Comparative Efficacy of CK2 Inhibitors CX-4945 and SGC-CK2-2 on CK2 Signaling
Abstract
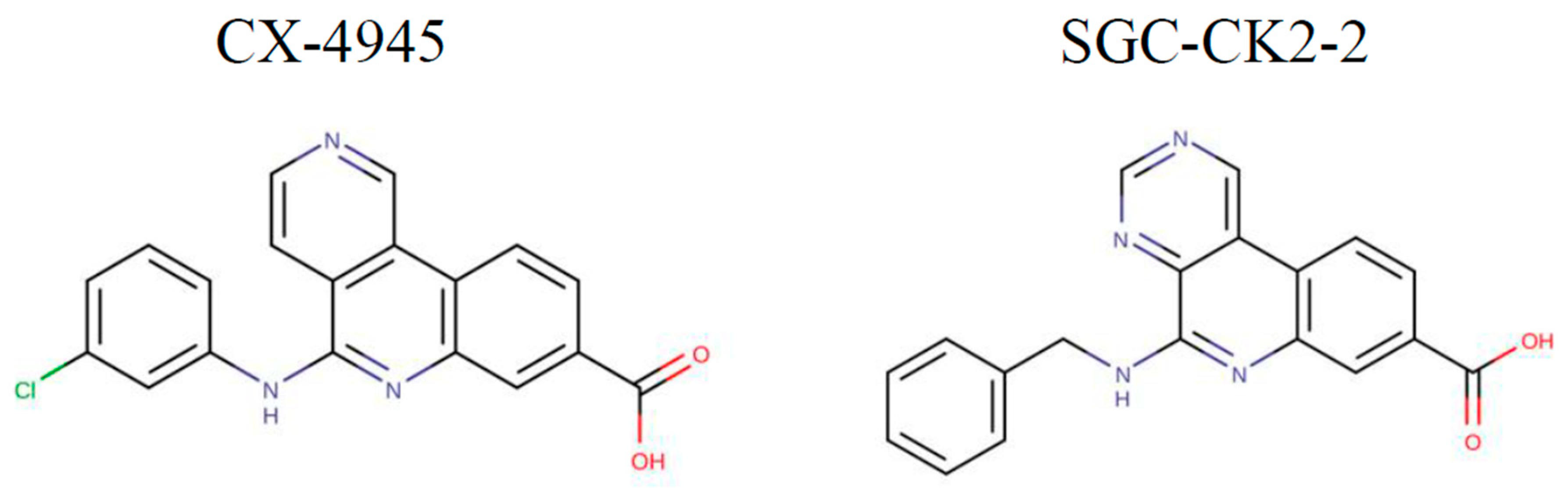
1. Introduction
2. Results
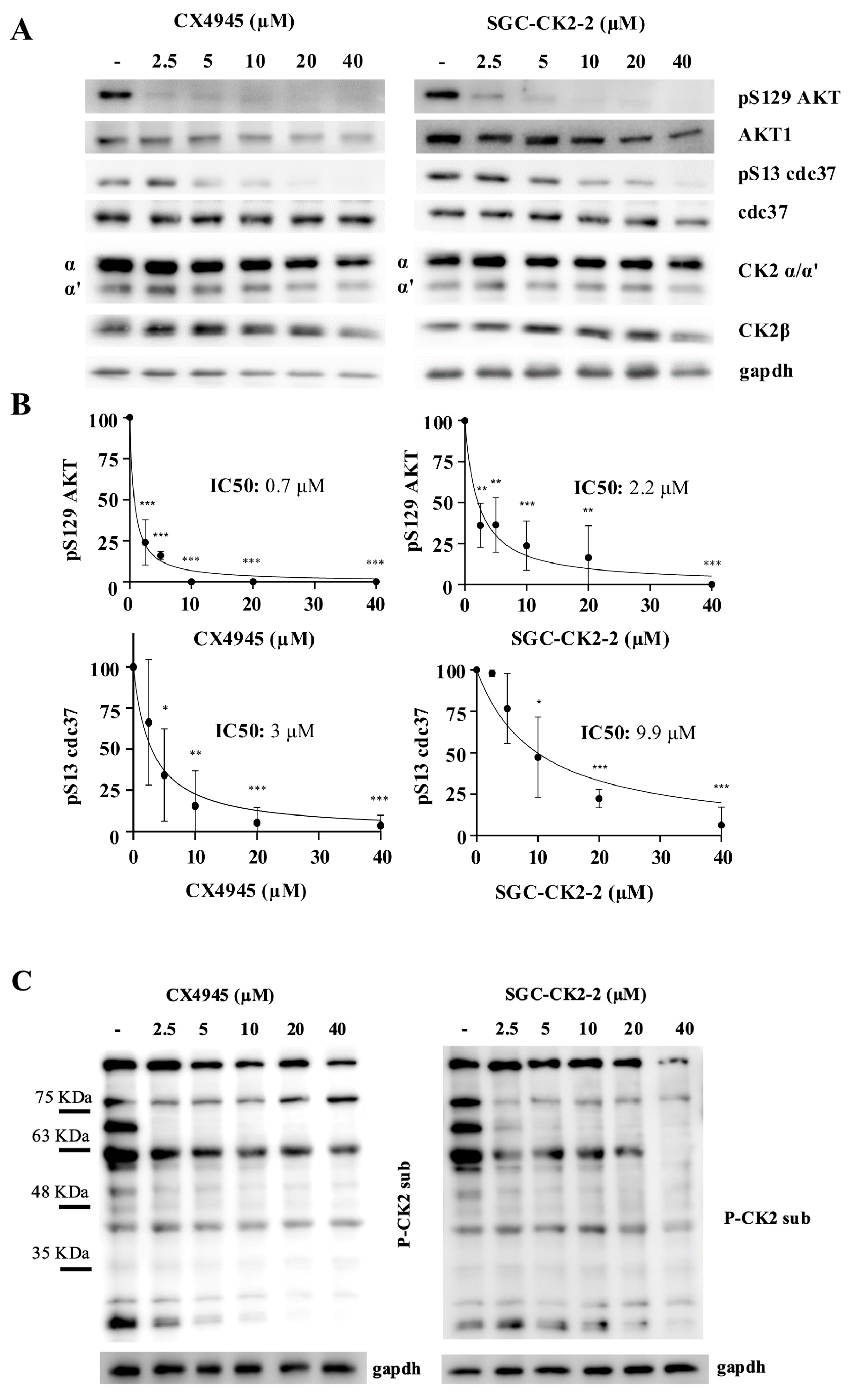
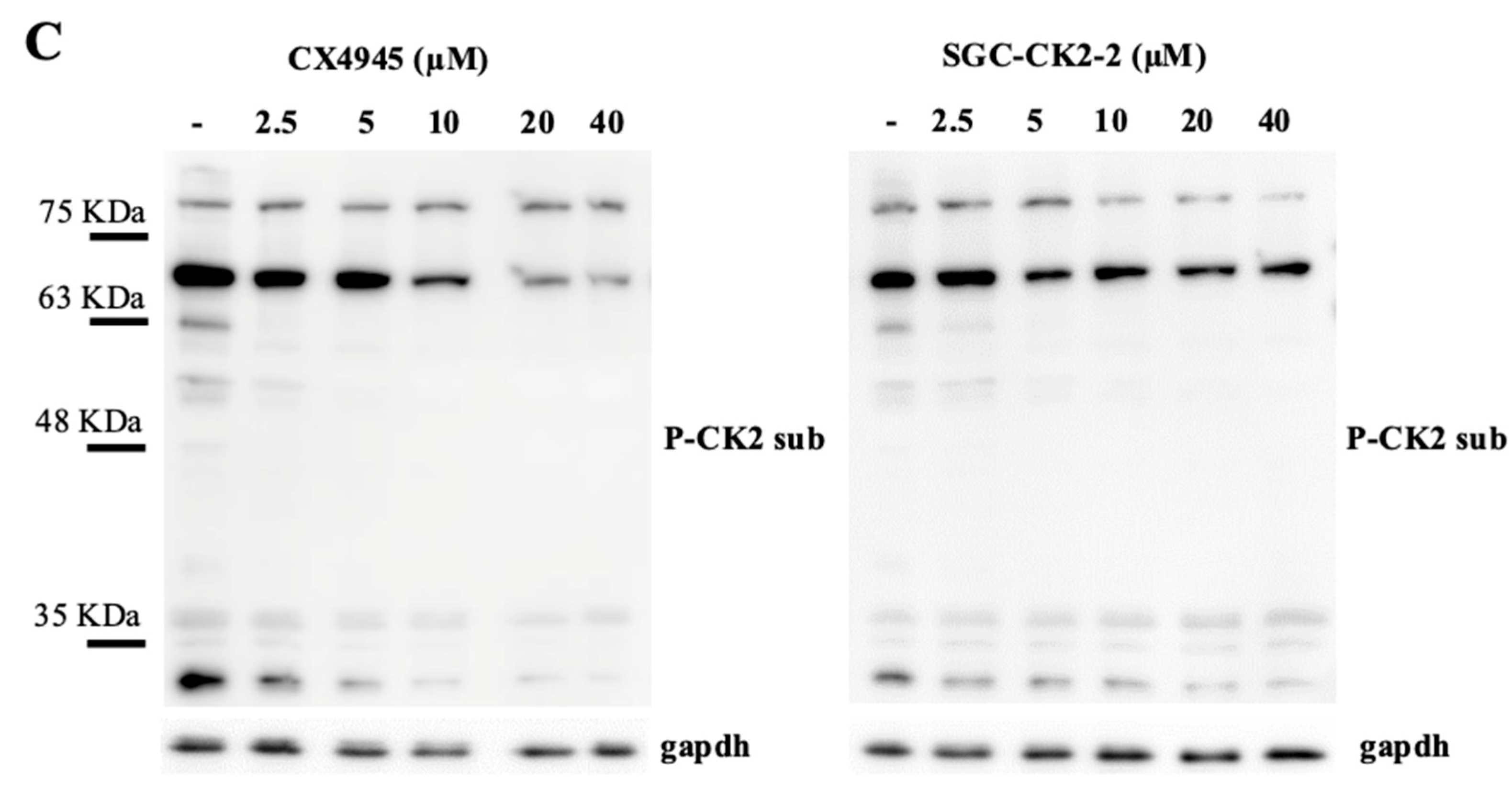
2.1. Inhibition of CK2 Substrates with CX-4945 and SGC-CK2-2 Reveals Target-Specific Sensitivities
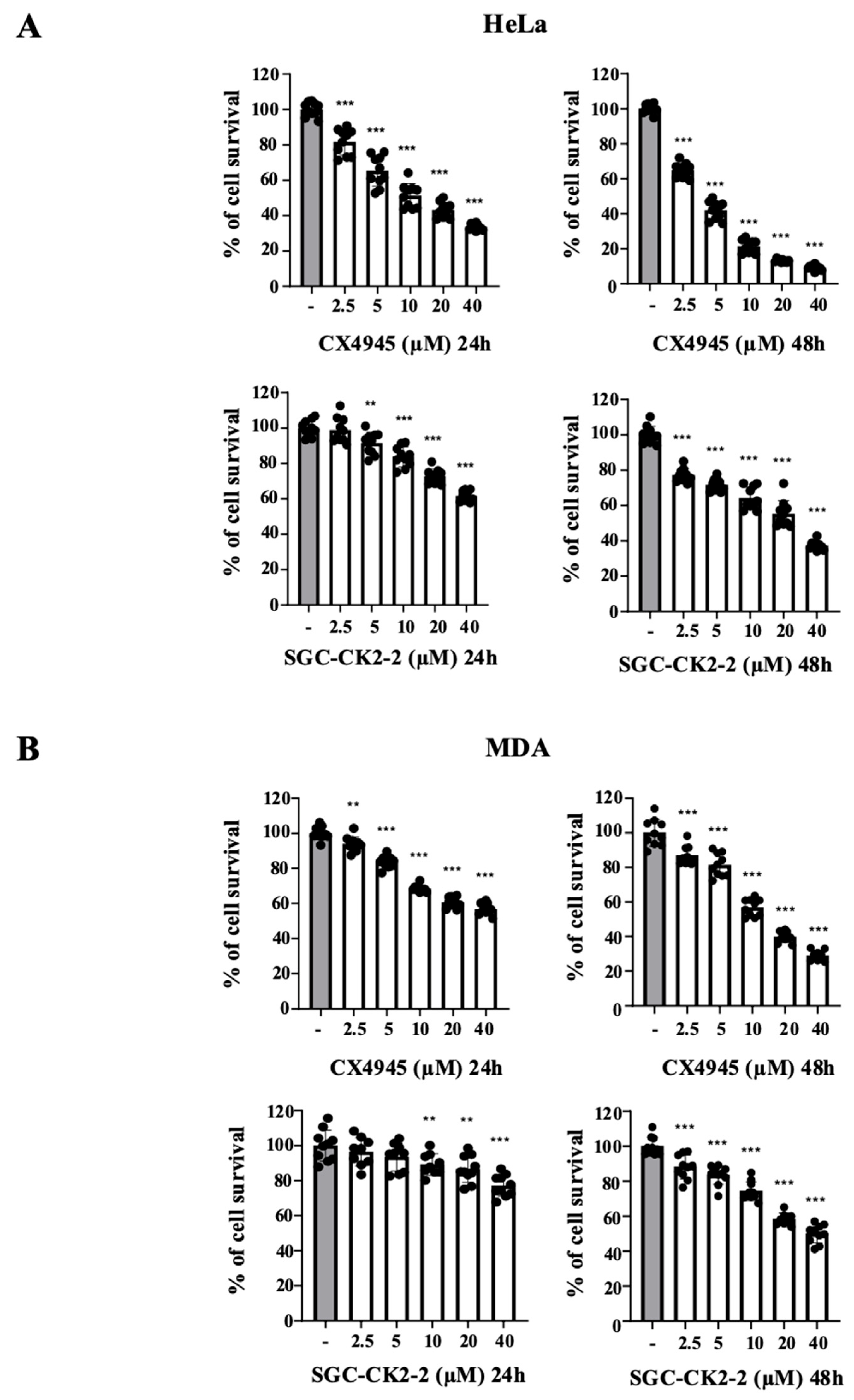
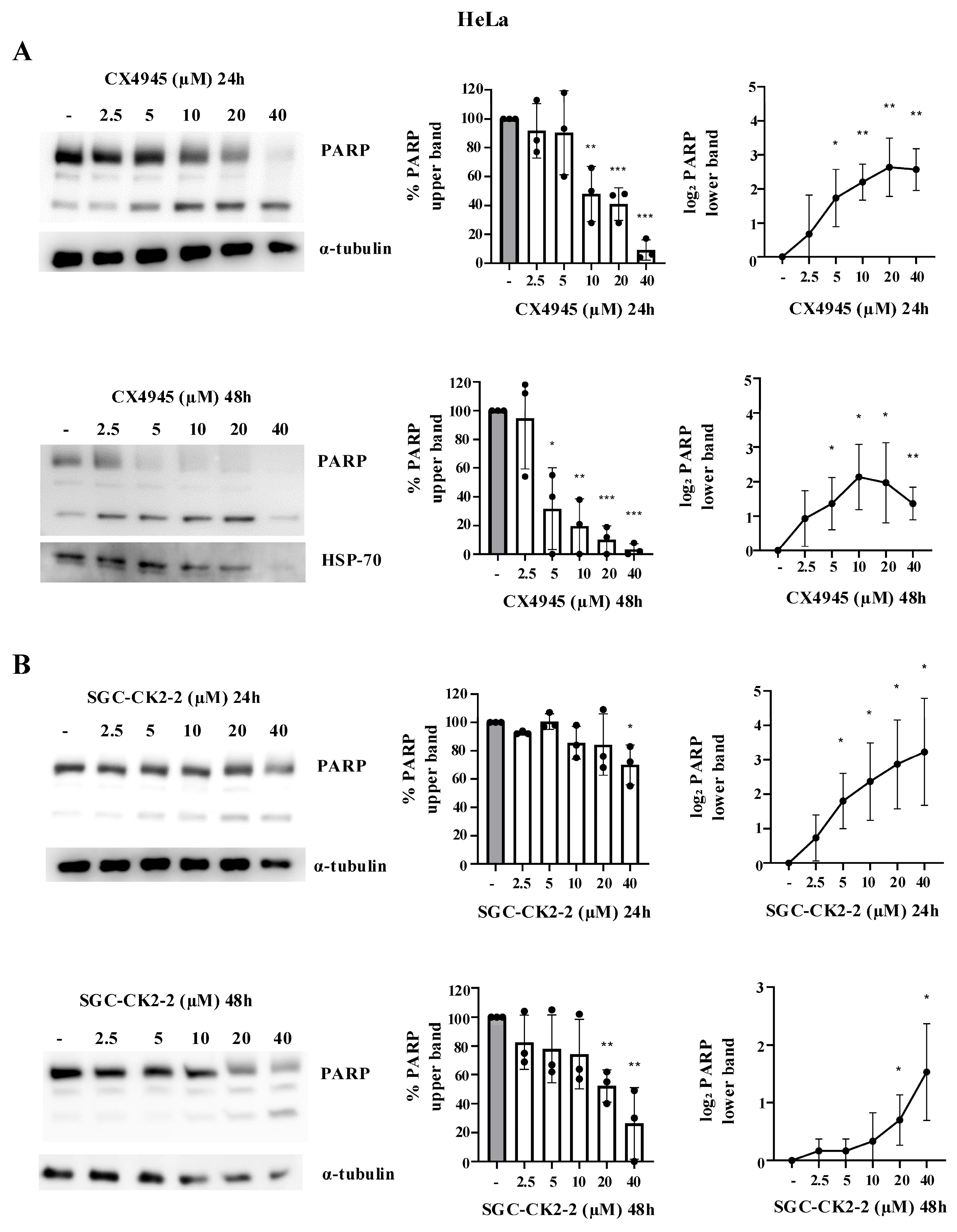
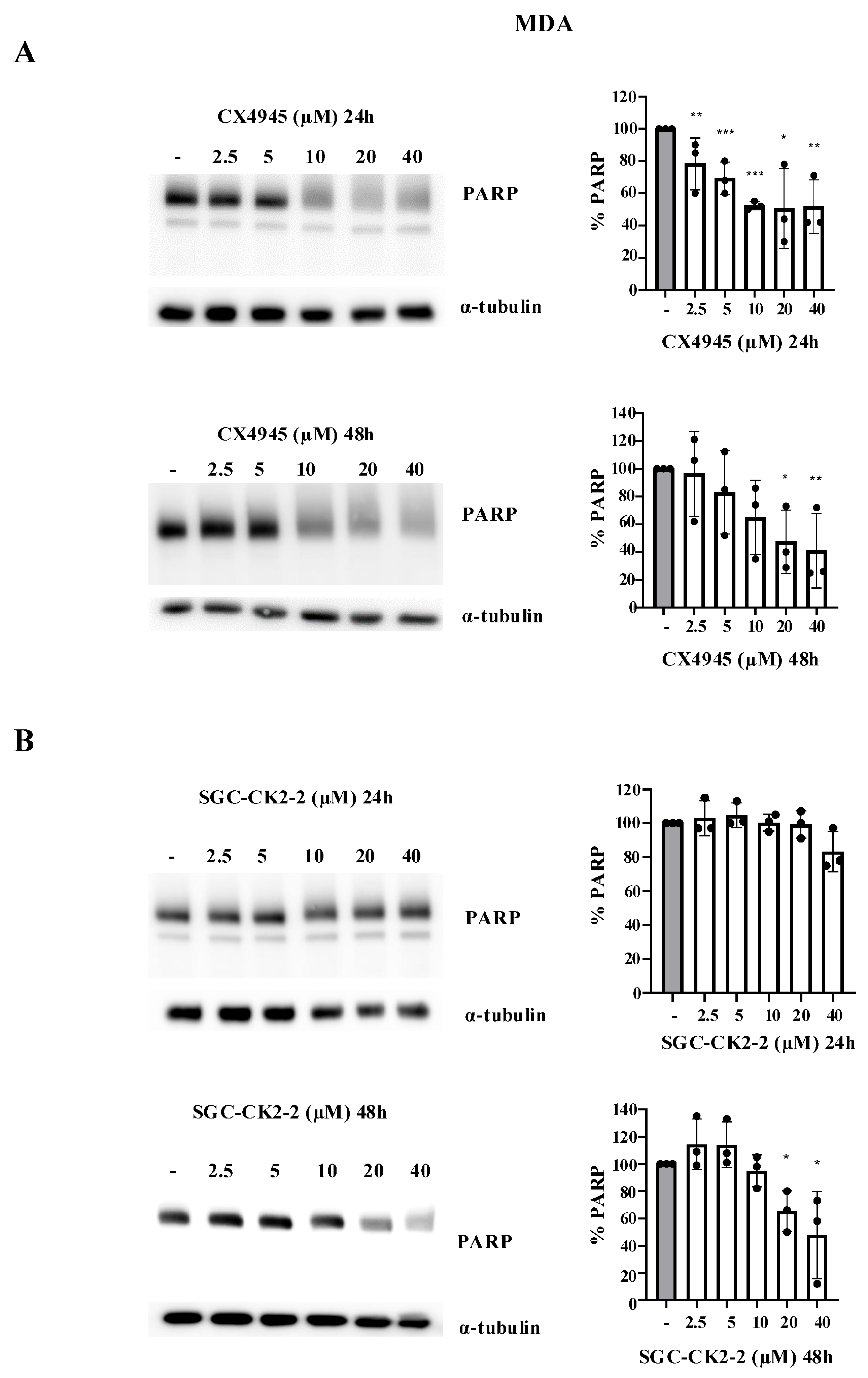
2.2. CX-4945 Strongly Affects Cell Viability in a Dose-Dependent Manner Compared with SGC-CK2-2
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Lysis and Western Blotting
4.4. MTT Assay
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borgo, C.; D’Amore, C.; Sarno, S.; Salvi, M.; Ruzzene, M. Protein Kinase CK2: A Potential Therapeutic Target for Diverse Human Diseases. Signal Transduct. Target. Ther. 2021, 6, 183. [Google Scholar] [CrossRef]
- Strum, S.W.; Gyenis, L.; Litchfield, D.W. CSNK2 in Cancer: Pathophysiology and Translational Applications. Br. J. Cancer 2022, 126, 994–1003. [Google Scholar] [CrossRef]
