Advances in Protein Kinase Regulation of Stress Responses in Fruits and Vegetables
Abstract
1. Introduction
2. Classification of Protein Kinases
2.1. AGC
2.2. CaMK
2.3. CMGC
2.4. CK1
2.5. STE
2.6. TK
2.7. TKL
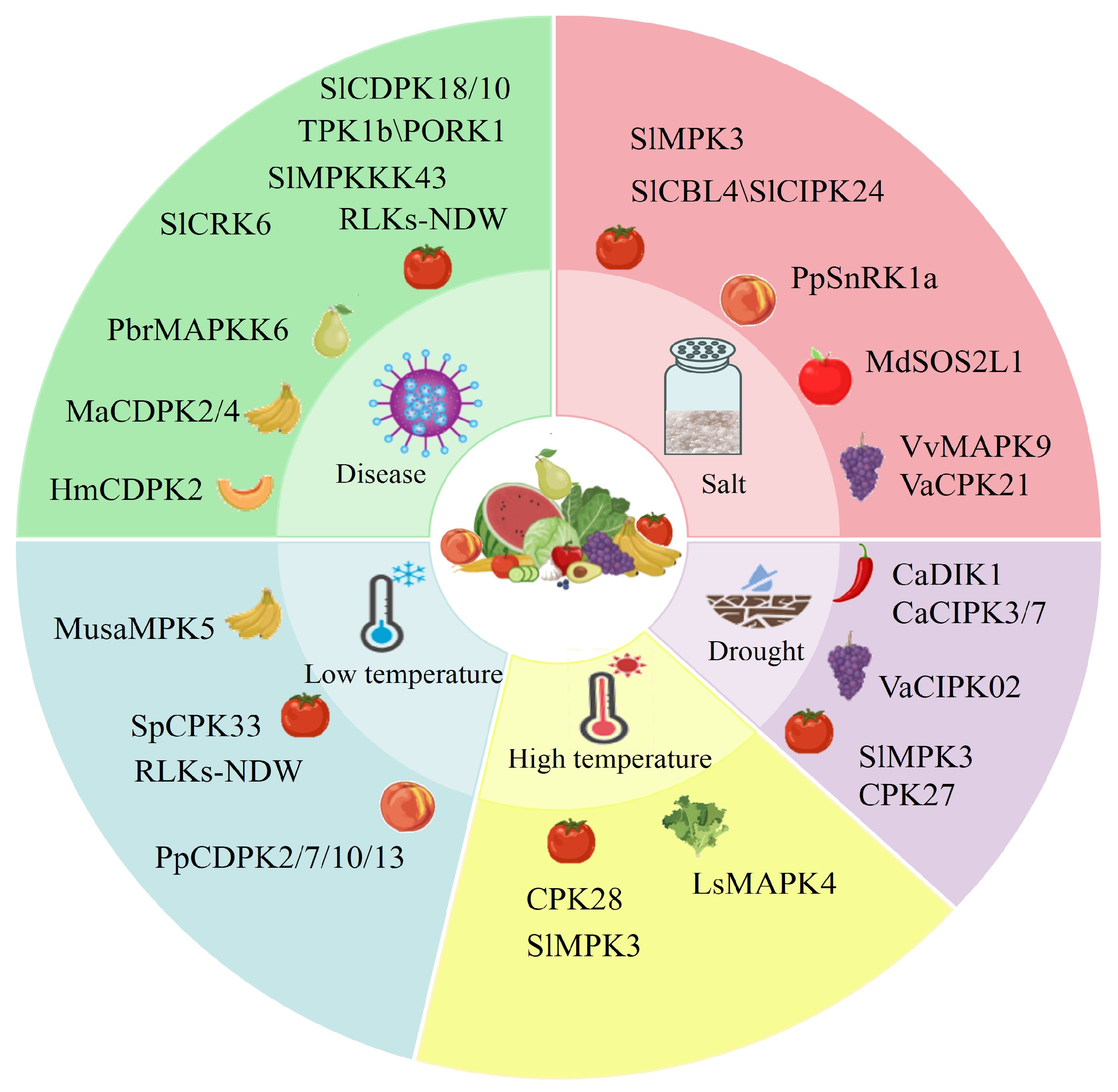
3. Regulation of Protein Kinase in F&V Stress Responses
3.1. The Role of Protein Kinases in the Salt Stress Response of F&Vs
3.2. The Role of Protein Kinases in the Temperature Stress Response of F&Vs
3.3. The Role of Protein Kinases in the Drought Tolerance of F&Vs
3.4. The Role of Protein Kinases in Disease Resistance of F&Vs
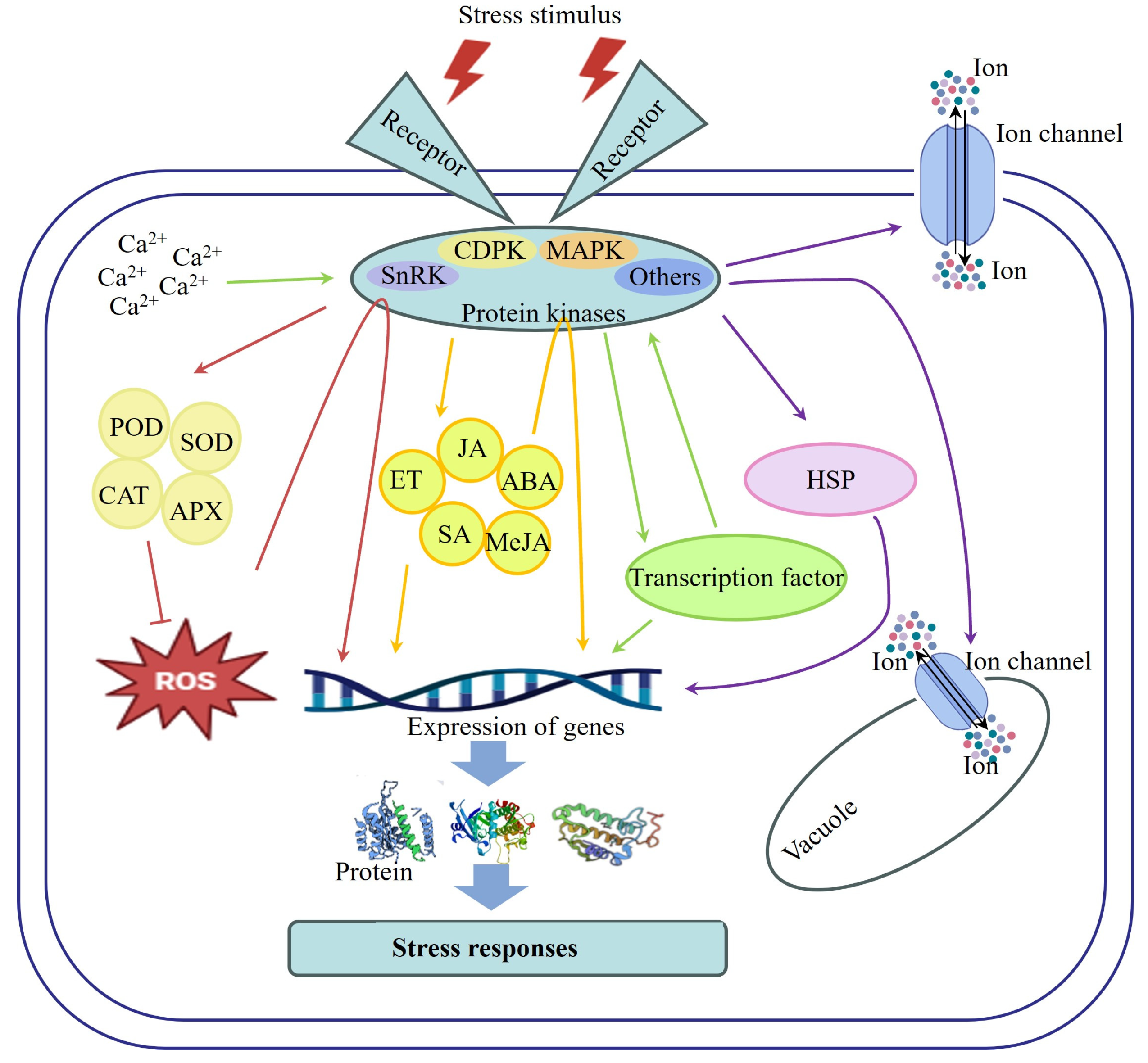
4. The Mechanisms of Protein Kinases Regulate the Response of F&Vs to Stress
4.1. Regulation of Protein Kinases on the Antioxidant System
4.2. Interaction of Protein Kinases with Hormone Signaling Pathways
4.3. Interaction of Protein Kinases with Transcription Factors
4.4. Regulation of Protein Kinases on Specific Proteins
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sardar, A. Genetic amelioration of fruit and vegetable crops to increase biotic and abiotic stress resistance through CRISPR genome editing. Front. Plant Sci. 2023, 14, 1260102. [Google Scholar] [CrossRef] [PubMed]
- Majeed, Y.; Zhu, X.; Cho, N.; UI-Ain, N.; Raza, A.; Haider, F.U.; Si, H.J. Harnessing the role of mitogen-activated protein kinases against abiotic stresses in plants. Front. Plant Sci. 2023, 14, 932923. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, P.; Kumar, A.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Grafting tomato as a tool to improve salt tolerance. Agronomy 2020, 10, 263. [Google Scholar] [CrossRef]
- Hendrawan, V.S.; Komori, D.; Kim, W. Possible factors determining global-scale patterns of crop yield sensitivity to drought. PLoS ONE 2023, 18, e0281287. [Google Scholar] [CrossRef] [PubMed]
- Aleem, S.; Sharif, I.; Amin, E.; Tahir, M.; Parveen, N.; Aslam, R.; Najeebullah, M.; Shahid, M.T.H. Heat tolerance in vegetables in the current genomic era: An overview. Plant Growth Regul. 2020, 92, 497–516. [Google Scholar] [CrossRef]
- Hoshikawa, K.; Pham, D.; Ezura, H.; Schafleitner, R.; Nakashima, K. Genetic and molecular mechanisms conferring heat stress tolerance in tomato plants. Front. Plant Sci. 2021, 12, 786688. [Google Scholar] [CrossRef]
- Xiao, L.; Jiang, X.Y.; Deng, Y.C.; Xu, K.H.; Duan, X.W.; Wan, K.; Tang, X.M. Study on characteristics and lignification mechanism of postharvest banana fruit during chilling injury. Foods 2023, 12, 1097. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Bi, Y. The role of signal production and transduction in induced resistance of harvested fruit and vegetables. Food Qual. Saf. 2021, 5, fyab011. [Google Scholar] [CrossRef]
- Lardon, R.; Trinh, H.K.; Xu, X.Y.; Vu, L.D.; van de Cotte, B.; Pernisova, M.; Vanneste, S.; De Smet, I.; Geelen, D. Histidine kinase inhibitors impair shoot regeneration in Arabidopsis thaliana via cytokinin signaling and SAM patterning determinants. Front. Plant Sci. 2022, 13, 894208. [Google Scholar] [CrossRef]
- Seok, S.H. Structural insights into protein regulation by phosphorylation and substrate recognition of protein kinases/phosphatases. Life 2021, 11, 957. [Google Scholar] [CrossRef]
- He, X.W.; Wang, C.Z.; Wang, H.B.; Li, L.G.; Wang, C. The function of MAPK cascades in response to various stresses in horticultural plants. Front. Plant Sci. 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Lehti-Shiu, M.D.; Shiu, S.H. Diversity, classification and function of the plant protein kinase superfamily. Philos. Trans. R. Soc. Lond. B-Biol. Sci. 2012, 367, 2619–2639. [Google Scholar] [CrossRef]
- Zhu, X.L.; Yang, K.; Wei, X.N.; Zhang, Q.F.; Rong, W.; Du, L.P.; Ye, X.J.; Qi, L.; Zhang, Z.Y. The wheat AGC kinase TaAGC1 is a positive contributor to host resistance to the necrotrophic pathogen Rhizoctonia cerealis. J. Exp. Bot. 2015, 66, 6591–6603. [Google Scholar] [CrossRef]
