Exploring the Link Between Renal Function Fluctuations Within the Physiological Range and Serum/CSF Levels of NfL, GFAP, tTAU, and UCHL1
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Relationship Between Biomarker Concentrations and Kidney Function
3. Discussion
4. Methods and Materials
4.1. Study Design and Biomarker Measurement
4.2. Statistical Analysis
4.3. Standard Protocol Approvals and Patient Consents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| BMI | Body mass index |
| CIS | Clinically isolated syndrome |
| CKD | Chronic kidney disease |
| CKD-Epi | Chronic Kidney Disease Epidemiology Collaboration |
| CSF | Cerebrospinal fluid |
| EDSS | Expanded Disability Status Scale |
| eGFR | Estimated glomerular filtration rate |
| GFAP | Glial fibrillary acidic protein |
| IQR | Interquartile range |
| MOGAD | Myelin oligodendrocyte glycoprotein antibody disease |
| MS | Multiple sclerosis |
| NfL | Neurofilament light chain |
| NID | Neuroinflammatory disease |
| NMOSD | Neuromyelitis optica spectrum disease |
| OCBs | Oligoclonal bands |
| PMS | Progressive multiple sclerosis |
| RIS | Radiologically isolated syndrome |
| ROC | Receiver operating characteristic |
| RRMS | Relapsing-remitting multiple sclerosis |
| SD | Standard deviation |
| SIMOA | Single molecule array |
| tTAU | Total tau |
| UCHL1 | Ubiquitin C-terminal hydrolase L1 |
References
- Khalil, M.; Teunissen, C.E.; Lehmann, S.; Otto, M.; Piehl, F.; Ziemssen, T.; Bittner, S.; Sormani, M.P.; Gattringer, T.; Abu-Rumeileh, S.; et al. Neurofilaments as Biomarkers in Neurological Disorders—Towards Clinical Application. Nat. Rev. Neurol. 2024, 20, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Ladang, A.; Kovacs, S.; Lengelé, L.; Locquet, M.; Reginster, J.-Y.; Bruyère, O.; Cavalier, E. Neurofilament Light Chain Concentration in an Aging Population. Aging Clin. Exp. Res. 2022, 34, 331–339. [Google Scholar] [CrossRef]
- Koini, M.; Pirpamer, L.; Hofer, E.; Buchmann, A.; Pinter, D.; Ropele, S.; Enzinger, C.; Benkert, P.; Leppert, D.; Kuhle, J.; et al. Factors Influencing Serum Neurofilament Light Chain Levels in Normal Aging. Aging 2021, 13, 25729–25738. [Google Scholar] [CrossRef]
