Advances and Future Trends in Nanozyme-Based SERS Sensors for Food Safety, Environmental and Biomedical Applications
Abstract
1. Introduction
2. Synthesis and Surface Modification of Nanozymes
2.1. Synthesis of Nanozymes
2.2. Techniques for Surface Modification
2.3. Catalytic Performance and Multienzyme-Like Activities
3. Nanozyme-Based SERS Sensors
3.1. Principles of SERS and Nanozyme Interaction
3.2. Design and Fabrication of Nanozyme-Based SERS Sensors
4. Application of Nanozyme-Based SERS Sensors
4.1. Food Contaminants Detection
4.1.1. Mycotoxin Detection
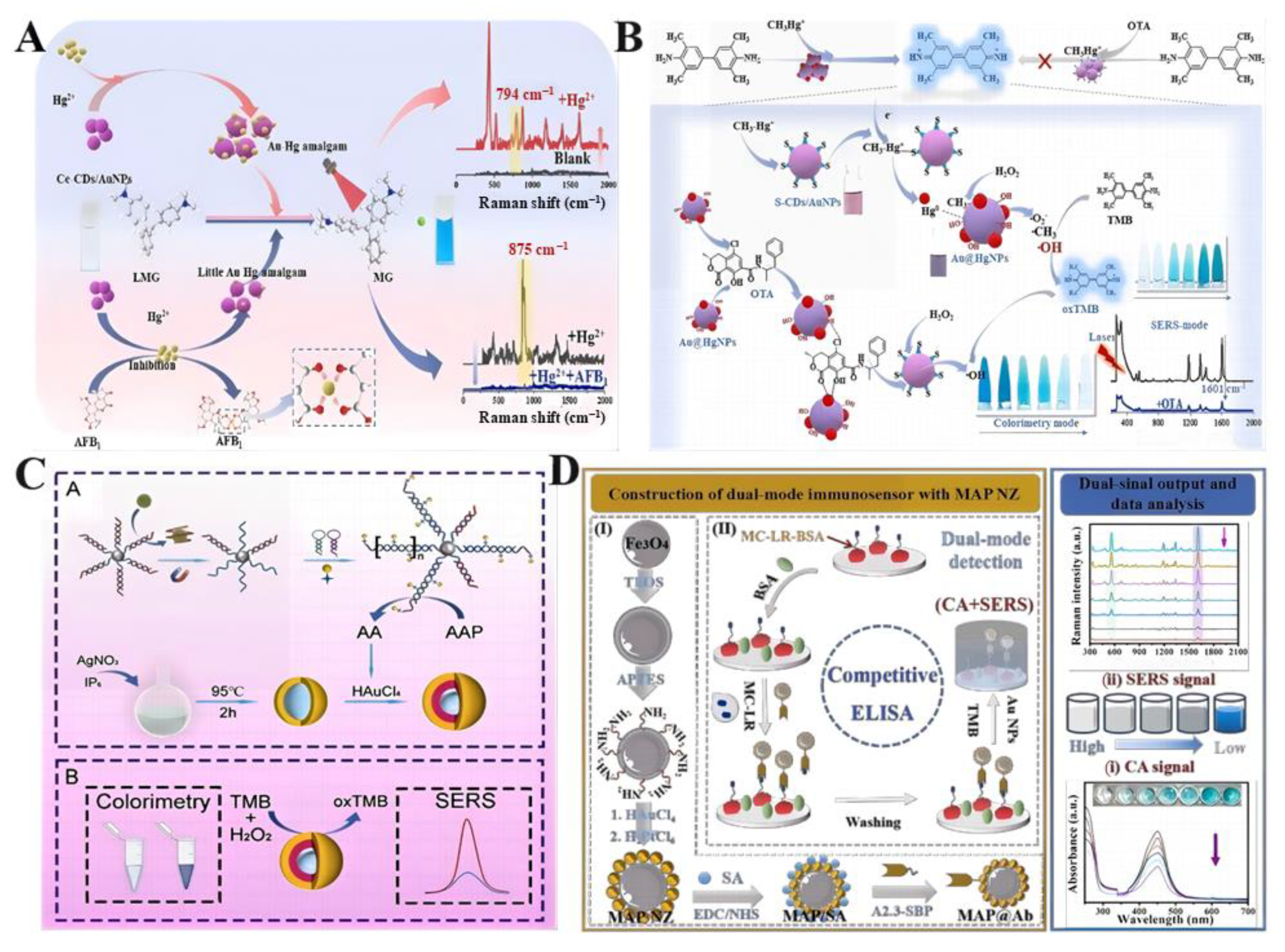
4.1.2. Pesticide and Veterinary Drug Detection
4.1.3. Pathogen Detection
4.1.4. Biogenic Amine Detection
4.1.5. Others
4.2. Environmental Pollutant Detection

4.3. Biomedical Marker Identification
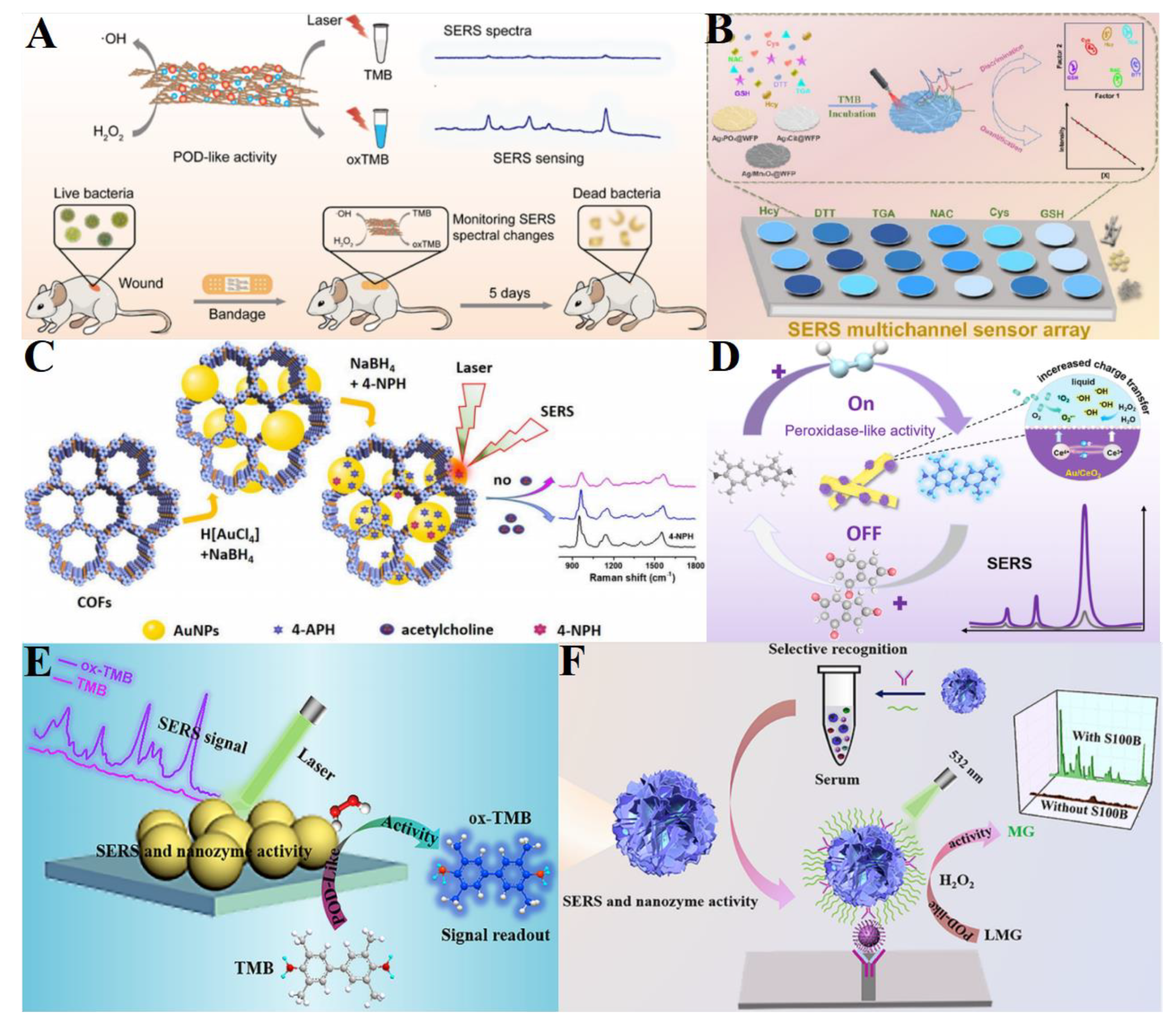
5. Perspectives and Challenges
- (I)
- Deepening understanding of nanozyme principles and mechanisms: A deeper understanding of the principles and mechanisms underlying nanozyme activity is essential. While significant progress has been made in the synthesis and application of nanozymes, the theoretical work and mechanism clarification remain limited. Future research should focus on elucidating the structure-activity relationships of nanozymes to guide their precise design for specific applications. This includes understanding how the physicochemical properties of nanozymes, such as size, morphology, and surface groups, influence their catalytic performance and selectivity.