- Götz, F.; Roske, Y.; Schulz, M.S.; Autenrieth, K.; Bertinetti, D.; Faelber, K.; Zühlke, K.; Kreuchwig, A.; Kennedy, E.J.; Krause, G.; et al. AKAP18: PKA-RIIα structure reveals crucial anchor points for recognition of regulatory subunits of PKA. Biochem. J. 2016, 473, 1881–1894. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Kong, Q.B.; Wang, J.; Jiang, Y.F.; Hua, H. Complex roles of Camp-PKA-CREB signaling in cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.W.; Zhan, X.Q.; Yang, P.; Li, J.; Chen, J.; Tang, B.; Wang, X.; Hong, Y. Dual activities of plant cGMP-dependent protein kinase and its roles in gibberellin signaling and salt stress. Plant Cell 2019, 31, 3073–3091. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, V.; Bogomolovas, J.; Ehler, E.; Dos Remedios, C.G.; Yu, J.; Gao, C.; Lange, S. PKC and PKN in heart disease. J. Mol. Cell. Cardiol. 2019, 128, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Rui, M.; Nasti, R.; Bignardi, E.; Della Volpe, S.; Rossino, G.; Rossi, D.; Collina, S. PKC in regenerative therapy: New insights for old targets. Pharmaceuticals 2017, 10, 46. [Google Scholar] [CrossRef]
- Devarenne, T.P.; Ekengren, S.K.; Pedley, K.F.; Martin, G.B. Adi3 is a Pdk1-interacting AGC kinase that negatively regulates plant cell death. EMBO J. 2006, 25, 255–265. [Google Scholar] [CrossRef]
- Aono, A.H.; Pimenta, R.J.G.; Dambroz, C.M.D.; Costa, F.C.L.; Kuroshu, R.M.; de Souza, A.P.; Pereira, W.A. Genome-wide characterization of the common bean kinome: Catalog and insights into expression patterns and genetic organization. Gene 2023, 855, 147127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Yao, W.J.S.; Wang, F.; Su, Y.H.; Zhang, D.J.; Hu, S.W.; Zhang, X.S. AGC protein kinase AGC1-4 mediates seed size in Arabidopsis. Plant Cell Rep. 2020, 39, 825–837. [Google Scholar] [CrossRef]
- Wang, J.P.; Xu, Y.P.; Munyampundu, J.P.; Liu, T.Y.; Cai, X.Z. Calcium-dependent protein kinase (CDPK) and CDPK-related kinase (CRK) gene families in tomato: Genome-wide identification and functional analyses in disease resistance. Mol. Genet. Genom. 2016, 291, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Bredow, M.; Monaghan, J. Regulation of plant immune signaling by calcium-dependent protein kinases. Mol. Plant-Microbe Interact. 2019, 32, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Huang, Y.; Cui, L.; Gan, C.X.; Qiu, Z.M.; Yan, C.H.; Deng, X.H. Genome-wide identification of the CDPK gene family and their involvement in taproot cracking in radish. Int. J. Mol. Sci. 2023, 24, 15059. [Google Scholar] [CrossRef]
- Liu, Z.X.; Zhu, Y.X.; Wu, G.Q.; Wei, M. The role of SnRK2 in the response to stress, the growth and development of plants. Chin. J. Biotechnol. 2022, 38, 89–103. [Google Scholar] [CrossRef]
- Zheng, H.F.; Xie, Y.L.; Mu, C.H.; Cheng, W.L.; Bai, Y.C.; Gao, J. Deciphering the regulatory role of PheSnRK genes in Moso bamboo: Insights into hormonal, energy, and stress responses. BMC Genom. 2024, 25, 252. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Akash; Roychowdhury, A. Identification of phosphorus starvation inducible SnRK genes in tomato (Solanum lycopersicum L.). J. Plant Biochem. Biotechnol. 2021, 30, 987–998. [Google Scholar] [CrossRef]
- Yu, W.Q.; Wang, L.; Zhao, R.R.; Sheng, J.P.; Zhang, S.J.; Li, R.; Shen, L. Knockout of SlMAPK3 enhances tolerance to heat stress involving ROS homeostasis in tomato plants. BMC Plant Biol. 2019, 19, 354. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhang, H.X.; Liang, S.B.; Chen, X.L.; Liu, J.Y.; Zhang, Y.; Wang, A.X. Identification of CDPK gene family in Solanum habrochaites and its function analysis under stress. Int. J. Mol. Sci. 2022, 23, 4227. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, M.M.; Ren, L.; Liu, Y.; Chen, H.Y. Identification and characterization of CBL and CIPK gene families in eggplant (Solanum melongena L.). Mol. Genet. Genom. 2016, 291, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Han, Y.T.; Zhao, F.L.; Hu, Y.; Gao, Y.R.; Ma, Y.F.; Zheng, Y.; Wang, Y.J.; Wen, Y.Q. Genome-wide identification and expression analysis of the CDPK gene family in grape, Vitis spp. BMC Plant Biol. 2015, 15, 164. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.M.; Sui, C.C.; Yu, Y.Y.; Liu, X.L.; Li, B.; Sun, Q.H. Grape VvMAPK9 positively regulates salt tolerance in Arabidopsis and grape callus through regulating the antioxidative system. Plant Cell Tiss. Org. 2022, 148, 609–622. [Google Scholar] [CrossRef]
- Liu, J.Y.; Chen, N.N.; Cheng, Z.M.; Xiong, J.S. Genome-wide identification, annotation and expression profile analysis of SnRK2 gene family in grapevine. Aust. J. Grape Wine Res. 2016, 22, 478–488. [Google Scholar] [CrossRef]