- Khalil, M.; Pirpamer, L.; Hofer, E.; Voortman, M.M.; Barro, C.; Leppert, D.; Benkert, P.; Ropele, S.; Enzinger, C.; Fazekas, F.; et al. Serum Neurofilament Light Levels in Normal Aging and Their Association with Morphologic Brain Changes. Nat. Commun. 2020, 11, 812. [Google Scholar] [CrossRef]
- Rebelos, E.; Rissanen, E.; Bucci, M.; Jääskeläinen, O.; Honka, M.J.; Nummenmaa, L.; Moriconi, D.; Laurila, S.; Salminen, P.; Herukka, S.K.; et al. Circulating Neurofilament Is Linked with Morbid Obesity, Renal Function, and Brain Density. Sci. Rep. 2022, 12, 7841. [Google Scholar] [CrossRef]
- Akamine, S.; Marutani, N.; Kanayama, D.; Gotoh, S.; Maruyama, R.; Yanagida, K.; Sakagami, Y.; Mori, K.; Adachi, H.; Kozawa, J.; et al. Renal Function Is Associated with Blood Neurofilament Light Chain Level in Older Adults. Sci. Rep. 2020, 10, 20350. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Panizzon, M.S.; Elman, J.A.; Gillespie, N.A.; Hauger, R.L.; Rissman, R.A.; Lyons, M.J.; Neale, M.C.; Reynolds, C.A.; Franz, C.E.; et al. Association of Neurofilament Light Chain with Renal Function: Mechanisms and Clinical Implications. Alzheimers Res. Ther. 2022, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Sotirchos, E.S.; Smith, M.D.; Lord, H.N.; DuVal, A.; Mowry, E.M.; Calabresi, P.A. Contributors to Serum Nfl Levels in People without Neurologic Disease. Ann. Neurol. 2022, 92, 688–698. [Google Scholar] [CrossRef] [PubMed]
- van der Plas, E.; Lullmann, O.; Hopkins, L.; Schultz, J.L.; Nopoulos, P.C.; Harshman, L.A. Associations between Neurofilament Light-Chain Protein, Brain Structure, and Chronic Kidney Disease. Pediatr. Res. 2022, 91, 1735–1740. [Google Scholar] [CrossRef]
- Kohlhase, K.; Frank, F.; Wilmes, C.; Koerbel, K.; Schaller-Paule, M.A.; Miles, M.; Betz, C.; Steinmetz, H.; Foerch, C. Brain-Specific Biomarkers in Urine as a Non-Invasive Approach to Monitor Neuronal and Glial Damage. Eur. J. Neurol. 2023, 30, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Tortosa-Carreres, J.; Cubas-Núñez, L.; Sanz, M.T.; Castillo-Villalba, J.; Gasqué-Rubio, R.; Carratalá-Boscá, S.; Alcalá-Vicente, C.; Quintanilla-Bordás, C.; Gorriz, D.; Casanova, B.; et al. Renal Function’s Impact on Serum Neurofilament Levels in Patients with Multiple Sclerosis: An Exploratory Analysis. Neurol. Sci. 2024. Online ahead of print. [Google Scholar]
