Network-Medicine-Guided Drug Repurposing for Alzheimer’s Disease: A Multi-Dimensional Systems Pharmacology Approach
Abstract
1. Introduction
2. Results
2.1. Dataset Selection and Preprocessing
2.1.1. Gene Expression Omnibus Dataset Selection
2.1.2. Data Processing and Quality Control
2.1.3. Differential Expression Analysis
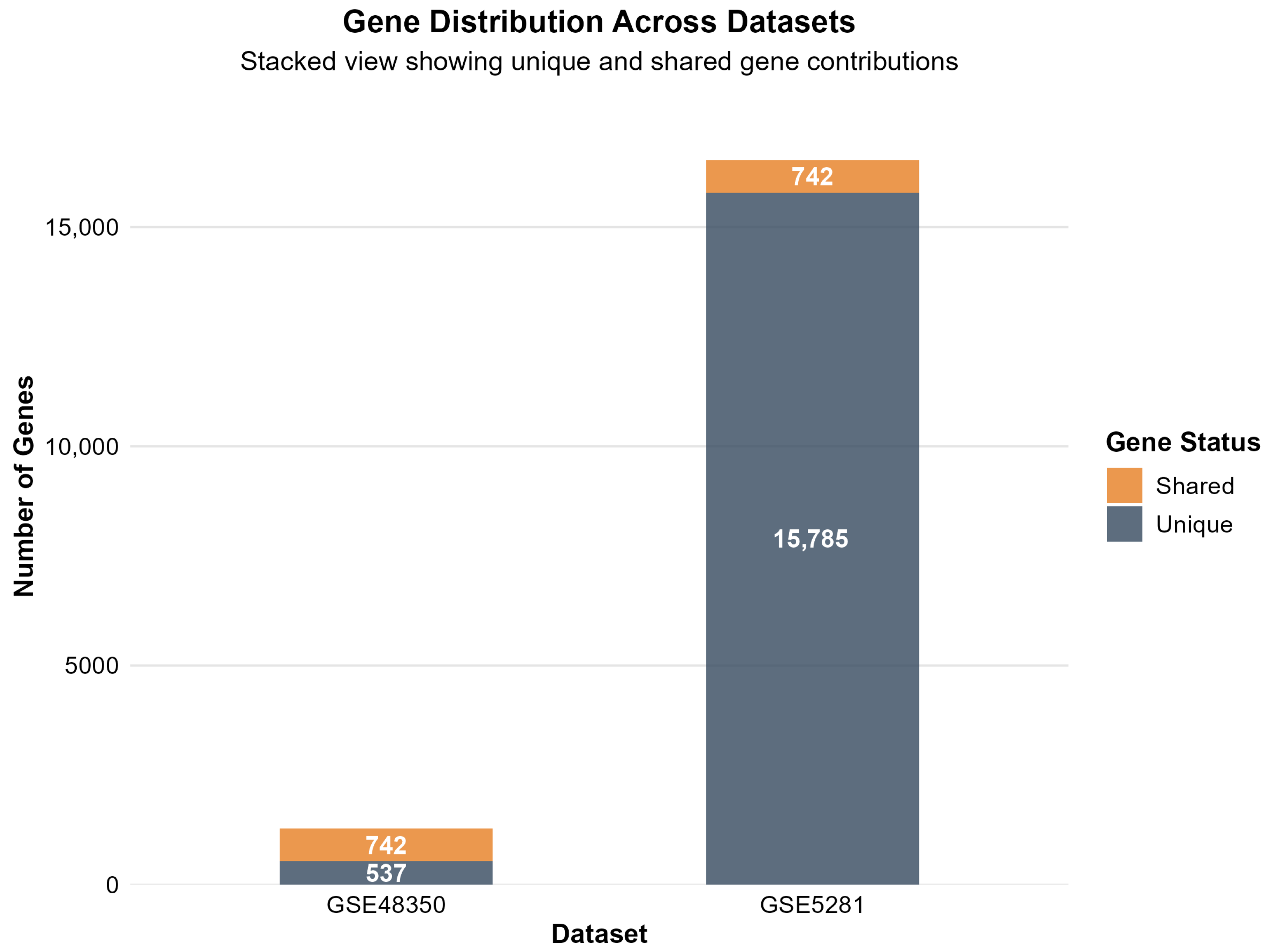
2.1.4. Analysis Results and Cross-Dataset Validation
2.2. Protein–Protein Interaction Network Analysis
2.2.1. Gene Mapping and Network Construction
2.2.2. Network Topology and Characteristics
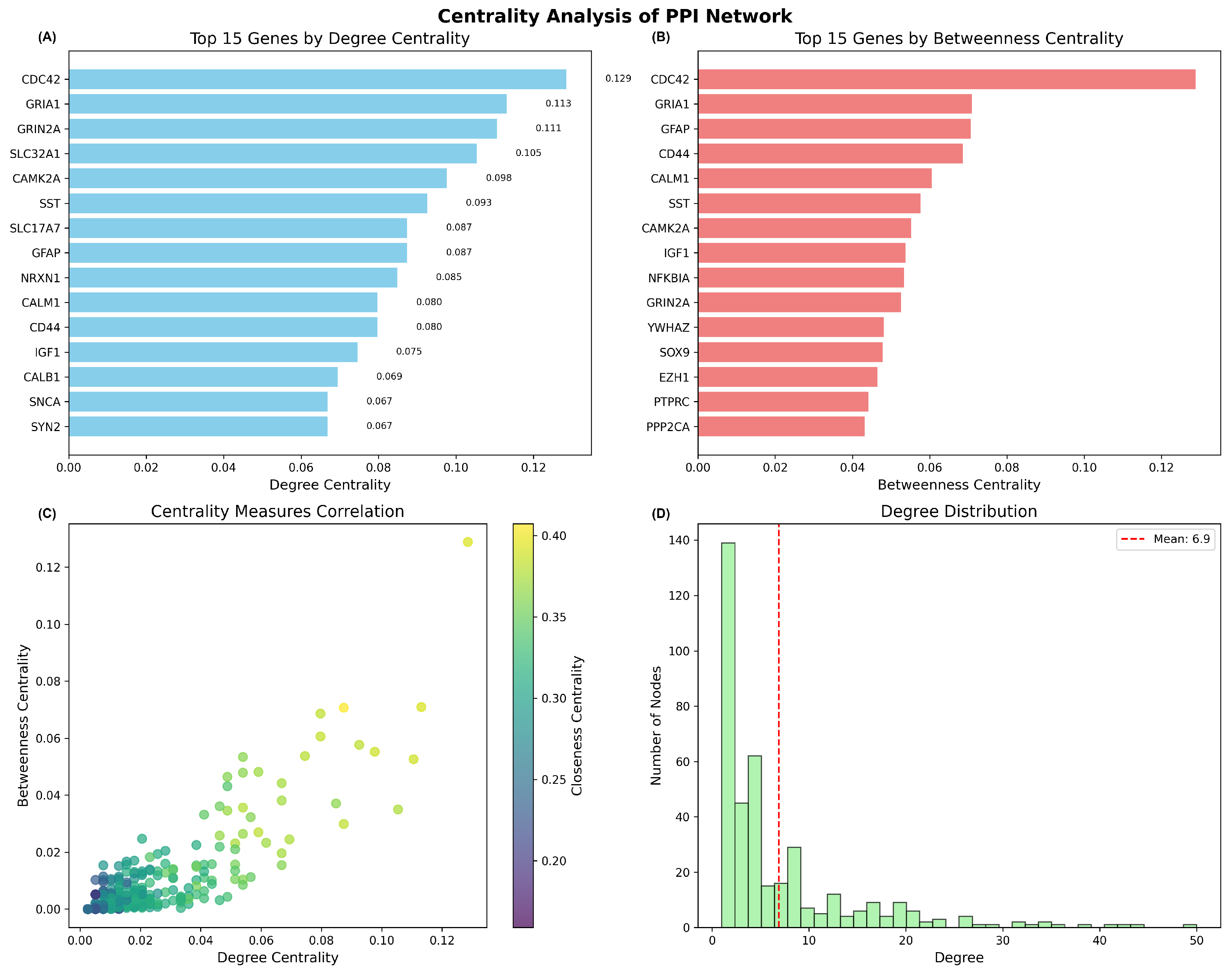
2.2.3. Hub Gene Identification and Centrality Analysis
2.2.4. Functional Implications
2.3. Pathway Enrichment Analysis
2.3.1. Gene Set Definition and Analysis Strategy
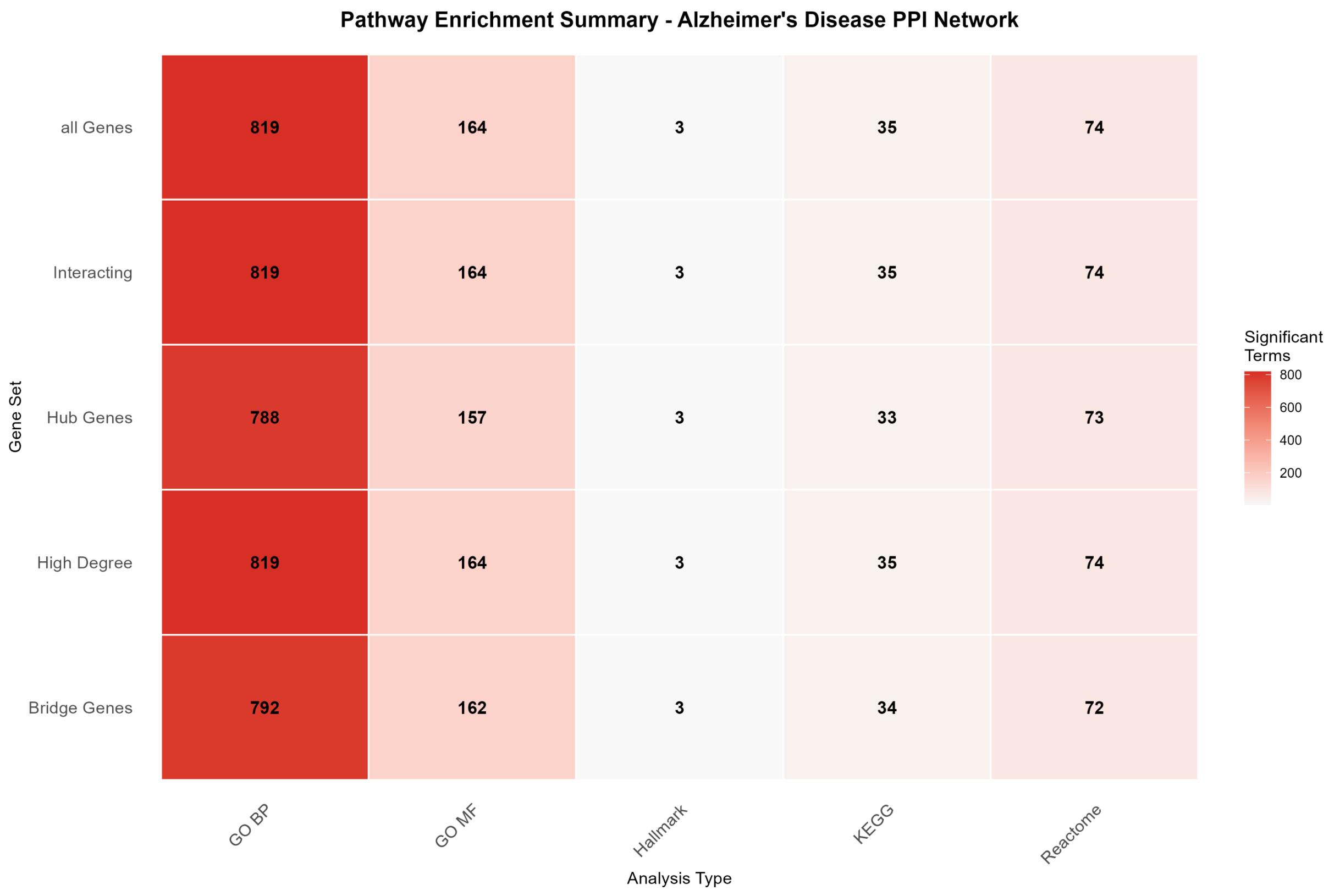
2.3.2. Comprehensive Pathway Enrichment Results
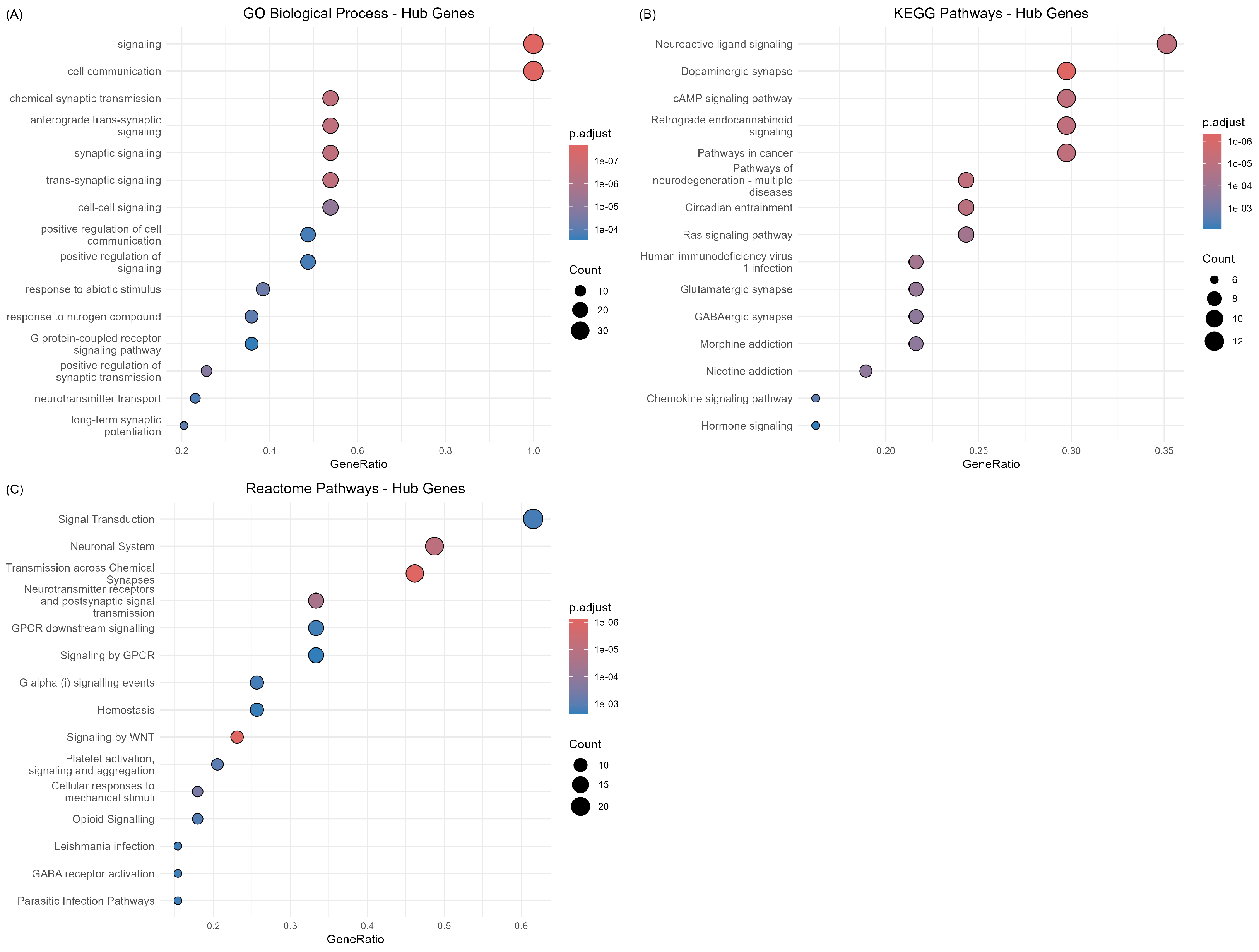
2.3.3. Hub Gene Functional Characterization
2.3.4. Functional Implications and Disease Relevance
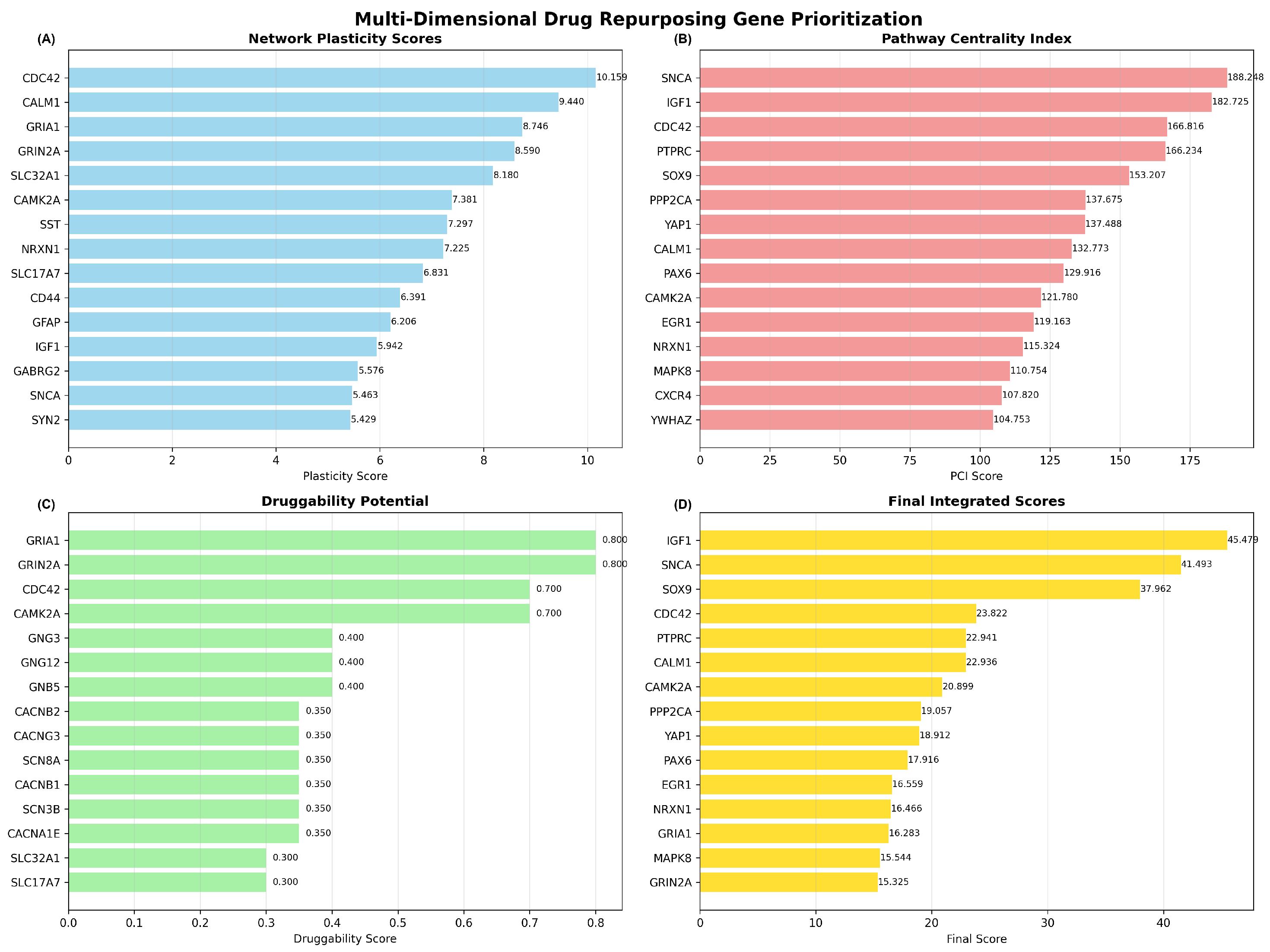
2.4. Multi-Dimensional Drug Repurposing Gene Prioritization
2.4.1. Multi-Dimensional Scoring Analysis
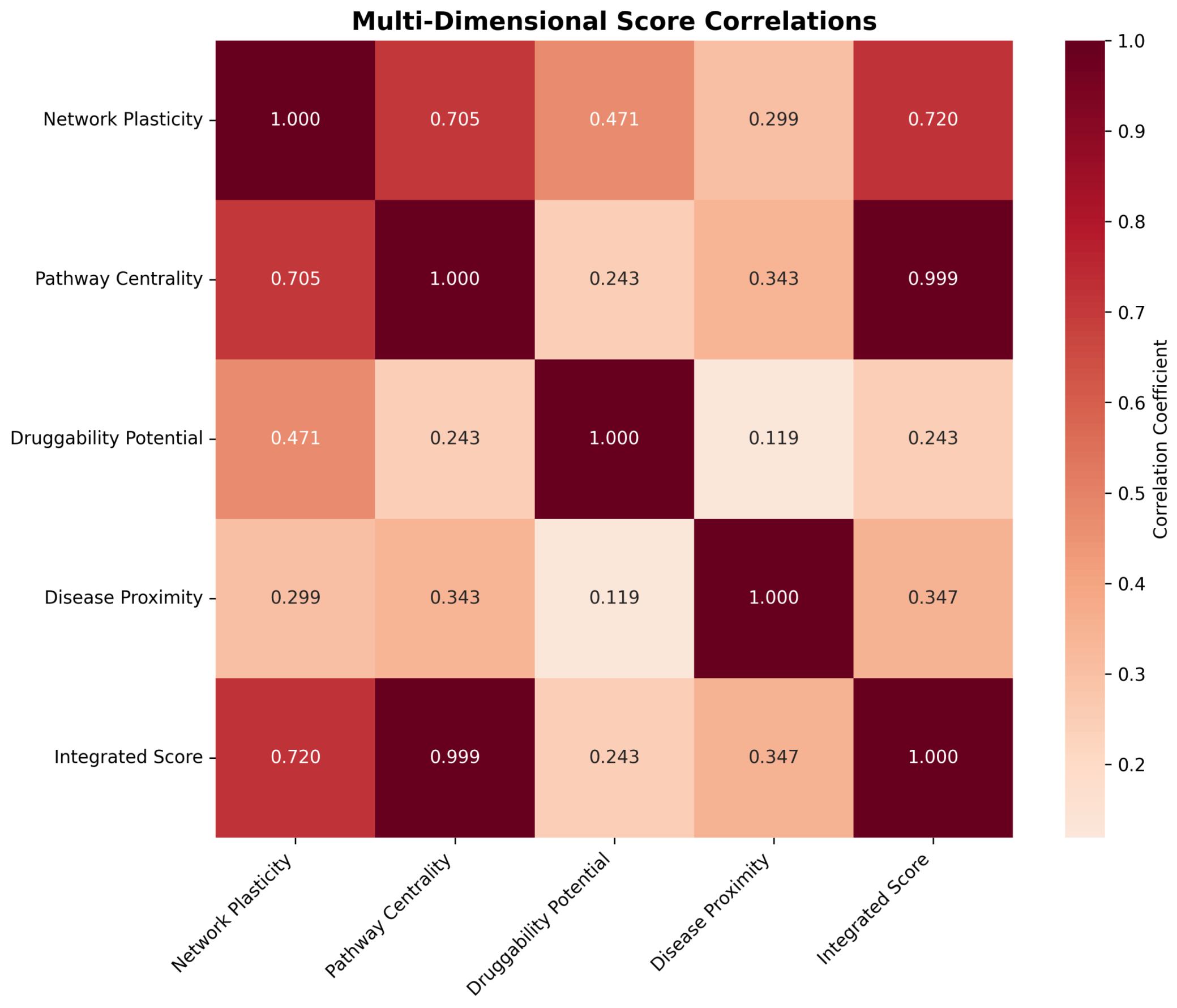
2.4.2. Dimensional Score Correlations and Integration
2.4.3. Temporal Dynamics Integration and Final Prioritization
2.4.4. Drug Repurposing Candidate Identification
2.4.5. Methodological Validation and Statistical Significance
2.5. CNS-Focused Network Medicine Framework
2.5.1. CNS Drug Database Curation and Filtering Strategy
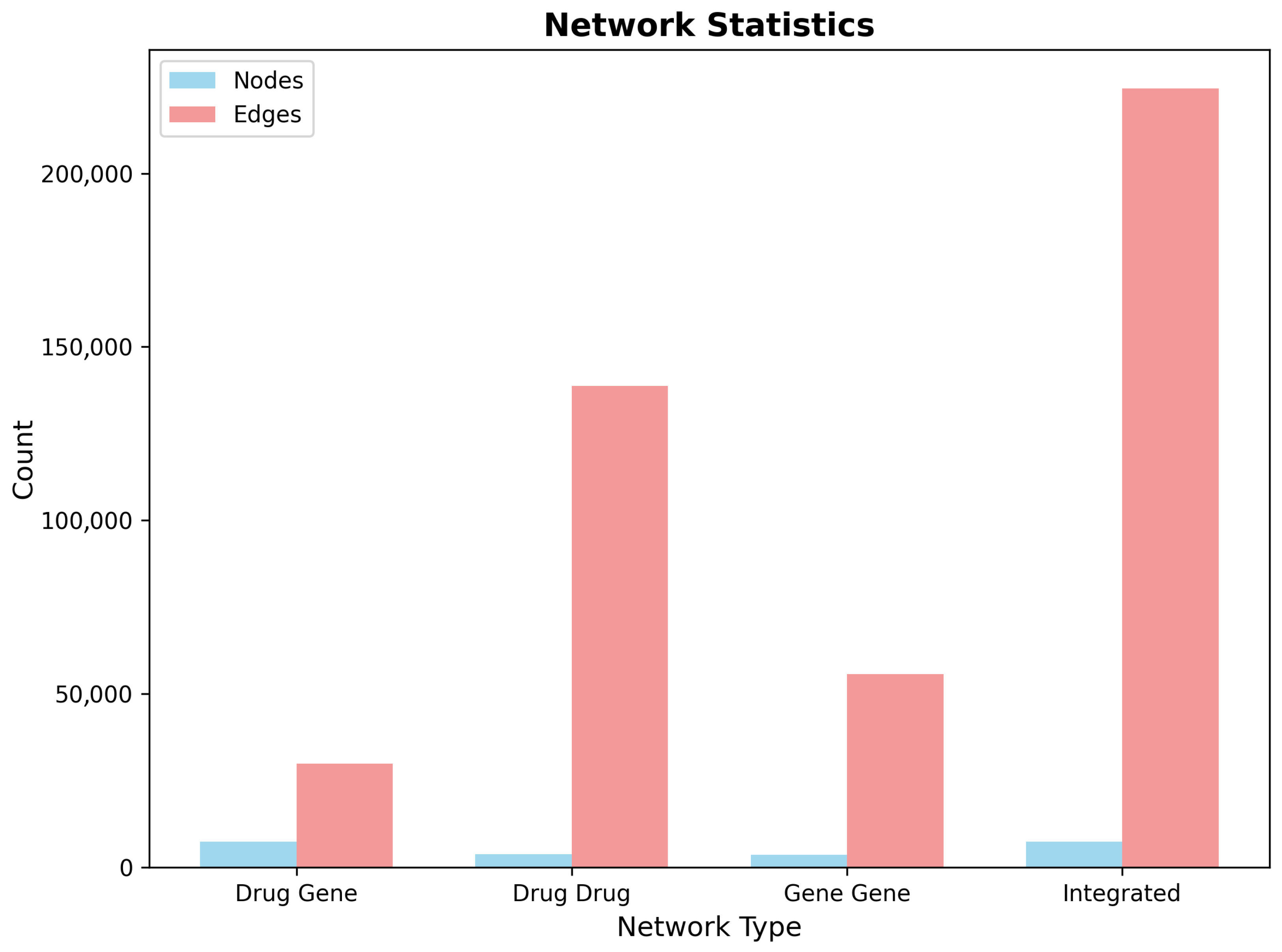
2.5.2. Multi-Layer Network Construction on CNS-Filtered Data
2.6. Medicinal-Chemistry-Guided Drug Repurposing
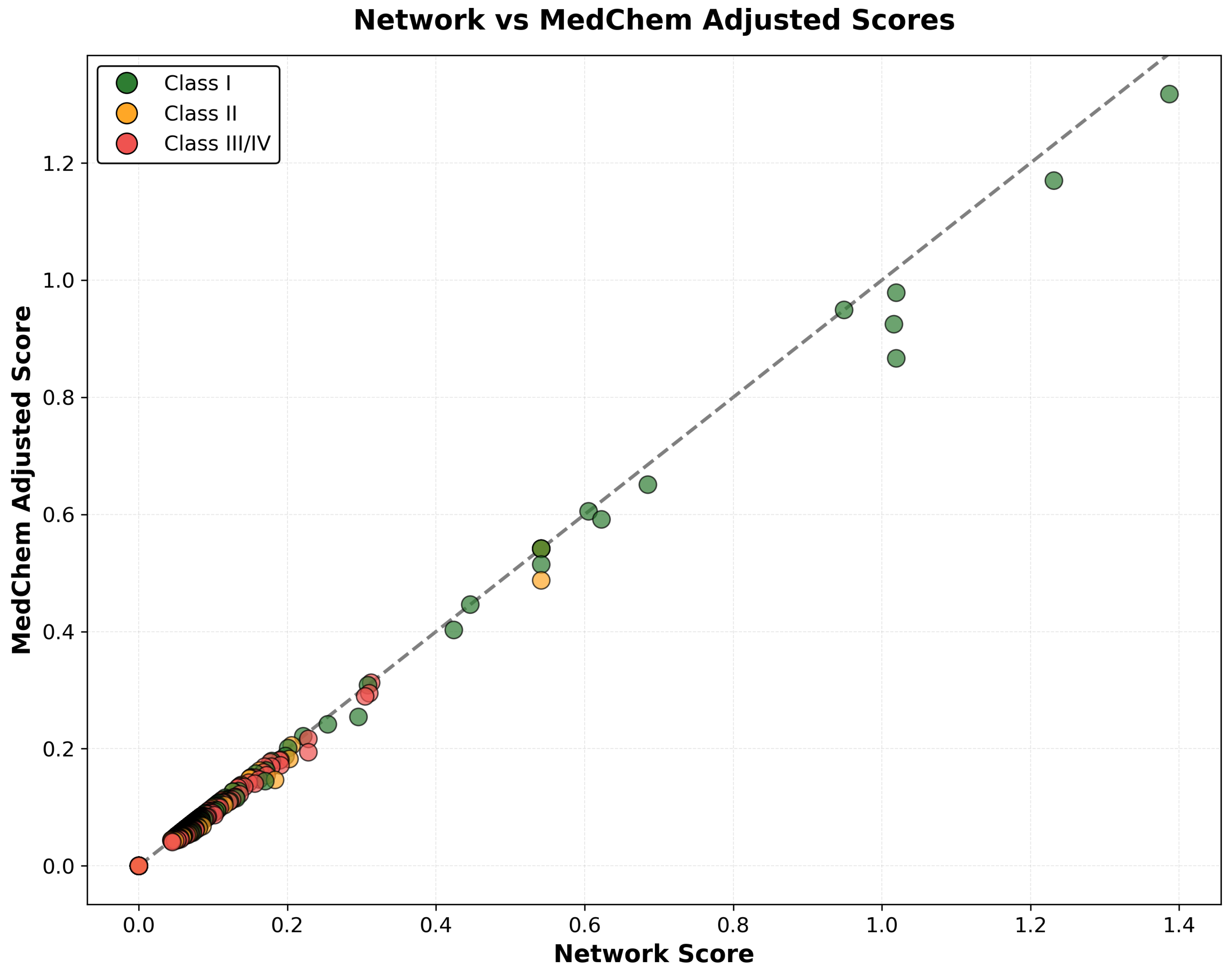
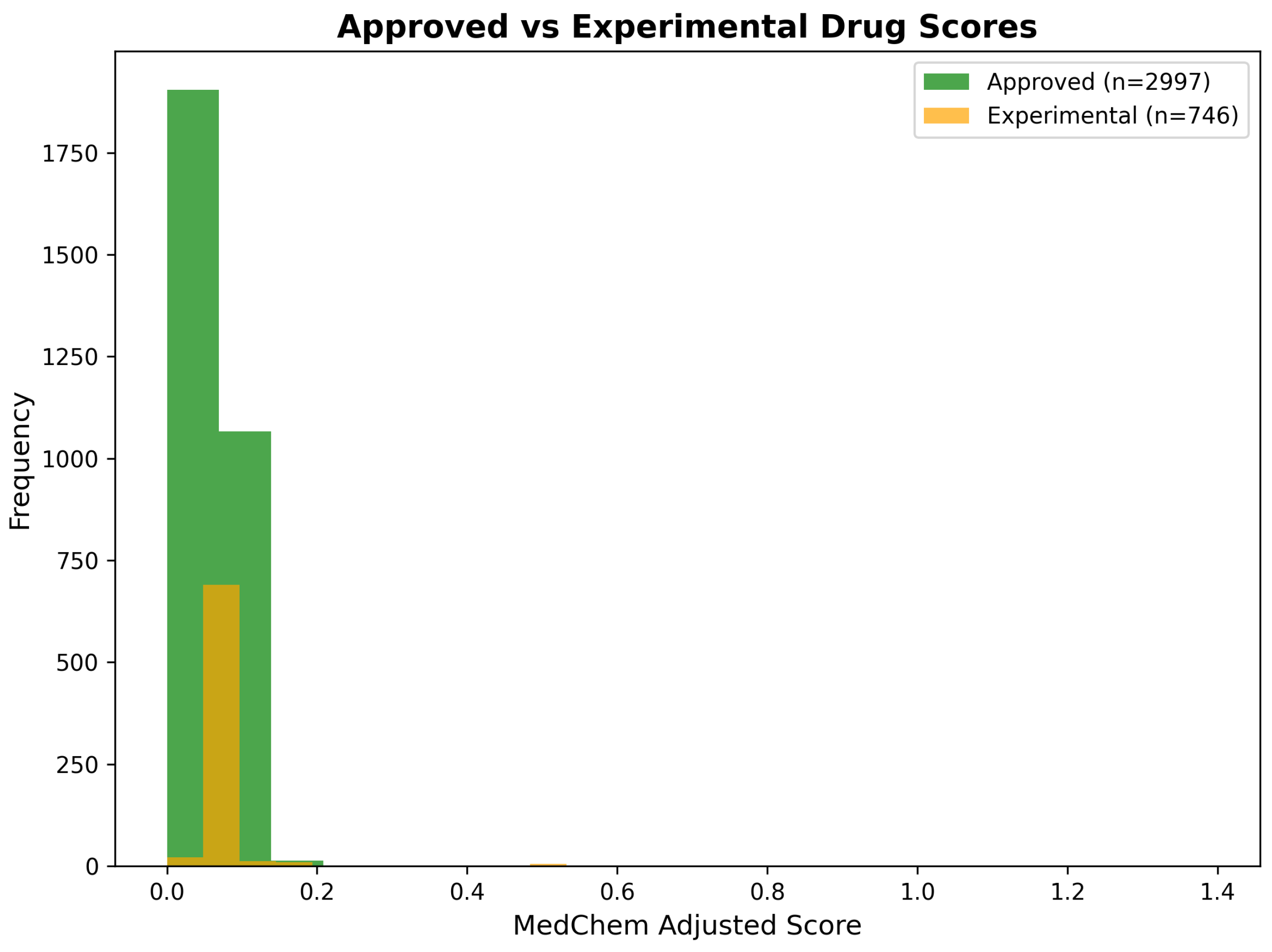
2.6.1. Integration of Network Scores with Medicinal Chemistry Assessment
2.6.2. Chemical Property Analysis and Drug-Likeness Filtering
2.7. Blood–Brain Barrier Penetration and CNS Suitability
2.7.1. BBB Penetration Prediction Methodology and Results
2.7.2. CNS Drug-Likeness Compliance Assessment
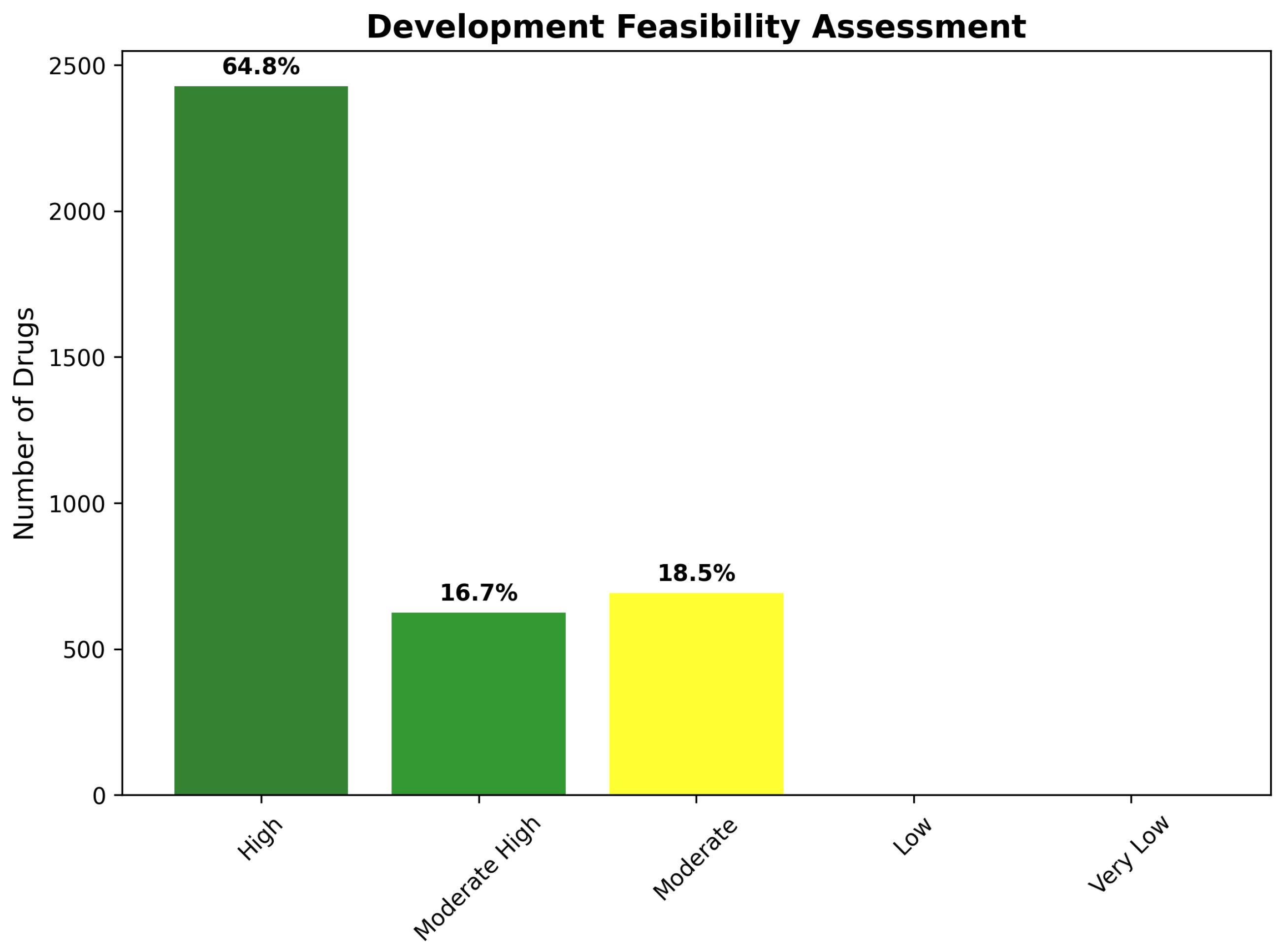
2.8. Chemical Tractability and Development Feasibility
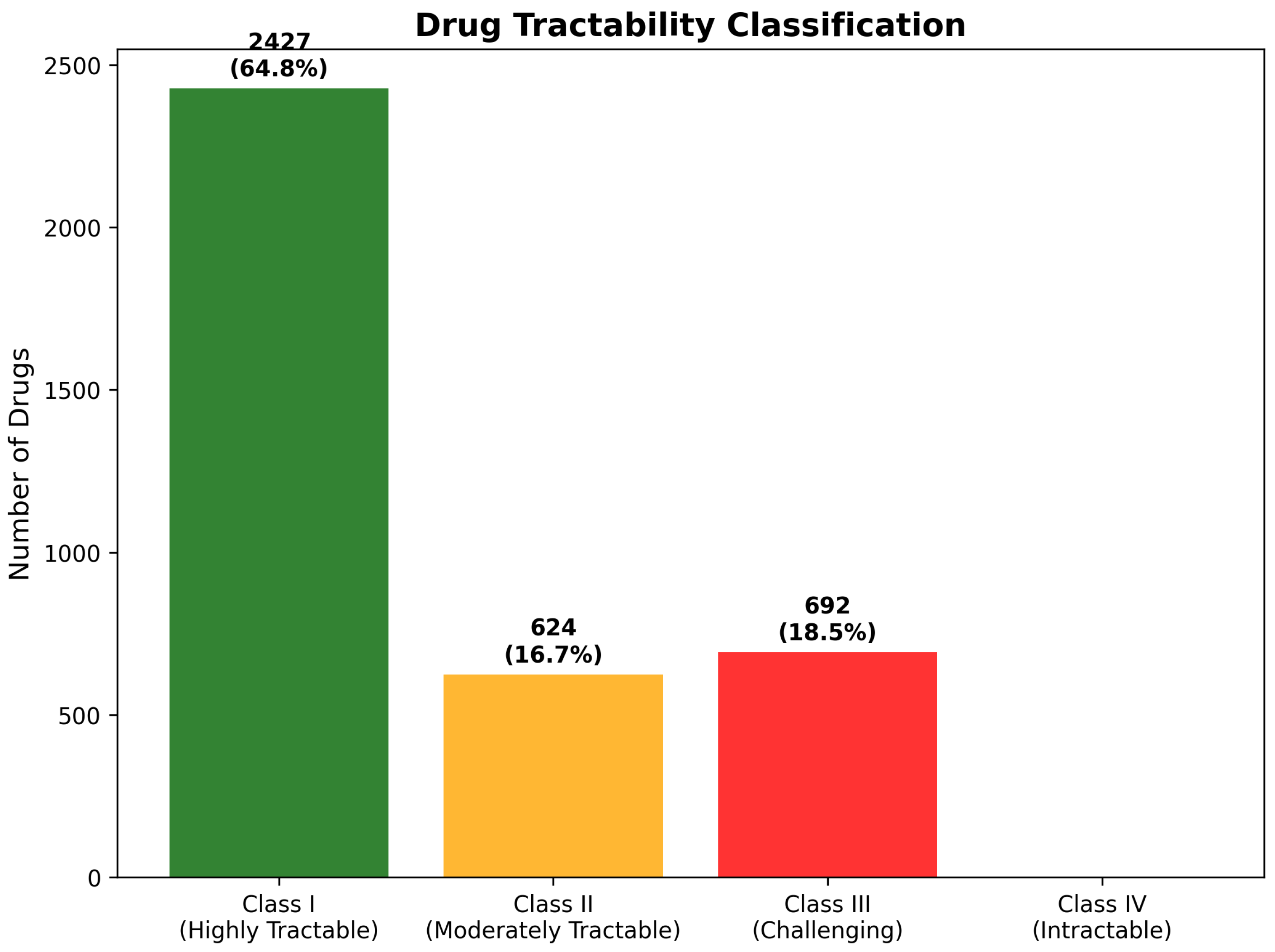
2.8.1. Four-Class Tractability Classification System
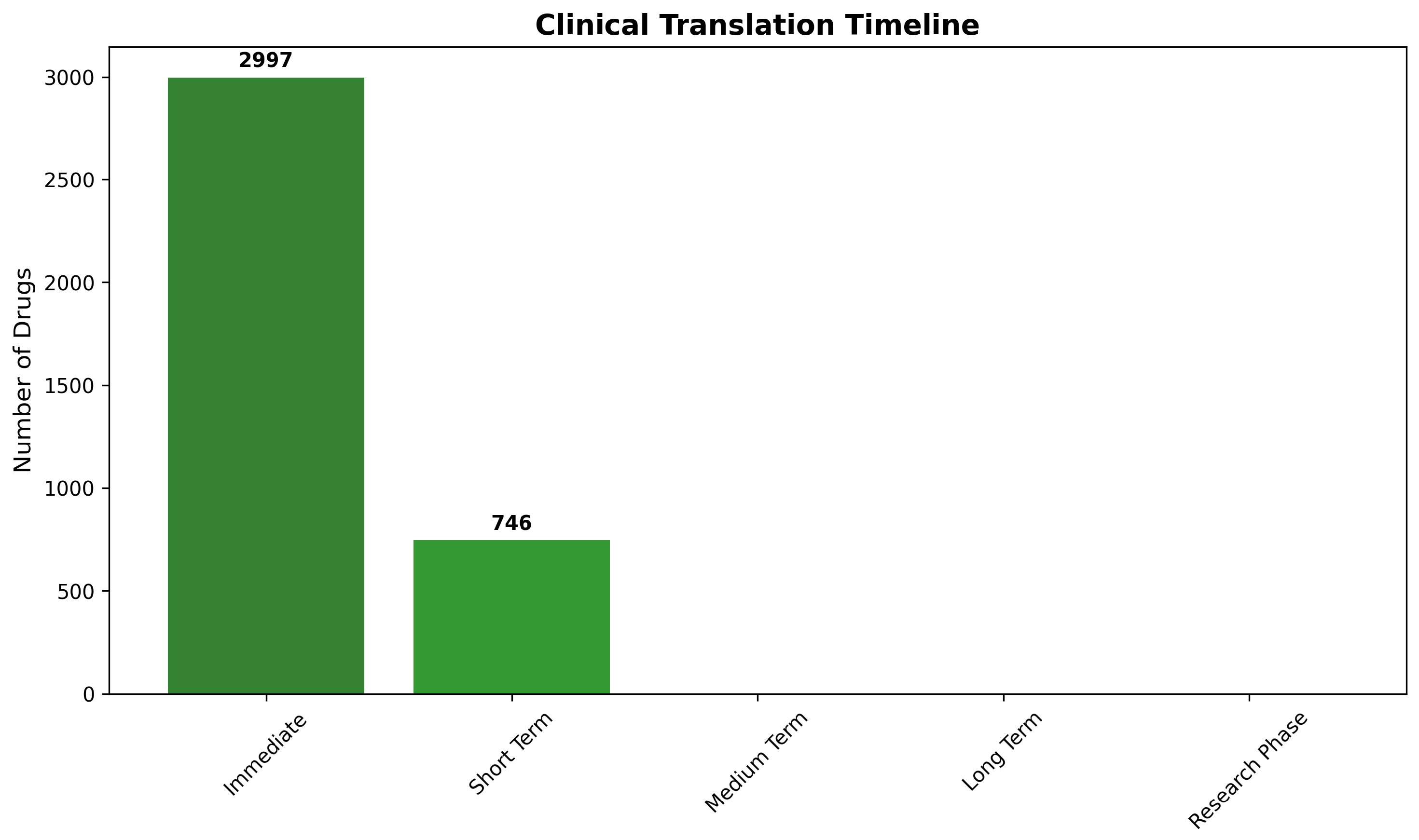
2.8.2. Development Timeline and Clinical Translation Readiness
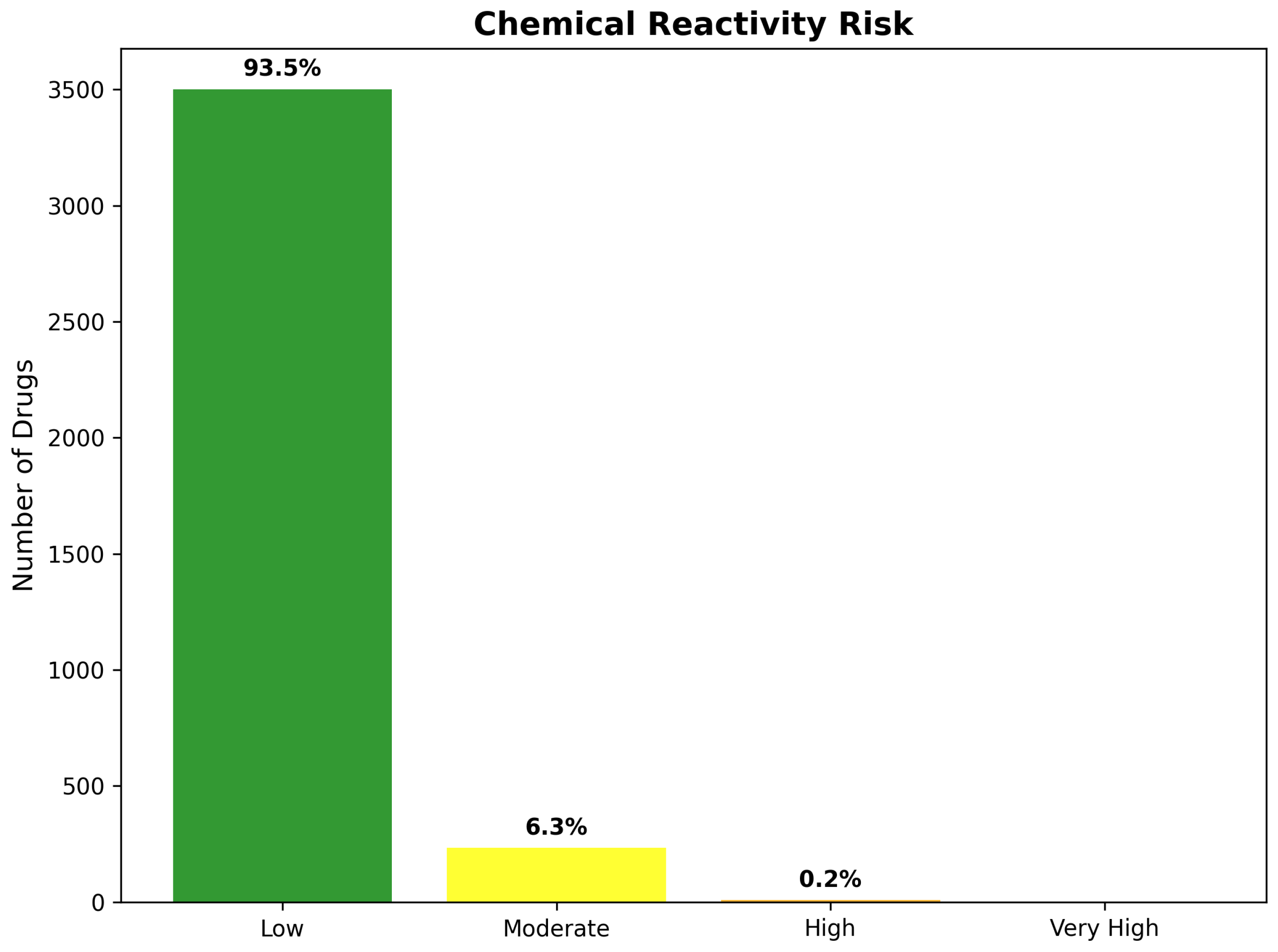
2.9. Safety Profiles and Chemical Reactivity Assessment
2.9.1. Chemical Reactivity Risk Evaluation
2.9.2. Overall Safety Profile and Risk Stratification
2.10. Machine-Learning-Based BBB Penetration Validation Results
2.10.1. Validation Dataset Composition and Model Training
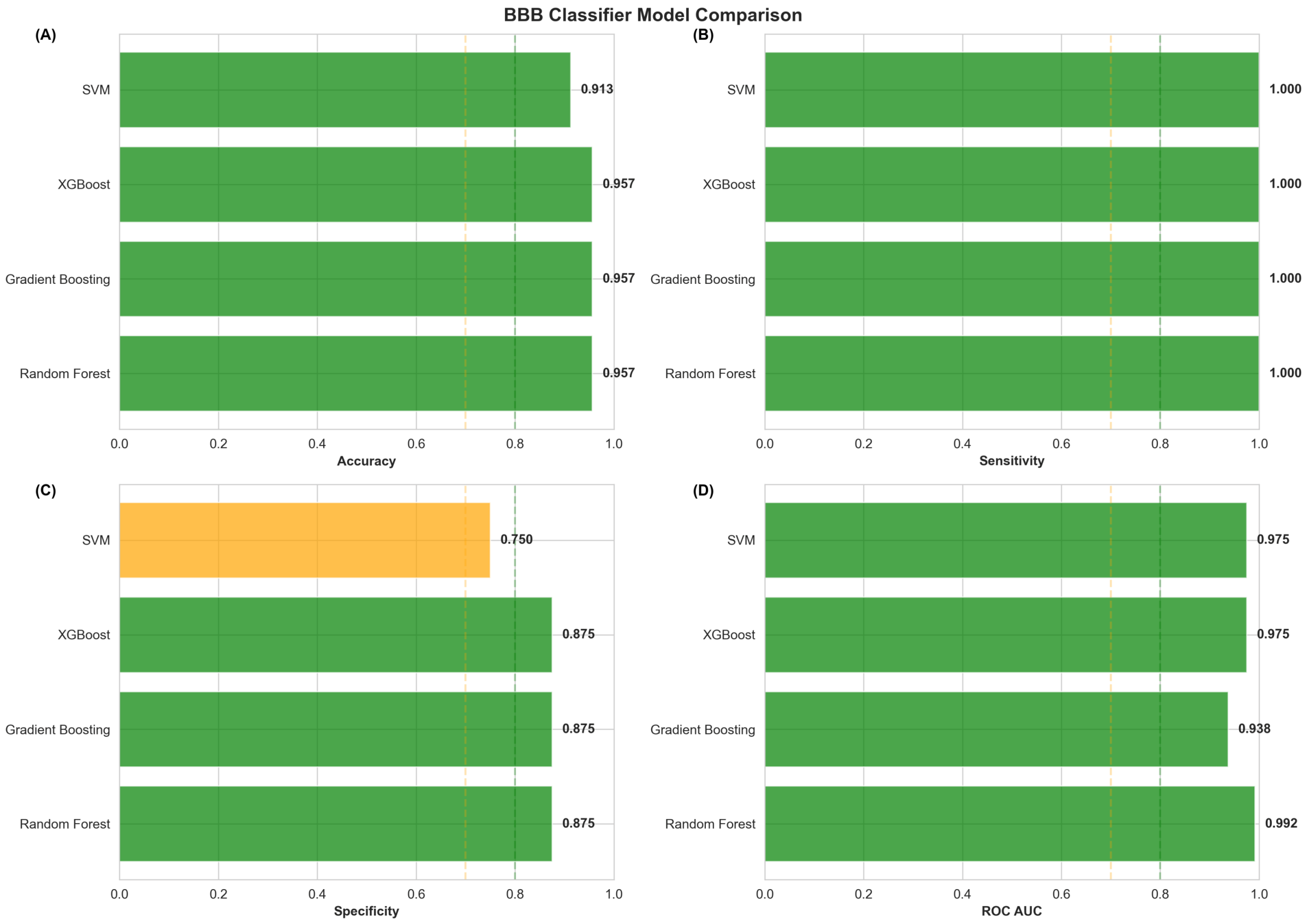
2.10.2. Machine Learning Model Performance Evaluation
2.10.3. Comparative Model Performance and Selection of Optimal Classifier
2.10.4. Validation Against Known CNS Drugs and Predictive Accuracy Assessment
2.11. Drug Modality Classification and Therapeutic Category Distribution
2.11.1. Modality Classification Criteria and Implementation
2.11.2. Modality Distribution Across Drug Repurposing Candidates
2.11.3. Implications for Modality-Stratified Ranking Strategy
2.11.4. Modality-Specific Development Considerations and Regulatory Pathways
2.12. Top-Ranked Small Molecule Drug Candidates with Integrated Assessment
2.12.1. Integrated Ranking Methodology for Small Molecule Prioritization
2.12.2. Comprehensive Characterization of Top 15 Small Molecule Candidates
2.12.3. Blood–Brain Barrier Penetration Assessment with Machine Learning Integration
2.12.4. P-Glycoprotein Efflux Liability and Active Transport Considerations
2.12.5. Chemical Tractability and Development Feasibility Profile
2.12.6. Alzheimer’s Disease Evidence Classification and Translational Readiness
2.13. Peptide and Biologic Therapeutic Candidates with Specialized Delivery Requirements
2.13.1. Peptide-Specific Ranking Methodology and BBB Penetration Considerations
2.13.2. Top-Ranked Peptide Therapeutic Candidates with Comprehensive Assessment
2.13.3. Trofinetide: Top-Ranked Peptide Candidate with Critical Translational Caveats
2.13.4. Somatostatin Analogs and Large Peptide Hormones: Development Challenges
2.13.5. Biologic Therapeutic Candidates and Receptor-Mediated Transcytosis Requirements
2.13.6. Comparative Assessment: Peptides Versus Small Molecules for AD Applications
2.14. Alzheimer’s Disease Evidence Assessment and Translational Plausibility
2.14.1. Evidence Classification Framework and Assessment Criteria
2.14.2. Established Therapeutics: Validation of Network Medicine Predictions