- (II)
- Development of standardized characterization systems: The development of standardized systems for characterizing nanozyme performance is a critical challenge. Nanozymes differ significantly from natural enzymes, and traditional characterization methods may not be directly applicable. Establishing uniform systems and standards will facilitate the comparison of different nanozymes and their catalytic activities. This is particularly important for the Michaelis-Menten kinetics, which are commonly used to discuss natural enzymes but may not fully capture the heterogeneous mechanisms of nanozymes on nanomaterial surfaces.
- (III)
- Engineering nanozymes with tailored properties: Another significant challenge is the engineering of nanozymes with tailored properties for specific applications. As size, morphology, and surface chemistry significantly influence enzymatic activity, it is crucial to achieve high-performance nanozymes by controlling these parameters. Research should focus on developing methods to controllably engineer nanozymes and extend their functions through surface modifications, such as the introduction of functional groups or the attachment of specific recognition elements like antibodies or aptamers.
- (IV)
- Evaluating high-performance nanozymes: The evaluation of high-performance nanozymes is essential for developing improved analytical techniques. While various nanozymes have been reported for signal production and amplification, their catalytic activity in real applications is still relatively low. There is a need for nanozymes with high catalytic activity, diverse enzymatic activities, and good substrate selectivity. This challenge requires the development of new materials and synthetic strategies to create nanozymes that can catalyze specific substrates efficiently.
- (V)
- Integrating diverse techniques: The integration of distinct techniques with nanozymes to create multi-modal detection platforms is a promising area for future research. Combining nanozymes with techniques such as molecular imprinting, fluorescence, and electrochemistry can enhance the detection specificity, selectivity, and sensitivity. This integration can lead to the development of next-generation analytical tools that are more powerful and versatile than current methods.
- (VI)
- Addressing real-world complexity: A significant challenge in the application of nanozyme-based SERS sensors is addressing the complexity of real-world samples. These sensors must be able to selectively detect target analytes in the presence of a multitude of interfering substances. Research should focus on improving the selectivity and robustness of nanozyme-SERS sensors to ensure accurate detection in complex matrices.
- (VII)
- Scaling up and commercialization: Finally, the challenge of scaling up the production of nanozymes and their integration into SERS sensors for commercial use cannot be overlooked. This involves not only the development of cost-effective and large-scale synthesis methods but also the standardization of sensor fabrication and performance. Commercialization will require addressing issues related to sensor stability, reproducibility, and user-friendliness.
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, L.; Qi, Z.; Yang, Y.; Lu, N.; Tang, Z. Enhanced “electronic tongue” for dental bacterial discrimination and elimination based on a DNA-encoded nanozyme sensor srray. ACS Appl. Mater. Interfaces 2024, 16, 11228–11238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, M.; Pan, Y.; Zhang, L.; Lei, H.; Zheng, Y.; Shi, Y.; Liu, S.; Li, N.; Wang, Y. Turning threat to therapy: A nanozyme-patch in surgical bed for convenient tumor vaccination by sustained in situ catalysis. Adv. Healthc. Mater. 2024, 13, 2304384. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Fu, L.; Wu, C.; Xu, S.; Bai, Y. Rapid trace detection of sulfite residue in white wine using a multichannel colorimetric nanozyme sensor. Foods 2023, 12, 3581. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Umapathi, A.; Patel, G.; Patra, C.; Malik, U.; Bhargava, S.K.; Daima, H.K. Nanozyme-based pollutant sensing and environmental treatment: Trends, challenges, and perspectives. Sci. Total Environ. 2023, 854, 158771. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, Z.; Zhang, X.; Ma, Z.; Yan, F.; Zhu, C.; Chen, Y. Single-atom nanozyme-like lanthanum moieties for high-performance electromagnetic energy absorption. Adv. Funct. Mater. 2024, 34, 2403508. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Liu, J.; Zi, X.; Zhu, H.; Sun, X.; Miao, Y.; Fu, Y. Revolutionizing nanozyme technology with metal-organic frameworks: Classification, catalytic mechanisms, regulation and applications in biotechnology. Chem. Eng. J. 2024, 500, 156850. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, S.; Wang, G.; Yun, Y.; Liu, G.; Zhang, W. Nanozyme applications: A glimpse of insight in food safety. Front. Bioeng. Biotechnol. 2021, 9, 727886. [Google Scholar] [CrossRef]
- Bilal, M.; Khaliq, N.; Ashraf, M.; Hussain, N.; Baqar, Z.; Zdarta, J.; Jesionowski, T.; Iqbal, H.M.N. Enzyme mimic nanomaterials as nanozymes with catalytic attributes. Colloids Surf. B. 2023, 221, 112950. [Google Scholar] [CrossRef]
- Peng, F.F.; Zhang, Y.; Gu, N. Size-dependent peroxidase-like catalytic activity of Fe3O4 nanoparticles. Chin. Chem. Lett. 2008, 19, 730–733. [Google Scholar] [CrossRef]
- Ju, J.; Chen, Y.; Liu, Z.; Huang, C.; Li, Y.; Kong, D.; Shen, W.; Tang, S. Modification and application of Fe3O4 nanozymes in analytical chemistry: A review. Chin. Chem. Lett. 2023, 34, 107820. [Google Scholar] [CrossRef]
- Liu, S.; Liao, Y.; Shu, R.; Sun, J.; Zhang, D.; Zhang, W.; Wang, J. Evaluation of the multidimensional enhanced lateral flow immunoassay in point-of-care nanosensors. ACS Nano 2024, 18, 27167–27205. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Nanomaterials exhibiting enzyme-like properties (nanozymes): Current advances and future perspectives. Front. Chem. 2019, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Li, Q.; Yang, D.; Yang, Y. Fluorescence detection of valence speciation of Cr(III) based on the catechol oxidase mimic enzyme activity of CuSeNP nanozymes. Microchim. Acta 2024, 191, 496. [Google Scholar] [CrossRef] [PubMed]
