Circulating Tumor Cell-Free DNA as Prognostic Biomarker in Non-Small Cell Lung Cancer Patients Undergoing Immunotherapy: The CORELAB Experience
Abstract
1. Introduction
2. Results
2.1. Clinicopathological Characteristics of Patients
2.2. Total Circulating Cell-Free DNA (cfDNA)
2.3. Circulating Tumor DNA and Detected Variants
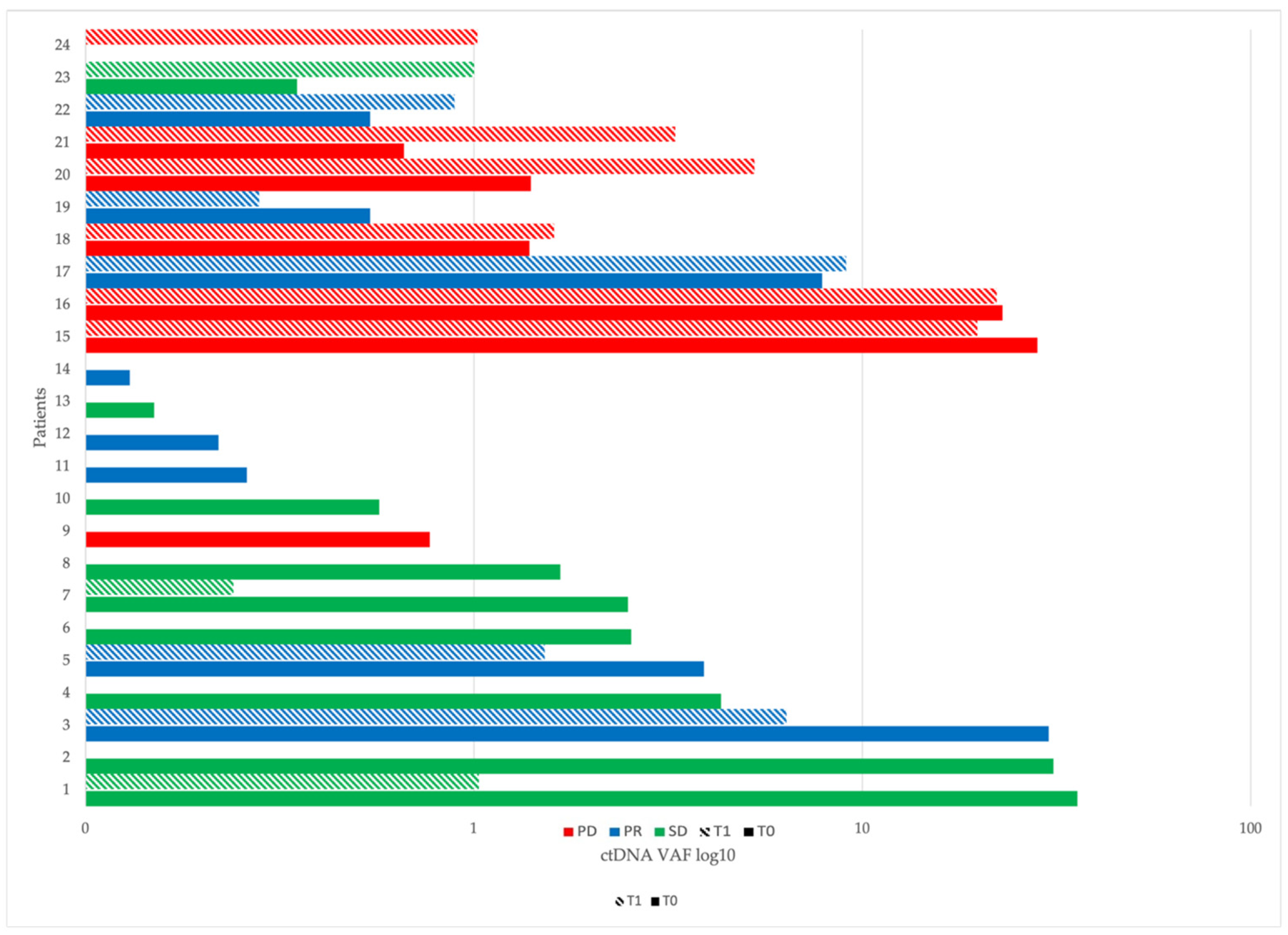
2.4. ctDNA at Baseline (T0) and After Two Months of Immunotherapy (T1)
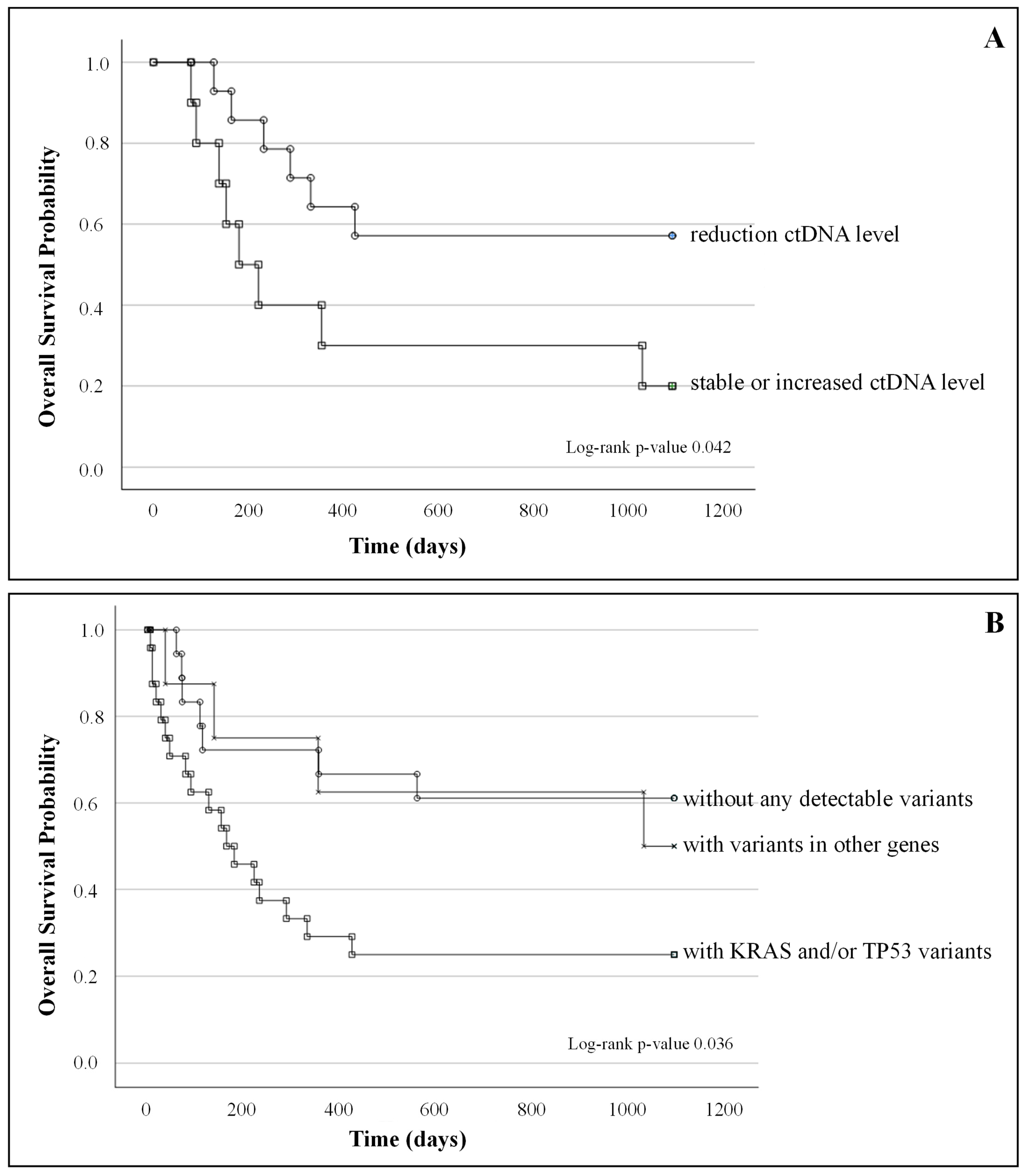
2.5. ctDNA Correlation with Clinical Outcome and Survival
- (A)
- Kaplan–Meier survival curves of patients with a reduction in ctDNA level between T0 and T1 (n = 14) and patients with stable or increased ctDNA level (n = 10); log-rank test p-value = 0.42;
- (B)
- Kaplan–Meier survival curves of patients without any detectable variants (n = 18), patients with KRAS and/or TP53 (n = 24) variants, and patients with variants in other genes (n = 8); log-rank test p-value 0.036.
