Involvement of ADAM17-Klotho Crosstalk in High Glucose-Induced Alterations of Podocyte Function
Abstract
1. Introduction
2. Results
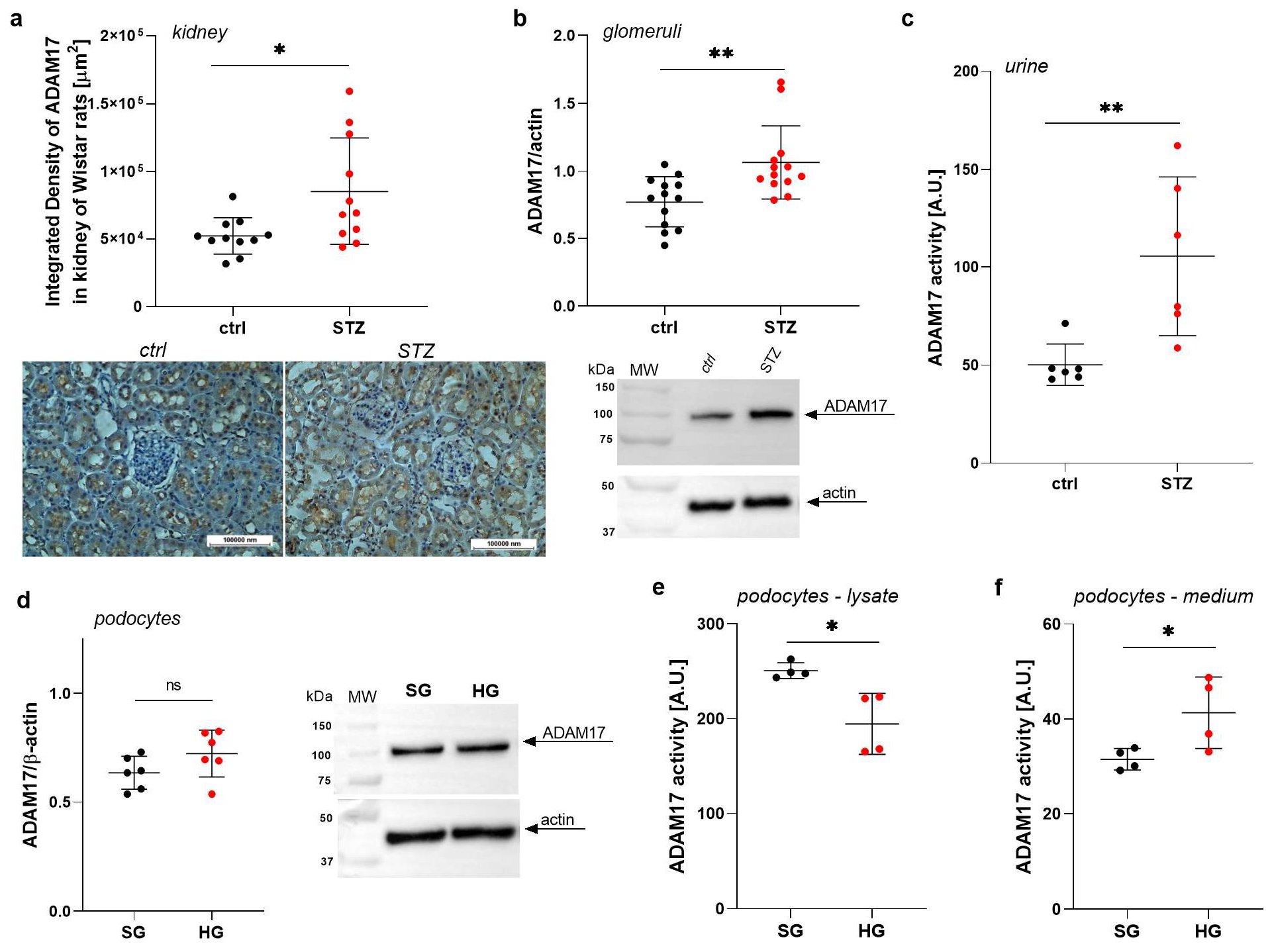
2.1. ADAM17 Levels Are Increased in Renal Tissues in Rats with STZ-Induced Diabetes
2.2. ADAM17 Levels and Activity Are Elevated in the Urine of Rats with STZ-Induced Diabetes
2.3. High Glucose Concentrations Impact the Extracellular Activity of ADAM17 in Podocytes
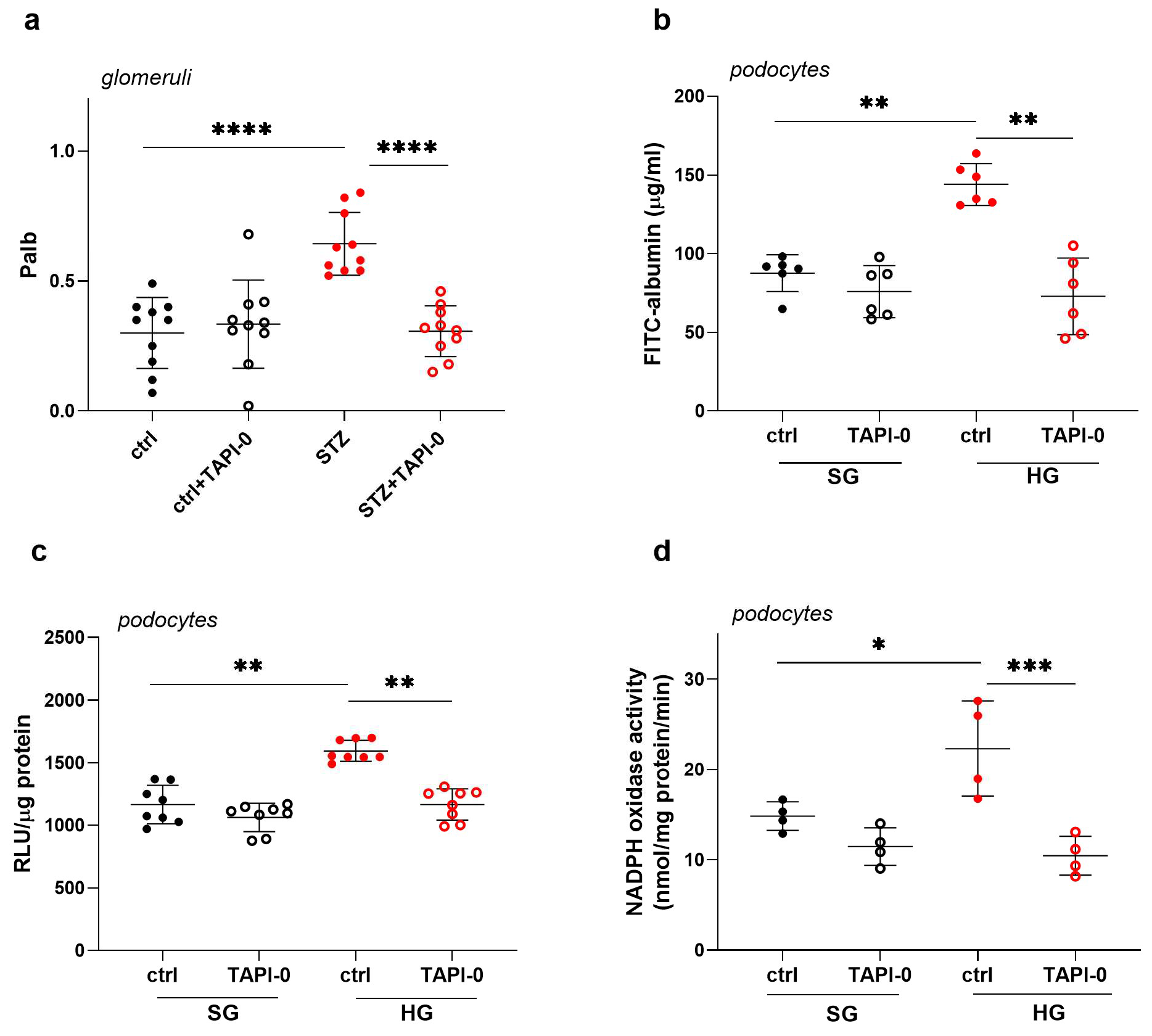
2.4. Inhibition of ADAM17 Improves Albumin Permeability Across the Glomerular Filtration Barrier in STZ Rats and Across a Monolayer of Podocytes Exposed to HG Concentrations
2.5. ADAM17 Inhibition Decreases HG-Induced Oxidative Stress in Podocytes
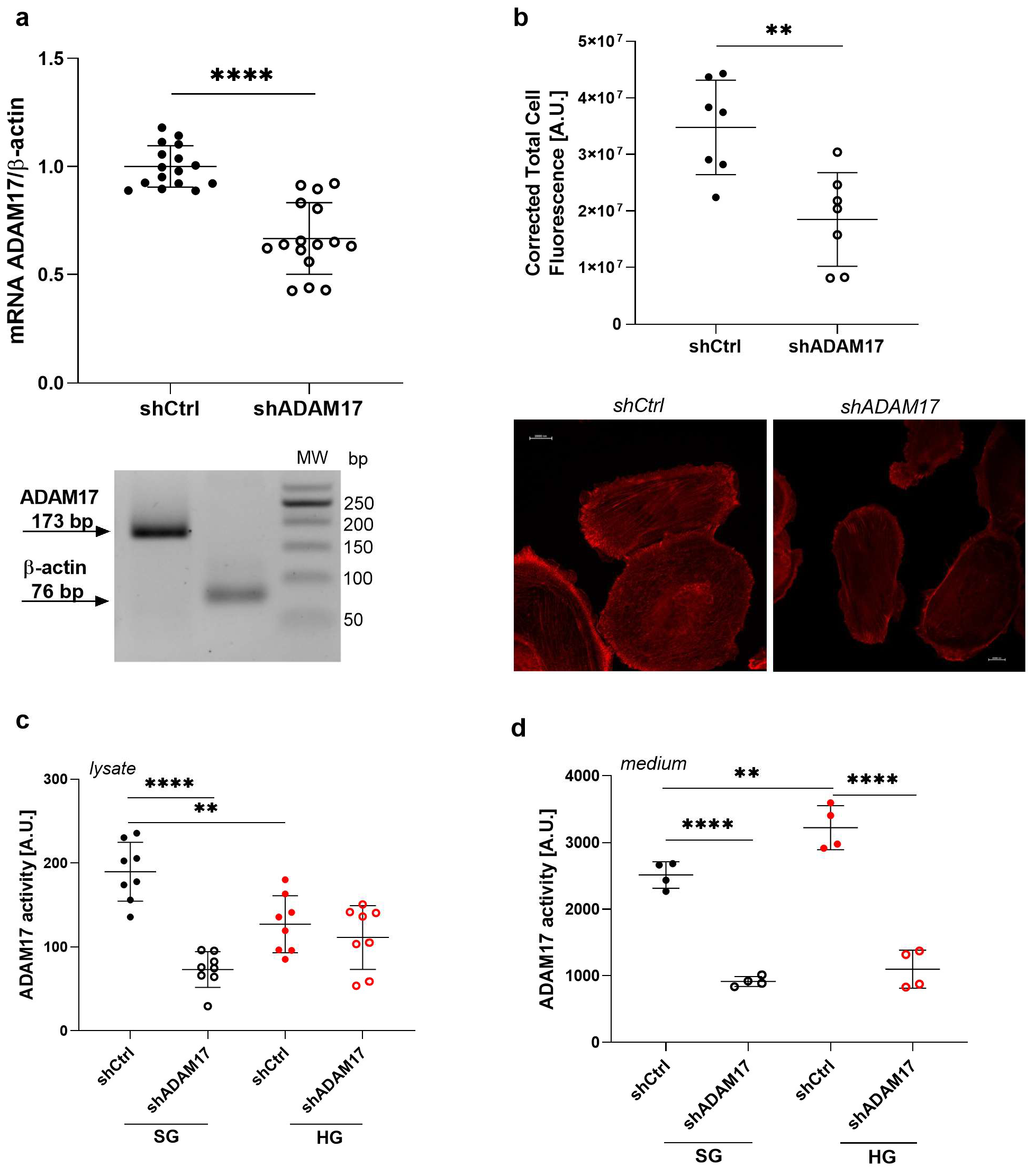
2.6. ADAM17 Downregulation Exerts a Beneficial Effect on the Function of Podocytes Under High Glucose Conditions
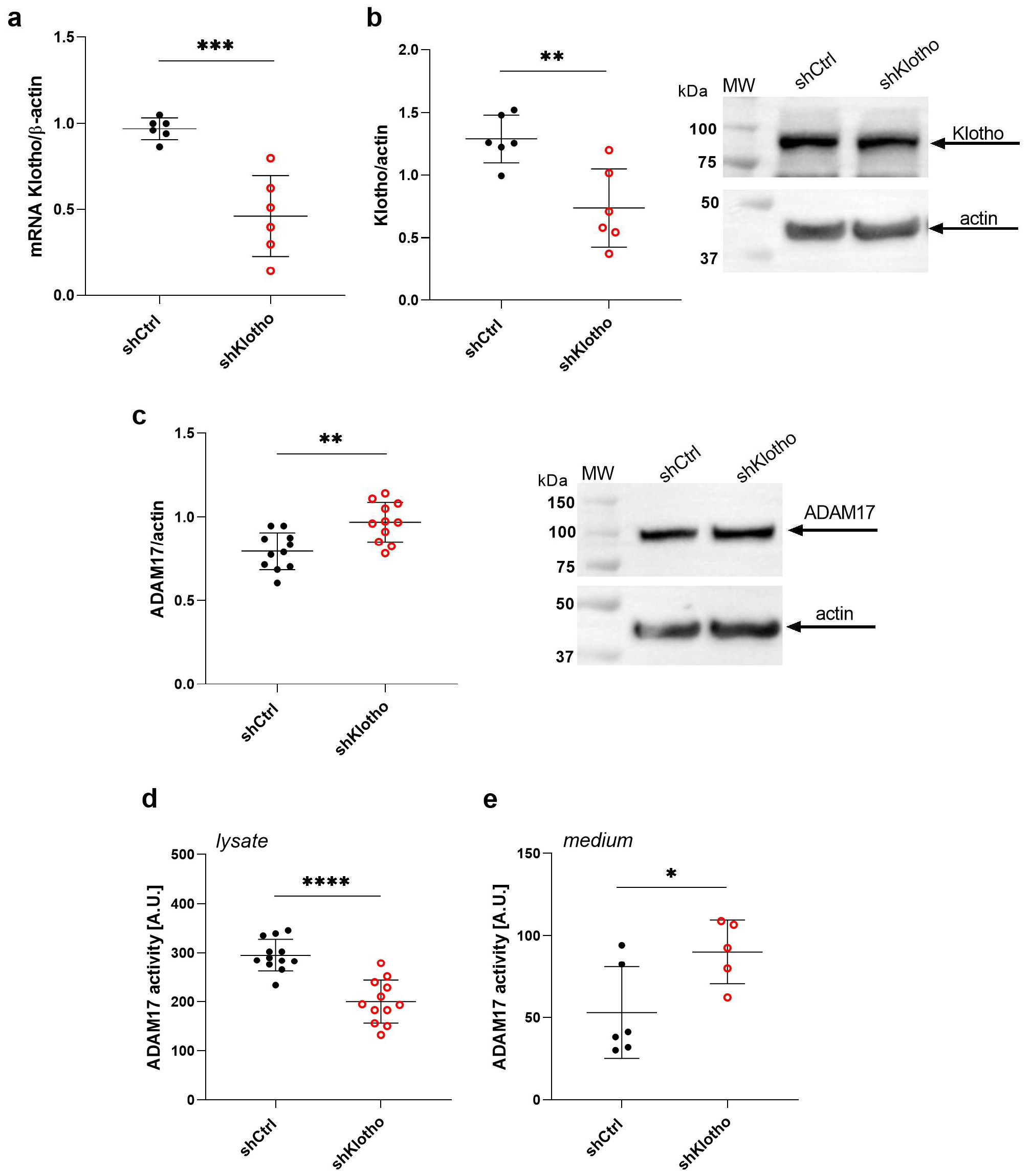
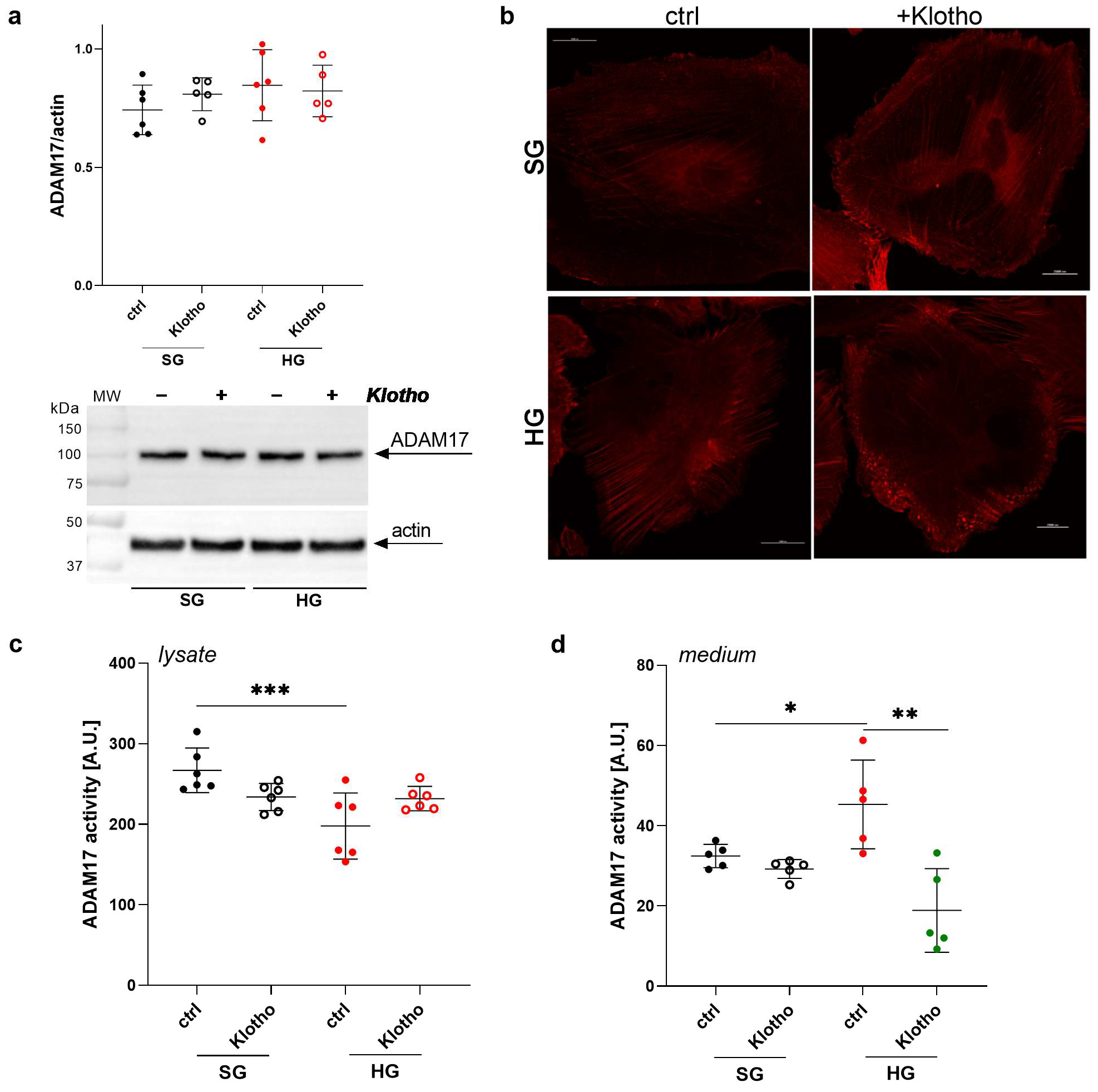
2.7. Klotho Deficiency Enhances ADAM17 Activity in the Extracellular Medium of Podocytes
2.8. Klotho Attenuates the HG-Induced Increase in ADAM17 Extracellular Activity in Podocytes
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Experimental Animals and Metabolic Cage Studies
4.3. Reagents
4.4. Human Podocyte Cell Culture
4.5. Antibodies
4.6. Western Blot
4.7. Immunofluorescence
4.8. Immunohistochemistry
4.9. ADAM17 Activity Assessment
4.10. Lentivirus-Derived Stable Gene Silencing
4.11. Quantitative Real-Time Polymerase Chain Reaction
4.12. Measurement of Intracellular ROS Levels
4.13. NADPH Oxidase Activity Assay
4.14. Isolation of Glomeruli
4.15. Glomerular Permeability Assay
4.16. Transepithelial Permeability Assay
4.17. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barisoni, L.; Mundel, P. Podocyte biology and the emerging understanding of podocyte diseases. Am. J. Nephrol. 2003, 23, 353–360. [Google Scholar] [CrossRef]
- Gutta, S.; Grobe, N.; Kumbaji, M.; Osman, H.; Saklayen, M.; Li, G.; Elased, K.M. Increased urinary angiotensin converting enzyme 2 and neprilysin in patients with type 2 diabetes. Am. J. Physiol. Ren. Physiol. 2018, 315, F263–F274. [Google Scholar] [CrossRef] [PubMed]