- Xi, Y.; Liu, J.Y.; Dong, C.; Cheng, Z.M. The CBL and CIPK gene family in grapevine (Vitis vinifera): Genome-wide analysis and expression profiles in response to various abiotic stresses. Front. Plant Sci. 2017, 8, 978. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.Y.; Cheng, J.B.; Yan, Y.; Xiao, Z.L.; Li, J.Z.; Mou, S.L.; Qiu, A.L.; Lai, Y.; Guan, D.Y.; He, S.L. Genome-wide identification and expression analysis of calcium-dependent protein kinase and its closely related kinase genes in Capsicum annuum. Front. Plant Sci. 2015, 6, 737. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Shi, L.P.; Liu, Y.Y.; Tang, Q.; Shen, L.; Yang, S.; Cai, J.S.; Yu, H.X.; Wang, R.Z.; Wen, J.Y.; et al. Genome-wide identification and transcriptional expression analysis of mitogen-activated protein kinase and mitogen-activated protein kinase kinase genes in Capsicum annuum. Front. Plant Sci. 2015, 6, 780. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Xie, B.; Hou, Y.Y.; Zhao, Y.Q.; Zheng, Y.H.; Jin, P. Genome-wide identification of the CDPK gene family reveals the CDPK-RBOH pathway potential involved in improving chilling tolerance in peach fruit. Plant Physiol. Biochem. 2022, 191, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Hu, W.; Ren, L.C.; Jia, C.H.; Liu, J.H.; Miao, H.X.; Guo, A.P.; Xu, B.Y.; Jin, Z.Q. Identification, expression, and interaction network analyses of the CDPK gene family reveal their involvement in the development, ripening, and abiotic stress response in banana. Biochem. Genet. 2020, 58, 40–62. [Google Scholar] [CrossRef]
- Hu, W.; Yan, Y.; Shi, H.T.; Liu, J.H.; Miao, H.X.; Tie, W.W.; Ding, Z.H.; Ding, X.P.; Wu, C.L.; Liu, Y.; et al. The core regulatory network of the abscisic acid pathway in banana: Genome-wide identification and expression analyses during development, ripening, and abiotic stress. BMC Plant Biol. 2017, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.H.; Lakhwani, D.; Pathak, S.; Bhambhani, S.; Bag, S.K.; Trivedi, P.K. Genome-wide identification and expression analysis of the mitogen-activated protein kinase gene family from banana suggest involvement of specific members in different stages of fruit ripening. Funct. Integr. Genom. 2014, 14, 161–175. [Google Scholar] [CrossRef]
- Wu, P.; Wang, W.L.; Duan, W.K.; Li, Y.; Hou, X.L. Comprehensive analysis of the CDPK-SnRK superfamily genes in chinese cabbage and its evolutionary implications in plants. Front. Plant Sci. 2017, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wang, Q.L.; Chen, Q.; Xiang, N.; Yang, Y.Q.; Yang, Y.P. Genome-wide identification and functional analysis of the calcineurin B-like protein and calcineurin B-like protein-interacting protein kinase gene families in turnip (Brassica rapa var. rapa). Front. Plant Sci. 2017, 8, 1191. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.N.; Tang, J.; Duan, W.K.; Wang, Z.; Song, X.M.; Hou, X.L. Molecular evolution, characterization, and expression analysis of SnRK2 gene family in Pak-choi (Brassica rapa ssp. chinensis). Front. Plant Sci. 2015, 6, 879. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.H.; He, Q.; Chai, M.N.; Huang, Y.M.; Chen, F.Q.; Wang, X.M.; Liu, Y.Q.; Cai, H.Y.; Qin, Y. Genome-wide investigation of calcium-dependent protein kinase gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2020, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Fakher, B.; Jakada, B.H.; Zhao, L.H.; Cao, S.J.; Cheng, Y.; Qin, Y. Genome-wide identification and expression profiling of CBL-CIPK gene family in pineapple (Ananas comosus) and the role of AcCBL1 in abiotic and biotic stress response. Biomolecules 2019, 9, 293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Ye, Y.Y.; Jiang, L.Y.; Lin, Y.X.; Gu, X.J.; Chen, Q.; Sun, B.; Zhang, Y.; Luo, Y.; Wang, Y.; et al. Genome-wide characterization of snf1-related protein kinases (SnRKs) and expression analysis of SnRK1.1 in strawberry. Genes 2020, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Crizel, R.L.; Perin, E.C.; Vighi, I.L.; Woloski, R.; Seixas, A.; da Silva Pinto, L.; Rombaldi, C.V.; Galli, V. Genome-wide identification, and characterization of the CDPK gene family reveal their involvement in abiotic stress response in Fragaria x ananassa. Sci. Rep. 2020, 10, 11040. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Li, B.H.; Yang, M.; Wang, L.X.; Hou, G.Y.; Lin, Y.X.; Zhang, Y.T.; Zhang, Y.; Chen, Q.; Wang, Y.; et al. Genome-wide identification and expression of MAPK gene family in cultivated strawberry and their involvement in fruit developing and ripening. Int. J. Mol. Sci. 2022, 23, 5201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Ren, S.Y.; Han, Y.F.; Zhang, Q.; Qin, L.; Xing, Y. Identification and analysis of mitogen-activated protein kinase (MAPK) cascades in Fragaria vesca. Int. J. Mol. Sci. 2017, 18, 1766. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.F.; Lin, D.H.; Ma, S.W.; Wang, C.H.; Lin, S.K. Genome-wide identification of the calcium-dependent protein kinase gene family in Fragaria vesca and expression analysis under different biotic stresses. Eur. J. Plant Pathol. 2022, 164, 283–298. [Google Scholar] [CrossRef]
