Early Immune Cell and Antibody Kinetics Following SARS-CoV-2 Vaccination in Healthy Adults and Low-Count Monoclonal B-Cell Lymphocytosis
Abstract
1. Introduction
2. Results
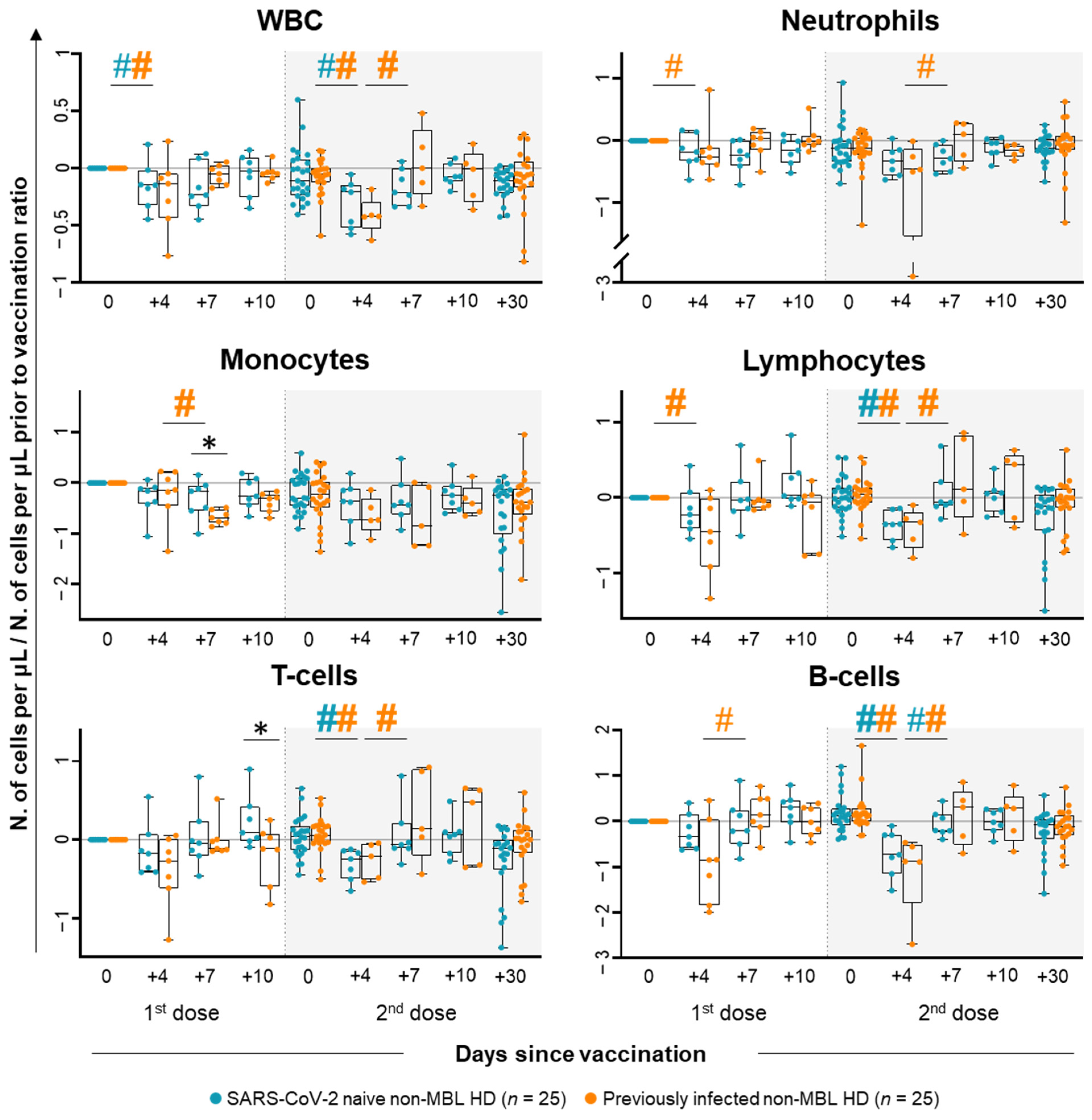
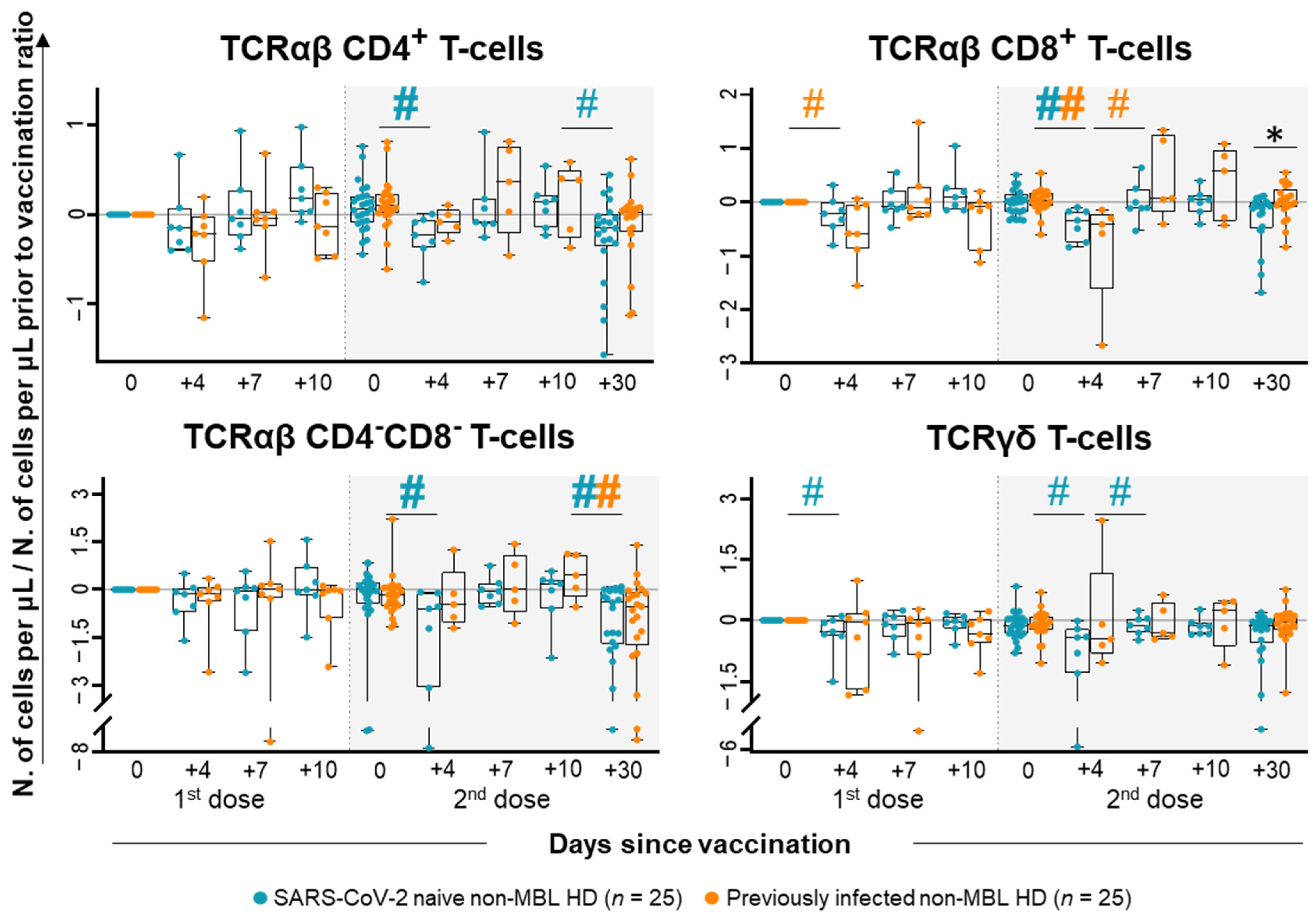
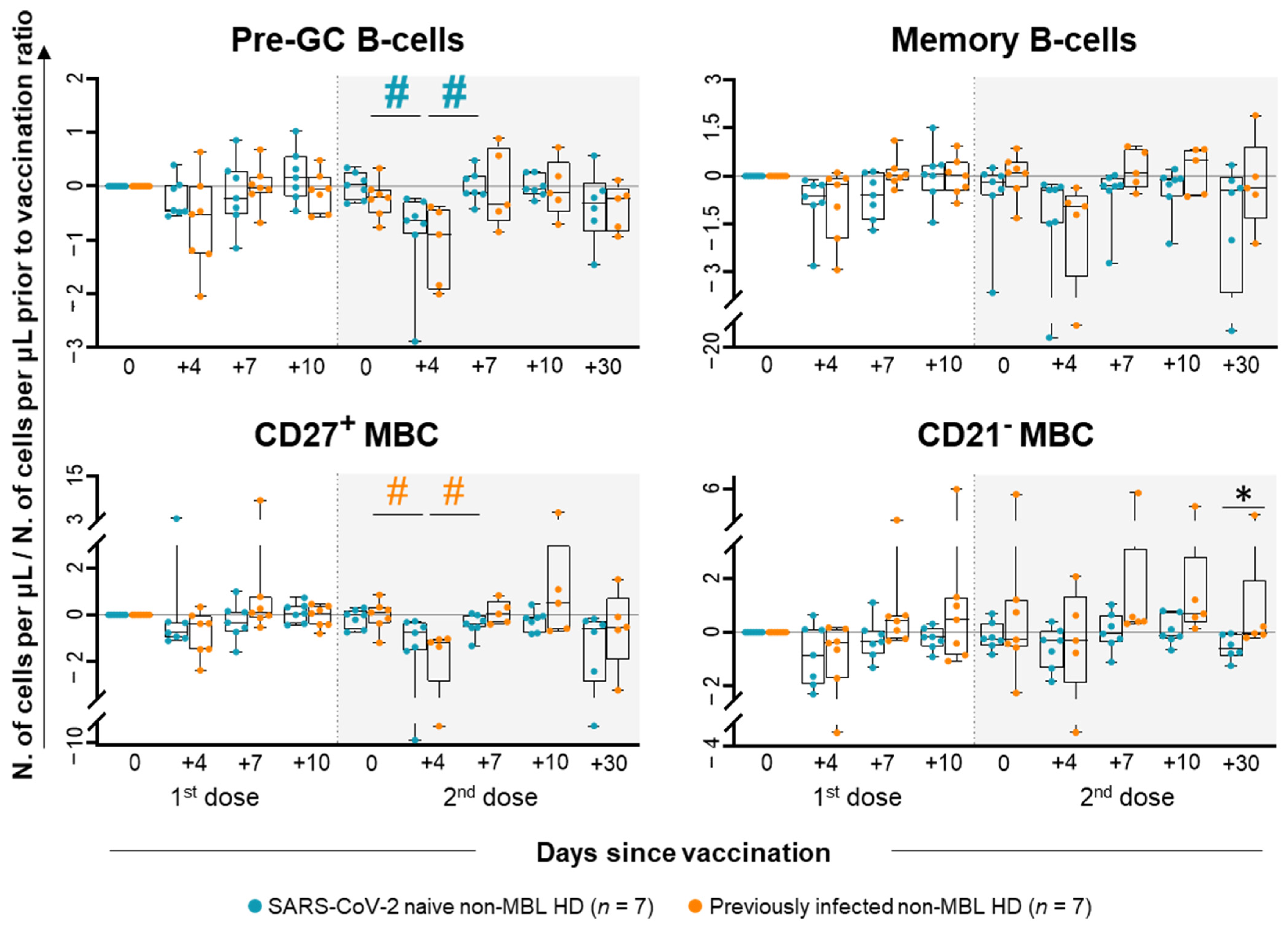
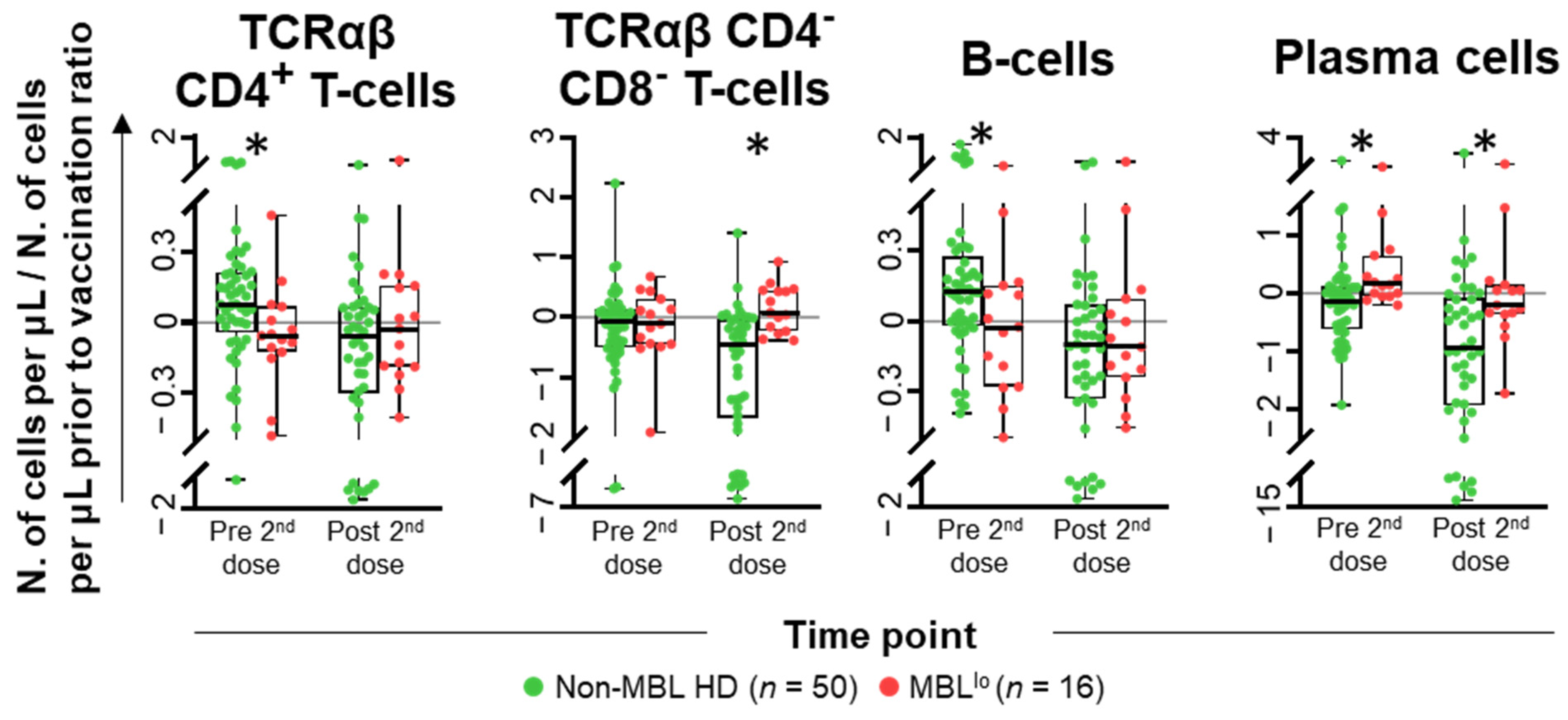
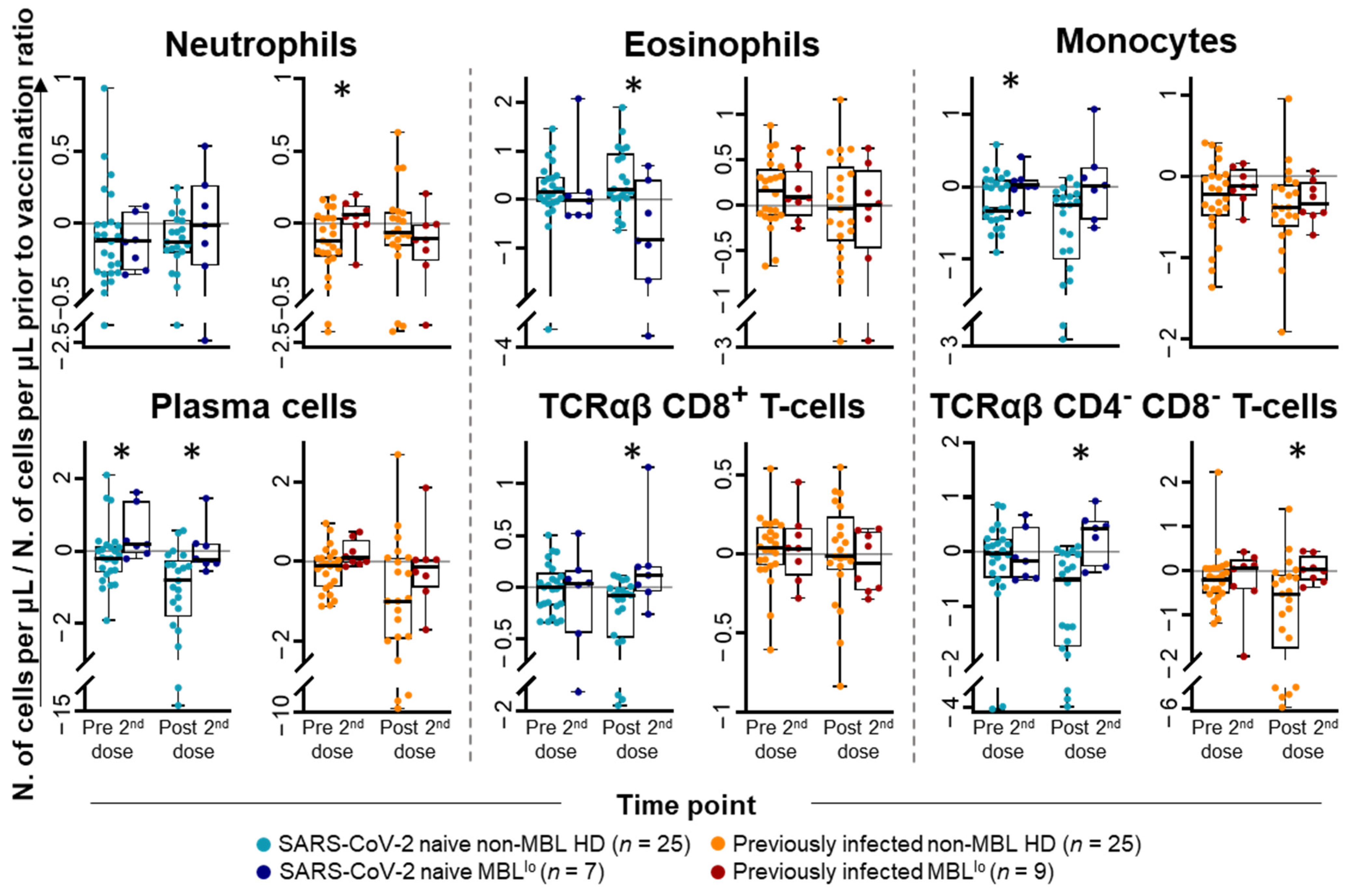
2.1. Kinetics of the Major Populations of Blood Leukocytes from Non-MBL HD Following Vaccination
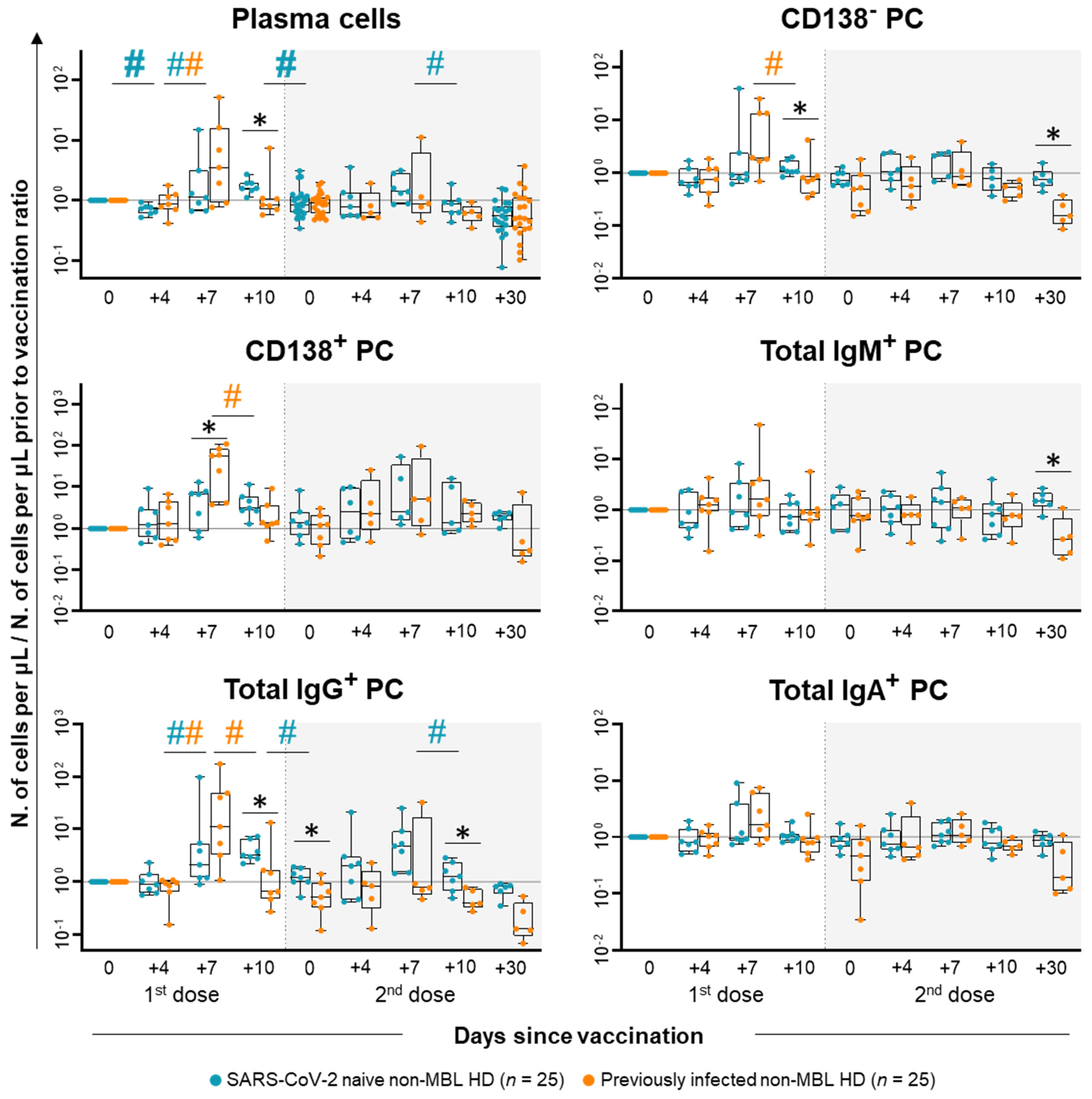
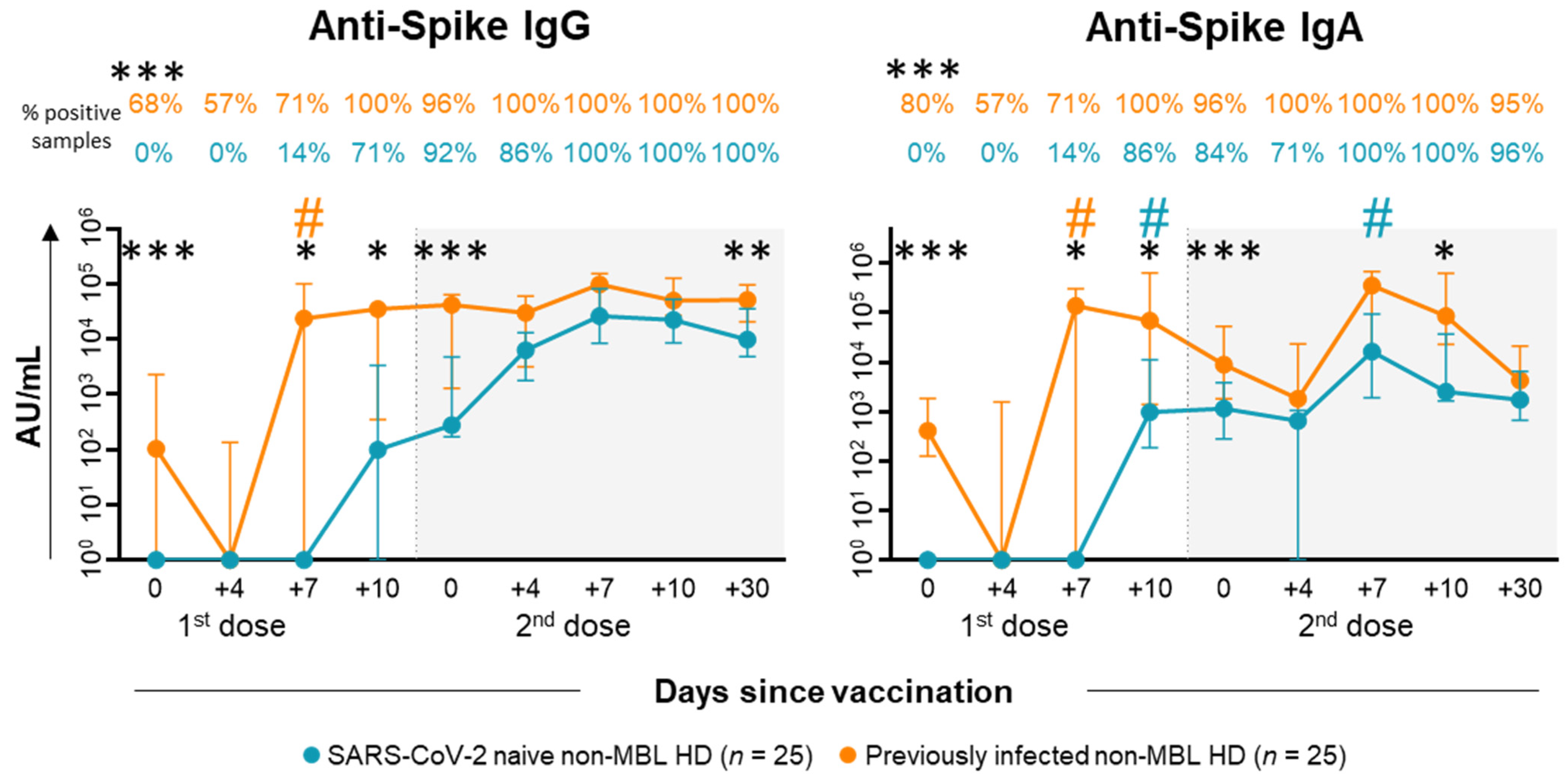
2.2. Anti-SARS-CoV-2 Antibody Kinetics in Plasma of Non-MBL HD After Vaccination
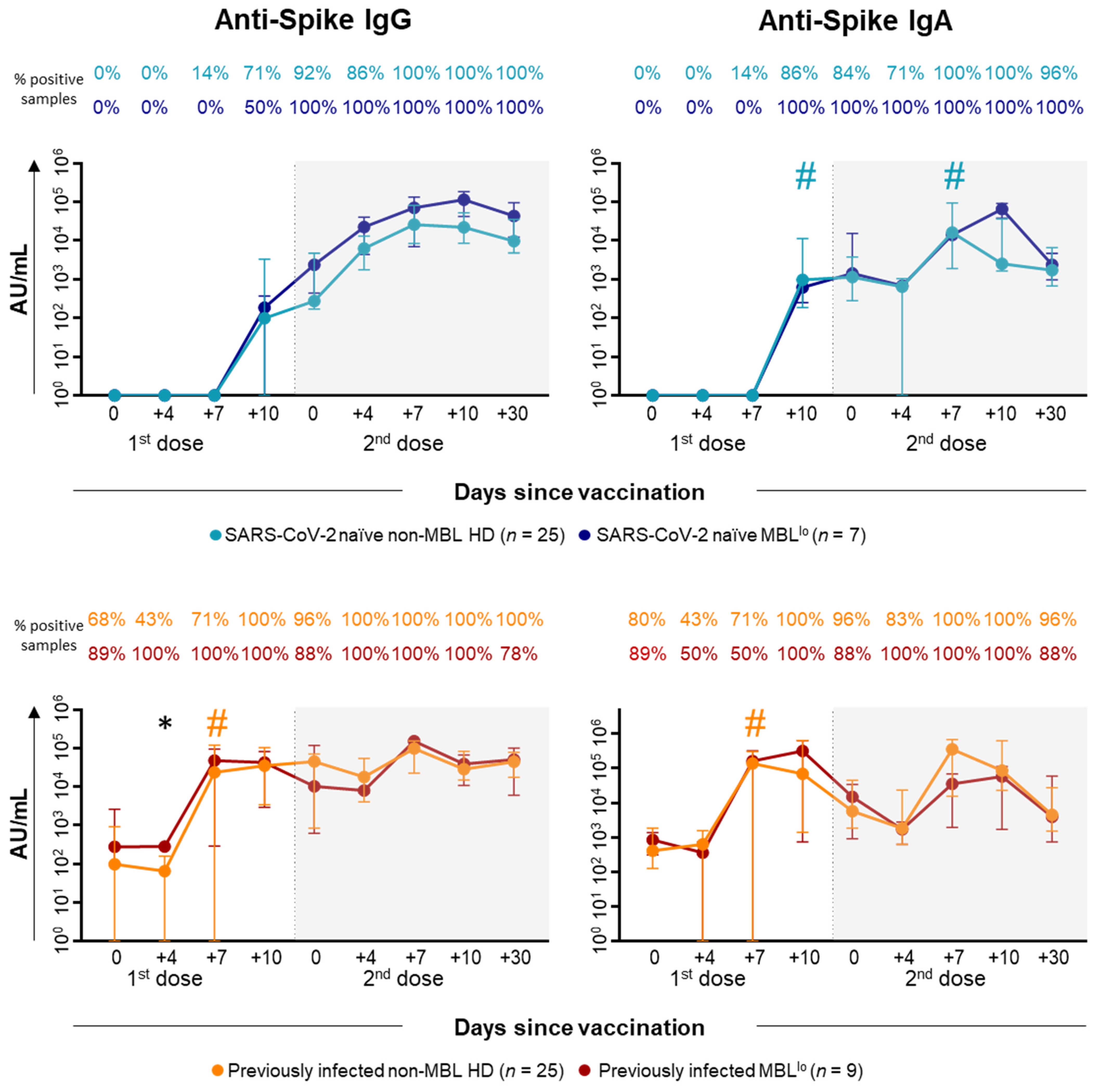
2.3. Kinetics of the Major Populations of Blood Leukocytes and Anti-SARS-CoV-2 Plasma Antibodies After Vaccination in MBLlo vs. Non-MBL HD
2.4. Kinetics of the B-Cell Clones After Vaccination in MBLlo Subjects
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. Immunophenotypic Studies
4.3. Measurement of Anti-SARS-CoV-2 IgG and IgA Antibody Plasma Levels
4.4. Statistical Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA J. Am. Med. Assoc. 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention. JAMA J. Am. Med. Assoc. 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Carsetti, R.; Zaffina, S.; Piano Mortari, E.; Terreri, S.; Corrente, F.; Capponi, C.; Palomba, P.; Mirabella, M.; Cascioli, S.; Palange, P.; et al. Different Innate and Adaptive Immune Responses to SARS-CoV-2 Infection of Asymptomatic, Mild, and Severe Cases. Front. Immunol. 2020, 11, 610300. [Google Scholar] [CrossRef]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Chen, R.; Sang, L.; Jiang, M.; Yang, Z.; Jia, N. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J. Allergy Clin. Immunol. 2020, 146, 89–100. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.; Perez Marc, G.; Moreira, E.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemer, D.; Spector, S.; Rouphael, N.; Creech, B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Thompson, M.G.; Burgess, J.L.; Naleway, A.L.; Tyner, H.; Yoon, S.K.; Meece, J.; Olsho, L.; Caban-Martinez, A.; Fowlkes, A.; Lutrick, K.; et al. Prevention and Attenuation of COVID-19 with the BNT162b2 and mRNA-1273 Vaccines. N. Engl. J. Med. 2021, 385, 320–329. [Google Scholar] [CrossRef]
