrol. 2016, 48, 1843–1853. [Google Scholar] [CrossRef]
- Negi, G.; Sharma, S.S. Inhibition of IκB Kinase (IKK) Protects against Peripheral Nerve Dysfunction of Experimental Diabetes. Mol. Neurobiol. 2015, 51, 591–598. [Google Scholar] [CrossRef]
- Shin, C.H.; Choi, D.-S. Essential Roles for the Non-Canonical IκB Kinases in Linking Inflammation to Cancer, Obesity, and Diabetes. Cells 2019, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT Pathway in Obesity and Type 2 Diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, W.; Zhang, Z.; He, M.; Zhang, Y.; Song, B.; Liu, J.; Zhang, H. FPS-ZM1 Attenuates the Deposition of Lipid in the Liver of Diabetic Mice by Sterol Regulatory Element Binding Protein-1c. BMC Endocr. Disord. 2024, 24, 164. [Google Scholar] [CrossRef]
- Im, K.; Maliakel, A.; Gopakumar, G.; Kumar, D.; Maliakel, B.; Kuttan, R. Improved Blood–Brain-Barrier Permeability and Tissue Distribution Following the Oral Administration of a Food-Grade Formulation of Curcumin with Fenugreek Fibre. J. Funct. Foods 2015, 14, 215–225. [Google Scholar] [CrossRef]
- Osete, J.R.; Akkouh, I.A.; Ievglevskyi, O.; Vandenberghe, M.; de Assis, D.R.; Ueland, T.; Kondratskaya, E.; Holen, B.; Szabo, A.; Hughes, T.; et al. Transcriptional and Functional Effects of Lithium in Bipolar Disorder iPSC-Derived Cortical Spheroids. Mol. Psychiatry 2023, 28, 3033–3043. [Google Scholar] [CrossRef]
- Kakkar, V.; Kaur, I.P. Evaluating Potential of Curcumin Loaded Solid Lipid Nanoparticles in Aluminium Induced Behavioural, Biochemical and Histopathological Alterations in Mice Brain. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2011, 49, 2906–2913. [Google Scholar] [CrossRef] [PubMed]
- Askarizadeh, A.; Barreto, G.E.; Henney, N.C.; Majeed, M.; Sahebkar, A. Neuroprotection by Curcumin: A Review on Brain Delivery Strategies. Int. J. Pharm. 2020, 585, 119476. [Google Scholar] [CrossRef] [PubMed]
| Chemical Reactions | Kf (nM−1·s−1 or s−1) | Kb (nM−1·s−1 or s−1) | References | |
|---|---|---|---|---|
| 1 | I + IR = IRL | k1_1: 3.33 × 10−4 | k1_2: 1 | [40] |
| 2 | IRL -> IRp | k2: 1.66 | - | [40] |
| 3 | IRp -> IRi | v = Vmax × IRp/(Km + IRp), Vmax = 333 nM−1s−1, Km = 266 nM | [40] | |
| 4 | IRi -> IRL | k4: 0.0166 | - | [40] |
| 5 | IRp -> Null | k5: 1.85 × 10−4 | - | [41] |
| 6 | IRp + IRS = IRp-IRS | k6_1: 0.1066 | k6_2: 3.75 | [40] |
| 7 | IRp-IRS -> IRS | k7: 1.85 × 10−4 | - | [41] |
| 8 | IRp-IRS -> IRp-IRSp | k8: 0.66 | - | [40] |
| 9 | IRp-IRSp + PTP1B -> IRp-IRS | k9: 8.75 × 10−5 | - | [37] |
| 10 | IRp-IRSp -> Null | k10: 0.001 | - | [37] |
| 11 | IRp-IRSp + PI3K = IRSp-PI3K | k11_1: 2.6 × 10−6 * | k11_2: 1.55 * | [37] |
| 12 | IRSp-PI3K + PIP2 -> PIP3 | k12: 0.055 | - | [37,41] |
| 13 | PIP3 + PTEN -> PIP2 | k13: 0.7025 | - | [41] |
| 14 | AKT + PIP3 -> pAKT | k14: 3.36 | - | [38] |