- Zunke, F.; Rose-John, S. The shedding protease ADAM17: Physiology and pathophysiology. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2059–2070. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.L.; Noy, P.J.; Reyat, J.S.; Tomlinson, M.G. Regulation of A disintegrin and metalloproteinase (ADAM) family sheddases ADAM10 and ADAM17: The emerging role of tetraspanins and rhomboids. Platelets 2017, 28, 333–341. [Google Scholar] [CrossRef]
- Lim, K.; Groen, A.; Molostvov, G.; Lu, T.; Lilley, K.S.; Snead, D.; James, S.; Wilkinson, I.B.; Ting, S.; Hsiao, L.L.; et al. α-Klotho Expression in Human Tissues. J. Clin. Endocrinol. Metab. 2015, 100, E1308–E1318. [Google Scholar] [CrossRef]
- Saar-Kovrov, V.; Donners, M.; van der Vorst, E.P.C. Shedding of Klotho: Functional Implications in Chronic Kidney Disease and Associated Vascular Disease. Front. Cardiovasc. Med. 2020, 7, 617842. [Google Scholar] [CrossRef] [PubMed]
- Richter, B.; Faul, C. FGF23 Actions on Target Tissues-With and Without Klotho. Front. Endocrinol. 2018, 9, 189. [Google Scholar] [CrossRef]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef]
- Liu, F.; Wu, S.; Ren, H.; Gu, J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat. Cell Biol. 2011, 13, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Drew, D.A.; Katz, R.; Kritchevsky, S.; Ix, J.; Shlipak, M.; Gutiérrez, O.M.; Newman, A.; Hoofnagle, A.; Fried, L.; Semba, R.D.; et al. Association Between Soluble Klotho and Change in Kidney Function: The Health Aging and Body Composition Study. J. Am. Soc. Nephrol. 2017, 28, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Griffith, C.; Kuro-o, M.; Moe, O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J. Am. Soc. Nephrol. 2011, 22, 124–136. [Google Scholar] [CrossRef]
- Fountoulakis, N.; Maltese, G.; Gnudi, L.; Karalliedde, J. Reduced Levels of Anti-Ageing Hormone Klotho Predict Renal Function Decline in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2018, 103, 2026–2032. [Google Scholar] [CrossRef]