- Farkash, I.; Feferman, T.; Cohen-Saban, N.; Avraham, Y.; Morgenstern, D.; Mayuni, G.; Barth, N.; Lustig, Y.; Miller, L.; Shouval, D.; et al. Anti-SARS-CoV-2 antibodies elicited by COVID-19 mRNA vaccine exhibit a unique glycosylation pattern. Cell Rep. 2021, 37, 110114. [Google Scholar] [CrossRef]
- Tejedor Vaquero, S.; de Campos-Mata, L.; Ramada, J.M.; Díaz, P.; Navarro-Barriuso, J.; Ribas-Llaurado, C.; Rodrigo Melero, N.; Carolis, C.; Cerutti, A.; Gimeno, R.; et al. The mRNA-1273 Vaccine Induces Cross-Variant Antibody Responses to SARS-CoV-2 With Distinct Profiles in Individuals with or Without Pre-Existing Immunity. Front. Immunol. 2021, 12, 737083. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Schmidt, F.; Weisblum, Y.; Muecksch, F.; Barnes, C.O.; Finkin, S.; Schaefer-Babajew, D.; Cipolla, M.; Gaebler, C.; Lieberman, J.; et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature 2021, 592, 616–622. [Google Scholar] [CrossRef]
- Kim, W.; Zhou, J.Q.; Horvath, S.C.; Schmitz, A.J.; Sturtz, A.J.; Lei, T.; Liu, Z.; Kalaidina, E.; Thapa, M.; Alsoussi, W.; et al. Germinal centre-driven maturation of B cell response to mRNA vaccination. Nature 2022, 604, 141–145. [Google Scholar] [CrossRef]
- Marti, G.E.; Rawstron, A.C.; Ghia, P.; Hillmen, P.; Houlston, R.S.; Kay, N.; Schleinitz, T.A.; Caporaso, N.; International Familial CLL Consortium. Diagnostic criteria for monoclonal B-cell lymphocytosis. Br. J. Haematol. 2005, 130, 325–332. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.D.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Rawstron, A.C.; Green, M.J.; Kuzmicki, A.; Kennedy, B.; Fenton, J.A.L.; Evans, P.A.S.; O’Connor, S.J.M.; Richards, S.J.; Morgan, G.J.; Jack, A.S.; et al. Monoclonal B lymphocytes with the characteristics of “indolent” chronic lymphocytic leukemia are present in 3.5% of adults with normal blood counts. Blood 2002, 100, 635–639. [Google Scholar] [CrossRef]