- Polymeris, A.A.; Helfenstein, F.; Benkert, P.; Aeschbacher, S.; Leppert, D.; Coslovsky, M.; Willemse, E.; Schaedelin, S.; Blum, M.R.; Rodondi, N.; et al. Renal Function and Body Mass Index Contribute to Serum Neurofilament Light Chain Levels in Elderly Patients with Atrial Fibrillation. Front. Neurosci. 2022, 16, 819010. [Google Scholar] [CrossRef]
- Pajewski, N.M.; Elahi, F.M.; Tamura, M.K.; Hinman, J.D.; Nasrallah, I.M.; Ix, J.H.; Miller, L.M.; Launer, L.J.; Wright, C.B.; Supiano, M.A.; et al. Plasma Amyloid Beta, Neurofilament Light Chain, and Total Tau in the Systolic Blood Pressure Intervention Trial (Sprint). Alzheimer’s Dement. 2022, 18, 1472–1483. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, A.; Ashton, N.J.; Zetterberg, H.; Blennow, K.; Zettergren, A.; Simrén, J.; Skillbäck, T.; Shams, S.; Machado, A.; Westman, E.; et al. Association of Chronic Kidney Disease with Plasma Nfl and Other Biomarkers of Neurodegeneration: The H70 Birth Cohort Study in Gothenburg. Neurology 2023, 101, e277–e288. [Google Scholar] [CrossRef] [PubMed]
- Hermansson, L.; Yilmaz, A.; Price, R.W.; Nilsson, S.; McCallister, S.; Makadzange, T.; Das, M.; Zetterberg, H.; Blennow, K.; Gisslen, M. Plasma Concentration of Neurofilament Light Chain Protein Decreases after Switching from Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide Fumarate. PLoS ONE 2019, 14, e0226276. [Google Scholar] [CrossRef] [PubMed]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood Gfap as an Emerging Biomarker in Brain and Spinal Cord Disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Bishop, P.; Rocca, D.; Henley, J.M. Ubiquitin C-Terminal Hydrolase L1 (Uch-L1): Structure, Distribution and Roles in Brain Function and Dysfunction. Biochem. J. 2016, 473, 2453–2462. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G.; et al. Csf and Blood Biomarkers for the Diagnosis of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef]
- Sarto, J.; Esteller-Gauxax, D.; Tort-Merino, A.; Guillén, N.; Pérez-Millan, A.; Falgàs, N.; Borrego-Écija, S.; Fernández-Villullas, G.; Bosch, B.; Juncà-Parella, J.; et al. Impact of Demographics and Comorbid Conditions on Plasma Biomarkers Concentrations and Their Diagnostic Accuracy in a Memory Clinic Cohort. J. Neurol. 2024, 271, 1973–1984. [Google Scholar] [CrossRef]