| 15 | pAKT -> AKT | k15: 1.188 × 10−6 | - | [37] |
| 16 | ppAKT + GSK3β -> pGSK3β | k16: 0.05 | - | [36] |
| 17 | pGSK3β -> GSK3β | k17: 0.015 | - | [36] |
| 18 | PIP2 -> IP3 + DAG | k18: 500.25 | - | [42] |
| 19 | IP3 -> Ins | k19: 10 | - | [43] |
| 20 | Ins -> PI | k20: 0.7081 | - | [42] |
| 21 | PI = PIP | k21_1: 0.4 | [44] | |
| 22 | PIP = PIP2 | k22_1: 60 | k22_2: 5 | [44] |
| 23 | G = G-a | k23_1: 5.18 × 10−6 | k23_2: 0.085 | [42] |
| 24 | PIP2 + G-a -> DAG | k24: 77.16 | - | [42] |
| 25 | DAG -> PIP2 | k25: 0.0077 | - | [42] |
| 26 | DAG + PKC -> PKC-a | k26: 1.311 | - | [42] |
| 27 | PKC-a -> PKC | k27: 0.0295 | - | [42] |
| 28 | PIP3 -> Null | 3.33 | [45] |
| PI3K_PI Normal | PI3K_PI Li | PI3K Normal | PI3K Li | |
|---|---|---|---|---|
| AKT | 473.58 | 818.24 | 162.00 | 571.93 |
| pAKT | 8.92 | 19.26 | 9.37 | 10.48 |
| pAKT% | 1.88% | 2.35% | 5.78% | 1.83% |
| GSK3β | 3931.82 | 2327.35 | 3924.94 | 3896.44 |
| pGSK3β | 268.18 | 327.48 | 275.06 | 303.57 |
| pGSK3β% | 6.82% | 14.07% | 7.01% | 7.79% |
| Control | AKT Modulation | PIP2 Modulation | GSK3β-Li Binding | Total Effects | |
|---|---|---|---|---|---|
| GSK3β-Li Complex | 0.00 | 0.00 | 0.00 | 1568.92 | 1545.16 |
| AKT | 473.58 | 782.62 | 496.51 | 473.58 | 818.24 |
| pAKT | 8.92 | 11.66 | 8.90 | 14.82 | 19.26 |
| pAKT% | 1.88% | 1.49% | 1.79% | 1.88% | 2.35% |
| GSK3β | 3931.82 | 3865.00 | 3935.19 | 2363.27 | 2327.35 |
| pGSK3β | 268.18 | 335.00 | 264.81 | 267.81 | 327.48 |
| pGSK3β% | 6.82% | 8.67% | 6.73% | 11.33% | 14.07% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, W.; Wang, Y.; Hao, Y.; Fu, L.; Liu, X. Computational Modeling of PI3K/AKT Pathway in Bipolar Disorder and Type 2 Diabetes: Implications for Lithium Treatment and Curcumin as a Potential Alternative. Int. J. Mol. Sci. 2025, 26, 10026. https://doi.org/10.3390/ijms262010026
Li J, Wang W, Wang Y, Hao Y, Fu L, Liu X. Computational Modeling of PI3K/AKT Pathway in Bipolar Disorder and Type 2 Diabetes: Implications for Lithium Treatment and Curcumin as a Potential Alternative. International Journal of Molecular Sciences. 2025; 26(20):10026. https://doi.org/10.3390/ijms262010026
Chicago/Turabian StyleLi, Jing, Wenqing Wang, Yajunzi Wang, Yang Hao, Lei Fu, and Xin Liu. 2025. "Computational Modeling of PI3K/AKT Pathway in Bipolar Disorder and Type 2 Diabetes: Implications for Lithium Treatment and Curcumin as a Potential Alternative" International Journal of Molecular Sciences 26, no. 20: 10026. https://doi.org/10.3390/ijms262010026
APA StyleLi, J., Wang, W., Wang, Y., Hao, Y., Fu, L., & Liu, X. (2025). Computational Modeling of PI3K/AKT Pathway in Bipolar Disorder and Type 2 Diabetes: Implications for Lithium Treatment and Curcumin as a Potential Alternative. International Journal of Molecular Sciences, 26(20), 10026. https://doi.org/10.3390/ijms262010026