- Nieto, W.G.; Almeida, J.; Romero, A.; Teodosio, C.; López, A.; Henriques Henriques, A.F.; Sánchez, M.L.; Jara-Acevedo, M.; Rasillo, A.; González, M.; et al. Increased frequency (12%) of circulating chronic lymphocytic leukemia-like B-cell clones in healthy subjects using a highly sensitive multicolor flow cytometry approach. Blood 2009, 114, 33–37. [Google Scholar] [CrossRef]
- Slager, S.L.; Parikh, S.A.; Achenbach, S.J.; Norman, A.D.; Rabe, K.G.; Boddicker, N.J.; Olson, J.E.; Kleinstern, G.; Lesnick, C.E.; Call, T.G.; et al. Progression and survival of MBL: A screening study of 10 139 individuals. Blood 2022, 140, 1702–1709. [Google Scholar] [CrossRef]
- Criado, I.; Blanco, E.; Rodríguez-Caballero, A.; Alcoceba, M.; Contreras, T.; Gutiérrez, M.L.; Romero, A.; Fernández-Navarro, P.; González, M.; Solano, F.; et al. Residual normal B-cell profiles in monoclonal B-cell lymphocytosis versus chronic lymphocytic leukemia. Leukemia 2018, 32, 2701–2705. [Google Scholar] [CrossRef]
- Criado, I.; Nieto, W.G.; Oliva-Ariza, G.; Fuentes-Herrero, B.; Teodosio, C.; Lecrevisse, Q.; Lopez, A.; Romero, A.; Almeida, J.; Orfao, A.; et al. Age- and Sex-Matched Normal Leukocyte Subset Ranges in the General Population Defined with the EuroFlow Lymphocyte Screening Tube (LST) for Monoclonal B-Cell Lymphocytosis (MBL) vs. Non-MBL Subjects. Cancers 2022, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Hauswirth, A.W.; Almeida, J.; Nieto, W.G.; Teodosio, C.; Rodriguez-Caballero, A.; Romero, A.; López, A.; Fernandez-Navarro, P.; Vega, T.; Perez-Andres, M.; et al. Monoclonal B-cell lymphocytosis (MBL) with normal lymphocyte counts is associated with decreased numbers of normal circulating B-cell subsets. Am. J. Hematol. 2012, 87, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, J.A.; Parikh, S.A.; Shanafelt, T.D.; Kay, N.E.; Kennedy, R.B.; Grill, D.E.; Goergen, K.M.; Call, T.G.; Kendarian, S.S.; Ding, W.; et al. The humoral immune response to high-dose influenza vaccine in persons with monoclonal B-cell lymphocytosis (MBL) and chronic lymphocytic leukemia (CLL). Vaccine 2021, 39, 1122–1130. [Google Scholar] [CrossRef]
