Cardiac Troponin I Antibodies Induce Cardiomyocyte Damage and Alter Cell Morphology
Abstract
1. Introduction
2. Results
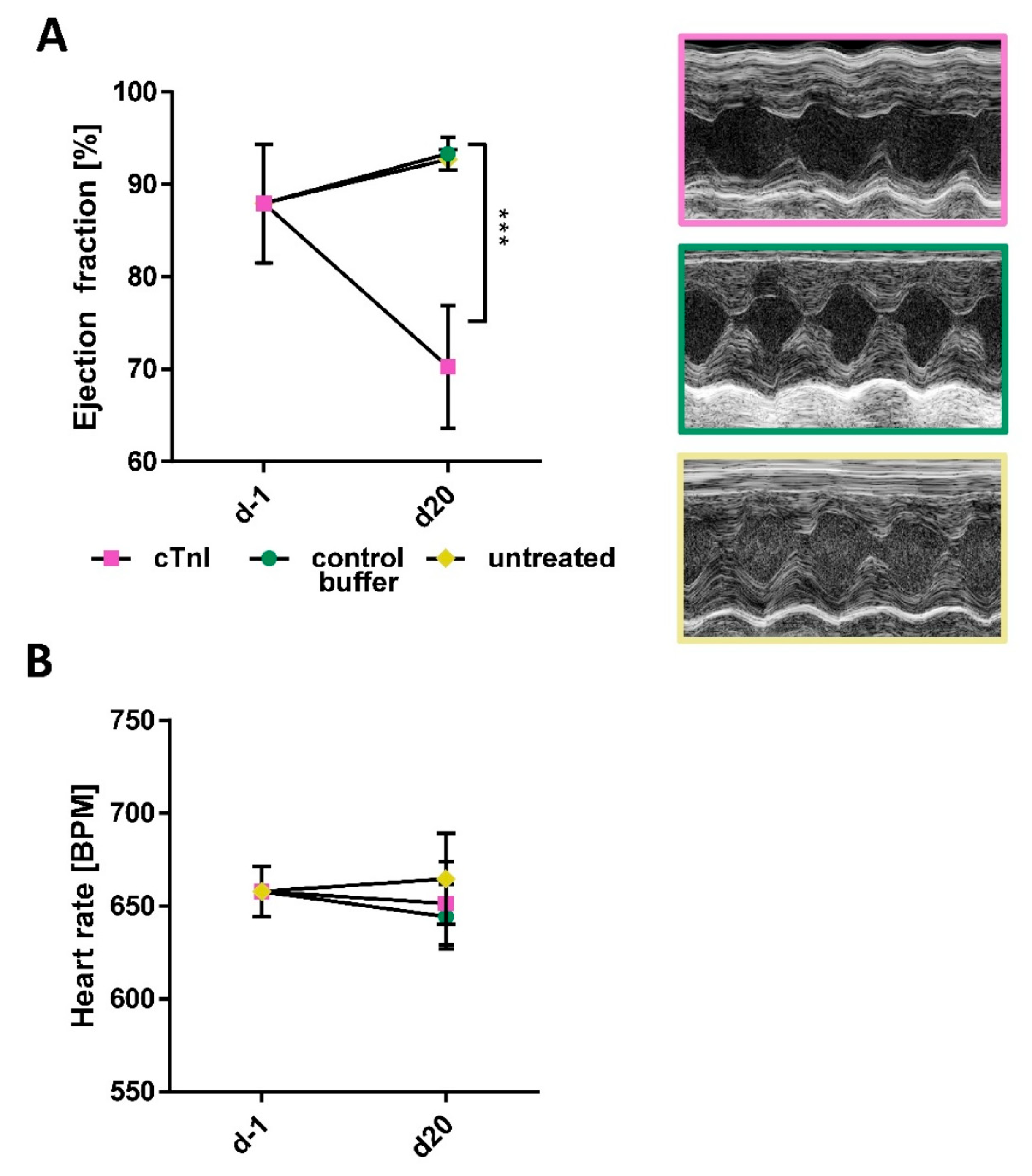
2.1. Immunization with cTnI Leads to the Production of High Anti-cTnI-aAb Titers Followed by Myocardial Inflammation, Fibrosis, and Reduction in Ejection Fraction
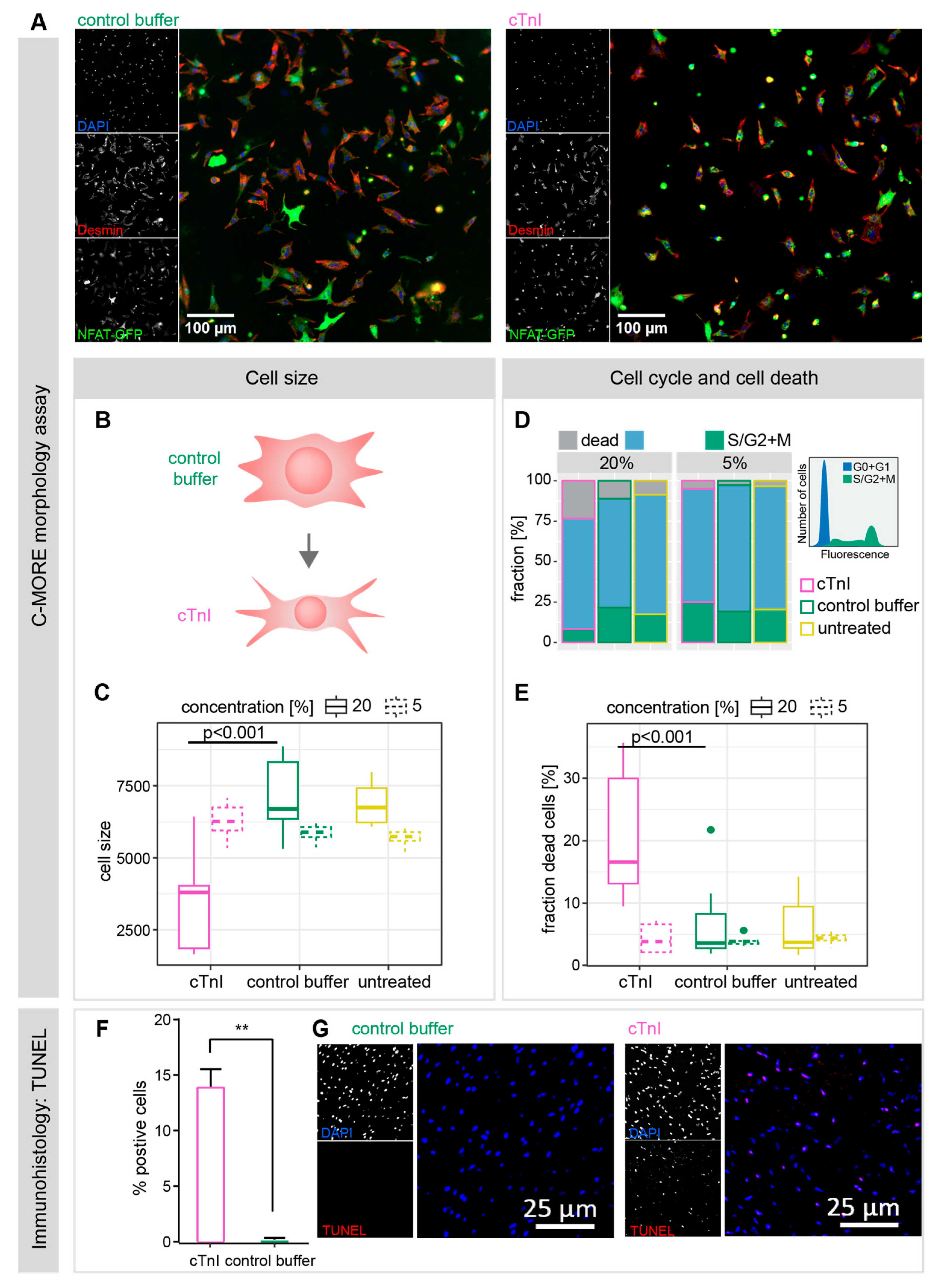
2.2. Plasma from cTnI-Immunized Mice Alters NRCM Morphology and Induces Apoptotic Cell Death
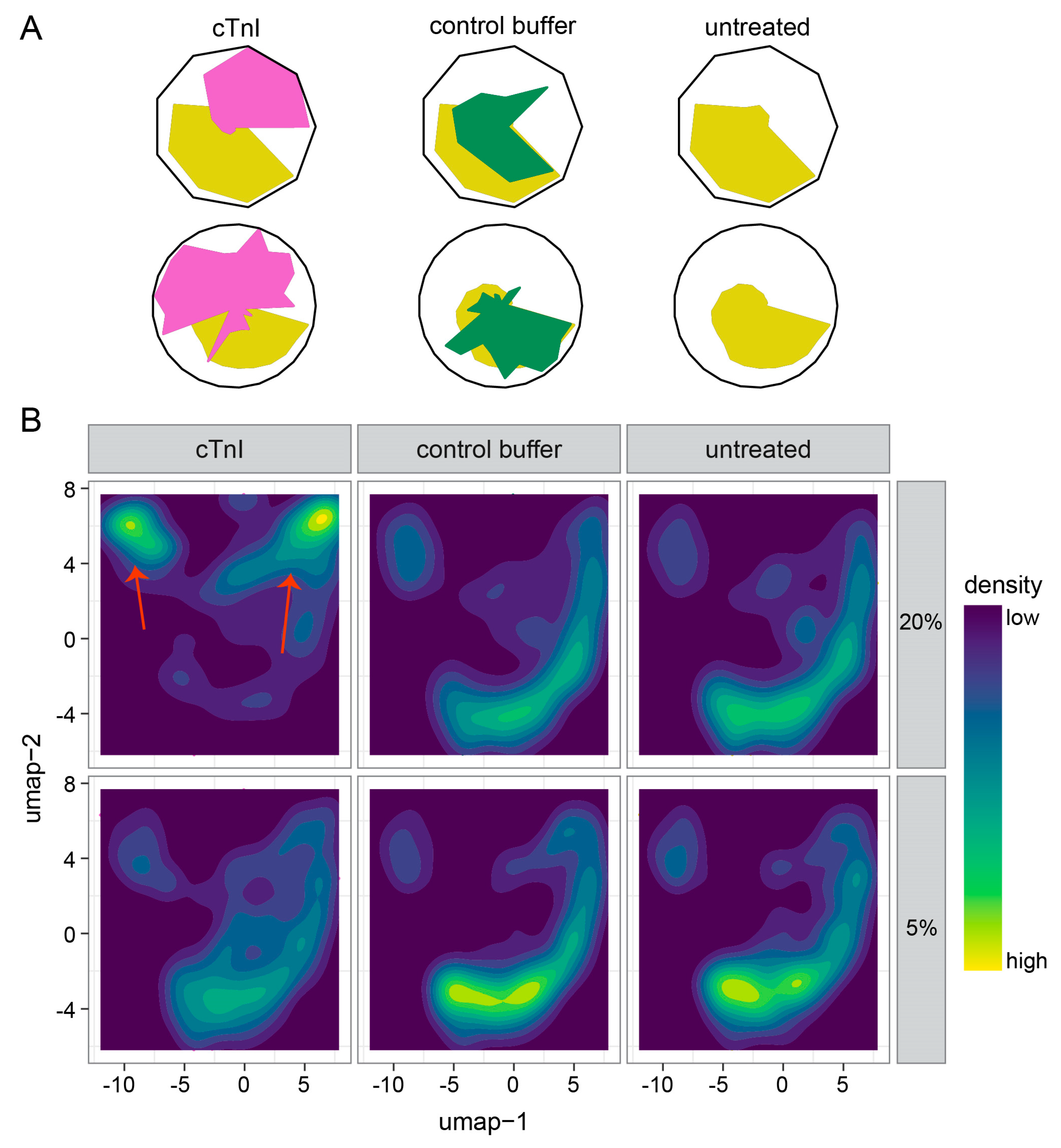
2.3. Single-Cell Morphology Analysis Identifies Two Distinct cTnI Plasma NRCM Subtypes
3. Discussion
4. Materials and Methods
4.1. Mice and Induction of Autoimmune Myocarditis
4.2. Determination of Highly Sensitive Troponin T (hsTnT) and Anti-cTnI-aAbs
4.3. Histopathological Preparation and Manual Analysis
4.4. Automated Fibrosis Area Quantification on Histopathological Sections
4.5. Echocardiography
4.6. NRCM Isolation and Treatment
4.7. NRCM Immunocytochemistry
4.8. NRCM Image Acquisition (IN Cell Analyzer 2200)
4.9. Data Processing
4.10. Single-Cell Phenotyping
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aAb | autoantibody |
| AFOG | acid fuchsin orange G-stain |
| cTnI | cardiac troponin I |
| cTnT | cardiac troponin T |
| DCMI | inflammatory dilated cardiomyopathy |
| EAM | experimental autoimmune myocarditis |
| HE | hematoxylin and eosin |
| hsTnT | high-sensitive troponin T |
| NRCM | neonatal rat cardiomyocyte |
| UMAP | uniform manifold approximation and projection |
References
- Roger, V.L. Epidemiology of Heart Failure: A Contemporary Perspective. Circ. Res. 2021, 128, 1421–1434. [Google Scholar] [CrossRef]
- Goser, S.; Andrassy, M.; Buss, S.J.; Leuschner, F.; Volz, C.H.; Ottl, R.; Zittrich, S.; Blaudeck, N.; Hardt, S.E.; Pfitzer, G.; et al. Cardiac troponin I but not cardiac troponin T induces severe autoimmune inflammation in the myocardium. Circulation 2006, 114, 1693–1702. [Google Scholar] [CrossRef]
