A Review of Immunological Evaluation of Patients with Recurrent Spontaneous Abortion (RSA)
Abstract
1. Introduction
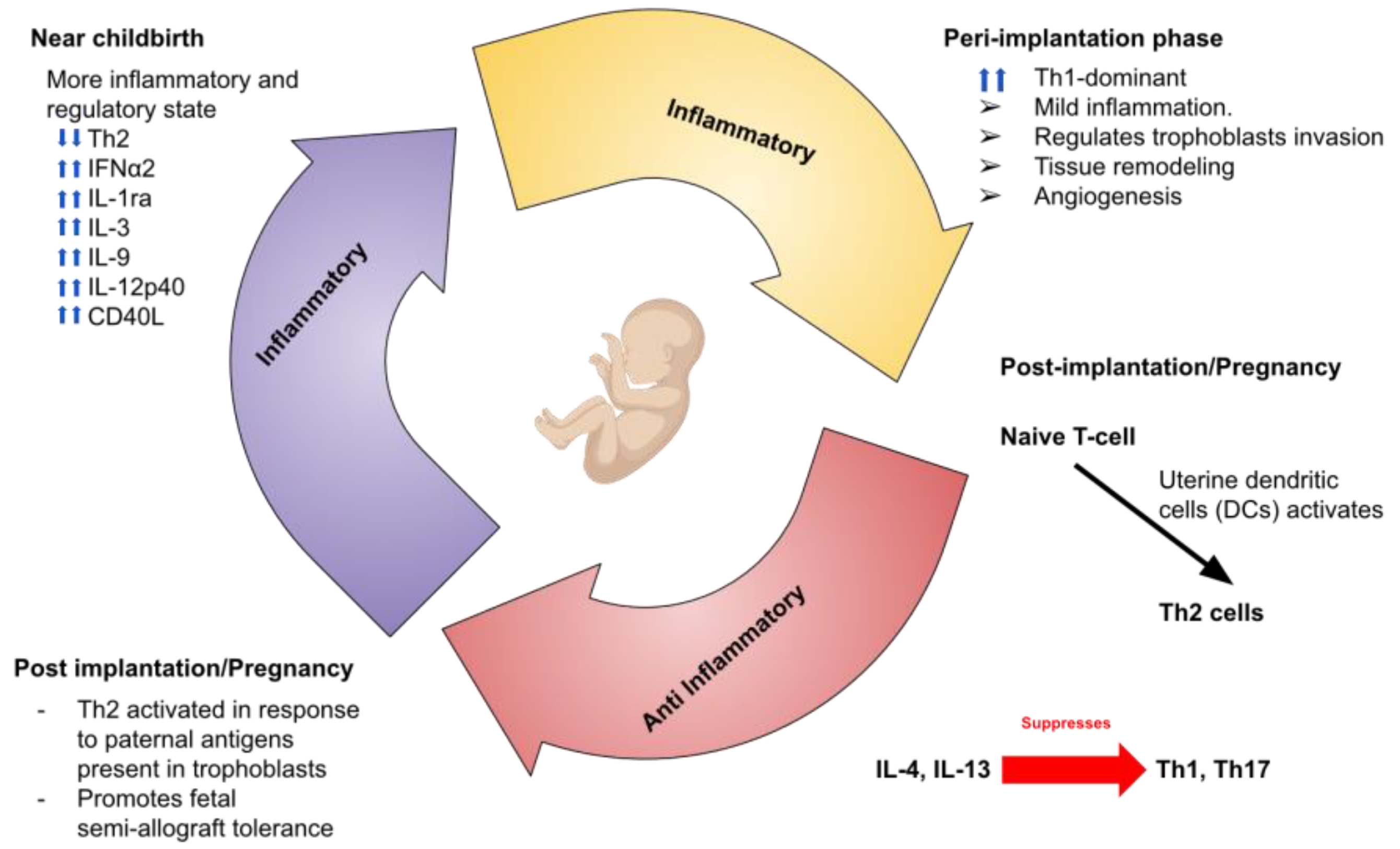
2. Immune System and Pregnancy
3. Main Players in RSA
3.1. Natural Killer Cells
3.2. T Cells
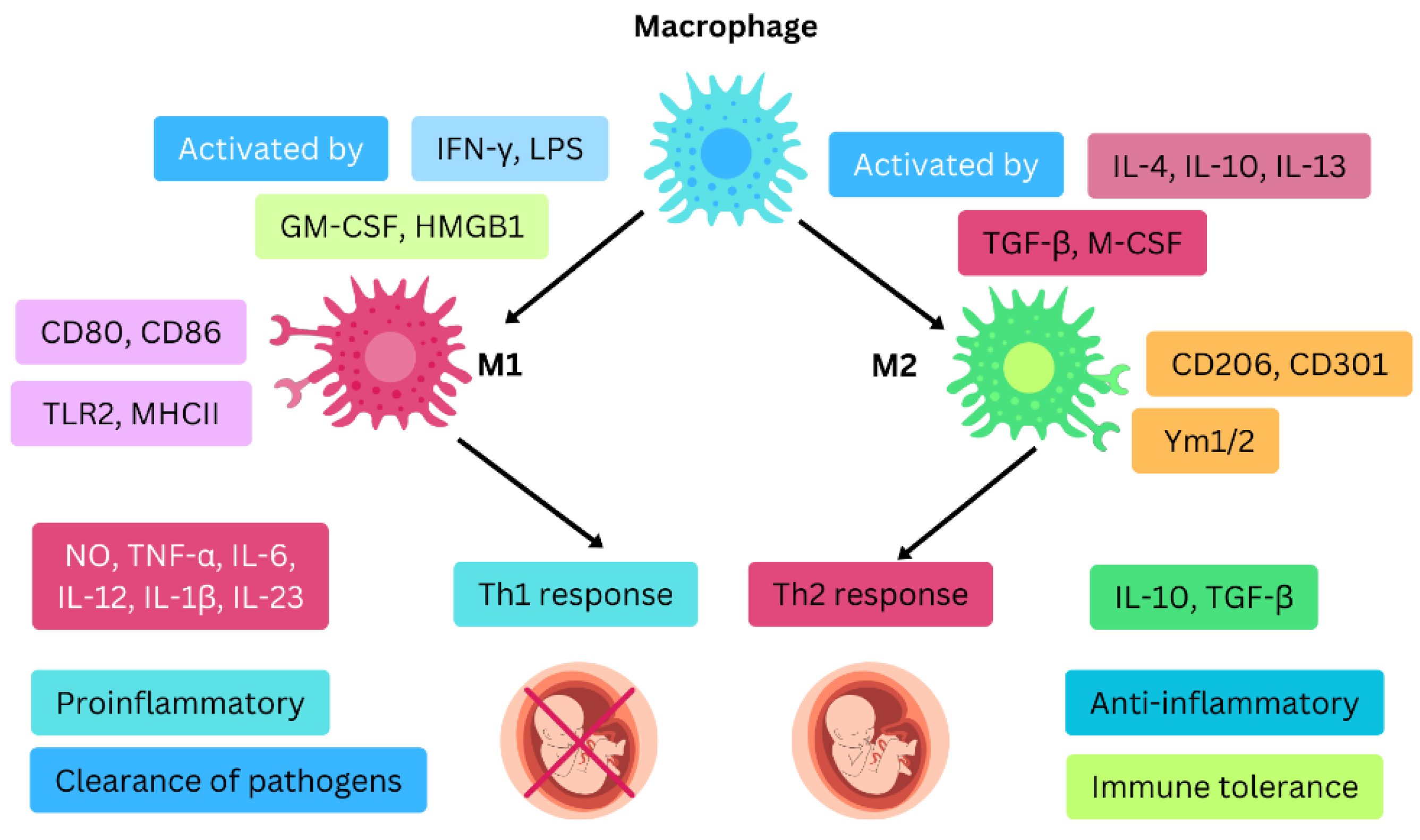
3.3. Macrophages
3.4. Dendritic Cells
4. Cytokines and Chemokines in RSA
5. Autoantibodies and RSA
5.1. Antiphospholipid Antibodies (aPL)
5.2. Antinuclear Antibodies (ANA)
5.3. Anti-Thyroid Antibodies
5.4. Anti-Cardiolipin Antibodies (aCL)
6. Clinical Recommendations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deng, T.; Liao, X.; Zhu, S. Recent Advances in Treatment of Recurrent Spontaneous Abortion. Obstet. Gynecol. Surv. 2022, 77, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Bender Atik, R.; Christiansen, O.B.; Elson, J.; Kolte, A.M.; Lewis, S.; Middeldorp, S.; Nelen, W.; Peramo, B.; Quenby, S.; Vermeulen, N.; et al. ESHRE guideline: Recurrent pregnancy loss. Hum. Reprod. Open 2018, 2018, hoy004. [Google Scholar] [CrossRef] [PubMed]
- Evaluation and treatment of recurrent pregnancy loss: A committee opinion. Fertil. Steril. 2012, 98, 1103–1111. [CrossRef] [PubMed]
- Luu, T.; AlSubki, L.; Wolf, K.; Thees, A.; Ganieva, U.; Dambaeva, S.; Beaman, K.; Kwak-Kim, J. Natural killer cell-mediated immunopathology in recurrent pregnancy losses. Explor. Immunol. 2022, 2, 693–722. [Google Scholar] [CrossRef]
- Abdollahi, E.; Tavasolian, F.; Ghasemi, N.; Mirghanizadeh, S.A.; Azizi, M.; Ghoryani, M.; Samadi, M. Association between lower frequency of R381Q variant (rs11209026) in IL-23 receptor gene and increased risk of recurrent spontaneous abortion (RSA). J. Immunotoxicol. 2015, 12, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Madani, J.; Aghebati-Maleki, L.; Gharibeh, N.; Pourakbari, R.; Yousefi, M. Fetus, as an allograft, evades the maternal immunity. Transpl. Immunol. 2022, 75, 101728. [Google Scholar] [CrossRef]
- Maxwell, A.J.; You, Y.; Aldo, P.B.; Zhang, Y.; Ding, J.; Mor, G. The role of the immune system during pregnancy: General concepts. In Reproductive Immunology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–21. [Google Scholar]
- Andreescu, M. The impact of the use of immunosuppressive treatment after an embryo transfer in increasing the rate of live birth. Front. Med. 2023, 10, 1167876. [Google Scholar] [CrossRef]
- Chakraborty, D.; Rumi, M.A.K.; Konno, T.; Soares, M.J. Natural killer cells direct hemochorial placentation by regulating hypoxia-inducible factor dependent trophoblast lineage decisions. Proc. Natl. Acad. Sci. USA 2011, 108, 16295–16300. [Google Scholar] [CrossRef]
- Kim, S.-G.; Paek, M.-Y.; Ko, I.G. Peripheral Blood Level of Natural Killer Cells in Pregnant Women with Recurrent Spontaneous Abortion during the 6–12 Weeks Gestation. Arch. Med. Health Sci. 2019, 7, 191. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Andreescu, M.; Frîncu, F.; Plotogea, M.; Mehedințu, C. Recurrent Abortion and the Involvement of Killer-Cell Immunoglobulin-like Receptor (KIR) Genes, Activated T Cells, NK Abnormalities, and Cytokine Profiles. J. Clin. Med. 2023, 12, 1355. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, Q.; Jin, L. Dynamic Function and Composition Changes of Immune Cells During Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2317. [Google Scholar] [CrossRef] [PubMed]
- Aldo, P.B.; Racicot, K.; Craviero, V.; Guller, S.; Romero, R.; Mor, G. Trophoblast induces monocyte differentiation into CD14+/CD16+ macrophages. Am. J. Reprod. Immunol. 2014, 72, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Svensson-Arvelund, J.; Mehta, R.B.; Lindau, R.; Mirrasekhian, E.; Rodriguez-Martinez, H.; Berg, G.; Lash, G.E.; Jenmalm, M.C.; Ernerudh, J. The human fetal placenta promotes tolerance against the semiallogeneic fetus by inducing regulatory T cells and homeostatic M2 macrophages. J. Immunol. 2015, 194, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, H. Role of Decidual Natural Killer Cells in Human Pregnancy and Related Pregnancy Complications. Front. Immunol. 2021, 12, 728291. [Google Scholar] [CrossRef]
- Male, V.; Moffett, A. Natural Killer Cells in the Human Uterine Mucosa. Annu. Rev. Immunol. 2023, 41, 127–151. [Google Scholar] [CrossRef]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.E.; Stephenson, E.; Polański, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef]