- Berry, K.; Asken, B.M.; Grab, J.D.; Chan, B.; Lario Lago, A.; Wong, R.; Seetharaman, S.; LaHue, S.C.; Possin, K.L.; Rojas, J.C.; et al. Hepatic and Renal Function Impact Concentrations of Plasma Biomarkers of Neuropathology. Alzheimer’s Dement. 2022, 14, e12321. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, M.G.; Altenburg, M.K.; Sanford, R.; Willett, J.D.; Bleasdale, B.; Ballou, B.; Wilder, J.; Li, F.; Miner, J.H.; Berg, U.B.; et al. Permeation of Macromolecules into the Renal Glomerular Basement Membrane and Capture by the Tubules. Proc. Natl. Acad. Sci. USA 2017, 114, 2958–2963. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Barthélemy, N.R.; He, Y.; Bateman, R.J.; Hansson, O. Mitigating the Associations of Kidney Dysfunction with Blood Biomarkers of Alzheimer Disease by Using Phosphorylated Tau to Total Tau Ratios. JAMA Neurol. 2023, 80, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.L.; Yao, X.Q.; Lei, L.; Jin, W.S.; Bai, Y.D.; Zeng, G.H.; Shi, A.Y.; Liang, J.; Zhu, L.; Liu, Y.H.; et al. Associations of Blood and Cerebrospinal Fluid Abeta and Tau Levels with Renal Function. Mol. Neurobiol. 2023, 60, 5343–5351. [Google Scholar] [CrossRef]
- Olczak, M.; Poniatowski, A.; Niderla-Bielińska, J.; Kwiatkowska, M.; Chutorański, D.; Tarka, S.; Wierzba-Bobrowicz, T. Concentration of Microtubule Associated Protein Tau (Mapt) in Urine and Saliva as a Potential Biomarker of Traumatic Brain Injury in Relationship with Blood-Brain Barrier Disruption in Postmortem Examination. Forensic Sci. Int. 2019, 301, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Benkert, P.; Meier, S.; Schaedelin, S.; Manouchehrinia, A.; Yaldizli, Ö.; Maceski, A.; Oechtering, J.; Achtnichts, L.; Conen, D.; Derfuss, T.; et al. Serum Neurofilament Light Chain for Individual Prognostication of Disease Activity in People with Multiple Sclerosis: A Retrospective Modelling and Validation Study. Lancet Neurol. 2022, 21, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Mair, R.D.; Nguyen, H.; Huang, T.T.; Plummer, N.S.; Sirich, T.L.; Meyer, T.W. Accumulation of Uremic Solutes in the Cerebrospinal Fluid in Experimental Acute Renal Failure. Am. J. Physiol. Renal Physiol. 2019, 317, F296–F302. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the Mcdonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Schaller-Paule, M.A.; Yalachkov, Y.; Steinmetz, H.; Friedauer, L.; Hattingen, E.; Miesbach, W.; Weber, F.; Kirchmayr, K.; Schaefer, J.H.; Foerch, C. Analysis of Csf D-Dimer to Identify Intrathecal Fibrin-Driven Autoimmunity in Patients with Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2022, 9, e1150. [Google Scholar] [CrossRef] [PubMed]