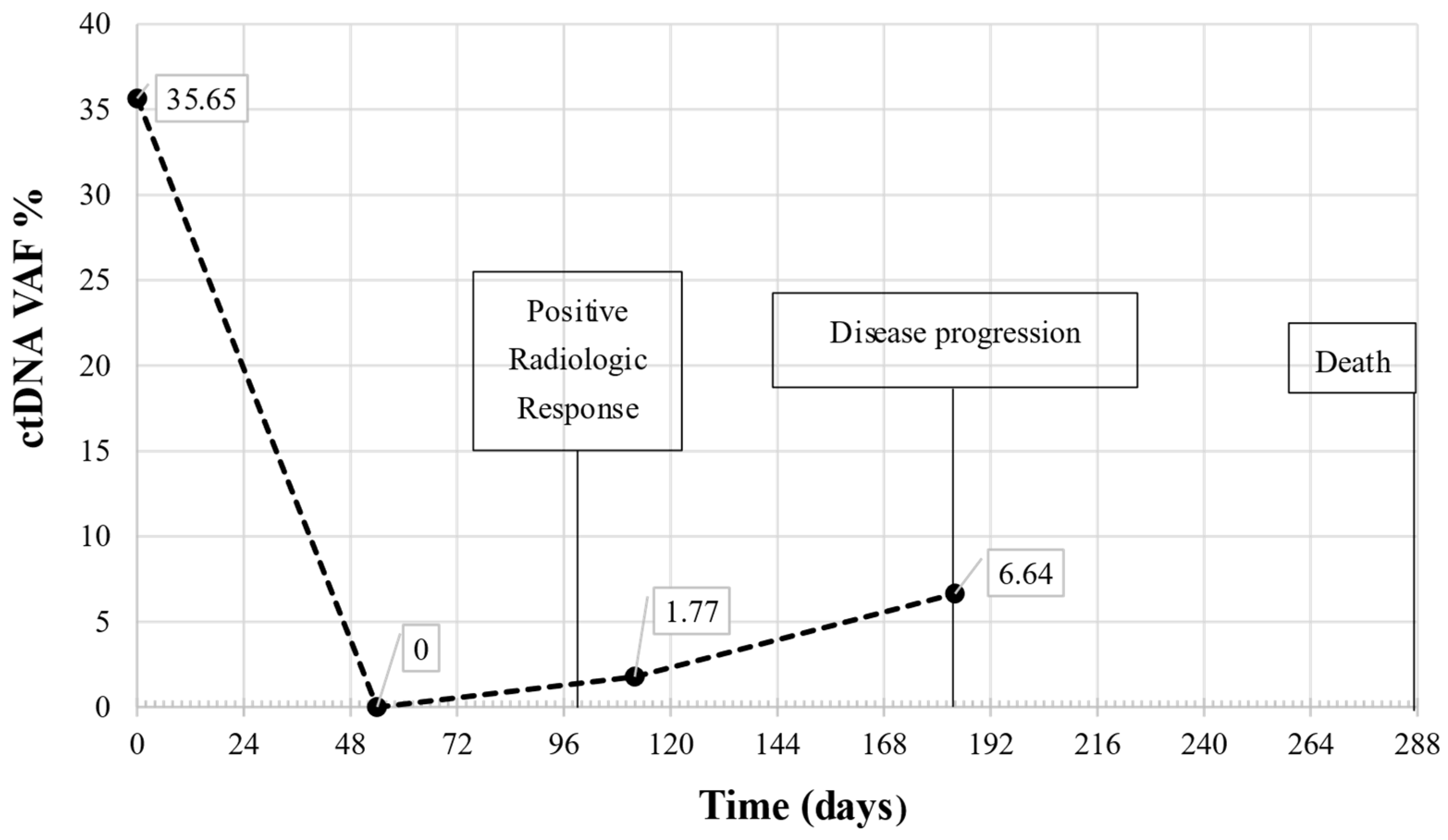
2.6. Longitudinal Monitoring by Liquid Biopsy
3. Discussion
4. Materials and Methods
4.1. Patients and Blood Collection
4.2. Plasma Separation, Cell-Free DNA Extraction and Quantification
4.3. Cell-Free DNA Analysis
4.4. ctDNA Assessment from Next Generation Sequencing Data and Molecular Response Measurement
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gulley, J.L.; Spigel, D.; Kelly, K.; Chandler, J.C.; Rajan, A.; Hassan, R.; Wong, D.J.L.; Leach, J.; Edenfield, W.J.; Wang, D.; et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in advanced NSCLC patients: A phase 1b, open-label expansion trial in patients progressing after platinum-based chemotherapy. J. Clin. Oncol. 2015, 33 (Suppl. 15), 8034. [Google Scholar] [CrossRef]
- Tang, S.; Qin, C.; Hu, H.; Liu, T.; He, Y.; Guo, H.; Yan, H.; Zhang, J.; Tang, S.; Zhou, H. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Progress, Challenges, and Prospects. Cells 2022, 11, 320. [Google Scholar] [CrossRef]
- Carlino, M.S.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, A.; Maji, A.; Potdar, P.D.; Singh, N.; Parikh, P.; Bisht, B.; Mukherjee, A.; Paul, M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 2023, 22, 40. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H.; Chen, Y.; Xiao, N.; Zheng, Z.; Liu, H.; Wan, J. Liquid biopsy on the horizon in immunotherapy of non-small cell lung cancer: Current status, challenges, and perspectives. Cell Death Dis. 2023, 14, 230. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.; Patel, S.P.; Kurzrock, R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat. Rev. Clin. Oncol. 2017, 14, 203–220. [Google Scholar] [CrossRef]
- Goh, K.Y.; Cheng, T.Y.; Tham, S.C.; Lim, D.W. Circulating Biomarkers for Prediction of Immunotherapy Response in NSCLC. Biomedicines 2023, 11, 508. [Google Scholar] [CrossRef]
- Alix-Panabiere, C.; Marchetti, D.; Lang, J.E. Liquid biopsy, from concept to clinical application. Sci. Rep. 2023, 13, 21685. [Google Scholar] [CrossRef] [PubMed]
- Alama, A.; Coco, S.; Genova, C.; Ross, G.; Fontana, V.; Tagliamento, M.; Dal Bello, G.M.; Rosa, A.; Boccardo, S.; Rijavec, E.; et al. Prognostic Relevance of Circulating Tumor Cells and Circulating Cell-Free DNA Association in Metastatic Non-Small Cell Lung Cancer Treated with Nivolumab. J. Clin. Med. 2019, 8, 1011. [Google Scholar] [CrossRef]