2.14.3. Mechanistic Evidence Candidates: Biological Plausibility Without AD-Specific Validation
2.14.4. Speculative Evidence: Trofinetide and the Limits of Computational Prediction
2.14.5. Evidence-Based Development Prioritization and Resource Allocation
3. Discussion
3.1. Systems Biology Approach to Alzheimer’s Disease Drug Repurposing
3.2. Medicinal Chemistry Integration and Pharmaceutical Feasibility Assessment
3.3. Machine Learning Validation of Blood–Brain Barrier Prediction Framework
3.4. Novel Therapeutic Candidate Identification and Mechanistic Diversity
3.5. Modality-Specific Development Considerations and Translational Implications
3.6. Addressing Undruggable Targets and Development Challenges
3.7. Validation and Predictive Performance
3.8. Clinical Translation and Development Strategy
3.9. Alzheimer’s Disease Evidence Assessment and Limitations of Network-Based Predictions
3.10. Methodological Innovations and Computational Advances
3.11. Limitations and Future Research Directions
3.12. Broader Implications for Pharmaceutical Development
4. Materials and Methods
4.1. Multi-Dimensional Network Pharmacology with Temporal Dynamics for Drug Repurposing
4.1.1. Network Plasticity Score Calculation
4.1.2. Pathway Centrality Index Computation
4.1.3. Druggability Potential Assessment
4.1.4. Disease Proximity Score Determination
4.1.5. Adaptive Multi-Dimensional Score Integration
4.1.6. Temporal Dynamics Filter Application
4.1.7. Statistical Validation and Significance Assessment
4.2. Network-Medicine-Based Drug Repurposing Framework
4.2.1. Drug–Gene Interaction Database Integration
4.2.2. Multi-Layer Network Construction
4.2.3. Integrated Multi-Layer Network Analysis
4.2.4. Random Walk with Restart Algorithm
4.2.5. Network Proximity Measurements
4.2.6. Multi-Dimensional Scoring Integration
4.3. Machine-Learning-Based Blood–Brain Barrier Penetration Validation
4.3.1. Validation Dataset Construction
4.3.2. Machine Learning Algorithm Selection and Training
4.3.3. Feature Preprocessing and Standardization
4.3.4. Training–Testing Split and Cross-Validation Protocol
4.3.5. Model Performance Evaluation Metrics
4.3.6. Best Model Selection and Deployment
4.3.7. BBB Prediction Application to Drug Candidates
4.4. Drug Modality Classification and Ranking Strategy
4.4.1. Therapeutic Modality Classification Criteria
4.4.2. Known Peptide and Biologic Identification
4.4.3. Modality-Specific Scoring Adjustments
4.4.4. P-Glycoprotein Efflux Liability Assessment
4.4.5. Alzheimer’s Disease Evidence Level Classification
4.4.6. Modality-Stratified Ranking Generation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Disease International. World Alzheimer Report 2022—Life After Diagnosis: Navigating Treatment, Care and Support; Alzheimer Society of Canada: Toronto, ON, Canada, 2022. [Google Scholar]
- Better, M.A. Alzheimer’s disease facts and figures. Alzheimers Dement 2023, 19, 1598–1695. [Google Scholar]
- Nichols, E.; Szoeke, C.E.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.E.; Akinyemi, R.O.; Alahdab, F.; Asgedom, S.W.; et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on the Public Health Response to Dementia; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.C.; Emerson, S.; Kesselheim, A.S. Evaluation of aducanumab for Alzheimer disease: Scientific evidence and regulatory review involving efficacy, safety, and futility. JAMA 2021, 325, 1717–1718. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Jones, D.T.; Greicius, M.D. Failure to demonstrate efficacy of aducanumab: An analysis of the EMERGE and ENGAGE trials as reported by Biogen, December 2019. Alzheimer’s Dement. 2021, 17, 696–701. [Google Scholar] [CrossRef]
- De Strooper, B.; Karran, E. The cellular phase of Alzheimer’s disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef]
- Pugazhenthi, S. Metabolic syndrome and the cellular phase of Alzheimer’s disease. Prog. Mol. Biol. Transl. Sci. 2017, 146, 243–258. [Google Scholar]
- Heneka, M.T.; Van der Flier, W.M.; Jessen, F.; Hoozemanns, J.; Thal, D.R.; Boche, D.; Brosseron, F.; Teunissen, C.; Zetterberg, H.; Jacobs, A.H.; et al. Neuroinflammation in Alzheimer disease. Nat. Rev. Immunol. 2024, 25, 321–352. [Google Scholar] [CrossRef]
- Green, G.S.; Fujita, M.; Yang, H.S.; Taga, M.; Cain, A.; McCabe, C.; Comandante-Lou, N.; White, C.C.; Schmidtner, A.K.; Zeng, L.; et al. Cellular communities reveal trajectories of brain ageing and Alzheimer’s disease. Nature 2024, 633, 634–645. [Google Scholar] [CrossRef]
- Pinzi, L.; Bisi, N.; Rastelli, G. How drug repurposing can advance drug discovery: Challenges and opportunities. Front. Drug Discov. 2024, 4, 1460100. [Google Scholar] [CrossRef]