- Feyaerts, D.; Benner, M.; Comitini, G.; Shadmanfar, W.; van der Heijden, O.W.H.; Joosten, I.; van der Molen, R.G. NK cell receptor profiling of endometrial and decidual NK cells reveals pregnancy-induced adaptations. Front. Immunol. 2024, 15, 1353556. [Google Scholar] [CrossRef]
- Fu, B.; Li, X.; Sun, R.; Tong, X.; Ling, B.; Tian, Z.; Wei, H. Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface. Proc. Natl. Acad. Sci. USA 2013, 110, E231–E240. [Google Scholar] [CrossRef]
- Mahajan, D.; Sharma, N.R.; Kancharla, S.; Kolli, P.; Tripathy, A.; Sharma, A.K.; Singh, S.; Kumar, S.; Mohanty, A.K.; Jena, M.K. Role of Natural Killer Cells during Pregnancy and Related Complications. Biomolecules 2022, 12, 68. [Google Scholar] [CrossRef]
- Kieffer, T.E.; Faas, M.M.; Scherjon, S.A.; Prins, J.R. Pregnancy persistently affects memory T cell populations. J. Reprod. Immunol. 2017, 119, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, T.E.; Laskewitz, A.; Scherjon, S.A.; Faas, M.M.; Prins, J.R. Memory T cells in pregnancy. Front. Immunol. 2019, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Jafarpour, R.; Pashangzadeh, S.; Mehdizadeh, S.; Bayatipoor, H.; Shojaei, Z.; Motallebnezhad, M. Functional significance of lymphocytes in pregnancy and lymphocyte immunotherapy in infertility: A comprehensive review and update. Int. Immunopharmacol. 2020, 87, 106776. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [CrossRef]
- Monteiro, C.; Kasahara, T.M.; Castro, J.R.; Sacramento, P.M.; Hygino, J.; Centurião, N.; Cassano, T.; Lopes, L.M.F.; Leite, S.; Gupta, S. Pregnancy favors the expansion of circulating functional follicular helper T Cells. J. Reprod. Immunol. 2017, 121, 1–10. [Google Scholar] [CrossRef]
- Logiodice, F.; Lombardelli, L.; Kullolli, O.; Haller, H.; Maggi, E.; Rukavina, D.; Piccinni, M.-P. Decidual interleukin-22-producing CD4+ T cells (Th17/Th0/IL-22+ and Th17/Th2/IL-22+, Th2/IL-22+, Th0/IL-22+), which also produce IL-4, are involved in the success of pregnancy. Int. J. Mol. Sci. 2019, 20, 428. [Google Scholar] [CrossRef]
- Billingham, R.E.; Brent, L.; Medawar, P.B. ‘Actively acquired tolerance’ of foreign cells. 1953. J. Immunol 2010, 184, 5–8. [Google Scholar] [CrossRef]
- Germain, S.J.; Sacks, G.P.; Sooranna, S.R.; Sargent, I.L.; Redman, C.W. Systemic inflammatory priming in normal pregnancy and preeclampsia: The role of circulating syncytiotrophoblast microparticles. J. Immunol. 2007, 178, 5949–5956. [Google Scholar] [CrossRef]
- Graham, J.J.; Longhi, M.S.; Heneghan, M.A. T helper cell immunity in pregnancy and influence on autoimmune disease progression. J. Autoimmun. 2021, 121, 102651. [Google Scholar] [CrossRef]
- Kim, B.; Kim, T.H. Fundamental role of dendritic cells in inducing Th2 responses. Korean J. Intern. Med. 2018, 33, 483–489. [Google Scholar] [CrossRef]
- Wang, J.; Han, T.; Zhu, X. Role of maternal-fetal immune tolerance in the establishment and maintenance of pregnancy. Chin. Med J. 2024, 137, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Chiasson, V.L.; Bounds, K.R.; Mitchell, B.M. Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy. Front. Immunol. 2014, 5, 253. [Google Scholar] [CrossRef] [PubMed]
- Iwaszko, M.; Biały, S.; Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef] [PubMed]
- Holtan, S.G.; Chen, Y.; Kaimal, R.; Creedon, D.J.; Enninga, E.A.L.; Nevala, W.K.; Markovic, S.N. Growth modeling of the maternal cytokine milieu throughout normal pregnancy: Macrophage-derived chemokine decreases as inflammation/counterregulation increases. J. Immunol. Res. 2015, 2015, 952571. [Google Scholar] [CrossRef]
- Zare, M.; Jahromi, B.N.; Gharesi-Fard, B. Analysis of the frequencies and functions of CD4+ CD25+ CD127low/neg, CD4+ HLA-G+, and CD8+ HLA-G+ regulatory T cells in pre-eclampsia. J. Reprod. Immunol. 2019, 133, 43–51. [Google Scholar] [CrossRef]
- Krop, J.; Heidt, S.; Claas, F.H.J.; Eikmans, M. Regulatory T Cells in Pregnancy: It Is Not All About FoxP3. Front. Immunol. 2020, 11, 1182. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Sun, H.-X. Immune checkpoint molecules in pregnancy: Focus on regulatory T cells. Eur. J. Immunol. 2020, 50, 160–169. [Google Scholar] [CrossRef]