- Chandio, I.; Ai, Y.; Wu, L.; Liang, Q. Recent progress in MOFs-based nanozymes for biosensing. Nano Res. 2023, 17, 39–64. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Nanozymes in electrochemical affinity biosensing. Microchim. Acta 2020, 187, 423. [Google Scholar] [CrossRef]
- Menbere Leul, M.; Ebrahim, M.A.; Andrea, C.; Wolfgang, F. Frontiers in laccase nanozymes-enabled colorimetric sensing: A review. Anal. Chim. Acta. 2024, 343333. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Q.; Miao, X.; Wen, S.; Yu, S.; Chu, Y.; Lu, X.; Jiang, L.-P.; Zhu, J. Spatially engineered janus hybrid nanozyme toward SERS liquid biopsy at nano/microscales. ACS Appl. Mater. Interfaces 2019, 11, 41979–41987. [Google Scholar] [CrossRef]
- Mu, M.; Wen, S.; Hu, S.; Zhao, B.; Song, W. Putting surface-enhanced Raman spectroscopy to work for nanozyme research: Methods, materials and applications. Trac Trend Anal. Chem. 2022, 152, 116603. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Kneipp, J. Surface-enhanced Raman scattering in local optical fields of silver and gold nanoaggregatesfrom single-molecule Raman spectroscopy to ultrasensitive probing in live cells. Acc. Chem. Res. 2006, 39, 443–450. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Lee, L.Y.S.; Wong, K.Y. An emerging direction for nanozyme design: From single-atom to dual-atomic-site catalysts. Nanoscale 2023, 15, 18173–18183. [Google Scholar] [CrossRef]
- Waris, N.; Hasnat, A.; Hasan, S.; Bano, S.; Sultana, S.; Ibhadon, A.O.; Khan, M.Z. Development of nanozyme based sensors as diagnostic tools in clinic applications: A review. J. Mater. Chem. B 2023, 11, 6762–6781. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Taheri-Ledari, R.; Saeidirad, M.; Qazi, F.S.; Kashtiaray, A.; Ganjali, F.; Tian, Y.; Maleki, A. Regulation of porosity in MOFs: A review on tunable scaffolds and related effects and advances in different applications. J. Environ. Chem. Eng. 2022, 10, 108836. [Google Scholar] [CrossRef]
- Mu, Z.; Wang, Y.; Guo, J.; Zhao, M. Active site-tuned high peroxidase-like activity nanozyme for on-the-spot detection of saliva total antioxidant capacity using smartphone devices. Talanta 2024, 276, 126207. [Google Scholar] [CrossRef] [PubMed]
- Kulandaivel, S.; Lin, C.-H.; Yeh, Y.-C. The bi-metallic MOF-919 (Fe–Cu) nanozyme capable of bifunctional enzyme-mimicking catalytic activity. Chem. Comm. 2021, 58, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Tong, P.H.; Wang, J.J.; Hu, X.L.; James, T.D.; He, X.P. Metal–organic framework (MOF) hybridized gold nanoparticles as a bifunctional nanozyme for glucose sensing. Chem. Sci. 2023, 14, 7762–7769. [Google Scholar] [CrossRef]
- Jiang, B.; Liang, M. Advances in single-atom nanozymes research. Chin. J. Chem. 2020, 39, 174–180. [Google Scholar] [CrossRef]
- Li, K.; Miao, Y.; Song, K.; He, S.; Zhang, G.; Waterhouse, G.I.N.; Guan, S.; Zhou, S. Collaborative CuMn diatomic nanozyme to boost nanocatalytic/mild photothermal/chemo-therapy through overcoming therapeutic resistance. Chem. Eng. J. 2023, 471, 144693. [Google Scholar] [CrossRef]
- Yan, X.; Liu, N.; Liu, W.; Zeng, J.; Liu, C.; Chen, S.; Yang, Y.; Gui, X.; Yang, G.; Yu, D.; et al. Recent advances on COF-based single-atom and dual-atom sites for oxygen catalysis. Chem. Comm. 2024, 60, 12787–12802. [Google Scholar] [CrossRef]
- Pudlarz, A.M.; Ranoszek-Soliwoda, K.; Czechowska, E.; Tomaszewska, E.; Celichowski, G.; Grobelny, J.; Szemraj, J. A Study of the activity of recombinant Mn-superoxide dismutase in the presence of gold and silver nanoparticles. Appl. Biochem. Biotechnol. 2018, 187, 1551–1568. [Google Scholar] [CrossRef]
- Nath, A.; Pal, R.; Singh, L.M.; Saikia, H.; Rahaman, H.; Ghosh, S.K.; Mazumder, R.; Sengupta, M. Gold-manganese oxide nanocomposite suppresses hypoxia and augments pro-inflammatory cytokines in tumor associated macrophages. Int. Immunopharmacol. 2018, 57, 157–164. [Google Scholar] [CrossRef]
- Mazrad, Z.A.I.; Lee, K.; Chae, A.; In, I.; Lee, H.; Park, S.Y. Progress in internal/external stimuli responsive fluorescent carbon nanoparticles for theranostic and sensing applications. J. Mater. Chem. B 2018, 6, 1149–1178. [Google Scholar] [CrossRef] [PubMed]
- Gusain, R.; Singhal, N.; Singh, R.; Kumar, U.; Khatri, O.P. Ionic-liquid-functionalized copper oxide nanorods for photocatalytic splitting of water. ChemPlusChem 2016, 81, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ovejero Paredes, K.; Díaz-García, D.; García-Almodóvar, V.; Lozano Chamizo, L.; Marciello, M.; Díaz-Sánchez, M.; Prashar, S.; Gómez-Ruiz, S.; Filice, M. Multifunctional silica-based nanoparticles with controlled release of organotin metallodrug for targeted theranosis of breast cancer. Cancers 2020, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.J.; Sun, T.Q.; Yang, L.P.; Sun, Y.G.; Bin, D.S.; Duan, S.Y.; Liu, Y.; Lv, R.W.; Cao, A.M. A facile synthetic strategy for the creation of hollow noble metal/transition metal oxide nanocomposites. Chem. Commun. 2018, 55, 1076–1079. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Surface modification of nanozymes. Nano Res. 2017, 10, 1125–1148. [Google Scholar] [CrossRef]
- Maddinedi, S.B.; Mandal, B.K.; Anna, K.K. Environment friendly approach for size controllable synthesis of biocompatible Silver nanoparticles using diastase. Environ. Toxicol. Pharmacol. 2016, 49, 131–136. [Google Scholar] [CrossRef]
- Moreira Da Silva, C.; Girard, A.; Le Bouar, Y.; Fossard, F.; Dragoe, D.; Ducastelle, F.; Loiseau, A.; Huc, V. Structural size effect in capped metallic nanoparticles. ACS Nano 2023, 17, 5663–5672. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, Z.; Zhao, Q.; Yue, Z.; Xiong, C.; Chen, G.; Wang, J.; Li, L. Lattice expansion in ruthenium nanozymes improves catalytic activity and electro-responsiveness for boosting cancer therapy. Nat. Commun. 2024, 15, 8097. [Google Scholar] [CrossRef]
- Pham, X.H.; Tran, V.K.; Hahm, E.; Kim, Y.H.; Kim, J.; Kim, W.; Jun, B.H. Synthesis of gold-platinum core-Shell nanoparticles assembled on a silica template and their peroxidase nanozyme properties. Int. J. Mol. Sci. 2022, 23, 6424. [Google Scholar] [CrossRef]