- Passiglia, F.; Galvano, A.; Castiglia, M.; Incorvaia, L.; Calò, V.; Listì, A.; Mazzarisi, S.; Perez, A.; Gallina, G.; Rizzo, S.; et al. Monitoring blood biomarkers to predict nivolumab effectiveness in NSCLC patients. Ther. Adv. Med. Oncol. 2019, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Izevbaye, I. RELAY phase 3 randomized study: A step closer to the clinic for ctDNA in non-small cell lung cancer treatment monitoring? Transl. Lung Cancer Res. 2023, 12, 2151–2156. [Google Scholar] [CrossRef]
- Aggarwal, C.; Leighl, N.B. Next-generation ctDNA-driven clinical trials in precision immuno-oncology. J. Immunother. Cancer 2023, 11, e006397. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, J.; Wu, S.; Si, H.; Gao, C.; Xu, W.; Abdullah, S.E.; Higgs, B.W.; Dennis, P.A.; van der Heijden, M.S.; et al. Prognostic and Predictive Impact of Circulating Tumor DNA in Patients with Advanced Cancers Treated with Immune Checkpoint Blockade. Cancer Discov. 2020, 10, 1842–1853. [Google Scholar] [CrossRef]
- Salvianti, F.; Gelmini, S.; Mancini, I.; Pazzagli, M.; Pillozzi, S.; Giommoni, E.; Brugia, M.; Di Costanzo, F.; Galardi, F.; De Luca, F.; et al. Circulating tumour cells and cell-free DNA as a prognostic factor in metastatic colorectal cancer: The OMITERC prospective study. Br. J. Cancer 2021, 125, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Villanueva, M.; Hidalgo-Pérez, L.; Rios-Romero, M.; Cedro-Tanda, A.; Ruiz-Villavicencio, C.A.; Page, K.; Hastings, R.; Fernandez-Garcia, D.; Allsopp, R.; Fonseca-Montaño, M.A.; et al. Cell-free DNA analysis in current cancer clinical trials: A review. Br. J. Cancer 2022, 126, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Mathai, R.A.; Vidya, R.V.S.; Reddy, B.S.; Thomas, L.; Udupa, K.; Kolesar, J.; Rao, M. Potential Utility of Liquid Biopsy as a Diagnostic and Prognostic Tool for the Assessment of Solid Tumors: Implications in the Precision Oncology. J. Clin. Med. 2019, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer 2011, 11, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.W.; Liu, Y.; Sha, H.H.; Wen, S.D.; Fang, Y.; Zhou, G.R. Relationship between plasma circulating cell-free DNA concentration and treatment outcomes including prognosis in patients with advanced non-small cell lung cancer. BMC Pulm. Med. 2023, 23, 348. [Google Scholar] [CrossRef] [PubMed]
- Guibert, N.; Jones, G.; Beeler, J.F.; Plagnol, V.; Morris, C.; Mourlanette, J.; Delaunay, M.; Keller, L.; Rouquette, I.; Favre, G.; et al. Targeted sequencing of plasma cell-free DNA to predict response to PD1 inhibitors in advanced non-small cell lung cancer. Lung Cancer 2019, 137, 1–6. [Google Scholar] [CrossRef]