- Samstein, R.M.; Josefowicz, S.Z.; Arvey, A.; Treuting, P.M.; Rudensky, A.Y. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 2012, 150, 29–38. [Google Scholar] [CrossRef]
- Xin, L.; Ertelt, J.M.; Rowe, J.H.; Jiang, T.T.; Kinder, J.M.; Chaturvedi, V.; Elahi, S.; Way, S.S. Cutting edge: Committed Th1 CD4+ T cell differentiation blocks pregnancy-induced Foxp3 expression with antigen-specific fetal loss. J. Immunol. 2014, 192, 2970–2974. [Google Scholar] [CrossRef]
- Figueiredo, A.S.; Schumacher, A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology 2016, 148, 13–21. [Google Scholar] [CrossRef]
- Hosseini, S.; Shokri, F.; Ansari Pour, S.; Jeddi-Tehrani, M.; Nikoo, S.; Yousefi, M.; Zarnani, A.-H. A shift in the balance of T17 and Treg cells in menstrual blood of women with unexplained recurrent spontaneous abortion. J. Reprod. Immunol. 2016, 116, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Pongcharoen, S.; Supalap, K. Interleukin-17 increased progesterone secretion by JEG-3 human choriocarcinoma cells. Am. J. Reprod. Immunol. 2009, 61, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.V.; Thees, A.; Ganieva, U.; Dambaeva, S.; Beaman, K.; Kwak-Kim, J. T regulatory, Th17, and treg/Th17 ratio, in pregnant women with recurrent pregnancy losses and normal pregnant women. Fertil. Steril. 2022, 118, e179–e180. [Google Scholar] [CrossRef]
- Nakashima, A.; Ito, M.; Yoneda, S.; Shiozaki, A.; Hidaka, T.; Saito, S. Circulating and decidual Th17 cell levels in healthy pregnancy. Am. J. Reprod. Immunol. 2010, 63, 104–109. [Google Scholar] [CrossRef]
- Tang, C.; Hu, W. The role of Th17 and Treg cells in normal pregnancy and unexplained recurrent spontaneous abortion (URSA): New insights into immune mechanisms. Placenta 2023, 142, 18–26. [Google Scholar] [CrossRef]
- Braga, A.; Neves, E.; Guimarães, J.; Braga, J.; Vasconcelos, C. Th17/Regulatory T cells ratio evolution: A prospective study in a group of healthy pregnant women. J. Reprod. Immunol. 2022, 149, 103468. [Google Scholar] [CrossRef]
- Romanowska-Próchnicka, K.; Felis-Giemza, A.; Olesińska, M.; Wojdasiewicz, P.; Paradowska-Gorycka, A.; Szukiewicz, D. The Role of TNF-α and Anti-TNF-α Agents during Preconception, Pregnancy, and Breastfeeding. Int. J. Mol. Sci. 2021, 22, 2922. [Google Scholar] [CrossRef]
- Wen, B.; Liao, H.; Lin, W.; Li, Z.; Ma, X.; Xu, Q.; Yu, F. The Role of TGF-β during Pregnancy and Pregnancy Complications. Int. J. Mol. Sci. 2023, 24, 16882. [Google Scholar] [CrossRef]
- Hadinedoushan, H.; Mirahmadian, M.; Aflatounian, A. Increased natural killer cell cytotoxicity and IL-2 production in recurrent spontaneous abortion. Am. J. Reprod. Immunol. 2007, 58, 409–414. [Google Scholar] [CrossRef]
- Karami, N.; Boroujerdnia, M.G.; Nikbakht, R.; Khodadadi, A. Enhancement of peripheral blood CD56(dim) cell and NK cell cytotoxicity in women with recurrent spontaneous abortion or in vitro fertilization failure. J. Reprod. Immunol. 2012, 95, 87–92. [Google Scholar] [CrossRef]
- Shakhar, K.; Ben-Eliyahu, S.; Loewenthal, R.; Rosenne, E.; Carp, H. Differences in number and activity of peripheral natural killer cells in primary versus secondary recurrent miscarriage. Fertil. Steril. 2003, 80, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Papúchová, H.; Meissner, T.B.; Li, Q.; Strominger, J.L.; Tilburgs, T. The Dual Role of HLA-C in Tolerance and Immunity at the Maternal-Fetal Interface. Front. Immunol. 2019, 10, 2730. [Google Scholar] [CrossRef] [PubMed]
- Ataei, M.; Mirzaei, M.; Inanloo, F.; Maleki, N.; Rad, S.S.; Noorbakhsh, S.M.; Atousa, K. KIR3DL1 gene genotype in patients with spontaneous recurrent abortion. Arch. Venez. De Farmacol. Y Ter. 2021, 40, 121–128. [Google Scholar]
- Akbari, S.; Shahsavar, F.; Karami, R.; Yari, F.; Anbari, K.; Ahmadi, S.A.Y. Recurrent Spontaneous Abortion (RSA) and Maternal KIR Genes: A Comprehensive Meta-Analysis. JBRA Assist Reprod 2020, 24, 197–213. [Google Scholar] [CrossRef]
- Mansour, L.; Alkhuriji, A.; Babay, Z.A.; Alqadheeb, S.; Al-Khulaifi, F.; Al-Talhi, R.; Alomar, S. Association of Killer Immunoglobulin-Like Receptor and Human Leukocyte Antigen Class I Ligand with Recurrent Abortion in Saudi Women. Genet. Test. Mol. Biomark. 2020, 24, 78–84. [Google Scholar] [CrossRef]