- Song, Q.M.; Li, D.Y.; Dai, Y.; Liu, S.X.; Huang, L.; Hong, Y.B.; Zhang, H.J.; Song, F.M. Characterization, expression patterns and functional analysis of the MAPK and MAPKK genes in watermelon (Citrullus lanatus). BMC Plant Biol. 2015, 15, 298. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, T.; Jia, Z.H.; Xuan, J.P.; Pan, D.L.; Guo, Z.R.; Zhang, J.Y. Genome-wide bioinformatics analysis of MAPK gene family in kiwifruit (Actinidia Chinensis). Int. J. Mol. Sci. 2018, 19, 2510. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.N.; Mo, Y.X.; Yang, Y.Y.; Liang, S.Q.; Xian, S.Q.; Deng, Z.X.; Zhao, M.Y.; Liu, S.Y.; Liu, K.D. Genome-wide identification of MAPK family in papaya (Carica papaya) and their involvement in fruit postharvest ripening. BMC Plant Biol. 2024, 24, 68. [Google Scholar] [CrossRef]
- Ren, Y.; Ge, D.P.; Dong, J.M.; Guo, L.H.; Yuan, Z.H. Genome-wide identification and expression analysis of MAPK and MAPKK gene family in pomegranate (Punica Granatum L.). Agronomy 2020, 10, 1015. [Google Scholar] [CrossRef]
- Wei, C.L.; Liu, X.Q.; Long, D.P.; Guo, Q.; Fang, Y.; Bian, C.K.; Zhang, D.Y.; Zeng, Q.W.; Xiang, Z.H.; Zhao, A.C. Molecular cloning and expression analysis of mulberry MAPK gene family. Plant Physiol. Biochem. 2014, 77, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Jue, D.W.; Zhang, F.; Zhang, D.J.; Liu, C.Y.; Wu, Q.S.; Luo, C. Genome-wide identification and expression analysis of the citrus calcium-dependent protein kinase (CDPK) genes in response to arbuscular mycorrhizal fungi colonization and drought. Biotechnol. Biotechnol. Equip. 2020, 34, 1304–1314. [Google Scholar] [CrossRef]
- Zhang, H.F.; Yang, B.; Liu, W.Z.; Li, H.W.; Wang, L.; Wang, B.Y.; Deng, M.; Liang, W.W.; Deyholos, M.K.; Jiang, Y.Q. Identification and characterization of CBL and CIPK gene families in canola (Brassica napus L). BMC Plant Biol. 2014, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.W.; Liu, M.; Lu, L.; He, M.; Qu, W.Q.; Xu, Q.; Qi, X.H.; Chen, X.H. Genome-wide analysis and expression of the calcium-dependent protein kinase gene family in cucumber. Mol. Genet. Genom. 2015, 290, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.Y.; Niu, Y.; Gao, R.; Wang, C.L.; Liao, W.B.A. Genome-wide identification and expression analysis of SnRK gene family under abiotic stress in cucumber (Cucumis sativus L.). Agronomy 2022, 12, 1550. [Google Scholar] [CrossRef]
- Wang, J.; Pan, C.T.; Wang, Y.; Ye, L.; Wu, J.; Chen, L.F.; Zou, T.; Lu, G. Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber. BMC Genom. 2015, 16, 386. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wei, C.H.; Yang, X.Z.; Chen, H.J.; Yang, Y.C.; Mo, Y.L.; Li, H.; Zhang, Y.; Ma, J.X.; Yang, J.Q.; et al. Genome-wide identification and expression analysis of calcium dependent protein kinase and its related kinase gene families in melon (Cucumis melo L.). PLoS ONE 2017, 12, e0176352. [Google Scholar] [CrossRef]
- Gao, Y.L.; Zhang, Y.F.; Ji, X.; Wang, J.X.; Suo, N.N.; Liu, J.C.; Huo, X.W. Identification of Dioscorea opposite Thunb CDPK gene family reveals that DoCDPK20 is related to heat resistance. Peer J. 2023, 11, e16110. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Wu, H.; Huang, W.F.; Liu, J.X.; Deng, J.X.; Li, C.H.; Mao, C.; Zhang, Y.; Wang, Y.K.; Zheng, J. The CDPK gene family in mustard (Brassica juncea L.): Genome-wide identification and expression analysis under cold stress. Horticulturae 2024, 10, 20. [Google Scholar] [CrossRef]