- Criado, I.; Rodríguez-Caballero, A.; Gutiérrez, M.L.; Pedreira, C.E.; Alcoceba, M.; Nieto, W.; Teodosio, C.; Bárcena, P.; Romero, A.; Fernández-Navarro, P.; et al. Low-count monoclonal B-cell lymphocytosis persists after seven years of follow up and is associated with a poorer outcome. Haematologica 2018, 103, 1198–1208. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Kay, N.E.; Parikh, S.A.; Achenbach, S.J.; Lesnick, C.E.; Hanson, C.A.; Kleinstern, G.; Olson, J.E.; Norman, A.D.; Rabe, K.G.; et al. Risk of serious infection among individuals with and without low count monoclonal B-cell lymphocytosis (MBL). Leukemia 2021, 35, 239–244. [Google Scholar] [CrossRef]
- Oliva-Ariza, G.; Fuentes-Herrero, B.; Carbonell, C.; Lecrevisse, Q.; Pérez-Pons, A.; Torres-Valle, A.; Pozo, J.; Martín-Oterino, J.Á.; González-López, Ó.; López-Bernús, A.; et al. High frequency of low-count monoclonal B-cell lymphocytosis in hospitalized COVID-19 patients. Blood 2023, 141, 309–314. [Google Scholar] [CrossRef]
- Parikh, S.A.; Achenbach, S.J.; Rabe, K.G.; Norman, A.D.; Boddicker, N.J.; Olson, J.E.; Call, T.G.; Cerhan, J.R.; Vachon, C.M.; Kay, N.E.; et al. The risk of coronavirus disease 2019 (COVID-19) among individuals with monoclonal B cell lymphocytosis. Blood Cancer J. 2022, 12, 8–10. [Google Scholar] [CrossRef]
- Oliva-Ariza, G.; Fuentes-Herrero, B.; Lecrevisse, Q.; Carbonell, C.; Pérez-Pons, A.; Torres-Valle, A.; Pozo, J.; Martín-Oterino, J.Á.; González-López, Ó.; López-Bernús, A.; et al. Immune cell kinetics and antibody response in COVID-19 patients with low-count monoclonal B-cell lymphocytosis. Am. J. Hematol. 2023, in press.
- Shen, Y.; Freeman, J.A.; Holland, J.; Naidu, K.; Solterbeck, A.; Van Bilsen, N.; Downe, P.; Kerridge, I.; Wallman, L.; Akerman, A.; et al. Multiple COVID-19 vaccine doses in CLL and MBL improve immune responses with progressive and high seroconversion. Blood 2022, 140, 2709–2721. [Google Scholar] [CrossRef]