- Han, X.; Zheng, B.; Ouyang, J.; Wang, X.; Kuang, Q.; Jiang, Y.; Xie, Z.; Zheng, L. Control of anatase TiO2 nanocrystals with a series of high-energy crystal facets via a fuorine-free strategy. Chem. Asian J. 2012, 7, 2538–2542. [Google Scholar] [CrossRef]
- Hashad, R.A.; Singla, R.; Kaur Bhangu, S.; Jap, E.; Zhu, H.; Peleg, A.Y.; Blakeway, L.; Hagemeyer, C.E.; Cavalieri, F.; Ashokkumar, M.; et al. Chemoenzymatic surface decoration of Nisin-shelled nanoemulsions: Novel targeted drug-nanocarriers for cancer applications. Ultrason. Sonochem. 2022, 90, 106183. [Google Scholar] [CrossRef] [PubMed]
- Jimenez Jimenez, A.M.; Rodrigo, M.A.M.; Milosavljevic, V.; Krizkova, S.; Kopel, P.; Heger, Z.; Adam, V. Gold nanoparticles-modified nanomaghemite and quantum dots-based hybridization assay for detection of HPV. Sens. Actuators B Chem. 2016, 240, 503–510. [Google Scholar] [CrossRef]
- Wang, Y.; Xianyu, Y. Nanobody and nanozyme-enabled immunoassays with enhanced specificity and sensitivity. Small Methods 2022, 6, 2101576. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Bu, H.; Zia, D.S.; Dai, P.; Liu, J. Nanomaterials for molecular recognition: Specific adsorption and regulation of nanozyme activities. Mater. Chem. Front. 2023, 7, 3625–3640. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Qu, X. Biosystem-inspired engineering of nanozymes for biomedical applications. Adv. Mater. 2023, 36, 2211147. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, X.; Zhang, W.; Zhang, L.; Li, L.; Gao, Y.; Wang, C. Photothermal amplified multizyme activity for synergistic photothermal-catalytic tumor therapy. J. Colloid Interface Sci. 2024, 679, 375–383. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, A.; Wang, R.; Zhang, Q.; Cui, D. A review on metal-and metal oxide-based nanozymes: Properties, mechanisms, and applications. Nano-Micro Lett. 2021, 13, 154. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Tian, X.; Jiang, Y.; Ma, H.Y.; Wang, W.; Zhang, W.S.; Martin, J.S.; Yan, Y.; Qin, D.-D.; Han, D.X.; et al. Advances in bimetallic materials and bimetallic oxide nanozymes: Synthesis, classification, catalytic mechanism and application in analytical chemistry. Trends Anal. Chem. 2024, 176, 117757. [Google Scholar] [CrossRef]
- Su, L.; Qin, S.; Xie, Z.; Wang, L.; Khan, K.; Tareen, A.K.; Li, D.; Zhang, H. Multi-enzyme activity nanozymes for biosensing and disease treatment. Coord. Chem. Rev. 2022, 473, 214784. [Google Scholar] [CrossRef]
- Ruan, H.; Zhang, S.; Wang, H.; Pei, J.; Zhao, R.; Mu, X.; Wang, H.; Zhang, X. Single-Atom Pd/CeO2 nanostructures for mimicking multienzyme activities. ACS Appl. Nano Mater. 2022, 5, 6564–6574. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.J.; Li, Q.; Liu, S.C.; Ma, H.; Ying, Y.L.; Long, Y.T. Simultaneous observation of the spatial and temporal dynamics of single enzymatic catalysis using a solid-state nanopore. Nanoscale 2023, 15, 7261–7266. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, Y.; Li, H.; Lin, H.; Cui, H.; Pan, Y.; Zhang, R.; Song, Y.; Shum, H.C. Heterogeneous self-assembly of a single type of nanoparticle modulated by skin formation. ACS Nano 2023, 17, 11645–11654. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, R.; Ai, C.; Wang, B.; Chu, R.; Wang, H.; Shui, L.; Zhou, F. Stick-slip-motion-assisted interfacial self-assembly of noble metal nanoparticles on tapered optical fiber surface and its application in SERS detection. Appl. Surf. Sci. 2022, 602, 154298. [Google Scholar] [CrossRef]
- Hu, P.; Yang, H.; Si, R.; Wei, B.; Wang, X.; Xu, Z.; Yang, X.; Guo, T.; Gebauer, R.; Teobaldi, G.; et al. Atomically thin Ag nanosheets for single-molecule SERS detection of BPF. Chem 2024, 10, 3364–3373. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Z.; Liao, H.; Li, R.; Feng, Z.; Li, Z.; Lin, L.; Li, J.; Chen, G. EM and CT synergistic SERS enhancement in Si/TiO2/Ag heterostructures via local interfacial effect. Chem. Eng. J. 2024, 493, 152316. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, X.Y.; Fan, W.; Guo, S.; Zhang, Y.; Liu, Y.; Chen, L.; Jung, Y.M. SERS study of Ag/FeS/4-MBA interface based on the SPR effect. Spectrochim. Acta A 2019, 219, 147–153. [Google Scholar] [CrossRef]
- Wen, P.; Yang, F.; Tang, L.; Hu, X.; Zhao, H.; Tang, B.; Chen, L. Precise regulation and control of hotspots in nanoparticle multilayer SERS substrates. Microchem. J. 2022, 187, 108371. [Google Scholar] [CrossRef]
- Li, P.; Wang, B.; Qi, M.; Jiang, H.; Li, Y.; Zhang, X. Construction of aptamer sensor based on Au nanozymes for ultrasensitive SERS detection of tobramycin. J. Food Compost. Anal. 2023, 123, 105617. [Google Scholar] [CrossRef]
- Rastogi, L.; Karunasagar, D.; Sashidhar, R.B.; Giri, A. Peroxidase-like activity of gum kondagogu reduced/stabilized palladium nanoparticles and its analytical application for colorimetric detection of glucose in biological samples. Sensor Actuators B Chem. 2016, 240, 1182–1188. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, L.; Xiao, D.; Li, N.; Li, Y.; Cao, D.; Shi, Z.; Cui, Z.; Lu, N. A silver nanoislands on silica spheres platform: Enriching trace amounts of analytes for ultrasensitive and reproducible SERS detection. Nanoscale 2017, 9, 16749–16754. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, P.; Chen, Y.; Wu, Y.; Wei, J. Interfacial deposition of Ag nanozyme on metal-polyphenol nanosphere for SERS detection of cellular glutathione. Biosens. Bioelectron. 2023, 228, 115200. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Niazi, J.H.; Qureshi, A. Mn3O4-Au nanozymes as peroxidase mimic and the surface-enhanced Raman scattering nanosensor for the detection of hydrogen peroxide. Mater. Today Chem. 2021, 22, 100560. [Google Scholar] [CrossRef]
- Xia, X.; Weng, Y.; Zhang, L.; Tang, R.; Zhang, X. A facile SERS strategy to detect glucose utilizing tandem enzyme activities of Au@Ag nanoparticles. Spectrochim. Acta A 2021, 259, 119889. [Google Scholar] [CrossRef]