- Shen, X.J.; Guo, X.; Zhao, D.; Zhang, Q.; Jiang, Y.Z.; Wang, Y.T.; Peng, X.; Wei, Y.; Zhai, Z.F.; Zhao, W.; et al. Cloning and expression profiling of the PacSnRK2 and PacPP2C gene families during fruit development, ABA treatment, and dehydration stress in sweet cherry. Plant Physiol. Biochem. 2017, 119, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Qin, Y.; Zou, Y.J.; Ma, F.W. Genome-wide identification and expression profiling of the SnRK2 gene family in Malus prunifolia. Gene 2014, 552, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Z.; Xu, R.R.; Luo, X.C.; Jiang, Z.S.; Shu, H.R. Genome-wide identification and expression analysis of MAPK and MAPKK gene family in Malus domestica. Gene 2013, 531, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Lin, J.; Li, H.; Li, X.G.; Yang, Q.S.; Cheng, Z.M.; Chang, Y.H. Characterization of CIPK family in Asian pear (Pyrus bretschneideri Rehd) and co-expression analysis related to salt and osmotic stress responses. Front. Plant Sci. 2016, 7, 1361. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Dong, X.M.; Yu, X.F.; Zhang, Q.Y. Characterization and tissue-specific as well as heat-stress expression analysis of CBL-interacting protein kinase genes in Dimocarpus longan Lour. Turk. J. Bot. 2019, 43, 724–736. [Google Scholar] [CrossRef]
- Babus, G.; Gohil, D.S.; Choudhury, S.R. Genome-wide identification, evolutionary and expression analysis of the cyclin-dependent kinase gene family in peanut. BMC Plant Biol. 2023, 23, 43. [Google Scholar] [CrossRef]
- Takatsuka, H.; Umeda-hara, C.; Umeda, M. Cyclin-dependent kinase-activating kinases CDKD;1 and CDKD;3 are essential for preserving mitotic activity in Arabidopsis thaliana. Plant J. 2015, 82, 1004–1017. [Google Scholar] [CrossRef]
- Liao, Y.F.; Feng, Y.; Shen, J.; Hornicek, F.J.; Duan, Z. The roles and therapeutic potential of cyclin-dependent kinases (CDKs) in sarcoma. Cancer Metastasis Rev. 2016, 35, 151–163. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Jia, C.; Xu, B.; Jin, Z. Molecular cloning and expression analysis of eight calcium-dependent protein kinase (CDPK) genes from banana (Musa acuminata L. AAA group, cv. Cavendish). S. Afr. J. Bot. 2016, 104, 134–141. [Google Scholar] [CrossRef]
- Bittner, T.; Nadler, S.; Schulze, E.; Fischer-Iglesias, C. Two homolog wheat glycogen synthase kinase 3/shaggy-like kinases are involved in brassinosteroid signaling. BMC Plant Biol. 2015, 15, 247. [Google Scholar] [CrossRef] [PubMed]
- Bullock, A.N.; Das, S.; Debreczeni, J.E.; Rellos, P.; Fedorov, O.; Niesen, F.H.; Guo, K.; Papagrigoriou, E.; Amos, A.L.; Cho, S.; et al. Kinase domain insertions define distinct roles of CLK kinases in SR protein phosphorylation. Cell Press. 2009, 17, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Sun, H.L.; Zhao, X.Y.; Liu, X.M. Arabidopsis casein kinase 1-like 8 enhances NaCl tolerance, early flowering, and the expression of flowering-related genes. J. Plant Interact. 2016, 11, 138–145. [Google Scholar] [CrossRef]
- Saito, A.N.; Matsuo, H.; Kuwata, K.; Ono, A.; Kinoshita, T.; Yamaguchi, J.; Nakamichi, N. Structure-function study of a novel inhibitor of the casein kinase 1 family in Arabidopsis thaliana. Plant Direct. 2019, 3, e00172. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, J.; Armando Casas-Mollano, J.; Dou, Y.C.; Jia, S.G.; Yang, Q.C.; Zhang, C.; Cerutti, H. MLK4-mediated phosphorylation of histone H3T3 promotes flowering by transcriptional silencing of FLC/MAF in Arabidopsis thaliana. Plant J. 2021, 105, 1400–1412. [Google Scholar] [CrossRef] [PubMed]
- Gaji, R.Y.; Sharp, A.K.; Brown, A.M. Protein kinases in Toxoplasma gondii. Int. J. Parasitol. 2021, 51, 415–429. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Kim, S.K.; Willis, S.D.; Cooper, K.F. The MAPKKKs Ste11 and Bck1 jointly transduce the high oxidative stress signal through the cell wall integrity MAP kinase pathway. Microb. Cell 2015, 2, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.F.; Chen, M.J.; Yan, Y.Q.; Gu, Q.N.; Huang, J.B.; Zheng, L. Chste7 is required for vegetative growth and various plant infection processes in Colletotrichum higginsianum. BioMed Res. Int. 2016, 2016, 7496569. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.Y.; Fu, T.; Song, Y.W.; Kim, K.S. MAPKK CsSTE7 is critical for appressorium formation and pathogenicity in pepper anthracnose fungus, Colletotrichum scovillei. J. Gen. Plant Pathol. 2024, 90, 108–119. [Google Scholar] [CrossRef]
- Siveen, K.S.; Prabhu, K.S.; Achkar, I.W.; Kuttikrishnan, S.; Shyam, S.; Khan, A.Q.; Merhi, M.; Dermime, S.; Uddin, S. Role of non receptor tyrosine kinases in hematological malignances and its targeting by natural products. Mol. Cancer 2018, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Ayrapetov, M.K. Dissection of the catalytic and regulatory structure-function relationships of csk protein tyrosine kinase. Front. Cell Dev. Biol. 2023, 11, 1148352. [Google Scholar] [CrossRef] [PubMed]