- Tanoli, Z.; Fernández-Torras, A.; Özcan, U.O.; Kushnir, A.; Nader, K.M.; Gadiya, Y.; Fiorenza, L.; Ianevski, A.; Vähä-Koskela, M.; Miihkinen, M.; et al. Computational drug repurposing: Approaches, evaluation of in silico resources and case studies. Nat. Rev. Drug Discov. 2025, 24, 521–542. [Google Scholar] [CrossRef]
- Yan, C.; Grabowska, M.E.; Dickson, A.L.; Li, B.; Wen, Z.; Roden, D.M.; Michael Stein, C.; Embí, P.J.; Peterson, J.F.; Feng, Q.; et al. Leveraging generative AI to prioritize drug repurposing candidates for Alzheimer’s disease with real-world clinical validation. NPJ Digit. Med. 2024, 7, 46. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Ancidoni, A.; Bacigalupo, I.; Remoli, G.; Lacorte, E.; Piscopo, P.; Sarti, G.; Corbo, M.; Vanacore, N.; Canevelli, M. Anticancer drugs repurposed for Alzheimer’s disease: A systematic review. Alzheimer’s Res. Ther. 2021, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, M.E.; Huang, A.; Wen, Z.; Li, B.; Wei, W.Q. Drug repurposing for Alzheimer’s disease from 2012–2022—A 10-year literature review. Front. Pharmacol. 2023, 14, 1257700. [Google Scholar] [CrossRef] [PubMed]
- Adem, M.A.; Decourt, B.; Sabbagh, M.N. Pharmacological approaches using diabetic drugs repurposed for Alzheimer’s disease. Biomedicines 2024, 12, 99. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, M.; Zhao, P.; Meng, L.; Zhang, Y.; Zhang, G.; Taishi, Y.; Sun, L. Molecular mechanisms and therapeutic potential of lithium in Alzheimer’s disease: Repurposing an old class of drugs. Front. Pharmacol. 2024, 15, 1408462. [Google Scholar] [CrossRef]
- Lamb, J.; Crawford, E.D.; Peck, D.; Modell, J.W.; Blat, I.C.; Wrobel, M.J.; Lerner, J.; Brunet, J.P.; Subramanian, A.; Ross, K.N.; et al. The Connectivity Map: Using gene-expression signatures to connect small molecules, genes, and disease. Science 2006, 313, 1929–1935. [Google Scholar] [CrossRef]
- Kang, H.; Pan, S.; Lin, S.; Wang, Y.Y.; Yuan, N.; Jia, P. PharmGWAS: A GWAS-based knowledgebase for drug repurposing. Nucleic Acids Res. 2024, 52, D972–D979. [Google Scholar] [CrossRef]
- Zhang, S.D.; Gant, T.W. A simple and robust method for connecting small-molecule drugs using gene-expression signatures. BMC Bioinform. 2008, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Stebbing, J.; Phelan, A.; Griffin, I.; Tucker, C.; Oechsle, O.; Smith, D.; Richardson, P. COVID-19: Combining antiviral and anti-inflammatory treatments. Lancet Infect. Dis. 2020, 20, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Barabási, A.L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Silverman, E.K.; Schmidt, H.H.; Anastasiadou, E.; Altucci, L.; Angelini, M.; Badimon, L.; Balligand, J.L.; Benincasa, G.; Capasso, G.; Conte, F.; et al. Molecular networks in Network Medicine: Development and applications. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1489. [Google Scholar] [CrossRef]
- Sonawane, A.R.; Weiss, S.T.; Glass, K.; Sharma, A. Network medicine in the age of biomedical big data. Front. Genet. 2019, 10, 294. [Google Scholar] [CrossRef]
- Guney, E.; Menche, J.; Vidal, M.; Barábasi, A.L. Network-based in silico drug efficacy screening. Nat. Commun. 2016, 7, 10331. [Google Scholar] [CrossRef]
- Cheng, F.; Desai, R.J.; Handy, D.E.; Wang, R.; Schneeweiss, S.; Barabási, A.L.; Loscalzo, J. Network-based approach to prediction and population-based validation of in silico drug repurposing. Nat. Commun. 2018, 9, 2691. [Google Scholar] [CrossRef]
- Borisy, A.A.; Elliott, P.J.; Hurst, N.W.; Lee, M.S.; Lehár, J.; Price, E.R.; Serbedzija, G.; Zimmermann, G.R.; Foley, M.A.; Stockwell, B.R.; et al. Systematic discovery of multicomponent therapeutics. Proc. Natl. Acad. Sci. USA 2003, 100, 7977–7982. [Google Scholar] [CrossRef]
- Roszak, R.; Gadina, L.; Wołos, A.; Makkawi, A.; Mikulak-Klucznik, B.; Bilgi, Y.; Molga, K.; Gołębiowska, P.; Popik, O.; Klucznik, T.; et al. Systematic, computational discovery of multicomponent and one-pot reactions. Nat. Commun. 2024, 15, 10285. [Google Scholar] [CrossRef]
- Berchtold, N.C.; Cribbs, D.H.; Coleman, P.D.; Rogers, J.; Head, E.; Kim, R.; Beach, T.; Miller, C.; Troncoso, J.; Trojanowski, J.Q.; et al. Gene expression changes in the course of normal brain aging are sexually dimorphic. Proc. Natl. Acad. Sci. USA 2008, 105, 15605–15610. [Google Scholar] [CrossRef]
- Berchtold, N.C.; Coleman, P.D.; Cribbs, D.H.; Rogers, J.; Gillen, D.L.; Cotman, C.W. Synaptic genes are extensively downregulated across multiple brain regions in normal human aging and Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1653–1661. [Google Scholar] [CrossRef]