- Alharbi, H.M.; Alkhuriji, A.F.; Alomar, S.Y.; Babay, Z.A.; Alnafjan, A.A.; Alobaid, H.M.; Allharbi, W.G.; Mansour, L.A. Association of recurrent spontaneous abortion with polycystic ovarian syndrome under the influence of killer immunoglobulin like receptors. J. King Saud Univ. Sci. 2022, 34, 102034. [Google Scholar] [CrossRef]
- Su, N.; Wang, H.; Zhang, B.; Kang, Y.; Guo, Q.; Xiao, H.; Yang, H.; Liao, S. Maternal natural killer cell immunoglobulin receptor genes and human leukocyte antigen-C ligands influence recurrent spontaneous abortion in the Han Chinese population. Exp. Ther. Med. 2018, 15, 327–337. [Google Scholar] [CrossRef]
- Alecsandru, D.; Barrio, A.; Garrido, N.; Aparicio, P.; Pellicer, A.; Moffett, A.; García-Velasco, J.A. Parental human leukocyte antigen-C allotypes are predictive of live birth rate and risk of poor placentation in assisted reproductive treatment. Fertil. Steril. 2020, 114, 809–817. [Google Scholar] [CrossRef]
- Yang, X.; Meng, T. Killer-cell immunoglobulin-like receptor/human leukocyte antigen-C combination and ‘great obstetrical syndromes’ (Review). Exp. Ther. Med. 2021, 22, 1178. [Google Scholar] [CrossRef]
- Akbari, S.; Ahmadi, S.A.Y.; Shahsavar, F. The relationship of maternal KIR and parental HLA-C genes with risk of recurrent spontaneous abortion: A regional study in Lorestan province, Iran. Crescent J. Med. Biol. Sci. 2018, 5, 194–197. [Google Scholar]
- Maftei, R.; Doroftei, B.; Popa, R.; Harabor, V.; Adam, A.-M.; Popa, C.; Harabor, A.; Adam, G.; Nechita, A.; Vasilache, I.-A.; et al. The Influence of Maternal KIR Haplotype on the Reproductive Outcomes after Single Embryo Transfer in IVF Cycles in Patients with Recurrent Pregnancy Loss and Implantation Failure—A Single Center Experience. J. Clin. Med. 2023, 12, 1905. [Google Scholar] [CrossRef] [PubMed]
- Elbaşı, M.O.; Tulunay, A.; Karagözoğlu, H.; Kahraman, S.; Ekşioğlu-Demiralp, E. Maternal killer-cell immunoglobulin-like receptors and paternal human leukocyte antigen ligands in recurrent pregnancy loss cases in Turkey. Clin. Exp. Reprod. Med. 2020, 47, 122. [Google Scholar] [CrossRef] [PubMed]
- Dambaeva, S.V.; Lee, D.H.; Sung, N.; Chen, C.Y.; Bao, S.; Gilman-Sachs, A.; Kwak-Kim, J.; Beaman, K.D. Recurrent Pregnancy Loss in Women with Killer Cell Immunoglobulin-Like Receptor KIR2DS1 is Associated with an Increased HLA-C2 Allelic Frequency. Am. J. Reprod. Immunol. 2016, 75, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.C.; Eikmans, M.; van der Hoorn, M.P.; Lashley, L. Recurrent miscarriages and the association with regulatory T cells; A systematic review. J. Reprod. Immunol. 2020, 139, 103105. [Google Scholar] [CrossRef]
- Luo, L.; Zeng, X.; Huang, Z.; Luo, S.; Qin, L.; Li, S. Reduced frequency and functional defects of CD4+CD25highCD127low/− regulatory T cells in patients with unexplained recurrent spontaneous abortion. Reprod. Biol. Endocrinol. 2020, 18, 62. [Google Scholar] [CrossRef]
- Winger, E.E.; Reed, J.L. Low circulating CD4(+) CD25(+) Foxp3(+) T regulatory cell levels predict miscarriage risk in newly pregnant women with a history of failure. Am. J. Reprod. Immunol. 2011, 66, 320–328. [Google Scholar] [CrossRef]
- Care, A.S.; Bourque, S.L.; Morton, J.S.; Hjartarson, E.P.; Robertson, S.A.; Davidge, S.T. Reduction in Regulatory T Cells in Early Pregnancy Causes Uterine Artery Dysfunction in Mice. Hypertension 2018, 72, 177–187. [Google Scholar] [CrossRef]
- Farshchi, M.; Abdollahi, E.; Saghafi, N.; Hosseini, A.; Fallahi, S.; Rostami, S.; Rostami, P.; Rafatpanah, H.; Habibagahi, M. Evaluation of Th17 and Treg cytokines in patients with unexplained recurrent pregnancy loss. J. Clin. Transl. Res. 2022, 8, 256–265. [Google Scholar]
- Yang, X.; Tian, Y.; Zheng, L.; Luu, T.; Kwak-Kim, J. The Update Immune-Regulatory Role of Pro- and Anti-Inflammatory Cytokines in Recurrent Pregnancy Losses. Int. J. Mol. Sci. 2023, 24, 132. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Hur, S.; Kim, C.; Na, B.; Lee, M.; Gilman-Sachs, A.; Kwak-Kim, J. An imbalance in interleukin-17-producing T and Foxp3+ regulatory T cells in women with idiopathic recurrent pregnancy loss. Hum. Reprod. 2011, 26, 2964–2971. [Google Scholar] [CrossRef]