- Pummerer, C.L.; Luze, K.; Grassl, G.; Bachmaier, K.; Offner, F.; Burrell, S.K.; Lenz, D.M.; Zamborelli, T.J.; Penninger, J.M.; Neu, N. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J. Clin. Investig. 1996, 97, 2057–2062. [Google Scholar] [CrossRef]
- Cihakova, D.; Sharma, R.B.; Fairweather, D.; Afanasyeva, M.; Rose, N.R. Animal models for autoimmune myocarditis and autoimmune thyroiditis. In Autoimmunity. Methods in Molecular Medicine; Humana Press: Totowa, NJ, USA, 2004; Volume 102, pp. 175–193. [Google Scholar] [CrossRef]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef]
- Maisch, B.; Richter, A.; Sandmoller, A.; Portig, I.; Pankuweit, S.; Network, B.M.-H.F. Inflammatory dilated cardiomyopathy (DCMI). Herz 2005, 30, 535–544. [Google Scholar] [CrossRef]
- Rose, N.R. Critical cytokine pathways to cardiac inflammation. J. Interferon Cytokine Res. 2011, 31, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Goser, S.; Ottl, R.; Brodner, A.; Dengler, T.J.; Torzewski, J.; Egashira, K.; Rose, N.R.; Katus, H.A.; Kaya, Z. Critical role for monocyte chemoattractant protein-1 and macrophage inflammatory protein-1alpha in induction of experimental autoimmune myocarditis and effective anti-monocyte chemoattractant protein-1 gene therapy. Circulation 2005, 112, 3400–3407. [Google Scholar] [CrossRef]
- Baldeviano, G.C.; Barin, J.G.; Talor, M.V.; Srinivasan, S.; Bedja, D.; Zheng, D.; Gabrielson, K.; Iwakura, Y.; Rose, N.R.; Cihakova, D. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ. Res. 2010, 106, 1646–1655. [Google Scholar] [CrossRef]
- Afanasyeva, M.; Georgakopoulos, D.; Rose, N.R. Autoimmune myocarditis: Cellular mediators of cardiac dysfunction. Autoimmun. Rev. 2004, 3, 476–486. [Google Scholar] [CrossRef]
- Leuschner, F.; Katus, H.A.; Kaya, Z. Autoimmune myocarditis: Past, present and future. J. Autoimmun. 2009, 33, 282–289. [Google Scholar] [CrossRef]
- O’Donohoe, T.J.; Ketheesan, N.; Schrale, R.G. Anti-troponin antibodies following myocardial infarction. J. Cardiol. 2017, 69, 38–45. [Google Scholar] [CrossRef]