- Liu, D.; Gao, H.; Jiang, W.; Yan, S.; Liu, H.; Chen, J.; Wen, S.; Zhang, W.; Wang, X.; Zhao, B.; et al. Ag aerogel-supported single-atom Hg nanozyme enables efficient SERS monitoring of enhanced oxidase-like catalysis. Anal. Chem. 2023, 95, 4335–4343. [Google Scholar] [CrossRef]
- Wen, S.; Ma, X.; Liu, H.; Chen, G.; Wang, H.; Deng, G.; Zhang, Y.; Song, W.; Zhao, B.; Ozaki, Y. Accurate monitoring platform for the surface catalysis of nanozyme validated by surface-enhanced Raman-kinetics model. Anal. Chem. 2020, 92, 11763–11770. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, C.; Yao, Q.; Jin, J.; Ye, T.; Chen, X.; Guo, Z.; Chen, X. Multifunction nanoenzyme-assisted ion-selective and oxidation catalysis SERS biosensors for point-of-care nitrite testing. Sensor Actuators B Chem. 2024, 405, 135352. [Google Scholar] [CrossRef]
- Yao, D.; Li, C.; Wang, H.; Wen, G.; Liang, A.; Jiang, Z. A new dual-mode SERS and RRS aptasensor for detecting trace organic molecules based on gold nanocluster-doped covalent-organic framework catalyst. Sensor Actuators B Chem. 2020, 319, 128308. [Google Scholar] [CrossRef]
- Arshad, F.; Nurul Azian Zakaria, S.; Ahmed, M.U. Gold-silver-platinum trimetallic nanozyme-based dual-mode immunosensor for ultrasensitive osteoprotegerin detection. Microchem. J. 2024, 201, 110523. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Majumder, S.; Senapati, D.; Banerjee, S.; Satpati, B. Core-shell gold @silver hollow nanocubes for higher SERS enhancement and non-enzymatic biosensor. Mater. Chem. Phys. 2020, 239, 122113. [Google Scholar] [CrossRef]
- Damborska, D.; Bertok, T.; Dosekova, E.; Holazova, A.; Lorencova, L.; Kasak, P.; Tkac, J. Nanomaterial-based biosensors for detection of prostate specific antigen. Microchim. Acta 2017, 184, 3049–3067. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.Y.; Kim, D.J.; Park, S.G.; Kim, S.H.; Kim, D.H. Nanostructured plasmonic substrates for use as SERS sensors. Nano Converg. 2016, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Liu, L.; Zhang, X.; Liu, X.; Xu, Y.; Li, S.; Zeng, M. A microfluidic-based SERS biosensor with multifunctional nanosurface immobilized nanoparticles for sensitive detection of MicroRNA. Anal. Chim. Acta 2022, 1221, 340139. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gu, Y.; Liu, X.; Deng, T.; Dai, S.; Qu, J.; Yang, G.; Qu, L. Reusable ring-like Fe3O4/Au nanozymes with enhanced peroxidase-like activities for colorimetric-SERS dual-mode sensing of biomolecules in human blood. Biosens. Bioelectron. 2022, 209, 114253. [Google Scholar] [CrossRef]
- Zhang, J.; Miao, X.; Song, C.; Chen, N.; Xiong, J.; Gan, H.; Ni, J.; Zhu, Y.; Cheng, K.; Wang, L. Non-enzymatic signal amplification-powered point-of-care SERS sensor for rapid and ultra-sensitive assay of SARS-CoV-2 RNA. Biosens. Bioelectron. 2022, 212, 114379. [Google Scholar] [CrossRef]
- Lin, Y.; Bariya, M.; Nyein, H.Y.Y.; Kivimäki, L.; Uusitalo, S.; Jansson, E.; Ji, W.; Yuan, Z.; Happonen, T.; Liedert, C.; et al. Porous enzymatic membrane for nanotextured glucose sweat sensors with high stability toward reliable noninvasive health monitoring. Adv. Funct. Mater. 2019, 29, 1902521. [Google Scholar] [CrossRef]
- Cottat, M.; D’Andrea, C.; Yasukuni, R.; Malashikhina, N.; Grinyte, R.; Lidgi-Guigui, N.; Fazio, B.; Sutton, A.; Oudar, O.; Charnaux, N.; et al. High sensitivity, high selectivity SERS detection of MnSOD using optical nanoantennas functionalized with aptamers. J. Phys. Chem. C 2015, 119, 15532–15540. [Google Scholar] [CrossRef]
- Wu, L.; Jiao, L.; Xue, D.; Li, Y.; Han, Y.; Wei, O.; Chen, Q. Nanozyme and bifunctional nanobody-based colorimetric-SERS dual-mode Immunosensor for microcystin-LR detection. Food Chem. 2024, 464, 141574. [Google Scholar] [CrossRef]
- Yu, H.; Wang, M.; Cao, J.; She, Y.; Zhu, Y.; Ye, J.; Wang, J.; Lao, S.; Abd El-Aty, A.M. Determination of dichlorvos in pears by surface-enhanced Raman scattering (SERS) with catalysis by platinum coated gold nanoparticles. Anal. Lett. 2021, 55, 427–437. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Zhou, X.; Yu, Y.; Liu, J.; Hu, N.; Wang, H.; Li, G.; Wu, Y. Recent progress on the construction of nanozymes-based biosensors and their applications to food safety assay. Trac Trend Anal. Chem. 2019, 121, 115668. [Google Scholar] [CrossRef]
- Li, Y.; Mu, Z.; Yuan, Y.; Zhou, J.; Bai, L.; Qing, M. An enzymatic activity regulation-based clusterzyme sensor array for high-throughput identification of heavy metal ions. J. Hazard. Mater. 2023, 454, 131501. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Niu, R.; Fan, S.; Jing, X.; Gao, R.; Yang, H.; Wang, H.; Wang, D.; Yang, Z.; Xie, Y.; et al. A dual-mode NADH biosensor based on gold nanostars decorated CoFe2 metal–organic frameworks to reveal dynamics of cell metabolism. ACS Sens. 2022, 7, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wen, R.; Ji, C.; Wei, J.; Han, Y.; Wu, L. Electrochemical potential enhanced EC-SERS sensor for sensitive and label-free detection of acetamiprid. Microchem. J. 2024, 206, 111524. [Google Scholar] [CrossRef]
- Liu, C.; Wu, T.; Zeng, W.; Liu, J.; Hu, B.; Wu, L. Dual-signal electrochemical aptasensor involving hybridization chain reaction amplification for aflatoxin B1 detection. Sens. Actuators B Chem. 2022, 371, 132494. [Google Scholar] [CrossRef]
- Long, W.; Shuhong, Z.; Yonghuan, Y.; Lin, Z.; Bei, L.; Weimin, Z. A multifunctional probe for lead(II) sensing using CdSe/ZnS-luminol-conjugated Fe3O4 magnetic nanocomposites. Sens. Actuators B Chem. 2021, 356, 131124. [Google Scholar]
- Wu, L.; Tang, X.; Wu, T.; Zeng, W.; Zhu, X.; Hu, B.; Zhang, S. A review on current progress of Raman-based techniques in food safety: From normal Raman spectroscopy to SESORS. Food Res. Int. 2023, 169, 112944. [Google Scholar] [CrossRef]
- Liu, J.; Hong, Z.; Yang, W.; Liu, C.; Lu, Z.; Wu, L.; Foda, M.F.; Yang, Z.; Han, H.; Zhao, Y. Bacteria inspired internal standard SERS substrate for quantitative detection. ACS Appl. Bio Mater. 2021, 4, 2009–2019. [Google Scholar] [CrossRef]
- Logan, N.; Cao, C.; Freitag, S.; Haughey, S.A.; Krska, R.; Elliott, C.T. Advancing mycotoxin detection in food and feed: Novel insights from surface-enhanced Raman spectroscopy (SERS). Adv. Mater. 2024, 36, 2309625. [Google Scholar] [CrossRef]