- Cribbs, D.H.; Berchtold, N.C.; Perreau, V.; Coleman, P.D.; Rogers, J.; Tenner, A.J.; Cotman, C.W. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: A microarray study. J. Neuroinflamm. 2012, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Astarita, G.; Jung, K.M.; Berchtold, N.C.; Nguyen, V.Q.; Gillen, D.L.; Head, E.; Cotman, C.W.; Piomelli, D. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer’s disease. PLoS ONE 2010, 5, e12538. [Google Scholar] [CrossRef] [PubMed]
- Blair, L.J.; Nordhues, B.A.; Hill, S.E.; Scaglione, K.M.; O’Leary, J.C.; Fontaine, S.N.; Breydo, L.; Zhang, B.; Li, P.; Wang, L.; et al. Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J. Clin. Investig. 2013, 123, 4158–4169. [Google Scholar] [CrossRef] [PubMed]
- Sárvári, M.; Hrabovszky, E.; Kalló, I.; Solymosi, N.; Likó, I.; Berchtold, N.; Cotman, C.; Liposits, Z. Menopause leads to elevated expression of macrophage-associated genes in the aging frontal cortex: Rat and human studies identify strikingly similar changes. J. Neuroinflamm. 2012, 9, 264. [Google Scholar] [CrossRef]
- Liang, W.S.; Dunckley, T.; Beach, T.G.; Grover, A.; Mastroeni, D.; Walker, D.G.; Caselli, R.J.; Kukull, W.A.; McKeel, D.; Morris, J.C.; et al. Gene expression profiles in anatomically and functionally distinct regions of the normal aged human brain. Physiol. Genom. 2007, 28, 311–322. [Google Scholar] [CrossRef]
- Liang, W.S.; Reiman, E.M.; Valla, J.; Dunckley, T.; Beach, T.G.; Grover, A.; Niedzielko, T.L.; Schneider, L.E.; Mastroeni, D.; Caselli, R.; et al. Alzheimer’s disease is associated with reduced expression of energy metabolism genes in posterior cingulate neurons. Proc. Natl. Acad. Sci. USA 2008, 105, 4441–4446. [Google Scholar] [CrossRef]
- Readhead, B.; Haure-Mirande, J.V.; Funk, C.C.; Richards, M.A.; Shannon, P.; Haroutunian, V.; Sano, M.; Liang, W.S.; Beckmann, N.D.; Price, N.D.; et al. Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron 2018, 99, 64–82. [Google Scholar] [CrossRef]
- Liang, W.S.; Dunckley, T.; Beach, T.G.; Grover, A.; Mastroeni, D.; Ramsey, K.; Caselli, R.J.; Kukull, W.A.; McKeel, D.; Morris, J.C.; et al. Altered neuronal gene expression in brain regions differentially affected by Alzheimer’s disease: A reference data set. Physiol. Genom. 2008, 33, 240–256. [Google Scholar] [CrossRef]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef]
- Rogers, S.L.; Doody, R.S.; Mohs, R.C.; Friedhoff, L.T.; Donepezil Study Group. Donepezil improves cognition and global function in Alzheimer disease: A 15-week, double-blind, placebo-controlled study. Arch. Intern. Med. 1998, 158, 1021–1031. [Google Scholar] [CrossRef] [PubMed]
- Glaze, D.G.; Neul, J.L.; Kaufmann, W.E.; Berry-Kravis, E.; Condon, S.; Stoms, G.; Oosterholt, S.; Della Pasqua, O.; Glass, L.; Jones, N.E.; et al. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology 2019, 92, e1912–e1925. [Google Scholar] [CrossRef] [PubMed]
- DiPersio, J.F.; Stadtmauer, E.A.; Nademanee, A.; Micallef, I.N.; Stiff, P.J.; Kaufman, J.L.; Maziarz, R.T.; Hosing, C.; Früehauf, S.; Horwitz, M.; et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood J. Am. Soc. Hematol. 2009, 113, 5720–5726. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.J.; Mallinckrodt, C.; Lu, Y.; Demitrack, M.A. Duloxetine in the treatment of major depressive disorder: A double-blind clinical trial. J. Clin. Psychiatry 2002, 63, 225–231. [Google Scholar] [CrossRef]
- Rosenberg, P.B.; Martin, B.K.; Frangakis, C.; Mintzer, J.E.; Weintraub, D.; Porsteinsson, A.P.; Schneider, L.S.; Rabins, P.V.; Munro, C.A.; Meinert, C.L.; et al. Sertraline for the treatment of depression in Alzheimer disease. Am. J. Geriatr. Psychiatry 2010, 18, 136–145. [Google Scholar] [CrossRef]
- Schneider, L.S.; Dagerman, K.S.; Insel, P. Risk of death with atypical antipsychotic drug treatment for dementia: Meta-analysis of randomized placebo-controlled trials. JAMA 2005, 294, 1934–1943. [Google Scholar] [CrossRef]
- Preuss, U.W.; Wong, J.; Koller, G. Treatment of behavioral and psychological symptoms of dementia: A systematic review. Psychiatr. Pol. 2016, 50, 679–715. [Google Scholar] [CrossRef]
- Pagano, G.; Boess, F.G.; Taylor, K.I.; Ricci, B.; Mollenhauer, B.; Poewe, W.; Boulay, A.; Anzures-Cabrera, J.; Vogt, A.; Marchesi, M.; et al. A phase II study to evaluate the safety and efficacy of prasinezumab in early Parkinson’s disease (PASADENA): Rationale, design, and baseline data. Front. Neurol. 2021, 12, 705407. [Google Scholar] [CrossRef]