- Kadoya, H.; Satoh, M.; Haruna, Y.; Sasaki, T.; Kashihara, N. Klotho attenuates renal hypertrophy and glomerular injury in Ins2Akita diabetic mice. Clin. Exp. Nephrol. 2016, 20, 671–678. [Google Scholar] [PubMed]
- Haruna, Y.; Kashihara, N.; Satoh, M.; Tomita, N.; Namikoshi, T.; Sasaki, T.; Fujimori, T.; Xie, P.; Kanwar, Y.S. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc. Natl. Acad. Sci. USA 2007, 104, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.C.; Shi, M.; Zhang, J.; Pastor, J.; Nakatani, T.; Lanske, B.; Razzaque, M.S.; Rosenblatt, K.P.; Baum, M.G.; Kuro-o, M.; et al. Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010, 24, 3438–3450. [Google Scholar]
- Kim, J.H.; Xie, J.; Hwang, K.H.; Wu, Y.L.; Oliver, N.; Eom, M.; Park, K.S.; Barrezueta, N.; Kong, I.D.; Fracasso, R.P.; et al. Klotho May Ameliorate Proteinuria by Targeting TRPC6 Channels in Podocytes. J. Am. Soc. Nephrol. 2017, 28, 140–151. [Google Scholar] [CrossRef]
- Stieger, N.; Worthmann, K.; Schiffer, M. The role of metabolic and haemodynamic factors in podocyte injury in diabetes. Diabetes. Metab. Res. Rev. 2011, 27, 207–215. [Google Scholar]
- Piwkowska, A.; Rogacka, D.; Audzeyenka, I.; Jankowski, M.; Angielski, S. High Glucose Concentration Affects the Oxidant-Antioxidant Balance in Cultured Mouse Podocytes. J. Cell. Biochem. 2011, 112, 1661–1672. [Google Scholar] [CrossRef]
- Jha, J.C.; Banal, C.; Chow, B.S.; Cooper, M.E.; Jandeleit-Dahm, K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid. Redox Signal. 2016, 25, 657–684. [Google Scholar] [CrossRef] [PubMed]
- Rogacka, D.; Piwkowska, A.; Audzeyenka, I.; Angielski, S.; Jankowski, M. Involvement of the AMPK-PTEN pathway in insulin resistance induced by high glucose in cultured rat podocytes. Int. J. Biochem. Cell Biol. 2014, 51, 120–130. [Google Scholar] [CrossRef]