- Baier, A.; Szyszka, R. Compounds from natural sources as protein kinase inhibitors. Biomolecules 2020, 10, 1546. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Fujita, M. Plant responses and tolerance to salt stress: Physiological and molecular interventions. Int. J. Mol. Sci. 2022, 23, 4810. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.J.; Feng, L.; Liu, Y.Y.; Liao, W.B. Mitogen-activated protein kinase is involved in salt stress response in tomato (Solanum lycopersicum) seedlings. Mol. Sci. 2022, 23, 7645. [Google Scholar] [CrossRef]
- Shu, P.; Li, Y.J.; Li, Z.Y.; Sheng, J.P.; Shen, L. SlMAPK3 enhances tolerance to salt stress in tomato plants by scavenging ROS accumulation and up-regulating the expression of ethylene signaling related genes. Environ. Exp. Bot. 2022, 193, 104698. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V.; Khristenko, V.S.; Aleynova, O.A. VaCPK21, a calcium-dependent protein kinase gene of wild grapevine Vitis amurensis Rupr. is involved in grape response to salt stress. Plant Cell Tiss. Org. 2016, 124, 137–150. [Google Scholar] [CrossRef]
- Cho, J.H.; Sim, S.C.; Kim, K.N. Calcium sensor SlCBL4 associates with SlCIPK24 protein kinase and mediates salt tolerance in Solanum lycopersicum. Plants 2021, 10, 2173. [Google Scholar] [CrossRef]
- Hu, D.G.; Ma, Q.J.; Sun, C.H.; Sun, M.H.; You, C.X.; Hao, Y.J. Overexpression of MdSOS2L1, a CIPK protein kinase, increases the antioxidant metabolites to enhance salt tolerance in apple and tomato. Physiol. Plantarum. 2016, 156, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.R.; Liang, J.H.; Wang, G.F.; Sun, M.X.; Peng, F.T.; Xiao, Y.S. Overexpression of PpSnRK1α in tomato enhanced salt tolerance by regulating ABA signaling pathway and reactive oxygen metabolism. BMC Plant Biol. 2020, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Evrard, A.; Kumar, M.; Lecourieux, D.; Lucks, J.; von Koskull-Döring, P.; Hirt, H. Regulation of the heat stress response in Arabidopsis by MPK6-targeted phosphorylation of the heat stress factor HsfA2. Peer J. 2013, 1, 59. [Google Scholar] [CrossRef]
- Geng, B.H.; Wang, Q.; Huang, R.S.; Liu, Y.J.; Guo, Z.F.; Lu, S.Y. A novel LRR-RLK (CTLK) confers cold tolerance through regulation on the C-repeat-binding factor pathway, antioxidants, and proline accumulation. Plant J. 2021, 108, 1679–1689. [Google Scholar] [CrossRef]
- Wang, T.Z.; Liu, M.J.; Wu, Y.; Tian, Y.F.; Han, Y.Y.; Liu, C.J.; Hao, J.H.; Fan, S.X. Genome-wide identification and expression analysis of MAPK gene family in lettuce (Lactuca sativa L.) and functional analysis of LsMAPK4 in high temperature-induced bolting. Int. J. Mol. Sci. 2022, 23, 11129. [Google Scholar] [CrossRef]
- Hu, Z.J.; Li, J.X.; Ding, S.T.; Cheng, F.; Li, X.; Jiang, Y.P.; Yu, J.Q.; Foyer, C.H.; Shi, K. The protein kinase CPK28 phosphorylates ascorbate peroxidase and enhances thermotolerance in tomato. Plant Physiol. 2021, 186, 1302–1317. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Gao, S.H.; Song, J.W.; Yang, Q.H.; Wang, T.T.; Zhang, Y.Y.; Zhang, J.H.; Li, H.X.; Yang, C.X.; Ye, Z.B. NDW, encoding a receptor-like protein kinase, regulates plant growth, cold tolerance and susceptibility to Botrytis cinerea in tomato. Plant Sci. 2020, 301, 110684. [Google Scholar] [CrossRef]
- Hu, J.H.; Wang, B.K.; Yang, T.; Li, N.; Yang, H.T.; Yu, Q.H.; Wang, J. A calcium-dependent protein kinase gene SpCPK33 from Solanum pennellii associated with increased cold tolerance in tomato. J. Plant Physiol. 2022, 279, 153834. [Google Scholar] [CrossRef]
- Tak, H.; Negi, S.; Rajpurohit, Y.S.; Misra, H.S.; Ganapathi, T.R. MusaMPK5, a mitogenactivated protein kinase is involved in regulation of cold tolerance in banana. Plant Physiol. Biochem. 2020, 146, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.A.; Jing, B.Y.; Lin, T.; Li, X.Y.; Zhang, M.; Zhou, Y.H.; Yu, J.Q.; Hu, Z.J. Phosphorylation of sugar transporter TST2 by protein kinase CPK27 enhances drought tolerance in tomato. Plant Physiol. 2024, 195, 1005–1024. [Google Scholar] [CrossRef]
- Lim, J.; Lim, C.W.; Lee, S.C. Pepper novel serine-threonine kinase CaDIK1 regulates drought tolerance via modulating ABA sensitivity. Front. Plant Sci. 2020, 11, 1133. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Gai, W.X.; Li, C.; Gong, Z.H. The CaCIPK3 gene positively regulates drought tolerance in pepper. Hortic. Res. 2021, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, Y.N.; Li, Y.; Gong, Z.H. The CBL-interacting protein kinase CaCIPK7 enhances drought resistance in pepper. Sci. Hortic. 2023, 310, 111726. [Google Scholar] [CrossRef]