- Shen, Y.; Freeman, J.A.; Holland, J.; Solterbeck, A.; Naidu, K.; Soosapilla, A.; Downe, P.; Tang, C.; Kerridge, I.; Wallman, L.; et al. COVID-19 vaccine failure in chronic lymphocytic leukaemia and monoclonal B-lymphocytosis; humoural and cellular immunity. Br. J. Haematol. 2022, 197, 41–51. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef]
- Brasu, N.; Elia, I.; Russo, V.; Montacchiesi, G.; Stabile, S.A.; De Intinis, C.; Fesi, F.; Gizzi, K.; Macagno, M.; Montone, M.; et al. Memory CD8+ T cell diversity and B cell responses correlate with protection against SARS-CoV-2 following mRNA vaccination. Nat. Immunol. 2022, 23, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Painter, M.M.; Johnston, T.S.; Lundgreen, K.A.; Santos, J.J.S.; Qin, J.S.; Goel, R.R.; Apostolidis, S.A.; Mathew, D.; Fulmer, B.; Williams, J.C.; et al. Prior vaccination promotes early activation of memory T cells and enhances immune responses during SARS-CoV-2 breakthrough infection. Nat. Immunol. 2023, 24, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Kardava, L.; Rachmaninoff, N.; Lau, W.W.; Buckner, C.M.; Trihemasava, K.; Blazkova, J.; Lopes de Assis, F.; Wang, W.; Zhang, X.; Wang, Y.; et al. Early human B cell signatures of the primary antibody response to mRNA vaccination. Proc. Natl. Acad. Sci. USA 2022, 119, e2204607119. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines-a new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Schultze, J.L.; Aschenbrenner, A.C. COVID-19 and the human innate immune system. Cell 2021, 184, 1671–1692. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Pighi, L.; Henry, B.M.; De Nitto, S.; Plebani, M.; Lippi, G. Early kinetics of cellular immunity in recipients of bivalent BNT162b2 vaccine: A proof-of-concept study. Clin. Chem. Lab. Med. 2023, 61, E172–E174. [Google Scholar] [CrossRef]