- Trembley, J.H.; Kren, B.T.; Afzal, M.; Scaria, G.A.; Klein, M.A.; Ahmed, K. Protein Kinase CK2—Diverse Roles in Cancer Cell Biology and Therapeutic Promise. Mol. Cell. Biochem. 2023, 478, 899–926. [Google Scholar] [CrossRef] [PubMed]
- Pandit, V.; DeGeorge, K.; Nohe, A. Scoping Pleiotropy of CK2 in Musculoskeletal Disorders for a Novel Targeting Approach. Kinases Phosphatases 2024, 2, 43–66. [Google Scholar] [CrossRef]
- Quezada Meza, C.P.; Ruzzene, M. Protein Kinase CK2 and SARS-CoV-2: An Expected Interplay Story. Kinases Phosphatases 2023, 1, 141–150. [Google Scholar] [CrossRef]
- Baier, A.; Szyszka, R. CK2 and Protein Kinases of the CK1 Superfamily as Targets for Neurodegenerative Disorders. Front. Mol. Biosci. 2022, 9, 916063. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, E.; Nalbach, L.; Menger, M.D.; Montenarh, M.; Götz, C. Protein Kinase CK2-A Putative Target for the Therapy of Diabetes Mellitus? Int. J. Mol. Sci. 2019, 20, 4398. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wei, H.; Benavides, G.A.; Turbitt, W.J.; Buckley, J.A.; Ouyang, X.; Zhou, L.; Zhang, J.; Harrington, L.E.; Darley-Usmar, V.M.; et al. Protein Kinase CK2 Controls CD8+ T Cell Effector and Memory Function during Infection. J. Immunol. 2022, 209, 896–906. [Google Scholar] [CrossRef]