- Piwkowska, A.; Rogacka, D.; Audzeyenka, I.; Angielski, S.; Jankowski, M. High glucose increases glomerular filtration barrier permeability by activating protein kinase G type Ialpha subunits in a Nox4-dependent manner. Exp. Cell Res. 2014, 320, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Typiak, M.; Kulesza, T.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Angielski, S.; Saleem, M.A.; Piwkowska, A. Role of Klotho in Hyperglycemia: Its Levels and Effects on Fibroblast Growth Factor Receptors, Glycolysis, and Glomerular Filtration. Int. J. Mol. Sci. 2021, 22, 7867. [Google Scholar] [CrossRef]
- Li, R.; Uttarwar, L.; Gao, B.; Charbonneau, M.; Shi, Y.; Chan, J.S.; Dubois, C.M.; Krepinsky, J.C. High Glucose Up-regulates ADAM17 through HIF-1α in Mesangial Cells. J. Biol. Chem. 2015, 290, 21603–21614. [Google Scholar] [CrossRef]
- Li, R.; Wang, T.; Walia, K.; Gao, B.; Krepinsky, J.C. Regulation of profibrotic responses by ADAM17 activation in high glucose requires its C-terminus and FAK. J. Cell Sci. 2018, 131, jcs208629. [Google Scholar] [CrossRef] [PubMed]
- Ford, B.M.; Eid, A.A.; Göőz, M.; Barnes, J.L.; Gorin, Y.C.; Abboud, H.E. ADAM17 mediates Nox4 expression and NADPH oxidase activity in the kidney cortex of OVE26 mice. Am. J. Physiol. Ren. Physiol. 2013, 305, F323–F332. [Google Scholar] [CrossRef]
- Sikora, H.; Gruba, N.; Wysocka, M.; Piwkowska, A.; Lesner, A. Optimization of fluorescent substrates for ADAM17 and their utility in the detection of diabetes. Anal. Biochem. 2023, 681, 115337. [Google Scholar] [CrossRef] [PubMed]
- Euler, G.; Locquet, F.; Kociszewska, J.; Osygus, Y.; Heger, J.; Schreckenberg, R.; Schlüter, K.D.; Kenyeres, É.; Szabados, T.; Bencsik, P.; et al. Matrix Metalloproteinases Repress Hypertrophic Growth in Cardiac Myocytes. Cardiovasc. Drugs Ther. 2021, 35, 353–365. [Google Scholar] [CrossRef]
- Szrejder, M.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Rychlowski, M.; Kreft, E.; Angielski, S.; Piwkowska, A. Metformin reduces TRPC6 expression through AMPK activation and modulates cytoskeleton dynamics in podocytes under diabetic conditions. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165610. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Niño, M.D.; Fernandez-Fernandez, B.; Ortiz, A. Klotho, the elusive kidney-derived anti-ageing factor. Clin. Kidney J. 2019, 13, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Piwkowska, A.; Rachubik, P.; Typiak, M.; Kulesza, T.; Audzeyenka, I.; Saleem, M.A.; Gruba, N.; Wysocka, M.; Lesner, A.; Rogacka, D. ADAM10 as a major activator of reactive oxygen species production and klotho shedding in podocytes under diabetic conditions. Biochem. Pharmacol. 2024, 225, 116328. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Kuro-o, M.; Moe, O.W. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 2010, 78, 1240–1251. [Google Scholar] [CrossRef]
- Neyra, J.A.; Hu, M.C. Potential application of klotho in human chronic kidney disease. Bone 2017, 100, 41–49. [Google Scholar] [CrossRef]
- Monroy, A.; Kamath, S.; Chavez, A.O.; Centonze, V.E.; Veerasamy, M.; Barrentine, A.; Wewer, J.J.; Coletta, D.K.; Jenkinson, C.; Jhingan, R.M.; et al. Impaired regulation of the TNF-alpha converting enzyme/tissue inhibitor of metalloproteinase 3 proteolytic system in skeletal muscle of obese type 2 diabetic patients: A new mechanism of insulin resistance in humans. Diabetologia 2009, 52, 2169–2181. [Google Scholar] [CrossRef] [PubMed]
- Welsh, G.I.; Hale, L.J.; Eremina, V.; Jeansson, M.; Maezawa, Y.; Lennon, R.; Pons, D.A.; Owen, R.J.; Satchell, S.C.; Miles, M.J.; et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010, 12, 329–340. [Google Scholar] [CrossRef]
- Rogacka, D. Insulin resistance in glomerular podocytes: Potential mechanisms of induction. Arch. Biochem. Biophys. 2021, 710, 109005. [Google Scholar] [CrossRef] [PubMed]
- Chodavarapu, H.; Grobe, N.; Somineni, H.K.; Salem, E.S.; Madhu, M.; Elased, K.M. Rosiglitazone treatment of type 2 diabetic db/db mice attenuates urinary albumin and angiotensin converting enzyme 2 excretion. PLoS ONE 2013, 8, e62833. [Google Scholar] [CrossRef]
- Cardellini, M.; Menghini, R.; Martelli, E.; Casagrande, V.; Marino, A.; Rizza, S.; Porzio, O.; Mauriello, A.; Solini, A.; Ippoliti, A.; et al. TIMP3 is reduced in atherosclerotic plaques from subjects with type 2 diabetes and increased by SirT1. Diabetes 2009, 58, 2396–2401. [Google Scholar] [CrossRef] [PubMed]