- Zhang, W.; Peng, Y.; Lin, C.; Xu, M.; Zhao, S.; Li, D.; Yang, Y.; Yang, Y. A novel ultra-sensitive semiconductor SERS substrate V5S4 nanopompons for the specific detection of antibiotics with AI technology. Chem. Eng. J. 2024, 502, 157907. [Google Scholar] [CrossRef]
- Wu, T.; Tang, X.; Zeng, W.; Han, Y.; Zhang, S.; Wei, J.; Wu, L. Potential powered EC-SERS for sensitive detection of acetamiprid. J. Food Eng. 2024, 378, 112109. [Google Scholar] [CrossRef]
- Xia, J.; Li, W.; Sun, M.; Wang, H. Application of SERS in the detection of Fungi, Bacteria and Viruses. Nanomaterials 2022, 12, 3572. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, P.; Yosri, N.; Chen, Q.; Elseedi, H.R.; Zou, X.; Yang, H. Detection of heavy metals in food and agricultural products by surface-enhanced Raman spectroscopy. Food Rev. Int. 2023, 39, 1440–1461. [Google Scholar] [CrossRef]
- Tan, X.; Kang, K.; Zhang, R.; Dong, J.; Wang, W.; Kang, W. An ultrasensitive dual-mode approach for AFB1 detection: Colorimetric/label-free SERS aptasensor based on a self-assembled core-shell structured Ag@Au IP6 bifunctional nanozyme. Sens. Actuators B Chem. 2024, 412, 135854. [Google Scholar] [CrossRef]
- Chen, P.; Li, S.; Jiang, C.; Wang, Z.; Ma, X. A surface-enhanced Raman scattering aptasensor for output-signal detection of aflatoxin B1 based on peroxidase-like Cu2O@Au hybrid nanozyme. Food Biosci. 2023, 54, 102885. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Q.; Li, H.; Wang, Y.; Xiao, F.; Yang, D.; Xia, Q.; Yang, Y. SERS detection of Hg2+ and aflatoxin B1 through on-off strategy of oxidase-like Au@HgNPs/carbon dots. Food Chem. 2023, 424, 136443. [Google Scholar] [CrossRef]
- Li, Q.; Han, Q.; Yang, D.; Li, K.; Wang, Y.; Chen, D.; Yang, Y.; Li, H. Methylmercury-sensitized “turn on” SERS-active peroxidase-like activity of carbon dots/Au NPs nanozyme for selective detection of ochratoxin A in coffee. Food Chem. 2024, 434, 137440. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Yu, X.; Jia, X.; Zhu, C.; Liu, J.; Zhang, F.; Chen, Z.; Yan, M.; Yang, Q. A sensitive and simple competitive nanozyme-linked apta-sorbent assay for the dual-mode detection of ochratoxin A. Analyst 2022, 147, 2215–2222. [Google Scholar] [CrossRef]
- Ma, J.; Feng, G.; Ying, Y.; Shao, Y.; She, Y.; Zheng, L.; Abd Ei-Aty, A.M.; Wang, J. Sensitive SERS assay for glyphosate based on the prevention of l-cysteine inhibition of a Au-Pt nanozyme. Analyst 2021, 146, 956–963. [Google Scholar] [CrossRef]
- Li, C.; Yu, F.; Yang, J.; Bai, H.; Ma, X.; Jiang, Z. SERS- and absorbance-based catalytic assay for determination of isocarbophos using aptamer-modified FeMOF nanozyme and in situ generated silver nanoparticles. Microchim. Acta 2023, 190, 4. [Google Scholar] [CrossRef]
- Zhang, R.; Xie, S.; Yang, J.; Zhang, L.; Xiong, R.; Sun, H.; Zhang, H.; Jiang, M.; He, Y. A dual-mode colorimetric and surface-enhanced Raman strategy for chloramphenicol detection based on the Au@Pt nanozyme and EXPAR. Microchem. J. 2024, 207, 111990. [Google Scholar] [CrossRef]
- Dai, J.; Li, J.; Jiao, Y.; Yang, X.; Yang, D.; Zhong, Z.; Li, H.; Yang, Y. Colorimetric-SERS dual-mode aptasensor for Staphylococcus aureus based on MnO2@AuNPs oxidase-like activity. Food Chem. 2024, 456, 139955. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, J.; Zhan, Y.; Shao, Z.; Gao, M.; Yao, Q.; Li, Z.; Sun, S.; Wang, L. Coupling bifunctional nanozyme-mediated catalytic signal amplification and label-free SERS with immunoassays for ultrasensitive detection of pathogens in milk samples. Anal. Chem. 2023, 95, 6417–6424. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chang, W.; Zhu, X.; Liu, G.; Liu, K.; Chen, W.; Wang, H.; Qin, P. Development of a colorimetric and SERS dual-signal platform via dCas9-mediated chain assembly of bifunctional Au@Pt nanozymes for ultrasensitive and robust salmonella assay. Anal. Chem. 2024, 96, 12684–12691. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Li, C.; He, R.; Zhang, Y.; Wang, B.; Zhang, Z.H.; Ho, C.T. Research advances on biogenic amines in traditional fermented foods: Emphasis on formation mechanism, detection and control methods. Food Chem. 2023, 405, 134911. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, N.; Wang, S.; Li, Z.; Sun, W.; Zhou, M.; Zhang, Y.; Ma, J.; Wu, L. Turn on fluorescent detection of biogenic amines in fish based on MnO2-coated and rhodamine 6G-loaded mesoporous silica nanospheres. Microchem. J. 2023, 195, 108664. [Google Scholar] [CrossRef]
- Patel, P.; Komorowski, A.S.; Mack, D.P. An allergist’s approach to food poisoning. Ann. Allergy Asthma Immunol. 2023, 130, 444–451. [Google Scholar] [CrossRef]
- Ferrante, M.C.; Mercogliano, R. Focus on histamine production during cheese manufacture and processing: A review. Food Chem. 2023, 419, 136046. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Wang, C.; Chen, S.; Lian, R.; Wang, W.; Fu, L.; Wang, Y. Immunological disturbance effect of exogenous histamine towards key immune cells. Food Sci. Hum. Wellness 2024, 13, 1856–1863. [Google Scholar] [CrossRef]
- Ma, X.; Xu, S.; Pan, Y.; Jiang, C.; Wang, Z. Construction of SERS output-signal aptasensor using MOF/noble metal nanoparticles based nanozyme for sensitive histamine detection. Food Chem. 2024, 440, 138227. [Google Scholar] [CrossRef]
- Wang, B.; Jiang, H.; Tang, R.; Tan, Y.; Xia, X.; Zhang, X. Construction of histamine aptamer sensor based on Au NPs nanozyme for ultrasensitive SERS detection of histamine. J. Food Compost. Anal. 2023, 120, 105337. [Google Scholar] [CrossRef]
- Su, Y.; Wu, D.; Chen, J.; Chen, G.; Hu, N.; Wang, H.; Wang, P.; Han, H.; Li, G.; Wut, Y. Ratiometric surface enhanced Raman scattering immunosorbent assay of allergenic proteins via covalent organic framework composite material based nanozyme tag triggered Raman signal “Turn-on” and amplification. Anal. Chem. 2019, 91, 11687–11695. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Wang, B.; Jiang, H.; Li, Y.; Li, P.; Zhang, X.; Han, L. Smartphone readable colorimetry and surface-enhanced Raman scattering (SERS) dual-mode sensing platform for ascorbic acid detection based on GeO2 composite nanozymes. J. Food Compos. Anal. 2024, 125, 105740. [Google Scholar] [CrossRef]