- Silva-Pavez, E.; Tapia, J.C. Protein Kinase CK2 in Cancer Energetics. Front. Oncol. 2020, 10, 893. [Google Scholar] [CrossRef]
- Buontempo, F.; McCubrey, J.A.; Orsini, E.; Ruzzene, M.; Cappellini, A.; Lonetti, A.; Evangelisti, C.; Chiarini, F.; Evangelisti, C.; Barata, J.T.; et al. Therapeutic Targeting of CK2 in Acute and Chronic Leukemias. Leukemia 2018, 32, 1–10. [Google Scholar] [CrossRef]
- Villalobos-Nova, K.; Toro, M.d.l.Á.; Pérez-Moreno, P.; Niechi, I.; Tapia, J.C. The CK2/ECE1c Partnership: An Unveiled Pathway to Aggressiveness in Cancer. Kinases Phosphatases 2024, 2, 1–8. [Google Scholar] [CrossRef]
- Chua, M.M.J.; Ortega, C.E.; Sheikh, A.; Lee, M.; Abdul-Rassoul, H.; Hartshorn, K.L.; Dominguez, I. CK2 in Cancer: Cellular and Biochemical Mechanisms and Potential Therapeutic Target. Pharmaceuticals 2017, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.W.; Drewry, D.H.; Axtman, A.D. CK2 Chemical Probes: Past, Present, and Future. Kinases Phosphatases 2023, 1, 288–305. [Google Scholar] [CrossRef]
- Day-Riley, S.; West, R.M.; Brear, P.D.; Hyvönen, M.; Spring, D.R. CK2 Inhibitors Targeting Inside and Outside the Catalytic Box. Kinases Phosphatases 2024, 2, 110–135. [Google Scholar] [CrossRef]