- Wang, W.; Sung, N.; Gilman-Sachs, A.; Kwak-Kim, J. T helper (Th) cell profiles in pregnancy and recurrent pregnancy losses: Th1/Th2/Th9/Th17/Th22/Tfh cells. Front. Immunol. 2020, 11, 2025. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Xie, Q.; Chen, S.; Li, Q.; Dong, X.; Zhang, T.; Fu, S.; Lei, Q.; Huang, D. Research progress of immune balance and genetic polymorphism in unexplained recurrent abortion. Explor. Immunol. 2023, 3, 453–474. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, P. Increased CD56 (+) NK cells and enhanced Th1 responses in human unexplained recurrent spontaneous abortion. Genet. Mol. Res. 2015, 14, 18103–18109. [Google Scholar] [CrossRef] [PubMed]
- Jena, M.K.; Nayak, N.; Chen, K.; Nayak, N.R. Role of macrophages in pregnancy and related complications. Arch. Immunol. Et Ther. Exp. 2019, 67, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Yin, T.; Yan, N.; Cheng, Y.; Yang, J. FasL on decidual macrophages mediates trophoblast apoptosis: A potential cause of recurrent miscarriage. Int. J. Mol. Med. 2019, 43, 2376–2386. [Google Scholar] [CrossRef]
- Wang, H.; He, M.; Hou, Y.; Chen, S.; Zhang, X.; Zhang, M.; Ji, X. Role of decidual CD14+ macrophages in the homeostasis of maternal–fetal interface and the differentiation capacity of the cells during pregnancy and parturition. Placenta 2016, 38, 76–83. [Google Scholar] [CrossRef]
- Zhao, Q.-Y.; Li, Q.-H.; Fu, Y.-Y.; Ren, C.-E.; Jiang, A.-F.; Meng, Y.-H. Decidual macrophages in recurrent spontaneous abortion. Front. Immunol. 2022, 13, 994888. [Google Scholar] [CrossRef]
- Pérez, S.; Rius-Pérez, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Jaiswal, M.K.; Mallers, T.M.; Larsen, B.; Kwak-Kim, J.; Chaouat, G.; Gilman-Sachs, A.; Beaman, K.D. V-ATPase upregulation during early pregnancy: A possible link to establishment of an inflammatory response during preimplantation period of pregnancy. Reproduction 2012, 143, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.; Oomomian, Y.; Stephen, G.; Shynlova, O.; Tower, C.L.; Garrod, A.; Lye, S.J.; Jones, R.L. Macrophages infiltrate the human and rat decidua during term and preterm labor: Evidence that decidual inflammation precedes labor. Biol. Reprod. 2012, 86, 39. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.; Rajasingh, S.; Samanta, S.; Cao, T.; Dawn, B.; Rajasingh, J. Macrophage polarization in response to epigenetic modifiers during infection and inflammation. Drug Discov. Today 2017, 22, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Lampiasi, N.; Russo, R.; Zito, F. The Alternative Faces of Macrophage Generate Osteoclasts. Biomed Res. Int. 2016, 2016, 9089610. [Google Scholar] [CrossRef]
- Tsao, F.-Y.; Wu, M.-Y.; Chang, Y.-L.; Wu, C.-T.; Ho, H.-N. M1 macrophages decrease in the deciduae from normal pregnancies but not from spontaneous abortions or unexplained recurrent spontaneous abortions. J. Formos. Med. Assoc. 2018, 117, 204–211. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, M.; Ren, L.; Zhang, A.; Zhao, M.; Zhang, H.; Jiang, Y.; Hu, X. Decidual macrophage M1 polarization contributes to adverse pregnancy induced by Toxoplasma gondii PRU strain infection. Microb. Pathog. 2018, 124, 183–190. [Google Scholar] [CrossRef]
- Brown, M.B.; von Chamier, M.; Allam, A.B.; Reyes, L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front. Immunol. 2014, 5, 606. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y.; Cai, X.; Zhang, Y.; Yan, S.; Wang, J.; Zhang, S.; Yin, T.; Yang, C.; Yang, J. Extracellular vesicles derived from M1 macrophages deliver miR-146a-5p and miR-146b-5p to suppress trophoblast migration and invasion by targeting TRAF6 in recurrent spontaneous abortion. Theranostics 2021, 11, 5813. [Google Scholar] [CrossRef]
- Darmochwal-Kolarz, D.; Rolinski, J.; Tabarkiewicz, J.; Leszczynska-Gorzelak, B.; Buczkowski, J.; Wojas, K.; Oleszczuk, J. Myeloid and lymphoid dendritic cells in normal pregnancy and pre-eclampsia. Clin. Exp. Immunol. 2003, 132, 339–344. [Google Scholar] [CrossRef]