- Rogacka, D.; Audzeyenka, I.; Rychlowski, M.; Rachubik, P.; Szrejder, M.; Angielski, S.; Piwkowska, A. Metformin overcomes high glucose-induced insulin resistance of podocytes by pleiotropic effects on SIRT1 and AMPK. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 115–125. [Google Scholar] [CrossRef]
- Jiang, W.; Xiao, T.; Han, W.; Xiong, J.; He, T.; Liu, Y.; Huang, Y.; Yang, K.; Bi, X.; Xu, X.; et al. Klotho inhibits PKCα/p66SHC-mediated podocyte injury in diabetic nephropathy. Mol. Cell Endocrinol. 2019, 494, 110490. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.Y.; Chen, X.; Chen, S.R.; Li, Q.; Wang, Y.H.; Zou, J.; Cao, W.W.; Luo, J.N.; Gao, H.; Liu, P.Q. Upregulation of Nox4 promotes angiotensin II-induced epidermal growth factor receptor activation and subsequent cardiac hypertrophy by increasing ADAM17 expression. Can. J. Cardiol. 2013, 29, 1310–1319. [Google Scholar] [CrossRef]
- Guo, Y.; Zhuang, X.; Huang, Z.; Zou, J.; Yang, D.; Hu, X.; Du, Z.; Wang, L.; Liao, X. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-κB-mediated inflammation both In Vitro and In Vivo. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 238–251. [Google Scholar] [CrossRef]
- Takenaka, T.; Kobori, H.; Miyazaki, T.; Suzuki, H.; Nishiyama, A.; Ishii, N.; Yamashita, M.; Hayashi, M. Klotho protein supplementation reduces blood pressure and renal hypertrophy in db/db mice, a model of type 2 diabetes. Acta Physiol. 2019, 225, e13190. [Google Scholar] [CrossRef]
- Xing, L.; Guo, H.; Meng, S.; Zhu, B.; Fang, J.; Huang, J.; Chen, J.; Wang, Y.; Wang, L.; Yao, X.; et al. Klotho ameliorates diabetic nephropathy by activating Nrf2 signaling pathway in podocytes. Biochem. Biophys. Res. Commun. 2021, 534, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kuro-o, M.; Sun, Z. Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell 2012, 11, 410–417. [Google Scholar] [CrossRef]
- Olejnik, A.; Banaszkiewicz, M.; Krzywonos-Zawadzka, A.; Bil-Lula, I. The Klotho protein supports redox balance and metabolic functions of cardiomyocytes during ischemia/reperfusion injury. Cardiol. J. 2022, 29, 836–849. [Google Scholar] [CrossRef]