- Nishimura, H.; Okazaki, T.; Tanaka, Y.; Nakatani, K.; Hara, M.; Matsumori, A.; Sasayama, S.; Mizoguchi, A.; Hiai, H.; Minato, N.; et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 2001, 291, 319–322. [Google Scholar] [CrossRef]
- Leuschner, F.; Li, J.; Goser, S.; Reinhardt, L.; Ottl, R.; Bride, P.; Zehelein, J.; Pfitzer, G.; Remppis, A.; Giannitsis, E.; et al. Absence of auto-antibodies against cardiac troponin I predicts improvement of left ventricular function after acute myocardial infarction. Eur. Heart J. 2008, 29, 1949–1955. [Google Scholar] [CrossRef]
- Staudt, A.; Schaper, F.; Stangl, V.; Plagemann, A.; Bohm, M.; Merkel, K.; Wallukat, G.; Wernecke, K.D.; Stangl, K.; Baumann, G.; et al. Immunohistological changes in dilated cardiomyopathy induced by immunoadsorption therapy and subsequent immunoglobulin substitution. Circulation 2001, 103, 2681–2686. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.; Mahon, N.G.; Baig, M.K.; Tona, F.; Murphy, R.T.; Elliott, P.M.; McKenna, W.J. Prospective familial assessment in dilated cardiomyopathy: Cardiac autoantibodies predict disease development in asymptomatic relatives. Circulation 2007, 115, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.; Iliceto, S. Genetically determined myocarditis: Clinical presentation and immunological characteristics. Curr. Opin. Cardiol. 2008, 23, 219–226. [Google Scholar] [CrossRef]
- Furusawa, S.; Ikeda, M.; Ide, T.; Kanamura, T.; Miyamoto, H.D.; Abe, K.; Ishimaru, K.; Watanabe, M.; Tsutsui, Y.; Miyake, R.; et al. Cardiac Autoantibodies Against Cardiac Troponin I in Post-Myocardial Infarction Heart Failure: Evaluation in a Novel Murine Model and Applications in Therapeutics. Circ. Heart Fail. 2023, 16, e010347. [Google Scholar] [CrossRef]
- Kaya, Z.; Goser, S.; Buss, S.J.; Leuschner, F.; Ottl, R.; Li, J.; Volkers, M.; Zittrich, S.; Pfitzer, G.; Rose, N.R.; et al. Identification of cardiac troponin I sequence motifs leading to heart failure by induction of myocardial inflammation and fibrosis. Circulation 2008, 118, 2063–2072. [Google Scholar] [CrossRef]