- Qian, Z.-D.; Huang, L.-L.; Zhu, X.-M. An immunohistochemical study of CD83- and CD1a-positive dendritic cells in the decidua of women with recurrent spontaneous abortion. Eur. J. Med. Res. 2015, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, P.; Jia, N.; Wen, X.; Luo, L.; Wang, S.; Li, J. The expression of intracellular cytokines of decidual natural killer cells in unexplained recurrent pregnancy loss. J. Matern. Fetal Neonatal Med. 2022, 35, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- Brogin Moreli, J.; Cirino Ruocco, A.M.; Vernini, J.M.; Rudge, M.V.; Calderon, I.M. Interleukin 10 and tumor necrosis factor-alpha in pregnancy: Aspects of interest in clinical obstetrics. ISRN Obstet. Gynecol 2012, 2012, 230742. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Wen, X.; Liu, J.; Yan, C.Y.; Yuan, J.; Luo, L.R.; Hu, Q.F.; Li, J. Simultaneous detection of decidual Th1/Th2 and NK1/NK2 immunophenotyping in unknown recurrent miscarriage using 8-color flow cytometry with FSC/Vt extended strategy. Biosci. Rep. 2017, 37, BSR20170150. [Google Scholar] [CrossRef] [PubMed]
- Calleja-Agius, J.; Jauniaux, E.; Pizzey, A.R.; Muttukrishna, S. Investigation of systemic inflammatory response in first trimester pregnancy failure. Hum. Reprod. 2012, 27, 349–357. [Google Scholar] [CrossRef]
- Li, M.-M.; Lin, J.; Wu, H.-F.; Zheng, G.-J.; Cai, R.-N. Analysis of the risk factors in patients with unexplained recurrent spontaneous abortion. Am. J. Reprod. Immunol. 2023, 90, e13774. [Google Scholar] [CrossRef]
- Alkhuriji, A.F.; Al Omar, S.Y.; Babay, Z.A.; El-khadragy, M.F.; Mansour, L.A.; Alharbi, W.G.; Khalil, M.I. Association of IL-1β, IL-6, TNF-α, and TGFβ1 Gene Polymorphisms with Recurrent Spontaneous Abortion in Polycystic Ovary Syndrome. Dis. Markers 2020, 2020, 6076274. [Google Scholar] [CrossRef]
- Begum, A.; Mishra, A.; Das, C.R.; Das, S.; Dutta, R.; Kashyap, N.; Bose, P.D.; Bose, S. Impact of TNF-α profile in recurrent pregnancy loss pathogenesis: A patient based study from Assam. J. Reprod. Immunol. 2021, 148, 103430. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, Q.; Zhang, N.; Chen, J.; Chen, X.; You, Q.; Wu, H. Tumour necrosis factor inhibitor combined with intravenous immunoglobulin and heparin for treatment of recurrent spontaneous abortion: A two-centre, retrospective, cohort study. J. Clin. Pharm. Ther. 2022, 47, 2320–2324. [Google Scholar] [CrossRef]
- Piosik, Z.M.; Goegebeur, Y.; Klitkou, L.; Steffensen, R.; Christiansen, O.B. Plasma TNF-α levels are higher in early pregnancy in patients with secondary compared with primary recurrent miscarriage. Am. J. Reprod. Immunol. 2013, 70, 347–358. [Google Scholar] [CrossRef]
- Chen, L.M.; Liu, B.; Zhao, H.B.; Stone, P.; Chen, Q.; Chamley, L. IL-6, TNFalpha and TGFbeta promote nonapoptotic trophoblast deportation and subsequently causes endothelial cell activation. Placenta 2010, 31, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, H.; Liu, M.; Yuan, Y.; Wang, Z.; Chen, Y.; Wei, J.; Su, F.; Zhang, J. Treg/Th17 Cell Imbalance and IL-6 Profile in Patients with Unexplained Recurrent Spontaneous Abortion. Reprod. Sci. 2017, 24, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Thaker, R.; Oza, H.; Verma, V.; Gor, M.; Kumar, S. The Association of Circulatory Cytokines (IL-6 and IL-10) Level with Spontaneous Abortion—A Preliminary Observation. Reprod. Sci. 2021, 28, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, S.; Xu, H.; Zhang, X.; Chen, C.; Fu, R.; Li, C.; Guo, F.; Zhao, A. Prognostic analysis of antibody typing and treatment for antiphospholipid syndrome-related recurrent spontaneous abortion. Int. J. Gynecol. Obstet. 2022, 156, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Beltagy, A.; Trespidi, L.; Gerosa, M.; Ossola, M.W.; Meroni, P.L.; Chighizola, C.B. Anti-phospholipid antibodies and reproductive failures. Am. J. Reprod. Immunol. 2021, 85, e13258. [Google Scholar] [CrossRef]
- Yu, X.; He, L. Aspirin and heparin in the treatment of recurrent spontaneous abortion associated with antiphospholipid antibody syndrome: A systematic review and meta-analysis. Exp. Ther. Med. 2021, 21, 57. [Google Scholar] [CrossRef]
- Liu, T.; Guo, X.; Liao, Y.; Liu, Y.; Zhu, Y.; Chen, X. Correlation Between the Presence of Antinuclear Antibodies and Recurrent Pregnancy Loss: A Mini Review. Front. Endocrinol. 2022, 13, 873286. [Google Scholar] [CrossRef]
- Shankarkumar, U.; Pradhan, V.D.; Patwardhan, M.M.; Shankarkumar, A.; Ghosh, K. Autoantibody profile and other immunological parameters in recurrent spontaneous abortion patients. Niger. Med. J. 2011, 52, 163–166. [Google Scholar] [CrossRef]