- Siddiqui-Jain, A.; Drygin, D.; Streiner, N.; Chua, P.; Pierre, F.; O’Brien, S.E.; Bliesath, J.; Omori, M.; Huser, N.; Ho, C.; et al. CX-4945, an Orally Bioavailable Selective Inhibitor of Protein Kinase CK2, Inhibits Prosurvival and Angiogenic Signaling and Exhibits Antitumor Efficacy. Cancer Res. 2010, 70, 10288–10298. [Google Scholar] [CrossRef]
- CX-4945 Granted Orphan Drug Designation. Oncol. Times 2017, 39, 23. [CrossRef]
- Son, Y.H.; Song, J.S.; Kim, S.H.; Kim, J. Pharmacokinetic Characterization of CK2 Inhibitor CX-4945. Arch. Pharmacal Res. 2013, 36, 840–845. [Google Scholar] [CrossRef]
- D’Amore, C.; Borgo, C.; Sarno, S.; Salvi, M. Role of CK2 Inhibitor CX-4945 in Anti-Cancer Combination Therapy—Potential Clinical Relevance. Cell. Oncol. 2020, 43, 1003–1016. [Google Scholar] [CrossRef]
- Grygier, P.; Pustelny, K.; Nowak, J.; Golik, P.; Popowicz, G.M.; Plettenburg, O.; Dubin, G.; Menezes, F.; Czarna, A. Silmitasertib (CX-4945), a Clinically Used CK2-Kinase Inhibitor with Additional Effects on GSK3β and DYRK1A Kinases: A Structural Perspective. J. Med. Chem. 2023, 66, 4009–4024. [Google Scholar] [CrossRef]