- Caforio, A.L.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Helio, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Herskowitz, A.; Wolfgram, L.J.; Rose, N.R.; Beisel, K.W. Coxsackievirus B3 murine myocarditis: A pathologic spectrum of myocarditis in genetically defined inbred strains. J. Am. Coll. Cardiol. 1987, 9, 1311–1319. [Google Scholar] [CrossRef]
- Rose, N.R.; Wolfgram, L.J.; Herskowitz, A.; Beisel, K.W. Postinfectious autoimmunity: Two distinct phases of coxsackievirus B3-induced myocarditis. Ann. N. Y. Acad. Sci. 1986, 475, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Menger, J.; Collini, V.; Groschel, J.; Adler, Y.; Brucato, A.; Christian, V.; Ferreira, V.M.; Gandjbakhch, E.; Heidecker, B.; Kerneis, M.; et al. 2025 ESC Guidelines for the management of myocarditis and pericarditis. Eur. Heart J. 2025, ehaf192. [Google Scholar] [CrossRef] [PubMed]
- Furkel, J.; Knoll, M.; Din, S.; Bogert, N.V.; Seeger, T.; Frey, N.; Abdollahi, A.; Katus, H.A.; Konstandin, M.H. C-MORE: A high-content single-cell morphology recognition methodology for liquid biopsies toward personalized cardiovascular medicine. Cell Rep. Med. 2021, 2, 100436. [Google Scholar] [CrossRef]
- McQuin, C.; Goodman, A.; Chernyshev, V.; Kamentsky, L.; Cimini, B.A.; Karhohs, K.W.; Doan, M.; Ding, L.; Rafelski, S.M.; Thirstrup, D.; et al. CellProfiler 3.0: Next-generation image processing for biology. PLoS Biol. 2018, 16, e2005970. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Afanasyeva, M.; Hill, S.L.; Rose, N.R. Characterization of murine autoimmune myocarditis induced by self and foreign cardiac myosin. Autoimmunity 1999, 31, 151–162. [Google Scholar] [CrossRef]
- Afanasyeva, M.; Wang, Y.; Kaya, Z.; Park, S.; Zilliox, M.J.; Schofield, B.H.; Hill, S.L.; Rose, N.R. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. Am. J. Pathol. 2001, 159, 193–203. [Google Scholar] [CrossRef]
- Smith, S.C.; Allen, P.M. Myosin-induced acute myocarditis is a T cell-mediated disease. J. Immunol. 1991, 147, 2141–2147. [Google Scholar] [CrossRef]