- Yalachkov, Y.; Schäfer, J.H.; Jakob, J.; Friedauer, L.; Steffen, F.; Bittner, S.; Foerch, C.; Schaller-Paule, M.A. Effect of Estimated Blood Volume and Body Mass Index on Gfap and Nfl Levels in the Serum and Csf of Patients with Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2023, 10, e200045. [Google Scholar] [CrossRef] [PubMed]
- Yalachkov, Y.; Anschütz, V.; Jakob, J.; Schaller-Paule, M.A.; Schäfer, J.H.; Reiländer, A.; Friedauer, L.; Behrens, M.; Steffen, F.; Bittner, S.; et al. Brain-Derived Neurotrophic Factor and Neurofilament Light Chain in Cerebrospinal Fluid Are Inversely Correlated with Cognition in Multiple Sclerosis at the Time of Diagnosis. Mult. Scler. Relat. Disord. 2022, 63, 103822. [Google Scholar] [CrossRef]
- Koerbel, K.; Maiworm, M.; Schaller-Paule, M.; Schäfer, J.H.; Jakob, J.; Friedauer, L.; Steffen, F.; Bittner, S.; Foerch, C.; Yalachkov, Y. Evaluating the Utility of Serum Nfl, Gfap, Uchl1 and Ttau as Estimates of Csf Levels and Diagnostic Instrument in Neuroinflammation and Multiple Sclerosis. Mult. Scler. Relat. Disord. 2024, 87, 105644. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (Strobe) Statement: Guidelines for Reporting Observational Studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
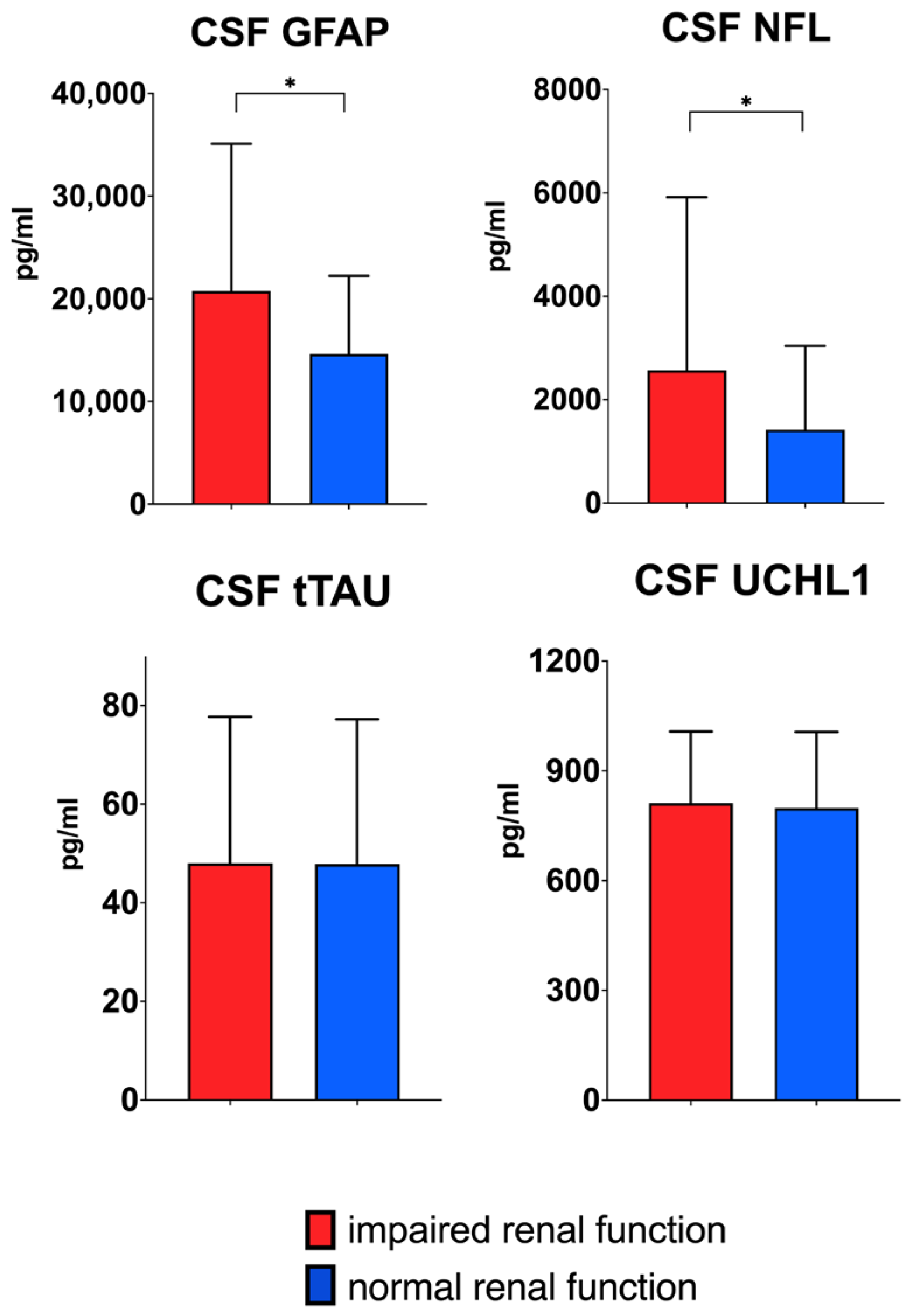
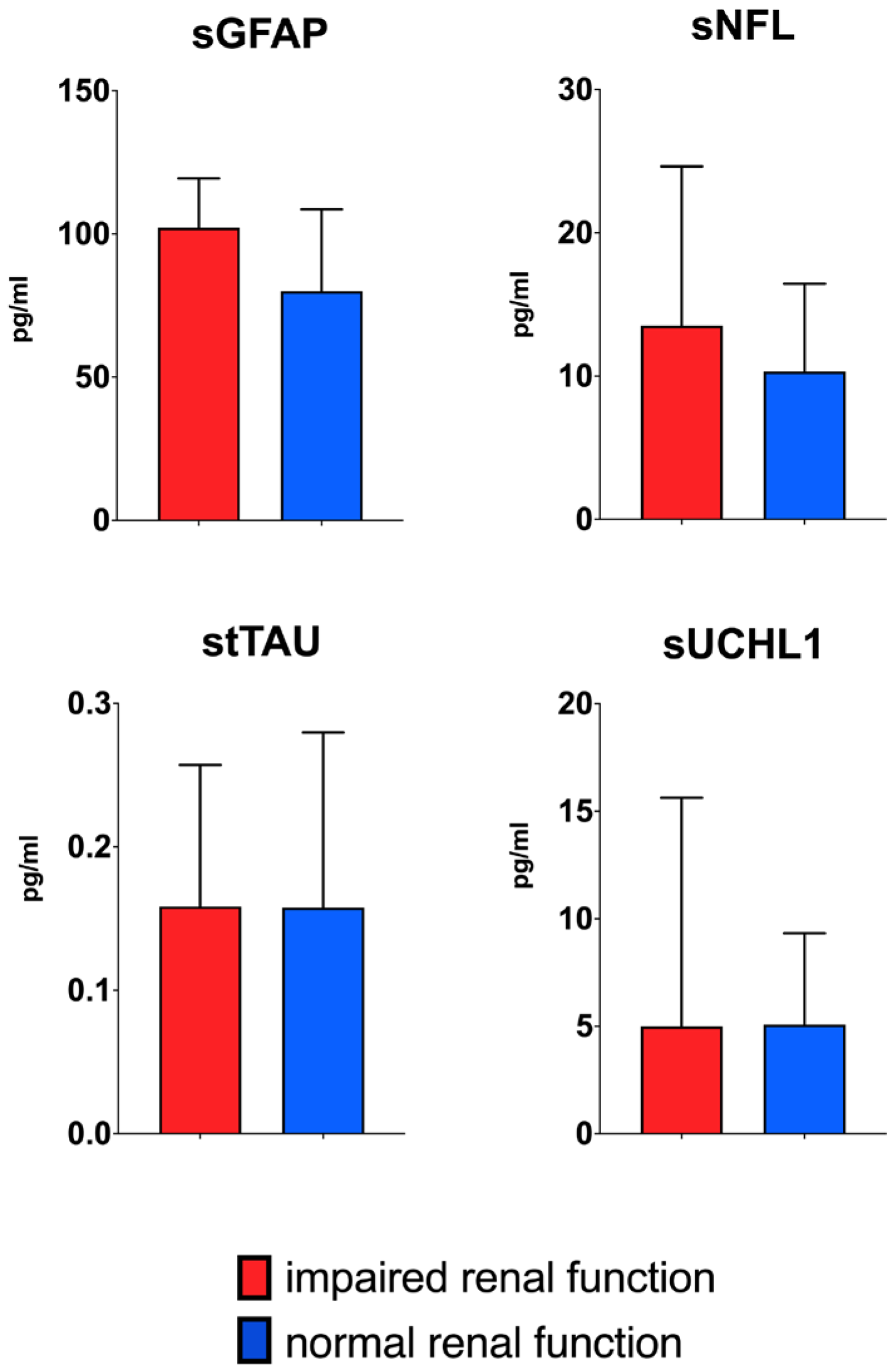

| Whole Cohort | Normal Kidney Function (eGFR > 90 mL/min/1.73 m2) | Impaired Kidney Function (eGFR < 90 mL/min/1.73 m2) | ||
|---|---|---|---|---|
| N (%) | 183 (100) | 167 (91.25) | 16 (8.74) | |
| Age (mean, SD) | 37.07 (12.25) | 35.24 (10.41) | 56.19 (13.91) | p < 0.01 |
| Sex (female, %) | 125 (68.7) | 119 (71.3) | 6 (37.5) | p = 0.05 |
| BMI kg/m2 (mean, SD) | 25.11 (5.94) | 24.87 (5.88) | 27.77 (6.18) | n.s. |
| Creatinine (mg/dL, mean, SD) | 0.68 (0.16) | 0.65 (0.14) | 0.95 (0.12) | |
| eGFR CKD-Epi (mL/min/1.73 m2, mean, SD) | 114.43 (16.16) | 117.71 (12.54) | 80.31 (7.77) | |
| sGFAP pg/mL (median, IQR) | 80.45 (48.05) | 79.98 (47.92) | 103.59 (77.45) | n.s. |