- Menyhart, D.; Gyenis, L.; Jurcic, K.; Roffey, S.E.; Puri, A.; Jovanovic, P.; Szkop, K.J.; Pittock, P.; Lajoie, G.; Axtman, A.D.; et al. Comparison of CX-4945 and SGC-CK2-1 as Inhibitors of CSNK2 Using Quantitative Phosphoproteomics: Triple SILAC in Combination with Inhibitor-Resistant CSNK2. Curr. Res. Chem. Biol. 2023, 3, 100041. [Google Scholar] [CrossRef]
- Kim, H.; Choi, K.; Kang, H.; Lee, S.-Y.; Chi, S.-W.; Lee, M.-S.; Song, J.; Im, D.; Choi, Y.; Cho, S. Identification of a Novel Function of CX-4945 as a Splicing Regulator. PLoS ONE 2014, 9, e94978. [Google Scholar] [CrossRef]
- Agnew, C.; Liu, L.; Liu, S.; Xu, W.; You, L.; Yeung, W.; Kannan, N.; Jablons, D.; Jura, N. The Crystal Structure of the Protein Kinase HIPK2 Reveals a Unique Architecture of Its CMGC-Insert Region. J. Biol. Chem. 2019, 294, 13545–13559. [Google Scholar] [CrossRef]
- Cesaro, L.; Zuliani, A.M.; Bosello Travain, V.; Salvi, M. Exploring Protein Kinase CK2 Substrate Recognition and the Dynamic Response of Substrate Phosphorylation to Kinase Modulation. Kinases Phosphatases 2023, 1, 251–264. [Google Scholar] [CrossRef]