- Chen, S.; Yang, G.; Wu, P.; Sun, Y.; Dai, F.; He, Y.; Qian, H.; Liu, Y.; Shi, G. Antinuclear antibodies positivity is a risk factor of recurrent pregnancy loss: A meta-analysis. Semin. Arthritis Rheum. 2020, 50, 534–543. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, L.; Sadhukhan, A.; Yang, S.; Yao, Q.; Zhou, P.; Rao, J.; Jin, M. Effect of antithyroid antibodies on women with recurrent miscarriage: A meta-analysis. Am. J. Reprod. Immunol. 2020, 83, e13238. [Google Scholar] [CrossRef]
- Song, H.; Cui, T.; Shi, S.; Xiao, H.; Wei, A. Effect of anti-thyroid antibodies on recurrent miscarriage: A meta-analysis. J. Obstet. Gynaecol. Res. 2024, 50, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, D.; Zhu, L.; Yin, J.; Ji, X.; Zhong, Y.; Gao, Y.; Zhang, J.; Liu, Y.; Zhang, R.; et al. Association of thyroid peroxidase antibodies with the rate of first-trimester miscarriage in euthyroid women with unexplained recurrent spontaneous abortion. Front. Endocrinol. 2022, 13, 966565. [Google Scholar] [CrossRef] [PubMed]
- Miko, E.; Meggyes, M.; Doba, K.; Farkas, N.; Bogar, B.; Barakonyi, A.; Szereday, L.; Szekeres-Bartho, J.; Mezosi, E. Characteristics of peripheral blood NK and NKT-like cells in euthyroid and subclinical hypothyroid women with thyroid autoimmunity experiencing reproductive failure. J. Reprod. Immunol. 2017, 124, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Chaurasia, S.; Mirza, S.; Resident, Y.P. To evaluate the prevalence of anticardiolipin antibodies among women with recurrent abortions and to determine any relation between anticardiolipin antibodies and number of abortions and their gestational age of abortions. J. Cardiovasc. Dis. Res. 2023, 14. [Google Scholar]
- Yokote, R.; Kuwabara, Y.; Kasano, S.; Yonezawa, M.; Ouchi, N.; Ichikawa, T.; Suzuki, S.; Takeshita, T. Risk factors for persistent positive anticardiolipin antibodies in women with recurrent pregnancy loss. J. Reprod. Immunol. 2023, 156, 103920. [Google Scholar] [CrossRef]
- Shaikhomar, O.A.; Ali, S.T. A Comparative Analysis of Anticardiolipin, Anti-Β2-Glycoprotein-1, and Lupus Anticoagulants in Saudi Women with Recurrent Spontaneous Abortions. J. Pers. Med. 2022, 13, 2. [Google Scholar] [CrossRef]
- Shi, Y.; Tan, D.; Hao, B.; Zhang, X.; Geng, W.; Wang, Y.; Sun, J.; Zhao, Y. Efficacy of intravenous immunoglobulin in the treatment of recurrent spontaneous abortion: A systematic review and meta-analysis. Am. J. Reprod. Immunol. 2022, 88, e13615. [Google Scholar] [CrossRef]
- Graphou, O.; Chioti, A.; Pantazi, A.; Tsukoura, C.; Kontopoulou, V.; Guorgiadou, E.; Balafoutas, C.; Koussoulakos, S.; Margaritis, L.H.; Varla-Leftherioti, M. Effect of intravenous immunoglobulin treatment on the Th1/Th2 balance in women with recurrent spontaneous abortions. Am. J. Reprod. Immunol. 2003, 49, 21–29. [Google Scholar] [CrossRef]
- Ahmadi, M.; Abdolmohammadi-vahid, S.; Ghaebi, M.; Aghebati-Maleki, L.; Afkham, A.; Danaii, S.; Abdollahi-Fard, S.; Heidari, L.; Jadidi-Niaragh, F.; Younesi, V.; et al. Effect of Intravenous immunoglobulin on Th1 and Th2 lymphocytes and improvement of pregnancy outcome in recurrent pregnancy loss (RPL). Biomed. Pharmacother. 2017, 92, 1095–1102. [Google Scholar] [CrossRef]
- Wu, H.; You, Q.; Jiang, Y.; Mu, F. Tumor necrosis factor inhibitors as therapeutic agents for recurrent spontaneous abortion (Review). Mol. Med. Rep. 2021, 24, 847. [Google Scholar] [CrossRef]
- Fu, J.; Li, L.; Qi, L.; Zhao, L. A randomized controlled trial of etanercept in the treatment of refractory recurrent spontaneous abortion with innate immune disorders. Taiwan. J. Obstet. Gynecol. 2019, 58, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Roumandeh, N.; Zare, A.; Saremi, A.T. Immunology of Recurrent Spontaneous Abortion. saremjm 2018, 3, 121–126. [Google Scholar] [CrossRef]
- Li, T.; Yuan, Y.; Liu, H.; Lu, Q.; Mu, R. Glucocorticoids Improve the Pregnancy Rate and Outcome in Women with Unexplained Positive Autoantibodies: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 819406. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Gu, Z.-D.; Diao, Q.-z. Meta-analysis on aspirin combined with low-molecular-weight heparin for improving the live birth rate in patients with antiphospholipid syndrome and its correlation with d-dimer levels. Medicine 2021, 100, e26264. [Google Scholar] [CrossRef]