- De Luca, G.M.; Breedijk, R.M.; Brandt, R.A.; Zeelenberg, C.H.; de Jong, B.E.; Timmermans, W.; Azar, L.N.; Hoebe, R.A.; Stallinga, S.; Manders, E.M. Re-scan confocal microscopy: Scanning twice for better resolution. BioMed Opt. Express 2013, 4, 2644–2656. [Google Scholar] [CrossRef]
- Kulesza, T.; Typiak, M.; Rachubik, P.; Audzeyenka, I.; Rogacka, D.; Angielski, S.; Saleem, M.A.; Piwkowska, A. Hyperglycemic environment disrupts phosphate transporter function and promotes calcification processes in podocytes and isolated glomeruli. J. Cell. Physiol. 2022, 237, 2478–2491. [Google Scholar] [CrossRef]
- Pubill, D.; Dayanithi, G.; Siatka, C.; Andres, M.; Dufour, M.N.; Guillon, G.; Mendre, C. ATP induces intracellular calcium increases and actin cytoskeleton disaggregation via P2x receptors. Cell Calcium 2001, 29, 299–309. [Google Scholar] [CrossRef]
- Piwkowska, A.; Rogacka, D.; Jankowski, M.; Dominiczak, M.H.; Stepiński, J.K.; Angielski, S. Metformin induces suppression of NAD(P)H oxidase activity in podocytes. Biochem. Biophys. Res. Commun. 2010, 393, 268–273. [Google Scholar] [CrossRef]
- Münzel, T.; Kurz, S.; Rajagopalan, S.; Thoenes, M.; Berrington, W.R.; Thompson, J.A.; Freeman, B.A.; Harrison, D.G. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J. Clin. Investig. 1996, 98, 1465–1470. [Google Scholar] [CrossRef]
- Savin, V.J.; Sharma, R.; Lovell, H.B.; Welling, D.J. Measurement of albumin reflection coefficient with isolated rat glomeruli. J. Am. Soc. Nephrol. 1992, 3, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Kasztan, M.; Piwkowska, A.; Kreft, E.; Rogacka, D.; Audzeyenka, I.; Szczepanska-Konkel, M.; Jankowski, M. Extracellular purines’ action on glomerular albumin permeability in isolated rat glomeruli: Insights into the pathogenesis of albuminuria. Am. J. Physiol. Ren. Physiol. 2016, 311, F103–F111. [Google Scholar] [CrossRef]


| Parameter | Control Rats (n = 5) | STZ Rats (n = 5) |
|---|---|---|