- Diao, Q.; Chen, X.; Tang, Z.; Li, S.; Tian, Q.; Bu, Z.; Liu, H.; Liu, J.; Niu, X. Nanozymes: Powerful catalytic materials for environmental pollutant detection and degradation. Environ. Sci. Nano 2024, 11, 766–796. [Google Scholar] [CrossRef]
- Wang, K.; Meng, X.; Yan, X.; Fan, K. Nanozyme-based point-of-care testing: Revolutionizing environmental pollutant detection with high efficiency and low cost. Nano Today 2024, 54, 102145. [Google Scholar] [CrossRef]
- Sun, Z.; Ji, X.; Lu, S.; Du, J. Shining a light on environmental science: Recent advances in SERS technology for rapid detection of persistent toxic substances. J. Environ. Sci. 2024. [Google Scholar] [CrossRef]
- Liu, H.; Guo, Y.; Wang, Y.; Zhang, H.; Ma, X.; Wen, S.; Jin, J.; Song, W.; Zhao, B.; Ozaki, Y. A nanozyme-based enhanced system for total removal of organic mercury and SERS sensing. J. Hazard. Mater. 2021, 405, 124642. [Google Scholar] [CrossRef]
- Li, Q.; Li, K.; Liu, J.; Chen, D.; Yang, D.; Yang, Y.; Li, H. Simulated enzyme catalytic strategy for surface-enhanced Raman scattering detection of methylmercury by using carbon dots/gold-based nanozyme. J. Environ. Chem. Eng. 2023, 11, 111156. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, J.; Cao, Y.; Zhang, Q.; Xie, C.; Xiong, W.; Luo, X.; He, Y. Efficient 2D MOFs nanozyme combining with magnetic SERS substrate for ultrasensitive detection of Hg2+. Spectrochim. Acta. A 2024, 312, 124062. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, C.; Chu, N.; Yang, J.; Lin, Y.; Chen, X. Colorimetric/fluorescent/SERS triple-readout sensing of Mercury(II) via in-situ growth of AgNPs in phenanthroline-functionalized covalent organic framework. Sens. Actuators B Chem. 2022, 369, 132361. [Google Scholar] [CrossRef]
- Xu, G.; Guo, N.; Zhang, Q.; Wang, T.; Song, P.; Xia, L. A sensitive surface-enhanced resonance Raman scattering sensor with bifunctional negatively charged gold nanoparticles for the determination of Cr(VI). Sci. Total Environ. 2022, 830, 154598. [Google Scholar] [CrossRef]
- Tang, R.; Xia, X.; Zhang, X.; Jiang, H.; Wang, B.; Zhang, P.; Zhang, Y.; Tang, Y.; Zhou, Y. Synergistic function of Au NPs/GeO2 nanozymes with enhanced peroxidase-like activity and SERS effect to detect choline iodide. Spectrochim. Acta A 2022, 266, 120467. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yang, T.; Wang, D.; Zhang, N.; Yang, H.; Jing, X.; Niu, R.; Yang, Z.; Xie, Y.; Meng, L. Gold nanorods/metal-organic framework hybrids: Photo enhanced peroxidase-like activity and SERS performance for organic dyestuff degradation and detection. Anal. Chem. 2022, 94, 4484–4494. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wang, Z.; Zong, S.; Yang, K.; Zhu, K.; Cui, Y. Peroxidase-like recyclable SERS probe for the detection and elimination of cationic dyes in pond water. J. Hazard. Mater. 2021, 408, 124426. [Google Scholar] [CrossRef] [PubMed]
- Hassanain, W.A.; Izake, E.L.; Sivanesan, A.; Ayoko, G.A. Towards interference free HPLC-SERS for the trace analysis of drug metabolites in biological fluids. J. Pharm. Biomed. Anal. 2017, 136, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Cialla-May, D.; Bonifacio, A.; Bocklitz, T.; Markin, A.; Markina, N.; Fornasaro, S.; Dwivedi, A.; Dib, T.; Farnesi, E.; Liu, C.; et al. Biomedical SERS—The current state and future trends. Chem. Soc. Rev. 2024, 53, 8957–8979. [Google Scholar] [CrossRef]
- Vazquez-Iglesias, L.; Casagrande, G.M.S.; Garcia-Lojo, D.; Leal, L.F.; Ngo, T.A.; Perez-Juste, J.; Reis, R.M.; Kant, K.; Pastoriza-Santos, I. SERS sensing for cancer biomarker: Approaches and directions. Bioact. Mater. 2024, 34, 248–268. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Jiang, H.; Qi, M.; Zhang, X.; Zhu, B.; Han, L. CeO2@nanogel/Au nanozymes to enhance peroxidase activity for a novel ultrasensitive SERS assay of H2O2 determination. Microchem. J. 2023, 195, 109467. [Google Scholar] [CrossRef]
- Jin, J.; Song, W.; Wang, J.; Li, L.; Tian, Y.; Zhu, S.; Zhang, Y.; Xu, S.; Yang, B.; Zhao, B. A highly sensitive SERS platform based on small-sized Ag/GQDs nanozyme for intracellular analysis. Chem. Eng. J. 2022, 430, 132687. [Google Scholar] [CrossRef]
- Qu, L.; Han, J.; Huang, Y.; Yang, G.; Liu, W.; Long, Z.; Gu, Y.; Zhang, Q.; Gao, M.; Dong, X. Peroxidase-like nanozymes for point-of-care SERS sensing and wound healing. ACS Appl. Nano Mater. 2023, 6, 1272–1282. [Google Scholar] [CrossRef]
- Cao, X.; Jiang, H.; Huang, X.; Sun, D.; Qi, G. Hydrogel patch doped with nanoenzyme for SERS detection of hydrogen peroxide in complex body fluids. Talanta 2025, 285, 127328. [Google Scholar] [CrossRef]
- Li, Y.; Li, P.; Qi, M.; Zhang, X.; Meng, J. SERS-based non-enzymatic detection of uric acid using Au/CeO2 nanorods. ACS Appl. Nano Mater. 2024, 7, 20920–20932. [Google Scholar] [CrossRef]
- Tan, Y.; Qi, M.; Jiang, H.; Wang, B.; Zhang, X. Determination of uric acid in serum by SERS system based on VO-MnCo2O4/Ag nanozyme. Anal. Chim. Acta 2023, 1274, 341584. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Guan, C.; Shan, G.; Chen, Y.; Wang, C.; Liu, Y. A nanocomposite prepared from silver nanoparticles and carbon dots with peroxidase mimicking activity for colorimetric and SERS-based determination of uric acid. Microchim. Acta 2019, 186, 644. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Li, J.; Hu, Y.; Ge, K.; Li, G.; Liu, S. Bifunctional Mo2N nanoparticles with nanozyme and SERS activity: A versatile platform for sensitive detection of biomarkers in serum samples. Anal. Chem. 2024, 96, 2998–3007. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, J.-Y.; He, W.-M. Surface-enhanced Raman spectroscopy biosensor based on silver nanoparticles@metal-organic frameworks with peroxidase-mimicking activities for ultrasensitive monitoring of blood cholesterol. Sens. Actuators B Chem. 2022, 365, 131939. [Google Scholar] [CrossRef]
- Xu, J.; Jian, X.; Guo, J.; Zhao, J.; Tang, J.; Zhao, Y.; Xu, J.; Gao, Z.; Song, Y.-Y. Selective SERS identification and quantification of glucose enantiomers on homochiral MOFs based enzyme-free nanoreactors. Chem. Eng. J. 2023, 459, 141650. [Google Scholar] [CrossRef]