| cGFAP pg/mL (median, IQR) | 15,326.80 (14,219.60) | 14,594.42 (13,362.91) | 21,048.68 (27,364.01) | p = 0.026 |
| sNfL pg/mL (median, IQR) | 10.57 (9.41) | 10.14 (9.45) | 13.16 (15.48) | n.s. |
| cNfL pg/mL (median, IQR) | 1453.30 (2456.86) | 1414.56 (2408.02) | 2948.88 (6538.82) | p = 0.036 |
| stTAU pg/mL (median, IQR) | 0.16 (0.18) | 0.15 (0.19) | 0.17 (0.14) | n.s. |
| ctTAU pg/mL (median, IQR) | 47.78 (45.89) | 47.58 (46.34) | 49.79 (42.54) | n.s. |
| sUCHL1 pg/mL (median, IQR) | 5.01 (6.86) | 5.05 (6.34) | 4.06 (13.75) | n.s. |
| cUCHL1 pg/mL (median, IQR) | 798.61 (365.02) | 796.12 (364.90) | 821.11 (601.65) | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koerbel, K.; Yalachkov, Y.; Rotter, T.; Schaller-Paule, M.A.; Schaefer, J.H.; Friedauer, L.; Jakob, J.; Steffen, F.; Bittner, S.; Foerch, C.; et al. Exploring the Link Between Renal Function Fluctuations Within the Physiological Range and Serum/CSF Levels of NfL, GFAP, tTAU, and UCHL1. Int. J. Mol. Sci. 2025, 26, 748. https://doi.org/10.3390/ijms26020748
Koerbel K, Yalachkov Y, Rotter T, Schaller-Paule MA, Schaefer JH, Friedauer L, Jakob J, Steffen F, Bittner S, Foerch C, et al. Exploring the Link Between Renal Function Fluctuations Within the Physiological Range and Serum/CSF Levels of NfL, GFAP, tTAU, and UCHL1. International Journal of Molecular Sciences. 2025; 26(2):748. https://doi.org/10.3390/ijms26020748
Chicago/Turabian StyleKoerbel, Kimberly, Yavor Yalachkov, Tabea Rotter, Martin A. Schaller-Paule, Jan Hendrik Schaefer, Lucie Friedauer, Jasmin Jakob, Falk Steffen, Stefan Bittner, Christian Foerch, and et al. 2025. "Exploring the Link Between Renal Function Fluctuations Within the Physiological Range and Serum/CSF Levels of NfL, GFAP, tTAU, and UCHL1" International Journal of Molecular Sciences 26, no. 2: 748. https://doi.org/10.3390/ijms26020748
APA StyleKoerbel, K., Yalachkov, Y., Rotter, T., Schaller-Paule, M. A., Schaefer, J. H., Friedauer, L., Jakob, J., Steffen, F., Bittner, S., Foerch, C., & Maiworm, M. (2025). Exploring the Link Between Renal Function Fluctuations Within the Physiological Range and Serum/CSF Levels of NfL, GFAP, tTAU, and UCHL1. International Journal of Molecular Sciences, 26(2), 748. https://doi.org/10.3390/ijms26020748







