Sequential Obtention of Blood–Brain Barrier-Permeable Non-Polar and Polar Compounds from Salvia officinalis L. and Eucalyptus globulus Labill. with Neuroprotective Purposes
Abstract
1. Introduction
2. Results
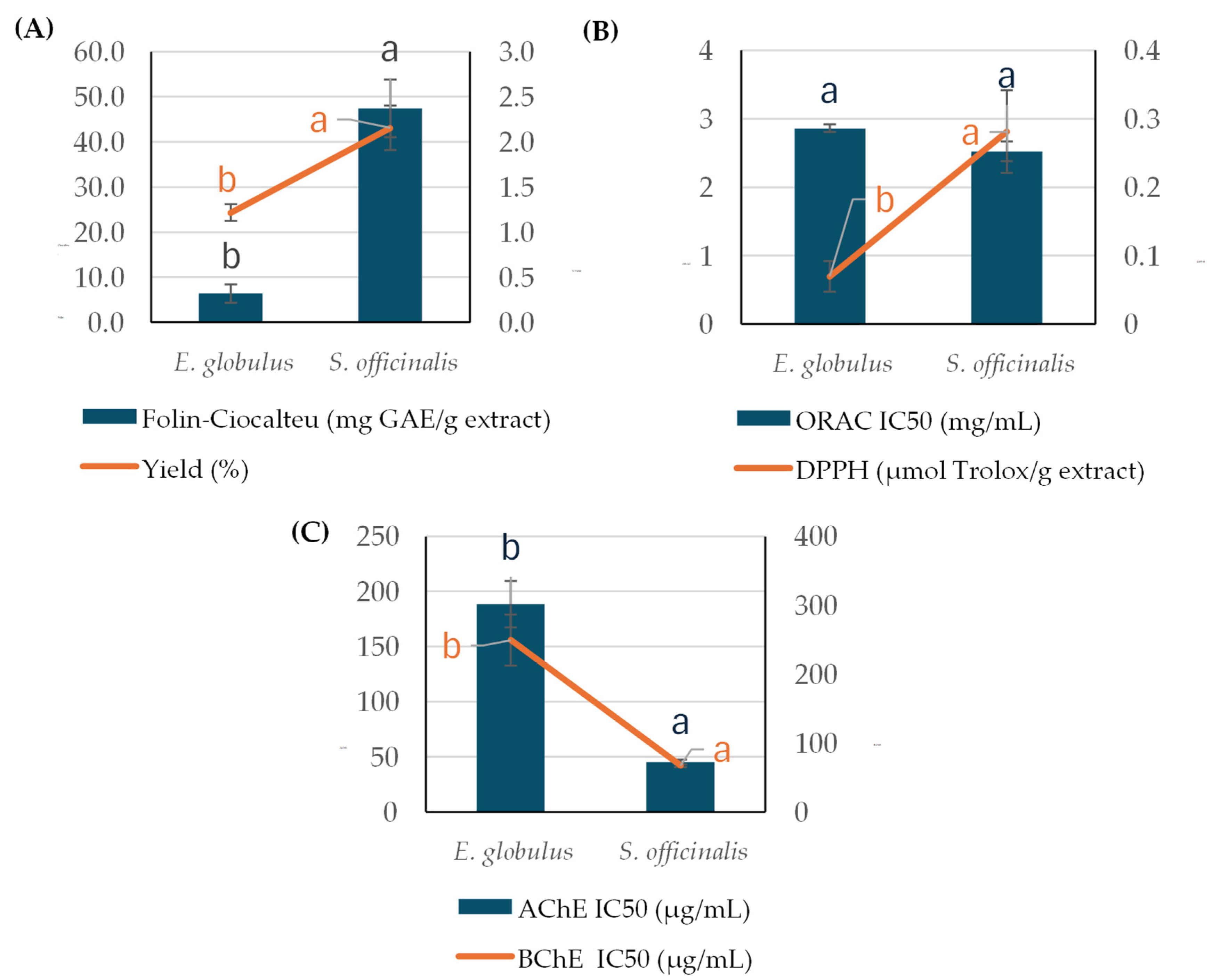
2.1. Bioactive Determination, Characterization, and BBB Permeability of Non-Polar Fractions Obtained from E. globulus and S. officinalis Leaves
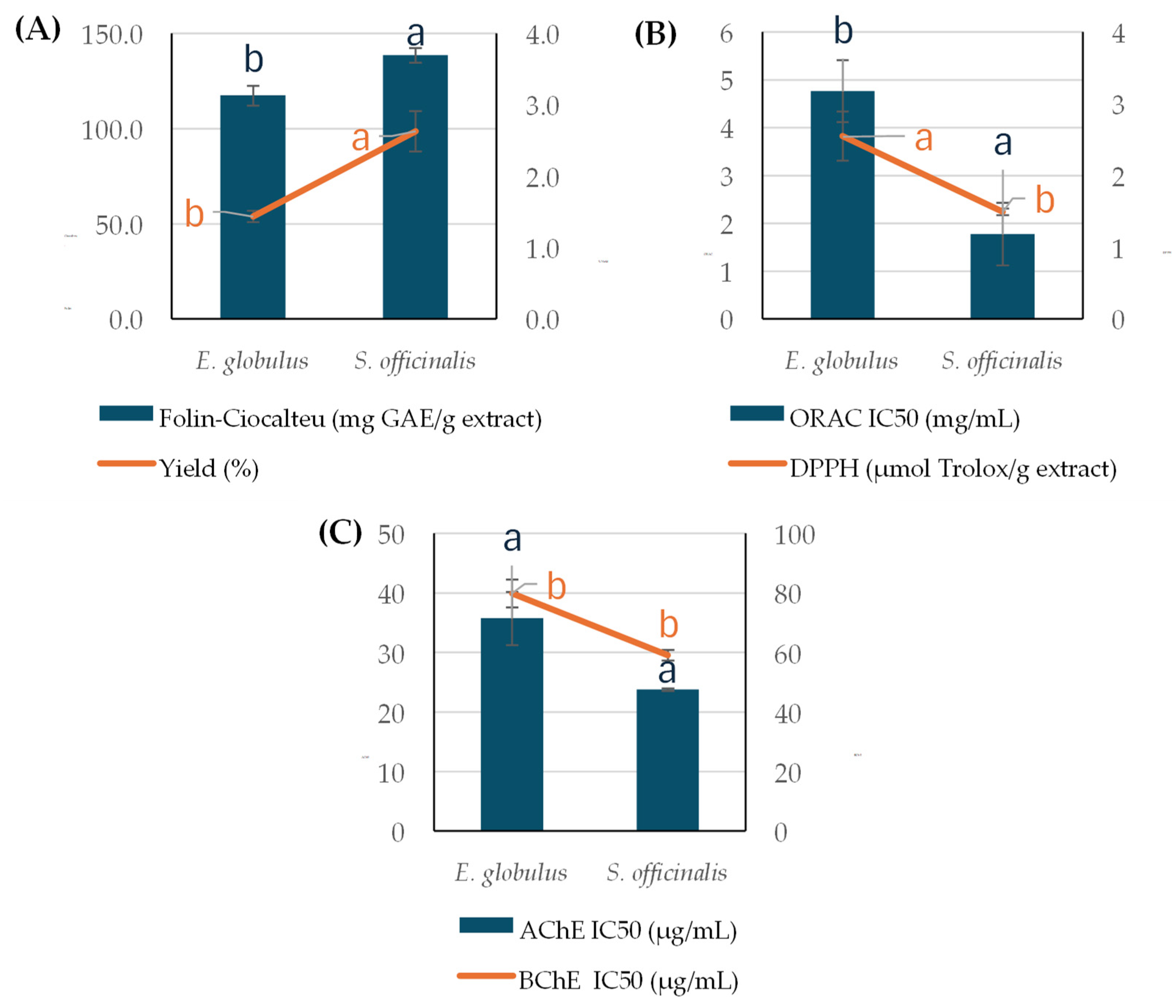
2.2. Bioactivity, Characterization, and BBB Permeability of Phenolic Compounds Obtained by Pressurized NaDES Extraction from the Residue of SC-CO2 Extraction
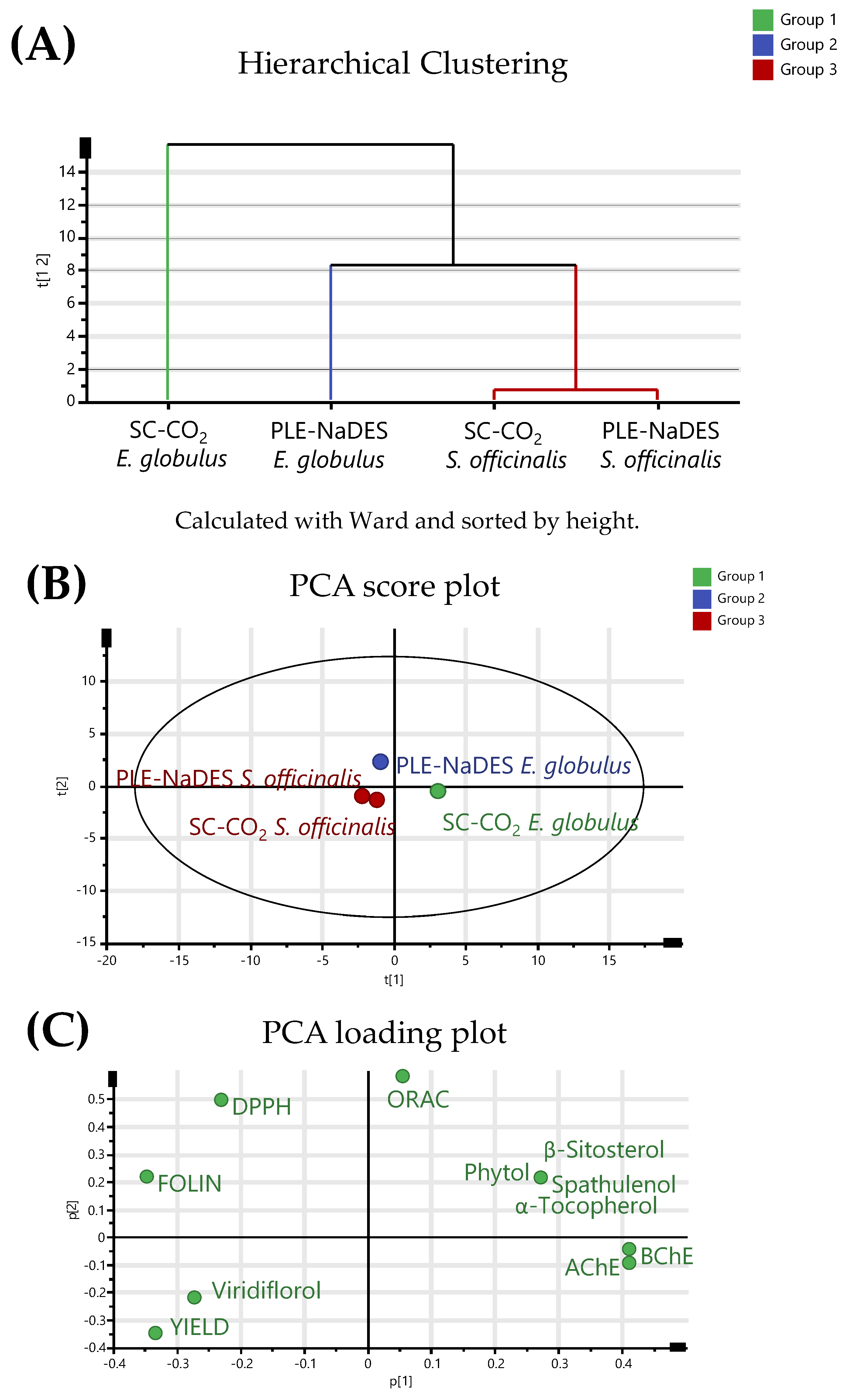
2.3. Relationship Between Individual Phenolic Compounds and Terpenoids, with the Antioxidant and Anticholinergic Capacities of SC-CO2 and PLE-NaDES Extracts from E. globulus and S. officinalis
3. Discussion
3.1. Elucidation of the Bioactive and Chemical Profiles of Non-Polar Compounds from E. globulus and S. officinalis SC-CO2 Extracts
3.2. Bioactive and Chemical Profiles of Polar Compounds from E. globulus and S. officinalis PLE-NaDES Extracts