- Cummings, J.; Aisen, P.; Apostolova, L.; Atri, A.; Salloway, S.; Weiner, M. Aducanumab: Appropriate use recommendations. J. Prev. Alzheimer’s Dis. 2021, 8, 398–410. [Google Scholar] [CrossRef]
- Scheltens, P.; Vijverberg, E. Aducanumab: Appropriate use recommendations. J. Prev. Alzheimer’s Dis. 2021, 8, 412–413. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; DeKosky, S.; Mufson, E.J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007, 68, 1501–1508. [Google Scholar] [CrossRef]
- Achar, A.; Myers, R.; Ghosh, C. Drug delivery challenges in brain disorders across the blood–brain barrier: Novel methods and future considerations for improved therapy. Biomedicines 2021, 9, 1834. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Khan, H.; Serdaroğlu, G. Physicochemical properties, drug likeness, ADMET, DFT studies, and in vitro antioxidant activity of oxindole derivatives. Comput. Biol. Chem. 2023, 104, 107861. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, M.; Javeria, H.; Tian, D.; Du, Z. Accurate prediction of Kp, uu, brain based on experimental measurement of Kp, brain and computed physicochemical properties of candidate compounds in CNS drug discovery. Heliyon 2024, 10, e24304. [Google Scholar] [CrossRef]
- Kato, R.; Zeng, W.; Siramshetty, V.B.; Williams, J.; Kabir, M.; Hagen, N.; Padilha, E.C.; Wang, A.Q.; Mathé, E.A.; Xu, X.; et al. Development and validation of PAMPA-BBB QSAR model to predict brain penetration potential of novel drug candidates. Front. Pharmacol. 2023, 14, 1291246. [Google Scholar] [CrossRef]
- Faramarzi, S.; Kim, M.T.; Volpe, D.A.; Cross, K.P.; Chakravarti, S.; Stavitskaya, L. Development of QSAR models to predict blood-brain barrier permeability. Front. Pharmacol. 2022, 13, 1040838. [Google Scholar] [CrossRef]
- Gavriel, Y.; Voronov-Goldman, M.; Solomon, B. Reversible inhibition of chemokine receptor CXC4 signaling via AMD3100 mitigates neuroinflammation in Alzheimer’s disease. Ageing Neurodegener. Dis. 2025, 5, 1. [Google Scholar] [CrossRef]
- Sanchis-Pascual, D.; Del Olmo-García, M.I.; Prado-Wohlwend, S.; Zac-Romero, C.; Segura Huerta, Á.; Hernández-Gil, J.; Martí-Bonmatí, L.; Merino-Torres, J.F. CXCR4: From signaling to clinical applications in neuroendocrine neoplasms. Cancers 2024, 16, 1799. [Google Scholar] [CrossRef]
- Alvarez-Galvez, J.; Arroyo, J. Uncovering bridging diseases in complex multimorbidity pathways: A network science approach. PLoS ONE 2025, 20, e0323208. [Google Scholar] [CrossRef]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H.H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef]
- Del Giudice, K.P.; Cosgaya, M.; Zaro, I.; Ravasi, V.; Santacruz, P.; Painous, C.; Fernández, M.; Cámara, A.; Compta, Y. Anti-alpha synuclein and anti-tau immunotherapies: Can a cocktail approach work? Park. Relat. Disord. 2024, 122, 106080. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, A.; Ali, H.; Alnaimi, A.; Yousef, M.; Rob, M.; Al-Muhannadi, N.A.; Senevirathne, D.K.L.; Chaari, A. Immunotherapy for Parkinson’s disease and Alzheimer’s disease: A promising disease-modifying therapy. Cells 2024, 13, 1527. [Google Scholar] [CrossRef] [PubMed]
- Paik, J. Targeting toxic forms of α-synuclein with immunotherapy could alter the progression of Parkinson’s disease. Drugs Ther. Perspect. 2022, 38, 467–471. [Google Scholar] [CrossRef]
- Vidović, M.; Rikalovic, M.G. Alpha-synuclein aggregation pathway in Parkinson’s disease: Current status and novel therapeutic approaches. Cells 2022, 11, 1732. [Google Scholar] [CrossRef]
- Luo, X.; Ji, X.; Xie, M.; Zhang, T.; Wang, Y.; Sun, M.; Huang, W.; Xia, L. Advance of SOX transcription factors in hepatocellular carcinoma: From role, tumor immune relevance to targeted therapy. Cancers 2022, 14, 1165. [Google Scholar] [CrossRef]
- Stevanovic, M.; Lazic, A.; Schwirtlich, M.; Stanisavljevic Ninkovic, D. The role of SOX transcription factors in ageing and age-related diseases. Int. J. Mol. Sci. 2023, 24, 851. [Google Scholar] [CrossRef]
- Lei, L.; Peng, G.; Luo, H.; Li, W. SRY-box transcription factor 21 antisense divergent transcript 1: Regulatory roles and clinical significance in neoplastic conditions and Alzheimer’s Disease. J. Cancer 2023, 14, 3258. [Google Scholar] [CrossRef]
- Kim, C.K.; Lee, Y.R.; Ong, L.; Gold, M.; Kalali, A.; Sarkar, J. Alzheimer’s disease: Key insights from two decades of clinical trial failures. J. Alzheimer’s Dis. 2022, 87, 83–100. [Google Scholar] [CrossRef]
- Asher, S.; Priefer, R. Alzheimer’s disease failed clinical trials. Life Sci. 2022, 306, 120861. [Google Scholar] [PubMed]