- Valaperti, A.; Marty, R.R.; Kania, G.; Germano, D.; Mauermann, N.; Dirnhofer, S.; Leimenstoll, B.; Blyszczuk, P.; Dong, C.; Mueller, C.; et al. CD11b+ monocytes abrogate Th17 CD4+ T cell-mediated experimental autoimmune myocarditis. J. Immunol. 2008, 180, 2686–2695. [Google Scholar] [CrossRef]
- Maisel, A.; Cesario, D.; Baird, S.; Rehman, J.; Haghighi, P.; Carter, S. Experimental autoimmune myocarditis produced by adoptive transfer of splenocytes after myocardial infarction. Circ. Res. 1998, 82, 458–463. [Google Scholar] [CrossRef]
- Dai, D.F.; Rabinovitch, P.S. Cardiac aging in mice and humans: The role of mitochondrial oxidative stress. Trends Cardiovasc. Med. 2009, 19, 213–220. [Google Scholar] [CrossRef]
- Ninh, V.K.; Brown, J.H. The contribution of the cardiomyocyte to tissue inflammation in cardiomyopathies. Curr. Opin. Physiol. 2021, 19, 129–134. [Google Scholar] [CrossRef]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef]
- Liao, L.; Sindhwani, R.; Rojkind, M.; Factor, S.; Leinwand, L.; Diamond, B. Antibody-mediated autoimmune myocarditis depends on genetically determined target organ sensitivity. J. Exp. Med. 1995, 181, 1123–1131. [Google Scholar] [CrossRef]
- Wallukat, G.; Reinke, P.; Dorffel, W.V.; Luther, H.P.; Bestvater, K.; Felix, S.B.; Baumann, G. Removal of autoantibodies in dilated cardiomyopathy by immunoadsorption. Int. J. Cardiol. 1996, 54, 191–195. [Google Scholar] [CrossRef]
- Felix, S.B.; Staudt, A.; Landsberger, M.; Grosse, Y.; Stangl, V.; Spielhagen, T.; Wallukat, G.; Wernecke, K.D.; Baumann, G.; Stangl, K. Removal of cardiodepressant antibodies in dilated cardiomyopathy by immunoadsorption. J. Am. Coll. Cardiol. 2002, 39, 646–652. [Google Scholar] [CrossRef]
- Nunez, R.; Sancho-Martinez, S.M.; Novoa, J.M.; Lopez-Hernandez, F.J. Apoptotic volume decrease as a geometric determinant for cell dismantling into apoptotic bodies. Cell Death Differ. 2010, 17, 1665–1671. [Google Scholar] [CrossRef]