| Authors | Year | Study Design | Study Population | Methodology | Results | Outcomes |
|---|---|---|---|---|---|---|
| Akbari et al. [61] | 2018 | Case-control study | 100 couples; RSA women | KIR genes and HLA allotypes with PCR-SSP for genotyping | Patients with RSA had elevated levels of KIR2DS1 and paternal HLA-C2 compared to the control. | KIR2DS1 and paternal HLA-C2 are risk factors for RSA |
| Mansour et al. [56] | 2020 | Case control | 75 RSA women; 65 controls | 17 KIR genes and HLA-C1 and HLA-C2 allotypes by PCR-SSP | KIR2DS2 and KIR2DL5A levels were lower in RSA compared to controls (p < 0.001) | KIR genes of the B haplotype can predict successful pregnancy. |
| Maftei et al. [62] | 2023 | Prospective study | 108 RSA or women with recurrent implantation failure (RIF) (RSA: 30, RIF:78) | KIR AA haplotype with PCR-SSP | Patients with a KIR AA haplotype who underwent IVF were more likely to have a miscarriage compared to spontaneous pregnancy (p = 0.032). | KIR haplotype assessment can help predict the risk of RSA. |
| Alecsandru et al. [59] | 2020 | Prospective observational cohort study | 204 RSA or RIF females | KIR and HLA-C genotypes assessed. HLA-C genes for all partners. | Higher rates of miscarriage were reported in KIR AA patients compared to KIR AB, and KIR BB. Birth rates significantly declined as HLA-C2 load increased in KIR AA women. | Increase in HLA-C levels in KIR AA women leads to poor outcomes for pregnancy. |
| Akbari et al. [55] | 2020 | Meta-analysis | 11 studies | Web of Science, PubMed, Scopus, Google Scholar | KIR3DL1 is a significant protective factor for RSA (p = 0.044) and KIR2DS2 and KIR2DS3 were risk factors for RSA. | Inhibitory KIR are protective in RSA and activating KIR are risk factors. |
| Ataei et al. [54] | 2021 | Case control | 80; 40 with RSA and 40 normal pregnant women | Genotypes of KIR genes assessed and KIR3DL1 genotype frequency compared | KIR3DL1 levels are lower in RSA group compared to healthy controls (p = 0.012). | KIR3DL1 inhibitory genotype is protective in RSA. |
| Su et al. [58] | 2018 | Case control | 110 RSA women, and 105 healthy controls | Genotypes of KIR genes and HLA-C assessed | KIR3DL1 levels were significantly lower in RSA whereas BB haplotype were significantly higher compared to control group. | RSA patients have reduced inhibitory genes and increased activating KIR genes. |
| Alharbi et al. [57] | 2022 | Case control | 199 women; 69 RSA, 65 polycystic ovarian syndrome (PCO), 65 healthy | KIR genes and HLA allotypes with PCR-SSP for genotyping | KIR3DL1, 2DS4ins, 2DL2, and KIR2DS2 levels were significantly low in RSA compared to controls (p < 0.01). | KIR2DL1 with HLA-C2 is risk factor for RSA. |
| Elbaşı et al. [63] | 2020 | Case control | 25 couples | KIR genes and HLA allotypes with PCR-SSP for genotyping | The KIR2DL5 levels were higher in both partners in RSA whereas KIR2DS3 levels were reduced in male RSA partners (p = 0.03). | Male HLA-C2 and female HLA-C1 ligand KIR receptors can affect the outcome of pregnancy. |
| Dambaeva et al. [64] | 2016 | Prospective study | 139 RSA women | Genomic DNA was extracted using QuickGene DNA | RSA patients with KIR2DS1 had a high frequency of HLA-C2 (45.3%). | KIR2DS1 with HLA-C2 is associated with RSA. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreescu, M.; Tanase, A.; Andreescu, B.; Moldovan, C. A Review of Immunological Evaluation of Patients with Recurrent Spontaneous Abortion (RSA). Int. J. Mol. Sci. 2025, 26, 785. https://doi.org/10.3390/ijms26020785
Andreescu M, Tanase A, Andreescu B, Moldovan C. A Review of Immunological Evaluation of Patients with Recurrent Spontaneous Abortion (RSA). International Journal of Molecular Sciences. 2025; 26(2):785. https://doi.org/10.3390/ijms26020785
Chicago/Turabian StyleAndreescu, Mihaela, Alina Tanase, Bogdan Andreescu, and Cosmin Moldovan. 2025. "A Review of Immunological Evaluation of Patients with Recurrent Spontaneous Abortion (RSA)" International Journal of Molecular Sciences 26, no. 2: 785. https://doi.org/10.3390/ijms26020785
APA StyleAndreescu, M., Tanase, A., Andreescu, B., & Moldovan, C. (2025). A Review of Immunological Evaluation of Patients with Recurrent Spontaneous Abortion (RSA). International Journal of Molecular Sciences, 26(2), 785. https://doi.org/10.3390/ijms26020785