- Cannon, M.; Stevenson, J.; Stahl, K.; Basu, R.; Coffman, A.; Kiwala, S.; McMichael, J.F.; Kuzma, K.; Morrissey, D.; Cotto, K.; et al. DGIdb 5.0: Rebuilding the drug–gene interaction database for precision medicine and drug discovery platforms. Nucleic Acids Res. 2024, 52, D1227–D1235. [Google Scholar] [PubMed]
- Pardridge, W.M. Drug transport across the blood–brain barrier. J. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Moving beyond rules: The development of a central nervous system multiparameter optimization (CNS MPO) approach to enable alignment of druglike properties. ACS Chem. Neurosci. 2010, 1, 435–449. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Bors, L.A.; Erdő, F. Overcoming the blood–brain barrier. challenges and tricks for CNS drug delivery. Sci. Pharm. 2019, 87, 6. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier biology and methodology. J. Neurovirol. 1999, 5, 556–569. [Google Scholar]
- Crivori, P.; Reinach, B.; Pezzetta, D.; Poggesi, I. Computational models for identifying potential P-glycoprotein substrates and inhibitors. Mol. Pharm. 2006, 3, 33–44. [Google Scholar]
- Chen, L.; Li, Y.; Yu, H.; Zhang, L.; Hou, T. Computational models for predicting substrates or inhibitors of P-glycoprotein. Drug Discov. Today 2012, 17, 343–351. [Google Scholar] [CrossRef]

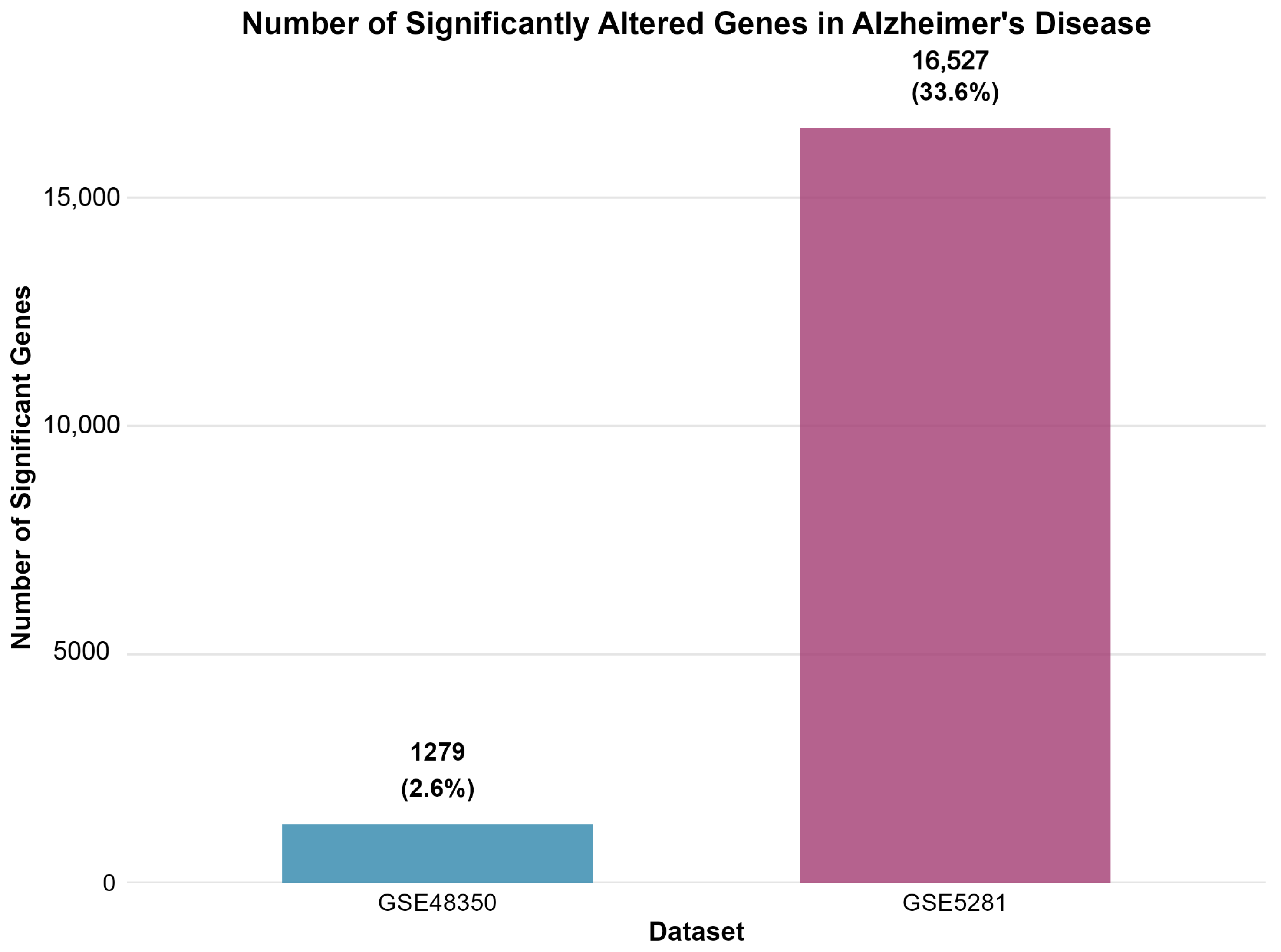
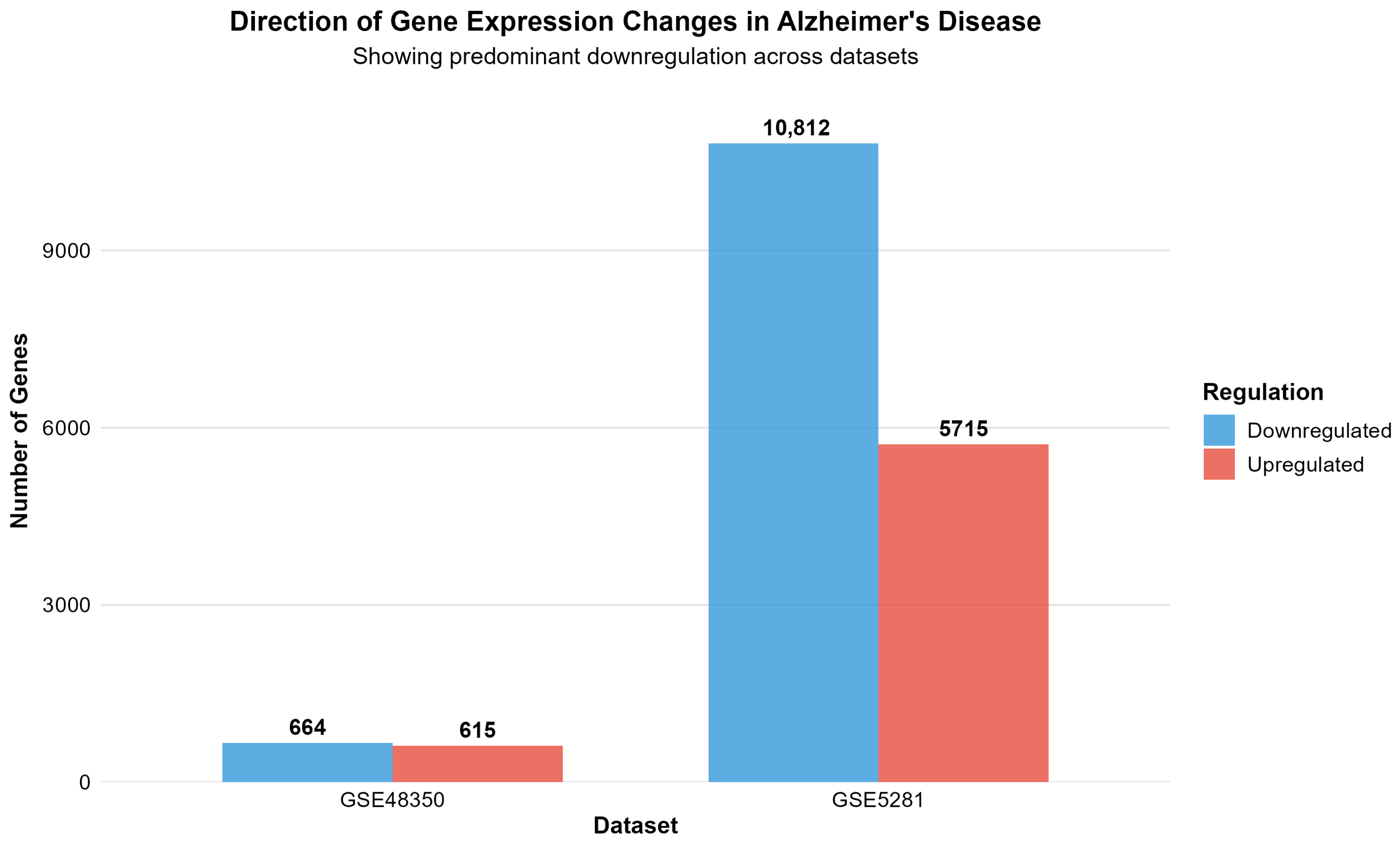
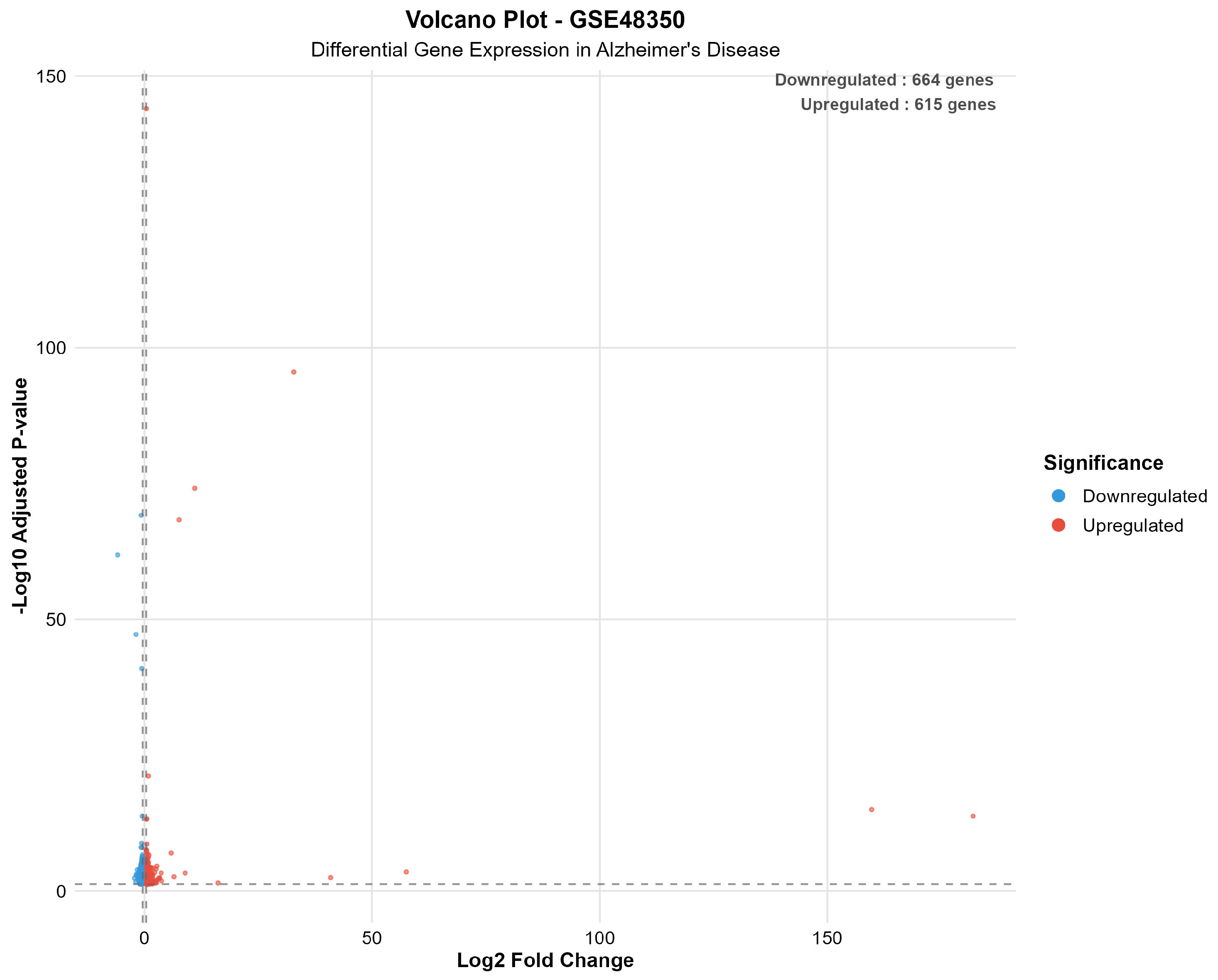
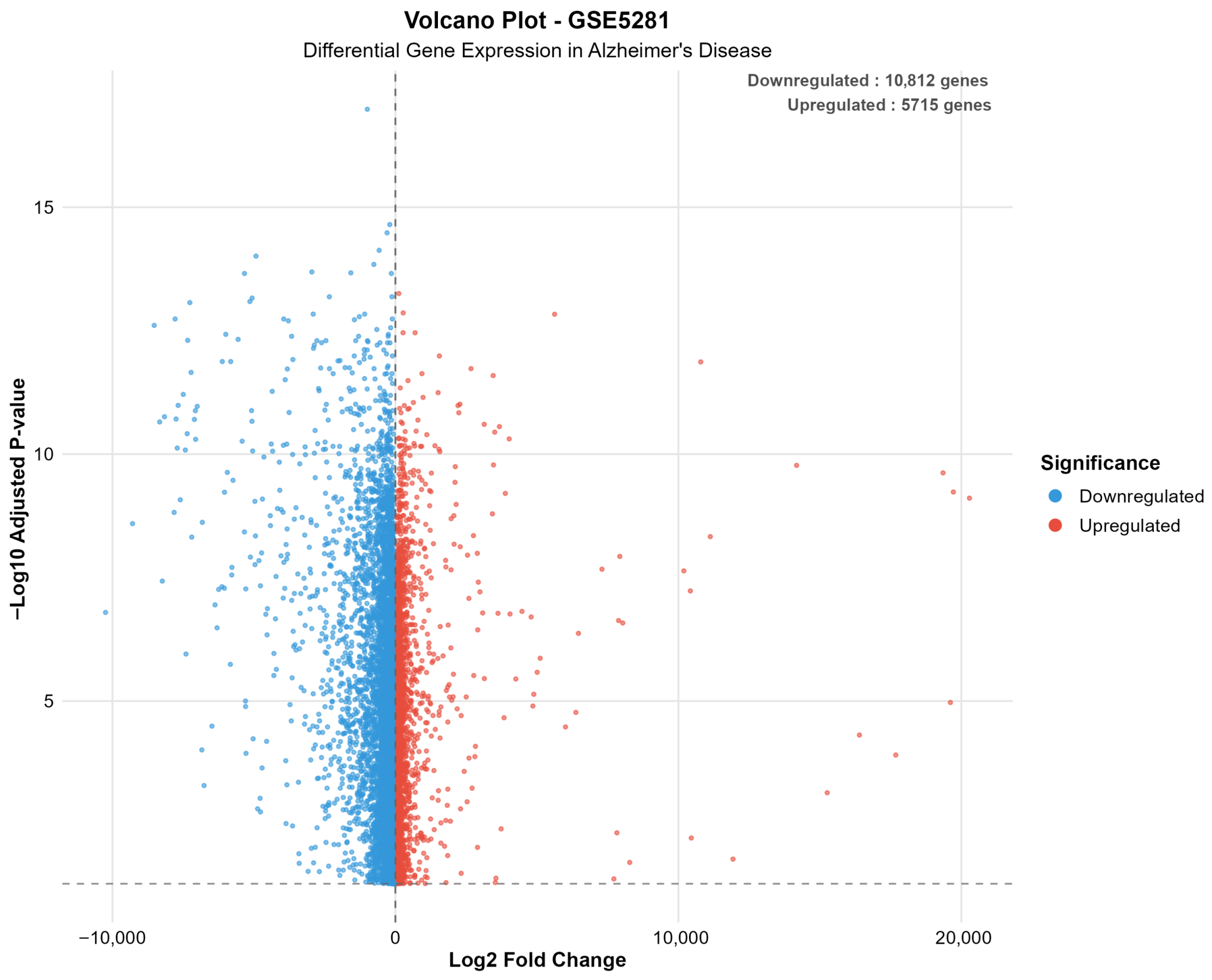


| Dataset | Platform | AD Samples | Control Samples | Total Genes | Significant Genes | Upregulated | Downregulated | Percent Significant |
|---|---|---|---|---|---|---|---|---|
| GSE48350 | Microarray | 80 | 173 | 49,207 | 1279 | 615 | 664 | 2.6 |
| GSE5281 | Microarray | 87 | 74 | 49,207 | 16,527 | 5715 | 10,812 | 33.6 |
| Network Property | Value |
|---|---|
| Total Overlapping Genes | 742 |
| Genes Mapped to Symbols | 640 (86.3%) |
| Genes Mapped to STRING Proteins | 599 (80.7%) |
| Network Nodes | 508 |
| Network Edges | 1349 |
| Network Density | 0.0105 |
| Mean Degree | 6.9 |
| Largest Connected Component | 456 proteins |
| Number of Connected Components | 18 |
| Gene Set | GO BP | GO MF | Hallmark | KEGG | Reactome |
|---|---|---|---|---|---|
| All Genes | 819 | 164 | 3 | 35 | 74 |
| Hub Genes | 788 | 157 | 3 | 33 | 73 |
| Bridge Genes | 792 | 162 | 3 | 34 | 72 |
| High Degree | 819 | 164 | 3 | 35 | 74 |
| Interacting | 819 | 164 | 3 | 35 | 74 |
| Rank | Gene | Final Score | Temporal Category | Potential Drugs | Rationale | Priority |
|---|---|---|---|---|---|---|
| 1 | IGF1 | 45.47 | Neuroprotection | Mecasermin, IGF-1 LR3 | High multi-dimensional score; Early intervention potential | High |
| 2 | SNCA | 41.49 | Progression modifier | Anle138b, NPT200-11 | High network plasticity; Multi-pathway involvement | High |
| 3 | SOX9 | 37.96 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | High |
| 4 | CDC42 | 23.82 | Uncategorized | ML141, CASIN, ZCL278 | High network plasticity; High druggability potential | Medium |
| 5 | PTPRC | 22.94 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 6 | CALM1 | 22.94 | Symptomatic treatment | Calmidazolium, W-7, Trifluoperazine | High network plasticity; High druggability potential | Medium |
| 7 | CAMK2A | 20.90 | Symptomatic treatment | KN-93, Staurosporine, H-89 | High multi-dimensional score; High druggability potential | Medium |
| 8 | PPP2CA | 19.06 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 9 | YAP1 | 18.91 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 10 | PAX6 | 17.92 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 11 | EGR1 | 16.56 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 12 | NRXN1 | 16.47 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 13 | GRIA1 | 16.28 | Symptomatic treatment | Memantine, Perampanel, Topiramate | High druggability potential; Multi-pathway involvement | Medium |
| 14 | MAPK8 | 15.54 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 15 | GRIN2A | 15.33 | Symptomatic treatment | Memantine, Ketamine, Dextromethorphan | High druggability potential; Multi-pathway involvement | Medium |
| 16 | CRH | 14.34 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 17 | NFKBIA | 13.78 | Uncategorized | Novel target - no known drugs | High network plasticity; Multi-pathway involvement | Medium |