- Bortner, C.D.; Cidlowski, J.A. Uncoupling cell shrinkage from apoptosis reveals that Na+ influx is required for volume loss during programmed cell death. J. Biol. Chem. 2003, 278, 39176–39184. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.C.; Stone, N.L.; Pittman, R.N. Extranuclear apoptosis. The role of the cytoplasm in the execution phase. J. Cell Biol. 1999, 146, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Doonan, F.; Cotter, T.G. Morphological assessment of apoptosis. Methods 2008, 44, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Qin, Y.H.; Liu, Y.; Zhu, L.; Zhao, X.X.; Liu, Y.Y.; Luo, S.W.; Tang, G.S.; Shen, Q. Cardiac troponin I autoantibody induces myocardial dysfunction by PTEN signaling activation. EBioMedicine 2019, 47, 329–340. [Google Scholar] [CrossRef]
- Al-Hillawi, E.; Minchin, S.D.; Trayer, I.P. Overexpression of human cardiac troponin-I and troponin-C in Escherichia coli and their purification and characterisation. Two point mutations allow high-level expression of troponin-I. Eur. J. Biochem. 1994, 225, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.; Pfitzer, G.; Stehle, R. Expression and purification of human cardiac troponin subunits and their functional incorporation into isolated cardiac mouse myofibrils. J. Chromatogr. B 2003, 786, 287–296. [Google Scholar] [CrossRef]
- Bangert, A.; Andrassy, M.; Muller, A.M.; Bockstahler, M.; Fischer, A.; Volz, C.H.; Leib, C.; Goser, S.; Korkmaz-Icoz, S.; Zittrich, S.; et al. Critical role of RAGE and HMGB1 in inflammatory heart disease. Proc. Natl. Acad. Sci. USA 2016, 113, E155–E164. [Google Scholar] [CrossRef]
- Andrassy, M.; Volz, H.C.; Maack, B.; Schuessler, A.; Gitsioudis, G.; Hofmann, N.; Laohachewin, D.; Wienbrandt, A.R.; Kaya, Z.; Bierhaus, A.; et al. HMGB1 is associated with atherosclerotic plaque composition and burden in patients with stable coronary artery disease. PLoS ONE 2012, 7, e52081, Correction in PLoS ONE 2014, 9, e99246. [Google Scholar] [CrossRef]