| Body weight (g) | 367.6 ± 44.3 | 255.6 ± 42.1 ** |
| Urine volume (mL/24 h) | 14.18 ± 1.72 | 217.8 ± 30.1 **** |
| Blood glucose concentration (mg/dL) | 168.8 ± 32.83 | 497.0 ± 66.7 **** |
| Urinary albumin excretion (mg/24 h) | 0.17 ± 0.07 | 4.15 ± 2.75 * |
| Gene | Accession No. | Primer Sequences | Probe Sequences | PCR Product Length [bp] |
|---|---|---|---|---|
| ADAM17 | NM_001382777.1 | Forward: TTTGTGGGAACTCGAGGGTG Reverse: GCACTTCTTCTGGGCAGTCT | ACCTGCTG | 173 |
| Klotho | NM_004795.4 | Forward: GCTCAACTCCCCCAGTCAGG Reverse: TGTGGGCTTTGAGAGCTTCG | CCAGGGCA | 193 |
| ACTB | NM_001101.5 | Forward: ATTGGCAATGAGCGGTTC Reverse: GGATGCCACAGGACTCCA | CTTCCAGC | 76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogacka, D.; Rachubik, P.; Typiak, M.; Kulesza, T.; Audzeyenka, I.; Saleem, M.A.; Sikora, H.; Gruba, N.; Wysocka, M.; Lesner, A.; et al. Involvement of ADAM17-Klotho Crosstalk in High Glucose-Induced Alterations of Podocyte Function. Int. J. Mol. Sci. 2025, 26, 731. https://doi.org/10.3390/ijms26020731
Rogacka D, Rachubik P, Typiak M, Kulesza T, Audzeyenka I, Saleem MA, Sikora H, Gruba N, Wysocka M, Lesner A, et al. Involvement of ADAM17-Klotho Crosstalk in High Glucose-Induced Alterations of Podocyte Function. International Journal of Molecular Sciences. 2025; 26(2):731. https://doi.org/10.3390/ijms26020731
Chicago/Turabian StyleRogacka, Dorota, Patrycja Rachubik, Marlena Typiak, Tomasz Kulesza, Irena Audzeyenka, Moin A. Saleem, Honorata Sikora, Natalia Gruba, Magdalena Wysocka, Adam Lesner, and et al. 2025. "Involvement of ADAM17-Klotho Crosstalk in High Glucose-Induced Alterations of Podocyte Function" International Journal of Molecular Sciences 26, no. 2: 731. https://doi.org/10.3390/ijms26020731
APA StyleRogacka, D., Rachubik, P., Typiak, M., Kulesza, T., Audzeyenka, I., Saleem, M. A., Sikora, H., Gruba, N., Wysocka, M., Lesner, A., & Piwkowska, A. (2025). Involvement of ADAM17-Klotho Crosstalk in High Glucose-Induced Alterations of Podocyte Function. International Journal of Molecular Sciences, 26(2), 731. https://doi.org/10.3390/ijms26020731