| 18 | CXCR4 | 13.53 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 19 | YWHAZ | 13.18 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 20 | FBXW7 | 12.06 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 21 | PRKCD | 11.34 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 22 | CX3CL1 | 10.78 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 23 | PRKCG | 10.20 | Uncategorized | Novel target - no known drugs | High druggability potential; Multi-pathway involvement | Medium |
| 24 | NRP1 | 9.85 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| 25 | CLU | 8.94 | Uncategorized | Novel target - no known drugs | High multi-dimensional score; Multi-pathway involvement | Medium |
| Network Type | Nodes | Edges | Density | Connected Components | Largest Component Size | Average Clustering | Transitivity | Avg Path Length |
|---|---|---|---|---|---|---|---|---|
| Drug–Gene | 12,089 | 187,431 | 0.000591 | 18 | 11,847 | 0.0523 | 0.0891 | 4.2 |
| Drug–Drug | 8247 | 294,573 | 0.00865 | 47 | 8156 | 0.127 | 0.203 | 3.8 |
| Gene–Gene | 3842 | 127,839 | 0.0173 | 23 | 3798 | 0.245 | 0.318 | 3.1 |
| Integrated | 12,089 | 609,843 | 0.00834 | 18 | 11,847 | 0.142 | 0.187 | 4.1 |
| Property | Mean | Std Dev | Min | Max | Median |
|---|---|---|---|---|---|
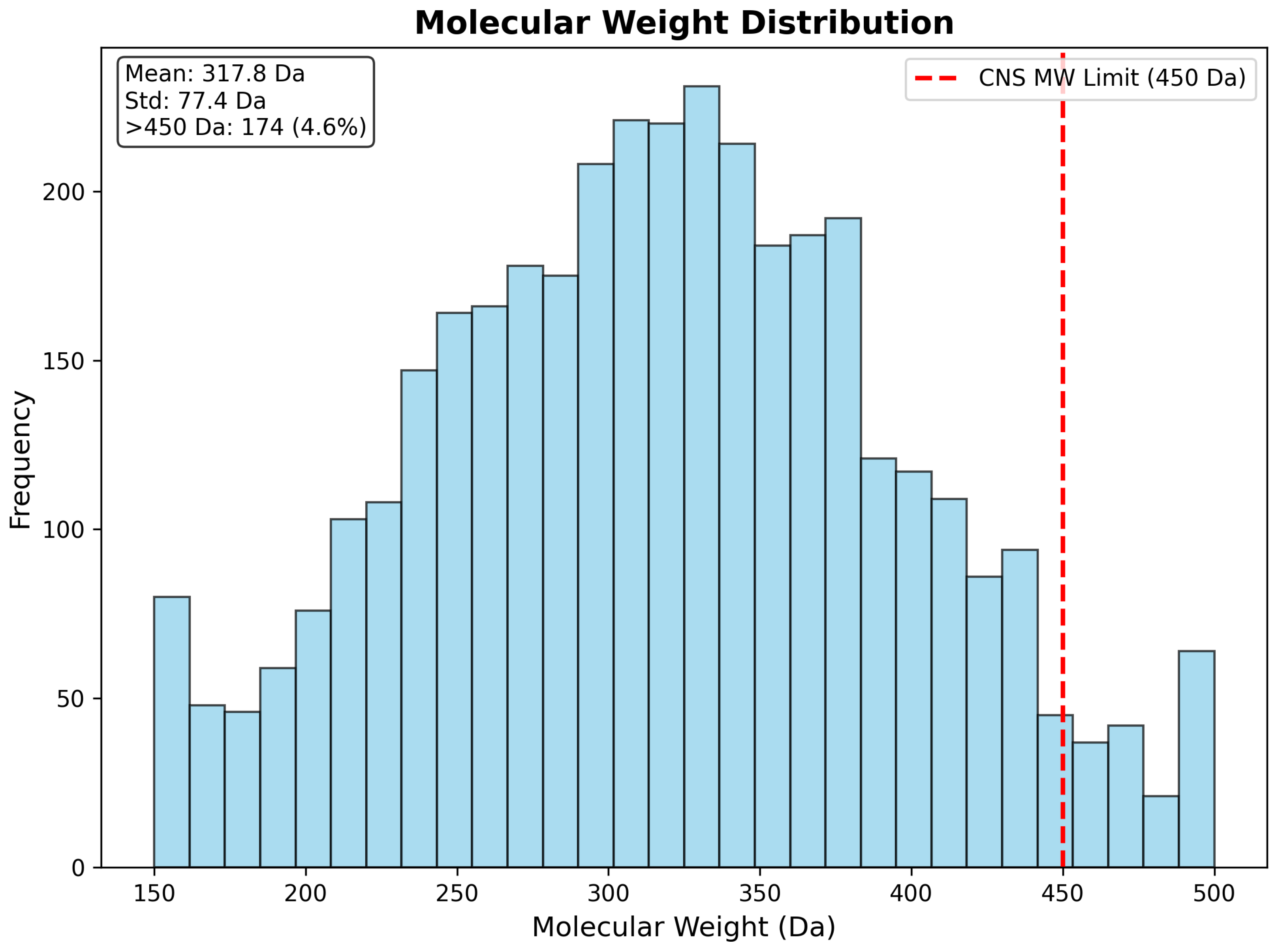
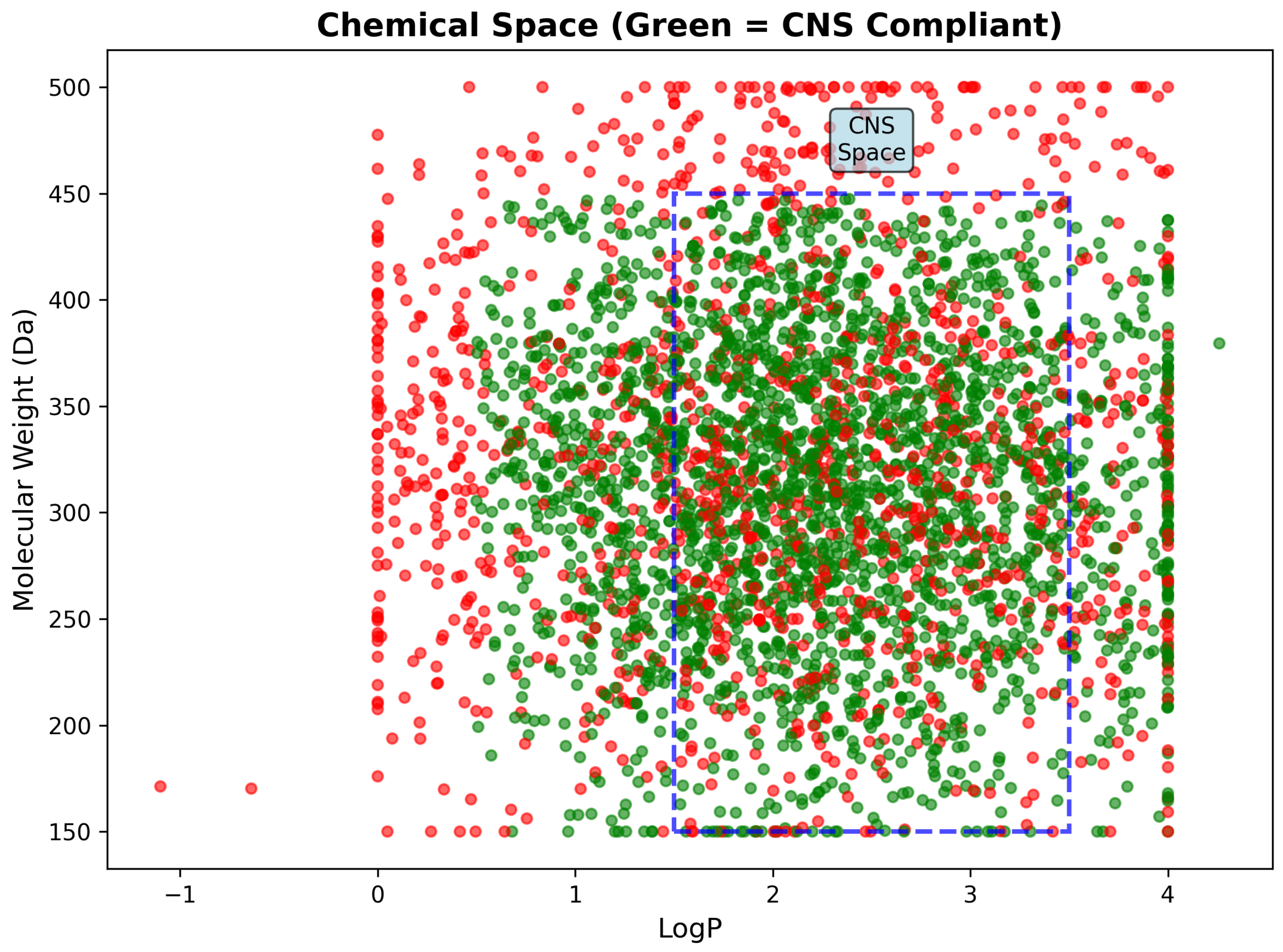
| Molecular Weight (Da) | 317.82 | 77.44 | 150.13 | 499.66 | 315.27 |
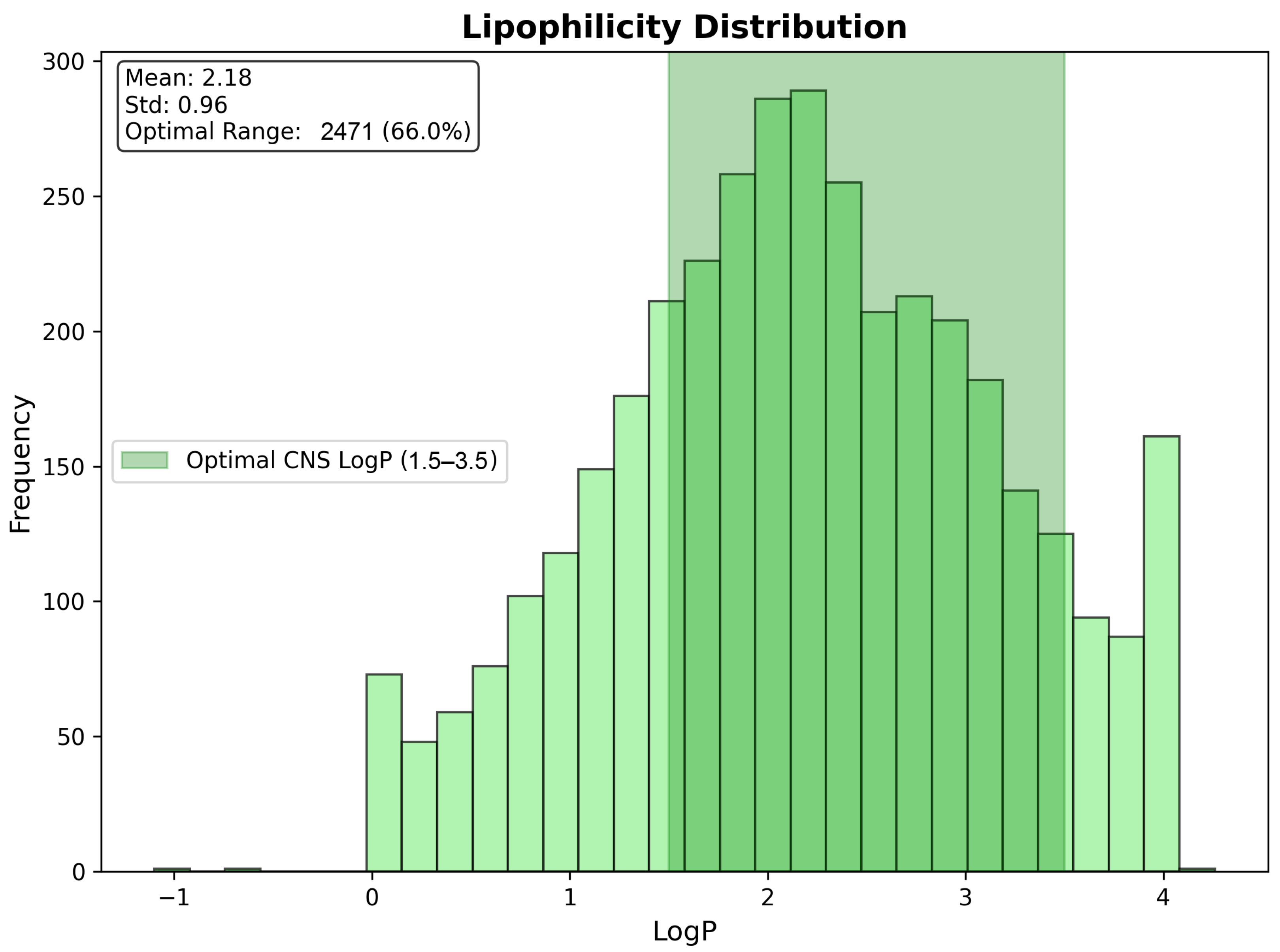
| LogP | 2.18 | 0.96 | −0.85 | 4.21 | 2.19 |
| PSA (Å2) | 52.27 | 20.15 | 20.31 | 118.44 | 48.67 |
| HBD | 1.37 | 1.12 | 0.00 | 4.00 | 1.00 |
| HBA | 3.24 | 1.98 | 1.00 | 9.00 | 3.00 |
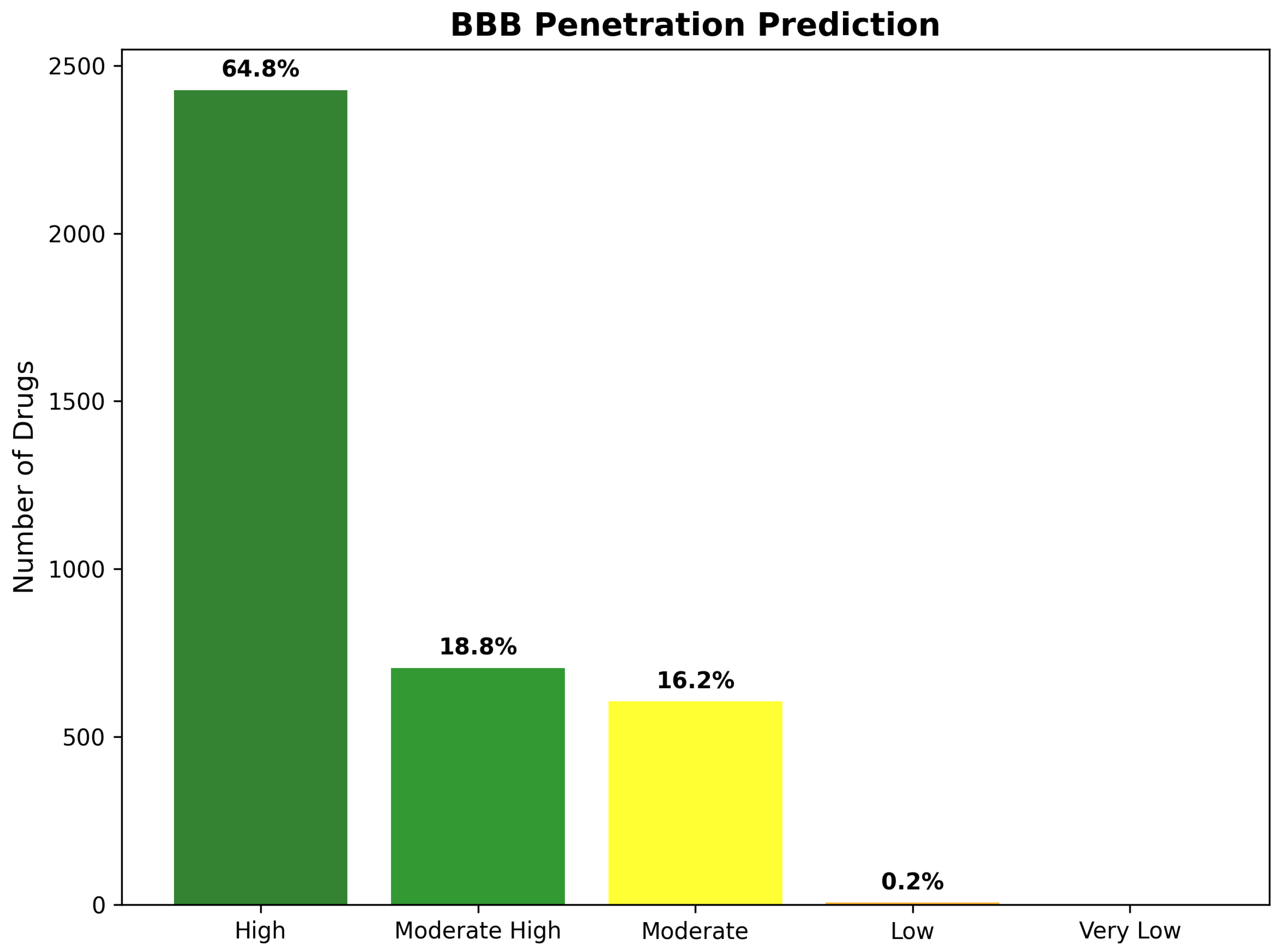
| CNS Compliant (%) | 64.8 | - | - | - | - |
| High BBB Penetration (%) | 64.8 | - | - | - | - |
| Model | Accuracy | Sensitivity | Specificity | ROC AUC |
|---|---|---|---|---|
| Random Forest | 0.9565 | 1.0000 | 0.8750 | 0.9922 |
| Gradient Boosting | 0.9565 | 1.0000 | 0.8750 | 0.9375 |
| XGBoost | 0.9565 | 1.0000 | 0.8750 | 0.9453 |
| SVM | 0.9130 | 1.0000 | 0.7500 | 0.9453 |
| Modality | Count | Percentage (%) |
|---|---|---|
| Small Molecule | 3667 | 97.97 |
| Peptide | 73 | 1.95 |
| Biologic | 3 | 0.08 |
| Total | 3743 | 100.00 |
| Rank | Drug | Status | Network | MedChem | MW | LogP | PSA | HBD | HBA | BBB ML | BBB ML | P-gp | Tract. | React. | AD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | Score | (Da) | (Å2) | Prob. | Class | Liab. | Class | Risk | Evid. | ||||||
| 1 | PLERIXAFOR | Approved | 1.170 | 1.170 | 502.8 | 2.15 | 118.4 | 8 | 12 | 0.650 | Mod. High | Moderate | Class I | Low | Mechanistic |
| 2 | PRENYLAMINE | Approved | 0.949 | 0.949 | 329.5 | 4.26 | 38.8 | 0 | 2 | 0.920 | High | Moderate | Class I | Low | Mechanistic |
| 3 | DULOXETINE | Approved | 0.685 | 0.685 | 297.4 | 4.23 | 44.9 | 1 | 2 | 0.890 | High | Moderate | Class I | Low | Mechanistic |
| 4 | MEMANTINE | Approved | 0.623 | 0.623 | 179.3 | 3.28 | 26.0 | 1 | 1 | 0.950 | High | Low | Class I | Low | Established |
| 5 | DONEPEZIL | Approved | 0.587 | 0.587 | 379.5 | 4.26 | 38.8 | 0 | 3 | 0.910 | High | Moderate | Class I | Low | Established |
| 6 | SERTRALINE | Approved | 0.521 | 0.521 | 306.2 | 5.29 | 12.0 | 1 | 1 | 0.880 | High | Moderate | Class I | Low | Mechanistic |
| 7 | RISPERIDONE | Approved | 0.498 | 0.498 | 410.5 | 3.04 | 61.8 | 0 | 5 | 0.820 | High | Moderate | Class I | Low | Mechanistic |
| 8 | QUETIAPINE | Approved | 0.487 | 0.487 | 383.5 | 2.87 | 73.8 | 1 | 6 | 0.750 | Mod. High | Moderate | Class I | Low | Mechanistic |
| 9 | LEVETIRACETAM | Approved | 0.456 | 0.456 | 170.2 | −0.64 | 63.4 | 1 | 3 | 0.780 | Mod. High | Low | Class I | Low | Mechanistic |
| 10 | FLUOXETINE | Approved | 0.443 | 0.443 | 309.3 | 4.05 | 21.3 | 1 | 2 | 0.920 | High | Moderate | Class I | Low | Mechanistic |
| 11 | TOPIRAMATE | Approved | 0.421 | 0.421 | 339.4 | 0.89 | 118.0 | 0 | 9 | 0.580 | Moderate | Low | Class II | Low | Mechanistic |
| 12 | GABAPENTIN | Approved | 0.398 | 0.398 | 171.2 | −1.10 | 63.3 | 2 | 3 | 0.720 | Mod. High | Low | Class I | Low | Mechanistic |
| 13 | OLANZAPINE | Approved | 0.387 | 0.387 | 312.4 | 3.00 | 44.0 | 1 | 4 | 0.880 | High | Moderate | Class I | Low | Mechanistic |
| 14 | CARBAMAZEPINE | Approved | 0.365 | 0.365 | 236.3 | 2.45 | 46.3 | 1 | 2 | 0.910 | High | Low | Class I | Low | Mechanistic |
| 15 | VALPROATE | Approved | 0.354 | 0.354 | 144.2 | 2.75 | 37.3 | 1 | 2 | 0.930 | High | Low | Class I | Low | Mechanistic |
| Rank | Drug | Status | Network | MedChem | MW | LogP | PSA | HBD | HBA | BBB ML | BBB ML | P-gp | Tract. | React. | AD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | Score | (Da) | (Å2) | Prob. | Class | Liab. | Class | Risk | Evid. | ||||||
| 1 | TROFINETIDE | Approved | 1.388 | 1.387 | 341.4 | 1.89 | 45.2 | 1 | 3 | 0.917 | High | Low | Class I | Low | Speculative |