- Wang, Y.; Afanasyeva, M.; Hill, S.L.; Kaya, Z.; Rose, N.R. Nasal administration of cardiac myosin suppresses autoimmune myocarditis in mice. J. Am. Coll. Cardiol. 2000, 36, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Kaya, Z.; Afanasyeva, M.; Wang, Y.; Dohmen, K.M.; Schlichting, J.; Tretter, T.; Fairweather, D.; Holers, V.M.; Rose, N.R. Contribution of the innate immune system to autoimmune myocarditis: A role for complement. Nat. Immunol. 2001, 2, 739–745. [Google Scholar] [CrossRef]
- Abramoff, M.; Magalhães, P.; Ram, S.J. Image Processing with ImageJ. Biophotonics Int. 2003, 11, 36–42. [Google Scholar]
- Vogel, B.; Siebert, H.; Hofmann, U.; Frantz, S. Determination of collagen content within picrosirius red stained paraffin-embedded tissue sections using fluorescence microscopy. MethodsX 2015, 2, 124–134. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furkel, J.; Zirkenbach, V.A.; Knoll, M.; Öttl, R.; Rein, K.; Abdollahi, A.; Frey, N.; Konstandin, M.H.; Kaya, Z. Cardiac Troponin I Antibodies Induce Cardiomyocyte Damage and Alter Cell Morphology. Int. J. Mol. Sci. 2025, 26, 10005. https://doi.org/10.3390/ijms262010005
Furkel J, Zirkenbach VA, Knoll M, Öttl R, Rein K, Abdollahi A, Frey N, Konstandin MH, Kaya Z. Cardiac Troponin I Antibodies Induce Cardiomyocyte Damage and Alter Cell Morphology. International Journal of Molecular Sciences. 2025; 26(20):10005. https://doi.org/10.3390/ijms262010005
Chicago/Turabian StyleFurkel, Jennifer, Vanessa A. Zirkenbach, Maximilian Knoll, Renate Öttl, Katrin Rein, Amir Abdollahi, Norbert Frey, Mathias H. Konstandin, and Ziya Kaya. 2025. "Cardiac Troponin I Antibodies Induce Cardiomyocyte Damage and Alter Cell Morphology" International Journal of Molecular Sciences 26, no. 20: 10005. https://doi.org/10.3390/ijms262010005
APA StyleFurkel, J., Zirkenbach, V. A., Knoll, M., Öttl, R., Rein, K., Abdollahi, A., Frey, N., Konstandin, M. H., & Kaya, Z. (2025). Cardiac Troponin I Antibodies Induce Cardiomyocyte Damage and Alter Cell Morphology. International Journal of Molecular Sciences, 26(20), 10005. https://doi.org/10.3390/ijms262010005