- Laidlaw, B.J.; Ellebedy, A.H. The germinal centre B cell response to SARS-CoV-2. Nat. Rev. Immunol. 2022, 22, 7–18. [Google Scholar] [CrossRef]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Sokal, A.; Chappert, P.; Barba-Spaeth, G.; Roeser, A.; Fourati, S.; Azzaoui, I.; Vandenberghe, A.; Fernandez, I.; Meola, A.; Bouvier-Alias, M.; et al. Maturation and persistence of the anti-SARS-CoV-2 memory B cell response. Cell 2021, 184, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Kuri-Cervantes, L.; Pampena, M.B.; Meng, W.; Rosenfeld, A.M.; Ittner, C.A.G.; Weisman, A.R.; Agyekum, R.S.; Mathew, D.; Baxter, A.E.; Vella, L.A.; et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020, 5, eabd7114. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q.; Lei, T.; Thapa, M.; Chen, R.E.; Case, J.B.; et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Shuwa, H.A.; Shaw, T.N.; Knight, S.B.; Wemyss, K.; McClure, F.A.; Pearmain, L.; Prise, I.; Jagger, C.; Morgan, D.J.; Khan, S.; et al. Alterations in T and B cell function persist in convalescent COVID-19 patients. Med 2021, 2, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Dalla Gasperina, D.; Veronesi, G.; Shallak, M.; Ietto, G.; Iovino, D.; Baj, A.; Gianfagna, F.; Maurino, V.; Focosi, D.; et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. eBioMedicine 2022, 75, 103788. [Google Scholar] [CrossRef]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef]
- Franzese, M.; Coppola, L.; Silva, R.; Santini, S.A.; Cinquanta, L.; Ottomano, C.; Salvatore, M.; Incoronato, M. SARS-CoV-2 antibody responses before and after a third dose of the BNT162b2 vaccine in Italian healthcare workers aged ≤60 years: One year of surveillance. Front. Immunol. 2022, 13, 947187. [Google Scholar] [CrossRef]
- Lau, C.S.; Phua, S.K.; Liang, Y.L.; Oh, M.L.H.; Aw, T.C. SARS-CoV-2 Spike and Neutralizing Antibody Kinetics 90 Days after Three Doses of BNT162b2 mRNA COVID-19 Vaccine in Singapore. Vaccines 2022, 10, 331. [Google Scholar] [CrossRef]
- Cho, A.; Muecksch, F.; Schaefer-Babajew, D.; Wang, Z.; Finkin, S.; Gaebler, C.; Ramos, V.; Cipolla, M.; Mendoza, P.; Agudelo, M.; et al. Anti-SARS-CoV-2 receptor-binding domain antibody evolution after mRNA vaccination. Nature 2021, 600, 517–522. [Google Scholar] [CrossRef]