- Ricciuti, B.; Jones, G.; Severgnini, M.; Aless, J.V.; Recondo, G.; Lawrence, M.; Forshew, T.; Lydon, C.; Nishino, M.; Cheng, M.; et al. Early plasma circulating tumor DNA (ctDNA) changes predict response to first-line pembrolizumab-based therapy in non-small cell lung cancer (NSCLC). J. Immunother. Cancer 2021, 9, e001504. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.C.; Carpenter, E.L.; Silva, B.A.; Rosenstein, J.; Chien, A.L.; Quinn, K.; Espenschied, C.R.; Mak, A.; Kiedrowski, L.A.; Lefterova, M.; et al. Serial Monitoring of Circulating Tumor DNA by Next-Generation Gene Sequencing as a Biomarker of Response and Survival in Patients With Advanced NSCLC Receiving Pembrolizumab-Based Therapy. JCO Precis. Oncol. 2021, 5, PO.20.00321. [Google Scholar] [CrossRef] [PubMed]
- Pavan, A.; Bragadin, A.B.; Calvetti, L.; Ferro, A.; Zulato, E.; Attili, I.; Nardo, G.; Dal Maso, A.; Frega, S.; Menin, A.G.; et al. Role of next generation sequencing-based liquid biopsy in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors: Impact of STK11, KRAS and TP53 mutations and co-mutations on outcome. Transl. Lung Cancer Res. 2021, 10, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Y.; Zhong, W.Z.; Zhang, X.C.; Su, J.; Xie, Z.; Liu, S.Y.; Tu, H.Y.; Chen, H.J.; Sun, Y.L.; Zhou, Q.; et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin. Cancer Res. 2017, 23, 3012–3024. [Google Scholar] [CrossRef]
- Frost, N.; Kollmeier, J.; Vollbrecht, C.; Grah, C.; Matthes, B.; Pultermann, D.; von Laffert, M.; Lüders, H.; Olive, E.; Raspe, M.; et al. KRASG12C/TP53 co-mutations identify long-term responders to first line palliative treatment with pembrolizumab monotherapy in PD-L1 high (≥50%) lung adenocarcinoma. Transl. Lung Cancer Res. 2021, 10, 737–752. [Google Scholar] [CrossRef]
- Jaharomi, A.H.; Zebel, M.; Okamura, R.; Hoh, C.K.; Kurzrock, R. Imaging variant allele fraction of genomic alterartions in circulating tumor DNA (% ctDNA) correlates with SUV max in PET scan. Am. J. Nucl. Med. Mol. Imaging 2021, 11, 307–312. [Google Scholar]
- Liu, B.; Hu, Z.; Ran, J.; Xie, N.; Tian, C.; Tang, Y.; Ouyang, Q. The circulating tumor DNA (ctDNA) alteration level predicts therapeutic response in metastatic breast cancer: Novel prognosis indexes based on, c.t.D.N.A. Breast 2022, 65, 116–123. [Google Scholar] [CrossRef]

| Total number of patients | 50 (100%) |
| Sex | |
| Male | 33 (66%) |
| Female | 17 (34%) |
| Age | (years) |
| Mean (SD) | 69 (7.86) |