- Marin, O.; Meggio, F.; Draetta, G.; Pinna, L.A. The Consensus Sequences for Cdc2 Kinase and for Casein Kinase-2 Are Mutually Incompatible. A Study with Peptides Derived from the Beta-Subunit of Casein Kinase-2. FEBS Lett. 1992, 301, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.I.; Drewry, D.H.; Pickett, J.E.; Tjaden, A.; Krämer, A.; Müller, S.; Gyenis, L.; Menyhart, D.; Litchfield, D.W.; Knapp, S.; et al. Development of a Potent and Selective Chemical Probe for the Pleiotropic Kinase CK2. Cell Chem. Biol. 2021, 28, 546–558.e10. [Google Scholar] [CrossRef]
- Yang, X.; Ong, H.W.; Dickmander, R.J.; Smith, J.L.; Brown, J.W.; Tao, W.; Chang, E.; Moorman, N.J.; Axtman, A.D.; Willson, T.M. Optimization of 3-Cyano-7-Cyclopropylamino-Pyrazolo[1,5-a]Pyrimidines toward the Development of an In Vivo Chemical Probe for CSNK2A. ACS Omega 2023, 8, 39546–39561. [Google Scholar] [CrossRef] [PubMed]
- Davis-Gilbert, Z.W.; Krämer, A.; Dunford, J.E.; Howell, S.; Senbabaoglu, F.; Wells, C.I.; Bashore, F.M.; Havener, T.M.; Smith, J.L.; Hossain, M.A.; et al. Discovery of a Potent and Selective Naphthyridine-Based Chemical Probe for Casein Kinase 2. ACS Med. Chem. Lett. 2023, 14, 432–441. [Google Scholar] [CrossRef]
- Salvi, M.; Borgo, C.; Pinna, L.A.; Ruzzene, M. Targeting CK2 in Cancer: A Valuable Strategy or a Waste of Time? Cell Death Discov. 2021, 7, 325. [Google Scholar] [CrossRef]