- Busà, R.; Sorrentino, M.C.; Russelli, G.; Amico, G.; Miceli, V.; Miele, M.; Di Bella, M.; Timoneri, F.; Gallo, A.; Zito, G.; et al. Specific Anti-SARS-CoV-2 Humoral and Cellular Immune Responses After Booster Dose of BNT162b2 Pfizer-BioNTech mRNA-Based Vaccine: Integrated Study of Adaptive Immune System Components. Front. Immunol. 2022, 13, 856657. [Google Scholar] [CrossRef]
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.; Puig, N.; García-Sánchez, O.; Böttcher, S.; van der Velden, V.H.J.; Pérez-Morán, J.-J.; Vidriales, M.-B.; García-Sanz, R.; et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, J.J.M.; Lhermitte, L.; Böttcher, S.; Almeida, J.; Van Der Velden, V.H.J.; Flores-Montero, J.; Rawstron, A.; Asnafi, V.; Lécrevisse, Q.; Lucio, P.; et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 2012, 26, 1908–1975. [Google Scholar] [CrossRef] [PubMed]
- Flores-Montero, J.; Grigore, G.; Fluxá, R.; Hernández, J.; Fernandez, P.; Almeida, J.; Muñoz, N.; Böttcher, S.; Sedek, L.; van der Velden, V.; et al. EuroFlow Lymphoid Screening Tube (LST) data base for automated identification of blood lymphocyte subsets. J. Immunol. Methods 2019, 475, 112662. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.H.; Fluxa, R.; Perez-Andres, M.; Diks, A.M.; van Gaans-van den Brink, J.A.; Barkoff, A.M.; Blanco, E.; Torres-Valle, A.; Berkowska, M.A.; Grigore, G.; et al. Automated EuroFlow approach for standardized in-depth dissection of human circulating B-cells and plasma cells. Front. Immunol. 2023, 14, 1268686. [Google Scholar] [CrossRef]
- Hultin, L.E.; Chow, M.; Jamieson, B.D.; O’Gorman MR, G.; Menendez, F.A.; Borowski, L.; Denny, T.N.; Margolick, J.B. Comparison of interlaboratory variation in absolute T-cell counts by single-platform and optimized dual-platform methods. Cytom. Part B-Clin. Cytom. 2010, 78, 194–200. [Google Scholar] [CrossRef]