- Fu, C.; Li, Y.; Lei, X.; Su, J.; Chen, Y.; Wu, Y.; Shi, W.; Tan, X.; Li, Y.; Jung, Y.M. SERS sensor for acetylcholine detection based on covalent organic framework hybridized gold nanoparticles as nanozymes. Anal. Chem. 2024, 96, 18585–18589. [Google Scholar] [CrossRef]
- Huang, X.; Yang, Y.; Zhou, H.; Hu, L.; Yang, A.; Jin, H.; Zheng, B.; Pi, J.; Xu, J.; Sun, P.; et al. Coupling of an Au@AgPt nanozyme array with an micrococcal nuclease-specific responsiveness strategy for colorimetric/SERS sensing of Staphylococcus aureus in patients with sepsis. J. Pharm. Anal. 2024, 101085. [Google Scholar] [CrossRef]
- Cai, J.; Lin, Y.; Yu, X.; Yang, Y.; Hu, Y.; Gao, L.; Xiao, H.; Du, J.; Wang, H.; Zhong, X.; et al. Multifunctional AuAg-doping Prussian Blue-based MOF: Enhanced colorimetric catalytic activities and amplified SERS signals for bacteria discrimination and detection. Sens. Actuators B Chem. 2023, 394, 134279. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Ji, Y.; Wang, L.; Liu, X.; Wang, F.; Li, C. Nanozyme-inhibited SERS multichannel paper-based sensor array for the quantification and identification of biothiols and cancer cells based on three Ag-based nanomaterials. Anal. Chem. 2024, 96, 11353–11365. [Google Scholar] [CrossRef]
- Zhao, L.; Du, X.; Xu, G.; Song, P. Nanozyme catalyzed-SERRS sensor for the recognition of dopamine based on AgNPs@PVP with oxidase-like activity. Spectrochim. Acta A 2024, 307, 123606. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Li, Y.; Li, G.; Hu, Y. Bifunctional MoO3–x/CuS heterojunction nanozyme-driven “Turn-On” SERS signal for the sensitive detection of cerebral infarction biomarker S100B. Anal. Chem. 2024, 96, 17711–17719. [Google Scholar] [CrossRef] [PubMed]


| Material | Linear Range | LOD | Detection Time (T)/ Reproducibility (RSD or CV)/Stability (S) | Target | Real Samples | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Food contaminants detection | |||||||
| Ag@AuIP6 | 2–200 pg/L | 0.58 pg/L | RSD: 8.09% | AFB1 | Corn | 79.49–105.09% | [93] |
| Cu2O@Au | 0.001–100 ng/mL | 0.7 pg/mL | RSD: 5.2% S: 10 day | AFB1 | Peanut | 93.6–102.3% | [94] |
| Ce-CDs/ AuNPs | 0.125–87.5 μg/L | 0.08 μg/L | / | AFB1 | Peanut oil | 93.97–109.30% | [95] |
| S-CDs/AuNPs | 0.25–18.75 μg/L | 0.29 μg/L | RSD: 4.88% | OTA | Coffee | 96.7–108.9% | [96] |
| MAP@Ab | 1.0–500 ng/mL | 0.032 ng/mL | RSD: 2% S: 10 day | MC-LR | Dongpo Lake water | 86.37–96.27% | [78] |
| FeMOF@OCTB | 0.02–1.2 nmol/L | 0.010 nmol/L | RSD: 4.5% | IPS | Rice | 97.7–104% | [99] |
| Au@Pt | 2.5 × 10−7–1.0 × 10−8 mol/L | 9.23 × 10−9 mol/L | RSD: 4.86% S: 10 day | CAP | Milk | 100.4–104.5% | [100] |
| Au@Apts | 10−10–10−1 mol/L | 2.04 × 10−11 mol/L | / | Tobramycin | Milk and eggs | 94.4–102% | [59] |
| MnO2@AuNPs | 101–107 CFU/mL | 1.561 CFU/mL | / | S. aureus | Milk, apple juice, milk tea, water, and human serum | 85–105% | [101] |
| Au@Pt | 10–104 CFU/mL | / | / | S. typhi | Milk | / | [102] |
| Au@Pt | 1–106 CFU/mL | 1 CFU/mL | RSD:0.55% | Salmonella | Lake water, egg, and cabbage | / | [103] |
| MIL-100(Fe)@AuNPs | 10−11–5 × 10−3 mol/L | 3.9 × 10−12 mol/L | RSD: 3.7% | HA | Fermented soybean products | 94.42–105.75% | [109] |
| Au NPs | 10−11–10−3 mol/L | 1.22 × 10−12 mol/L | RSD: 2.1% | Histamine | Fish samples and red wine | 93.7–108.4% | [110] |
| AuNPs doped COF | 25.65–6.2 × 104 ng/mL | 0.01 ng/mL | RSD: 6.35% S: 35 day | Allergenic proteins | Milk, yogurt, cookie, candy, PHF, and EHF | 98.81–101.49% | [111] |
| GeO2 @Fe3O4/Au NPs | 10−9–1 mol/L | 6.162 × 10−13 mol/L | RSD: 3.1% T: 42 day | AA | Oranges, vitamin C drinks and vitamin C tablets | 74.69–123.51% | [112] |
| Environmental pollutant detection | |||||||
| NP-CDs/Au NPs | 0.5–105.5 μg/L | 0.12 μg/L | RSD: 3.17% | MeHg | Water samples | 106.48–120.69%. | [117] |
| Fe3O4@Ag@OPD@S1 | 1.0 × 10−12–1.0 × 10−2 mol/L | 1.36 × 10−13 mol/L | RSD: 4.72 % | Hg2+ | River | 96.8–106.5 % | [118] |
| PA- COF@AgNPs | 0.05–100 μmol/L | 2 × 10−5 μmol/L | / | Hg2+ | Tap water samples | 86.00–105% | [119] |
| AuNPs | 10−5–10−9 mol/L | 0.4 nmol/L | RSD: 5.12% | Cr (VI) | River water and industrial wastewater. | 90.64–111.83% | [120] |
| Au NPs/GeO | 10−2–10−7 mol/L | 3.11 × 10−10 mol/L | RSD: 5% | ChI | Tap water | 91.11–107.37% | [121] |
| Au NRs/Fe-MOF | 10−9–10−5 mol/L | 9.3 × 10−12 mol/L | RSD: 2.2% | MB | Tap wate | 97.0–110.0% | [122] |
| Ni@Mil-100 (Fe) @Ag | 10−6–10−10 mol/L | 10−10 mol/L | RSD:9.27% | CV | / | / | [123] |
| Biomedical marker identification | |||||||
| Ag/Mn3O4, Ag3PO4 and Ag3Cit | 1–100 μmol/L | / | RSD: 1.97% | GSH | Tumor Cells | / | [140] |
| AuNPs@COF | 0.001–10.0 nmol/L | 0.3 pmol/L | RSD: 4.86% S: 30day | Ach | Serum | 97.2–104.5% | [137] |
| Au/CeO2 | 10−8–10−2mol/L | 3.29 × 10−10 mol/L | RSD: 0.018% S: 42 day | UA | Serum and urine | 98.6–102.5% | [131] |
| Mo2N | 0–100 µmol/L 0.1–1000 ng/mL 0.1–1000 ng/mL | 0.1 μmol/L, 89.1, 74.6 pg/mL | RSD: 7% | GSH, AFP, and CEA | Serum | 96.0–101% | [134] |
| MoO3−x/CuS | 1 × 10−6–1 ug/mL | 0.47 pg/mL | RSD:5.6% S:90 day | S100B | Serum | 93.5–108% | [142] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Tang, X.; Ji, C.; Wu, L.; Zhu, Y. Advances and Future Trends in Nanozyme-Based SERS Sensors for Food Safety, Environmental and Biomedical Applications. Int. J. Mol. Sci. 2025, 26, 709. https://doi.org/10.3390/ijms26020709
Wang X, Tang X, Ji C, Wu L, Zhu Y. Advances and Future Trends in Nanozyme-Based SERS Sensors for Food Safety, Environmental and Biomedical Applications. International Journal of Molecular Sciences. 2025; 26(2):709. https://doi.org/10.3390/ijms26020709
Chicago/Turabian StyleWang, Xingyu, Xuemei Tang, Chengzhen Ji, Long Wu, and Yongheng Zhu. 2025. "Advances and Future Trends in Nanozyme-Based SERS Sensors for Food Safety, Environmental and Biomedical Applications" International Journal of Molecular Sciences 26, no. 2: 709. https://doi.org/10.3390/ijms26020709
APA StyleWang, X., Tang, X., Ji, C., Wu, L., & Zhu, Y. (2025). Advances and Future Trends in Nanozyme-Based SERS Sensors for Food Safety, Environmental and Biomedical Applications. International Journal of Molecular Sciences, 26(2), 709. https://doi.org/10.3390/ijms26020709