- Di Maira, G.; Salvi, M.; Arrigoni, G.; Marin, O.; Sarno, S.; Brustolon, F.; Pinna, L.A.; Ruzzene, M. Protein Kinase CK2 Phosphorylates and Upregulates Akt/PKB. Cell Death Differ. 2005, 12, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Nishida, E. CK2 Controls Multiple Protein Kinases by Phosphorylating a Kinase-Targeting Molecular Chaperone, Cdc37. Mol. Cell. Biol. 2004, 24, 4065–4074. [Google Scholar] [CrossRef]
- Franchin, C.; Borgo, C.; Cesaro, L.; Zaramella, S.; Vilardell, J.; Salvi, M.; Arrigoni, G.; Pinna, L.A. Re-Evaluation of Protein Kinase CK2 Pleiotropy: New Insights Provided by a Phosphoproteomics Analysis of CK2 Knockout Cells. Cell. Mol. Life Sci. 2018, 75, 2011–2026. [Google Scholar] [CrossRef] [PubMed]
- Lettieri, A.; Borgo, C.; Zanieri, L.; D’Amore, C.; Oleari, R.; Paganoni, A.; Pinna, L.A.; Cariboni, A.; Salvi, M. Protein Kinase CK2 Subunits Differentially Perturb the Adhesion and Migration of GN11 Cells: A Model of Immature Migrating Neurons. Int. J. Mol. Sci. 2019, 20, 5951. [Google Scholar] [CrossRef] [PubMed]