- Muhammad, T.; Zhang, J.; Ma, Y.L.; Li, Y.S.; Zhang, F.; Zhang, Y.; Liang, Y. Overexpression of a mitogen-activated protein kinase SlMAPK3 positively regulates tomato tolerance to cadmium and drought stress. Molecules 2019, 24, 556. [Google Scholar] [CrossRef]
- Huang, X.; Wei, J.M.; Feng, W.Z.; Luo, Q.; Tan, G.F.; Li, Y.Z. Interaction between SlMAPK3 and SlASR4 regulates drought resistance in tomato (Solanum lycopersicum L.). Mol. Breed. 2023, 43, 73. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.R.; Shen, W.; Ma, J.J.; Ya, R.; Zheng, Q.L.; Wu, N.; Yu, Q.H.; Yao, W.K.; Zhang, N.B.; Zhang, J.X. Role of an Amur grape CBL-interacting protein kinase VaCIPK02 in drought tolerance by modulating ABA signaling and ROS production. Environ. Exp. Bot. 2020, 172, 103999. [Google Scholar] [CrossRef]
- Malik, J.; Moosa, A.; Zulfiqar, F.; Aslam, M.N.; Albalawi, M.A.; Almowallad, S.; Mahmood, T.; Alasmari, A.; Yong, J.W.H. Biocontrol potential of lipopeptides produced by the novel Bacillus altitudinis strain TM22A against postharvest Alternaria rot of tomato. LWT Food Sci. Technol. 2024, 191, 115541. [Google Scholar] [CrossRef]
- Shen, Q.; Li, H.S.; Wang, Q.F.; Wang, J.Q.; Ge, J.R.; Yang, X.Y.; Wang, X.Y.; Li, X.M.; Zhang, Y.; Zhang, R.M.; et al. Alleviating effects of linalool fumigation on Botrytis cinerea infections in postharvest tomato fruit. Horticulturae 2022, 8, 1074. [Google Scholar] [CrossRef]
- Xu, S.M.; Liao, C.J.; Jaiswal, N.; Lee, S.; Yun, D.J.; Lee, S.Y.; Garvey, M.; Kaplan, I.; Mengiste, T. Tomato pepr1 ortholog receptor-like kinase1 regulates responses to systemin, necrotrophic fungi, and insect herbivory. Plant Cell 2018, 30, 2214–2229. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.N.; Lu, H.M.; Zhao, L.Q.; He, B.Q.; Zhang, J.J.; Zhao, B.; Guo, Y.D.; Zhang, N. SlMAPKKK43 regulates tomato resistance to gray mold. Acta Hortic. Sin. 2024, 51, 309–320. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Q.M.; Jia, L.T.; Qian, M.; Qiao, Q.H.; Huang, X.S.; Zhang, S.L. Genome-wide identification of the mitogen-activated protein kinase kinases in pear (Pyrus bretschneideri) and their functional analysis in response to black spot. Hortic. Plant J. 2023, 9, 681–692. [Google Scholar] [CrossRef]
- Ning, M.; Tang, F.X.; Zhang, Q.; Zhao, X.X.; Yang, L.P.; Cai, W.C.; Shan, C.H. Effects of Penicillium infection on the expression and activity of CDPK2 in postharvest Hami melon treated with calcium chloride. Physiol. Mol. Plant Pathol. 2019, 106, 175–181. [Google Scholar] [CrossRef]
- Gupta, R.; Min, C.W.; Kim, S.W.; Yoo, J.S.; Moon, A.R.; Shin, A.Y.; Kwon, S.Y.; Kim, S.T. A TMT-based quantitative proteome analysis to elucidate the TSWV induced signaling cascade in susceptible and resistant cultivars of Solanum lycopersicum. Plants 2020, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Tyagi, A.; Bae, H. ROS interplay between plant growth and stress biology: Challenges and future perspectives. Plant Physiol. Biochem. 2023, 203, 108032. [Google Scholar] [CrossRef]
- Li, M.P.; Kim, C. Chloroplast ROS and stress signaling. Plant Commun. 2022, 3, 100264. [Google Scholar] [CrossRef] [PubMed]
- Hamel, L.P.; Nicole, M.C.; Sritubtim, S.; Morency, M.J.; Ellis, M.; Ehlting, J.; Beaudoin, N.; Barbazuk, B.; Klessig, D.; Lee, J.; et al. Ancient signals: Comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci. 2006, 11, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.W.; Huang, W.C. Calcium-dependent protein kinases in phytohormone signaling pathways. Int. J. Mol. Sci. 2017, 18, 2436. [Google Scholar] [CrossRef] [PubMed]
- Moosa, A.; Zulfiqar, F.; Siddique, K.H. Transcriptional and biochemical profiling of defense enzymes in Citrus sinensis during salicylic acid and cinnamon mediated suppression of green and blue mold. Front. Plant Sci. 2022, 13, 1048433. [Google Scholar] [CrossRef]
- Zhang, J.; Hafeez, M.T.; Di, D.W.; Wu, L.; Zhang, L. Precise control of ABA signaling through post-translational protein modification. Plant Growth Regul. 2019, 88, 99–111. [Google Scholar] [CrossRef]