| Median (range) | 70 (48–83) |
| Smoking history | |
| Current or former | 38 (76%) |
| Never | 12 (24%) |
| Histology | |
| Adenocarcinoma | 41 (82%) |
| Squamous cell carcinoma | 5 (10%) |
| Other | 4 (8%) |
| Clinical stage | |
| III | 6 (12%) |
| IV | 44 (88%) |
| Number of metastatic sites | |
| 1 | 11 (22%) |
| 2 | 23 (46%) |
| ≥3 | 16 (32%) |
| Metastatic location | |
| Contralateral lung | 19 |
| Pleura | 4 |
| Adrenal gland | 10 |
| Lymph nodes | 29 |
| Bones | 21 |
| Brain | 13 |
| Liver | 11 |
| Other | 7 |
| Tissue PD-L1% | |
| <1% | 20 (40%) |
| 1–49% | 11 (22%) |
| ≥50% | 11 (22%) |
| Unknown | 8 (16%) |
| Systemic Treatment | |
| Chemo-immunotherapy | 39 (78%) |
| Mono-immunotherapy | 11 (22%) |
| Immunotherapy | |
| Pembrolizumab | 50 (100%) |
| Gene (NM) | CDS and AA Mutation | Cosmic ID | Type | T0 (n) | T1 (n) | Classification |
|---|---|---|---|---|---|---|
| BRAF NM_004333.6 | c.1799T>A p.(Val600Glu) | COSM476 | missense | 2 | 3 | pathogenic/oncogenic |
| EGFR NM_005228.5 | c.2235_2249del p.(Glu746_Ala750del) | COSM6223 | del | 1 | 0 | pathogenic/oncogenic |
| c.2240_2257del p.(Leu747_Pro753delinsSer) | COSM12370 | del | 1 | 0 | likely pathogenic | |
| c.2155G>A p.(Gly719Ser) | COSM6252 | missense | 2 | 2 | likely pathogenic | |
| KRAS NM_004985.5 | c.34G>T p.(Gly12Cys) | COSM516 | missense | 7 | 5 | likely pathogenic/pathogenic |
| c.35G>T p.(Gly12Val) | COSM520 | missense | 3 | 0 | pathogenic/oncogenic | |
| c.35G>C p.(Gly12Ala) | COSM522 | missense | 3 | 0 | pathogenic/oncogenic | |
| c.37G>T p.(Gly13Cys) | COSM527 | missense | 1 | 1 | likely pathogenic/oncogenic | |
| MAP2K1 NM_002755.4 | c.167A>C p.(Gln56Pro) | COSM1235481 | missense | 1 | 2 | pathogenic |
| c.607G>A p.(Glu203Lys) | COSM232755 | missense | 1 | 0 | likely pathogenic | |
| c.371C>T p.(Pro124Leu) | COSM1315861 | missense | 1 | 0 | likely pathogenic/pathogenic | |
| c.158T>G p.(Phe53Cys) | COSM1562837 | missense | 0 | 2 | na | |
| MET NM_000245.4 | c.3028+1G>A (p. unknown) | COSM6108461 | substitution intronic | 1 | 0 | pathogenic |
| c.3802A>G p.(Met1268Val | na | na | 0 | 1 | na | |
| NRAS NM_002524.5 | c.38G>A p.(Gly13Asp) | COSM573 | missense | 1 | 1 | likely pathogenic/pathogenic |
| PIK3CA NM_006218.4 | c.1624G>A p.(Glu542Lys) | COSM760 | missense | 2 | 0 | pathogenic |
| c.3140A>G p.(His1047Arg) | COSM775 | missense | 1 | 3 | pathogenic | |
| c.1633G>A p.(Glu545Lys) | COSM763 | missense | 1 | 2 | likely pathogenic/pathogenic | |