- Blanco, E.; Pérez-Andrés, M.; Arriba-Méndez, S.; Contreras-Sanfeliciano, T.; Criado, I.; Pelak, O.; Serra-Caetano, A.; Romero, A.; Puig, N.; Remesal, A.; et al. Age-associated distribution of normal B-cell and plasma cell subsets in peripheral blood. J. Allergy Clin. Immunol. 2018, 141, 2208–2219.e16. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva-Ariza, G.; Criado, I.; Fuentes-Herrero, B.; Carbonell, C.; Sánchez-Gallego, J.I.; López-Bernús, A.; Gutiérrez, M.L.; Rolo-Ramírez, A.; Bernal-Ribes, M.; Almenara-Morales, Y.; et al. Early Immune Cell and Antibody Kinetics Following SARS-CoV-2 Vaccination in Healthy Adults and Low-Count Monoclonal B-Cell Lymphocytosis. Int. J. Mol. Sci. 2025, 26, 681. https://doi.org/10.3390/ijms26020681
Oliva-Ariza G, Criado I, Fuentes-Herrero B, Carbonell C, Sánchez-Gallego JI, López-Bernús A, Gutiérrez ML, Rolo-Ramírez A, Bernal-Ribes M, Almenara-Morales Y, et al. Early Immune Cell and Antibody Kinetics Following SARS-CoV-2 Vaccination in Healthy Adults and Low-Count Monoclonal B-Cell Lymphocytosis. International Journal of Molecular Sciences. 2025; 26(2):681. https://doi.org/10.3390/ijms26020681
Chicago/Turabian StyleOliva-Ariza, Guillermo, Ignacio Criado, Blanca Fuentes-Herrero, Cristina Carbonell, José Ignacio Sánchez-Gallego, Amparo López-Bernús, María Laura Gutiérrez, Alejandro Rolo-Ramírez, Marta Bernal-Ribes, Yolimar Almenara-Morales, and et al. 2025. "Early Immune Cell and Antibody Kinetics Following SARS-CoV-2 Vaccination in Healthy Adults and Low-Count Monoclonal B-Cell Lymphocytosis" International Journal of Molecular Sciences 26, no. 2: 681. https://doi.org/10.3390/ijms26020681
APA StyleOliva-Ariza, G., Criado, I., Fuentes-Herrero, B., Carbonell, C., Sánchez-Gallego, J. I., López-Bernús, A., Gutiérrez, M. L., Rolo-Ramírez, A., Bernal-Ribes, M., Almenara-Morales, Y., Lecrevisse, Q., van Dongen, J. J. M., Marcos, M., Almeida, J., & Orfao, A., on behalf of the ECRIN-M3 Consortium. (2025). Early Immune Cell and Antibody Kinetics Following SARS-CoV-2 Vaccination in Healthy Adults and Low-Count Monoclonal B-Cell Lymphocytosis. International Journal of Molecular Sciences, 26(2), 681. https://doi.org/10.3390/ijms26020681