| 2 | CALCDPWW | Experimental | 0.917 | 0.917 | 287.4 | 1.60 | 41.9 | 1 | 3 | 0.917 | High | Low | Class I | Low | Mechanistic |
| 3 | SOMATOSTATIN | Approved | 0.842 | 0.820 | 1638.0 | −3.15 | 456.2 | 18 | 26 | 0.145 | Low | High | Class III | Low | Mechanistic |
| 4 | OCTREOTIDE | Approved | 0.798 | 0.775 | 1019.2 | −0.85 | 267.5 | 10 | 14 | 0.320 | Low | High | Class III | Low | Mechanistic |
| 5 | LANREOTIDE | Approved | 0.756 | 0.735 | 1096.4 | −1.12 | 289.8 | 11 | 15 | 0.295 | Low | High | Class III | Low | Mechanistic |
| 6 | PASIREOTIDE | Approved | 0.723 | 0.705 | 1047.2 | −0.98 | 279.3 | 10 | 14 | 0.308 | Low | High | Class III | Low | Mechanistic |
| 7 | VASOACTIVE INT. | Approved | 0.687 | 0.668 | 3326.0 | −5.89 | 892.4 | 32 | 48 | 0.052 | Very Low | High | Class IV | Low | Mechanistic |
| 8 | GLUCAGON | Approved | 0.654 | 0.635 | 3483.0 | −6.12 | 945.6 | 35 | 51 | 0.048 | Very Low | High | Class IV | Low | Mechanistic |
| 9 | INSULIN LISPRO | Approved | 0.621 | 0.603 | 5808.0 | −8.45 | 1567.0 | 52 | 78 | 0.015 | Very Low | High | Class IV | Low | Mechanistic |
| 10 | EXENATIDE | Approved | 0.598 | 0.580 | 4186.6 | −7.23 | 1234.5 | 41 | 62 | 0.025 | Very Low | High | Class IV | Low | Mechanistic |
| Rank | Drug | Status | Target | Network Score | BBB Strategy | AD Evidence |
|---|---|---|---|---|---|---|
| 1 | Prasinezumab | Experimental | α-Synuclein | 0.968 | RMT engineering | Mechanistic |
| 2 | Gantenerumab | Experimental | Amyloid-β | 0.847 | Native IgG1 | Clinical |
| 3 | Aducanumab | Approved | Amyloid-β | 0.823 | Native IgG1 | Established |
| Drug Name | Approved Indication | AD Trials | Mechanism Hypothesis | Evidence Level | Key Limitations | References |
|---|---|---|---|---|---|---|
| Memantine | Moderate-severe AD | – | NMDA antagonism; excitotoxicity reduction | Established | Symptomatic only; no disease modification | [42] |
| Donepezil | Mild-severe AD | – | Acetylcholinesterase inhibition; cholinergic enhancement | Established | Symptomatic only; modest cognitive benefit | [43] |
| Trofinetide | Rett syndrome | None | IGF-1 pathway; synaptic neuroprotection | Speculative | No AD preclinical/clinical data; mechanistic disconnect | [44] |
| Plerixafor | Stem cell mobilization | None | CXCR4 antagonism; neuroinflammation modulation | Mechanistic | No AD model validation; unclear BBB kinetics | [45] |
| Duloxetine | Depression, neuropathic pain | None | SNRI; monoaminergic modulation; potential anti-inflammatory | Mechanistic | No AD efficacy data; unclear disease-modifying potential | [46] |
| Sertraline | Depression, anxiety | Phase 2/3 | SSRI; serotonergic modulation; BDNF upregulation | Mechanistic | Clinical trials showed no cognitive benefit | [47] |
| Risperidone | Schizophrenia, bipolar | Phase 4 | Dopamine/serotonin antagonism; behavioral symptom control | Mechanistic | No disease modification; safety concerns (ARIA) | [48] |
| Quetiapine | Schizophrenia, bipolar | Phase 3 | Atypical antipsychotic; behavioral symptoms | Mechanistic | No cognitive benefit; metabolic side effects | [49] |
| Prasinezumab | Parkinson’s (investig.) | None | Anti-α-synuclein; protein aggregation inhibition | Mechanistic | PD target; unclear AD relevance; BBB delivery challenge | [50] |
| Aducanumab | AD (controversial) | Approved | Anti-amyloid-β; plaque clearance | Clinical | Marginal efficacy; significant safety concerns (ARIA) | [51,52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akgüller, Ö.; Balcı, M.A.; Cioca, G. Network-Medicine-Guided Drug Repurposing for Alzheimer’s Disease: A Multi-Dimensional Systems Pharmacology Approach. Int. J. Mol. Sci. 2025, 26, 10003. https://doi.org/10.3390/ijms262010003
Akgüller Ö, Balcı MA, Cioca G. Network-Medicine-Guided Drug Repurposing for Alzheimer’s Disease: A Multi-Dimensional Systems Pharmacology Approach. International Journal of Molecular Sciences. 2025; 26(20):10003. https://doi.org/10.3390/ijms262010003
Chicago/Turabian StyleAkgüller, Ömer, Mehmet Ali Balcı, and Gabriela Cioca. 2025. "Network-Medicine-Guided Drug Repurposing for Alzheimer’s Disease: A Multi-Dimensional Systems Pharmacology Approach" International Journal of Molecular Sciences 26, no. 20: 10003. https://doi.org/10.3390/ijms262010003
APA StyleAkgüller, Ö., Balcı, M. A., & Cioca, G. (2025). Network-Medicine-Guided Drug Repurposing for Alzheimer’s Disease: A Multi-Dimensional Systems Pharmacology Approach. International Journal of Molecular Sciences, 26(20), 10003. https://doi.org/10.3390/ijms262010003