| 3TP53 NM_000546.6 | c.818G>C p.(Arg273Pro) | COSM43896 | missense | 1 | 0 | likely pathogenic/pathogenic |
| c.818G>T p.(Arg273Leu) | COSM10779 | missense | 1 | 0 | pathogenic | |
| c.817C>T p.(Arg273Cys) | COSM10659 | missense | 1 | 0 | likely pathogenic/pathogenic | |
| c.818G>A p.(Arg273His) | COSM10660 | missense | 0 | 2 | pathogenic | |
| c.659A>G p.(Tyr220Cys) | COSM10758 | missense | 3 | 1 | pathogenic/oncogenic | |
| c.742C>T p.(Arg248Trp) | COSM10656 | missense | 4 | 1 | pathogenic/oncogenic | |
| c.743G>A p.(Arg248Gln) | COSM10662 | missense | 1 | 1 | pathogenic/oncogenic | |
| c.733G>T p.Gly245Cys) | COSM11081 | missense | 1 | 0 | pathogenic/oncogenic | |
| c.734G>T p.(Gly245Val) | COSM11196 | missense | 1 | 1 | pathogenic/oncogenic | |
| c.524G>A p.(Arg175His) | COSM10648 | missense | 1 | 0 | pathogenic/oncogenic | |
| c.711G>A p.(Met237Ile) | COSM10834 | missense | 1 | 0 | pathogenic/oncogenic | |
| c.473G>T p.(Arg158Leu) | COSM10714 | missense | 1 | 1 | likely pathogenic/pathogenic | |
| c.517G>T p.(Val173Leu) | COSM43559 | missense | 1 | 0 | pathogenic/oncogenic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelmini, S.; Calabri, A.; Mancini, I.; Comin, C.E.; Pasini, V.; Banini, M.; Scotti, V.; Pinzani, P. Circulating Tumor Cell-Free DNA as Prognostic Biomarker in Non-Small Cell Lung Cancer Patients Undergoing Immunotherapy: The CORELAB Experience. Int. J. Mol. Sci. 2025, 26, 611. https://doi.org/10.3390/ijms26020611
Gelmini S, Calabri A, Mancini I, Comin CE, Pasini V, Banini M, Scotti V, Pinzani P. Circulating Tumor Cell-Free DNA as Prognostic Biomarker in Non-Small Cell Lung Cancer Patients Undergoing Immunotherapy: The CORELAB Experience. International Journal of Molecular Sciences. 2025; 26(2):611. https://doi.org/10.3390/ijms26020611
Chicago/Turabian StyleGelmini, Stefania, Adele Calabri, Irene Mancini, Camilla Eva Comin, Valeria Pasini, Marco Banini, Vieri Scotti, and Pamela Pinzani. 2025. "Circulating Tumor Cell-Free DNA as Prognostic Biomarker in Non-Small Cell Lung Cancer Patients Undergoing Immunotherapy: The CORELAB Experience" International Journal of Molecular Sciences 26, no. 2: 611. https://doi.org/10.3390/ijms26020611
APA StyleGelmini, S., Calabri, A., Mancini, I., Comin, C. E., Pasini, V., Banini, M., Scotti, V., & Pinzani, P. (2025). Circulating Tumor Cell-Free DNA as Prognostic Biomarker in Non-Small Cell Lung Cancer Patients Undergoing Immunotherapy: The CORELAB Experience. International Journal of Molecular Sciences, 26(2), 611. https://doi.org/10.3390/ijms26020611