- Hasan, M.M.; Liu, X.D.; Waseem, M.; Yao, G.Q.; Alabdallah, N.M.; Jahan, M.S.; Fang, X.W. ABA activated SnRK2 kinases: An emerging role in plant growth and physiology. Plant Signal. Behav. 2022, 17, e2071024. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.Y.; Zhan, M.H.; Li, C.R.; Pei, T.T.; Wang, Q.; Li, P.M.; Ma, F.W.; Liu, C.H. The apple FERONIA receptor-like kinase MdMRLK2 negatively regulates Valsa canker resistance by suppressing defence responses and hypersensitive reaction. Mol. Plant Pathol. 2022, 23, 1170–1186. [Google Scholar] [CrossRef]
- Hewedy, O.A.; Elsheery, N.I.; Karkour, A.M.; Elhamouly, N.; Arafa, R.A.; Mahmoud, G.A.; Dawood, M.F.A.; Hussein, W.E.; Mansour, A.; Amin, D.H.; et al. Jasmonic acid regulates plant development and orchestrates stress response during tough times. Environ. Exp. Bot. 2023, 208, 105260. [Google Scholar] [CrossRef]
- Grau, J.; Francozorrilla, J.M. TDTHub, a web server tool for the analysis of transcription factor binding sites in plants. Plant J. 2022, 111, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Song, J.N.; Lin, R.; Tang, M.J.; Wang, L.Y.; Fan, P.X.; Xia, X.J.; Yu, J.Q.; Zhou, Y.H. SlMPK1 and SlMPK2 mediated SlBBX17 phosphorylation positively regulates CBF-dependent cold tolerance in tomato. New Phytol. 2023, 239, 1887–1902. [Google Scholar] [CrossRef]
- Hahn, A.; Bublak, D.; Schleiff, E.; Scharf, K.D. Crosstalk between Hsp90 and Hsp70 chaperones and heat stress transcription factors in tomato. Plant Cell 2011, 23, 741–755. [Google Scholar] [CrossRef]
- Van Kleeff, P.J.M.; Gao, J.; Mol, S.; Zwart, N.; Zhang, H.; Li, K.W.; De Boer, A.H. The Arabidopsis GORK K+ -channel is phosphorylated by calcium-dependent protein kinase 21 (CPK21), which in turn is activated by 14-3-3 proteins. Plant Physiol. Biochem. 2018, 125, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J.M. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef] [PubMed]
| Species | Protein Kinase | Numbers | References |
|---|---|---|---|
| Solanum lycopersicum | AGC | 17 | [19] |
| CDPK | 29 | [22] | |
| SnRK1 | 2 | [27] | |
| SnRK2 | 8 | ||
| SnRK3 | 30 | ||
| MAPK | 16 | [28] | |
| Solanum habrochaites | CDPK | 33 | [29] |
| Solanum melongena | CIPK | 15 | [30] |
| Vitis vinifera | CDPK | 19 | [31] |
| MAPK | 14 | [32] | |
| SnRK2 | 8 | [33] | |
| CIPK | 20 | [34] | |
| Capsicum annuum | CDPK | 31 | [35] |
| MAPK | 19 | [36] | |
| Prunus persica | CDPK | 17 | [37] |
| Musa nana | CDPK | 44 | [38] |
| SnRK2 | 11 | [39] | |
| Musa acuminata | MAPK | 25 | [40] |
| Brassica rapa | CDPK | 41 | [41] |
| CIPK | 51 | [42] | |
| SnRK2 | 15 | [43] | |
| Ananas comosus | CDPK | 17 | [44] |
| CIPK | 21 | [45] | |
| Fragaria ananassa | SnRK1 | 1 | [46] |
| SnRK2 | 9 | ||
| SnRK3 | 16 | ||
| CDPK | 11 | [47] | |
| MAPK | 43 | [48] | |
| Fragaria vesca | MAPK | 12 | [49] |
| CDPK | 19 | [50] | |
| Citrullus lanatus | MAPK | 15 | [51] |
| Actinidia Chinensis | MAPK | 18 | [52] |
| Carica papaya | MAPK | 9 | [53] |
| Punica Granatum | MAPK | 18 | [54] |
| Morus alba | MAPK | 10 | [55] |
| Citrus reticulata | CDPK | 29 | [56] |
| Raphanus sativus | CDPK | 37 | [24] |
| Brassica napus | CDPK | 25 | [57] |
| Cucumis sativus | CDPK | 19 | [58] |
| SnRK1 | 1 | [59] | |
| SnRK2 | 10 | ||
| SnRK3 | 19 | ||
| Cucumis melo | MAPK | 14 | [60] |
| CDPK | 18 | [61] | |
| Dioscorea opposite | CDPK | 29 | [62] |
| Brassica juncea | CDPK | 101 | [63] |
| Prunus avium | SnRK2 | 6 | [64] |
| Malus prunifolia | SnRK2 | 12 | [65] |
| Malus domestica | MAPK | 26 | [66] |
| Pyrus bretschneideri | CIPK | 28 | [67] |
| Dimocarpus longan | CIPK | 8 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Li, F.; Ali, M.; Li, X.; Zhang, X.; Ahmed, Z.F.R. Advances in Protein Kinase Regulation of Stress Responses in Fruits and Vegetables. Int. J. Mol. Sci. 2025, 26, 768. https://doi.org/10.3390/ijms26020768
Song Y, Li F, Ali M, Li X, Zhang X, Ahmed ZFR. Advances in Protein Kinase Regulation of Stress Responses in Fruits and Vegetables. International Journal of Molecular Sciences. 2025; 26(2):768. https://doi.org/10.3390/ijms26020768
Chicago/Turabian StyleSong, Yanan, Fujun Li, Maratab Ali, Xiaoan Li, Xinhua Zhang, and Zienab F. R. Ahmed. 2025. "Advances in Protein Kinase Regulation of Stress Responses in Fruits and Vegetables" International Journal of Molecular Sciences 26, no. 2: 768. https://doi.org/10.3390/ijms26020768
APA StyleSong, Y., Li, F., Ali, M., Li, X., Zhang, X., & Ahmed, Z. F. R. (2025). Advances in Protein Kinase Regulation of Stress Responses in Fruits and Vegetables. International Journal of Molecular Sciences, 26(2), 768. https://doi.org/10.3390/ijms26020768