- Borgo, C.; D’Amore, C.; Cesaro, L.; Itami, K.; Hirota, T.; Salvi, M.; Pinna, L.A. A N-Terminally Deleted Form of the CK2α’ Catalytic Subunit Is Sufficient to Support Cell Viability. Biochem. Biophys. Res. Commun. 2020, 531, 409–415. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noventa, F.; Venerando, R.; Bosello Travain, V.; Salvi, M. Comparative Efficacy of CK2 Inhibitors CX-4945 and SGC-CK2-2 on CK2 Signaling. Int. J. Mol. Sci. 2025, 26, 10006. https://doi.org/10.3390/ijms262010006
Noventa F, Venerando R, Bosello Travain V, Salvi M. Comparative Efficacy of CK2 Inhibitors CX-4945 and SGC-CK2-2 on CK2 Signaling. International Journal of Molecular Sciences. 2025; 26(20):10006. https://doi.org/10.3390/ijms262010006
Chicago/Turabian StyleNoventa, Francesca, Rina Venerando, Valentina Bosello Travain, and Mauro Salvi. 2025. "Comparative Efficacy of CK2 Inhibitors CX-4945 and SGC-CK2-2 on CK2 Signaling" International Journal of Molecular Sciences 26, no. 20: 10006. https://doi.org/10.3390/ijms262010006
APA StyleNoventa, F., Venerando, R., Bosello Travain, V., & Salvi, M. (2025). Comparative Efficacy of CK2 Inhibitors CX-4945 and SGC-CK2-2 on CK2 Signaling. International Journal of Molecular Sciences, 26(20), 10006. https://doi.org/10.3390/ijms262010006