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Samples
4.3. Supercritical CO2 Extraction of Non-Polar Compounds
4.4. Pressurized NaDES Extraction
4.5. Solid-Phase Extraction to Remove NaDES from the Extracts
4.6. Total Phenolic Content (TPC)
4.7. Anticholinergic Activity Determination
4.8. Antioxidant Capacity Determination
4.9. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
4.10. HPLC-DAD-QTOF-MS Analysis
4.11. Parallel Artificial Membrane Permeability Assay for the Blood–Brain Barrier (PAMPA-BBB)
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rausch, H.; Schröder, S.; Friedemann, T.; Cameron, S.; Kuchta, K.; Konrad, M. History and Present of European Traditional Herbal Medicine (Phytotherapy). In History, Present and Prospect of World Traditional Medicine; World Scientific: Singapore, 2024; pp. 131–234. ISBN 978-981-12-8710-7. [Google Scholar]
- Herbs and Spices—New Processing Technologies; Ahmad, R.S., Ed.; IntechOpen: London, UK, 2021; ISBN 978-1-83969-608-4. [Google Scholar]
- Spadaccino, G.; Frabboni, L.; Petruzzi, F.; Disciglio, G.; Mentana, A.; Nardiello, D.; Quinto, M. Essential Oil Characterization of Prunus spinosa L., Salvia officinalis L., Eucalyptus globulus L., Melissa officinalis L. and Mentha x piperita L. by a Volatolomic Approach. J. Pharm. Biomed. Anal. 2021, 202, 114167. [Google Scholar] [CrossRef] [PubMed]
- Halder, M.; Jha, S. Medicinal Plants and Bioactive Phytochemical Diversity: A Fountainhead of Potential Drugs Against Human Diseases. In Medicinal Plants: Biodiversity, Biotechnology and Conservation; Jha, S., Halder, M., Eds.; Sustainable Development and Biodiversity; Springer Nature: Singapore, 2023; Volume 33, pp. 39–93. ISBN 978-981-19-9935-2. [Google Scholar]
- Min, S.L.S.; Liew, S.Y.; Chear, N.J.Y.; Goh, B.H.; Tan, W.-N.; Khaw, K.Y. Plant Terpenoids as the Promising Source of Cholinesterase Inhibitors for Anti-AD Therapy. Biology 2022, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Penumala, M.; Zinka, R.B.; Shaik, J.B.; Gangaiah, D.A. In Vitro Screening of Three Indian Medicinal Plants for Their Phytochemicals, Anticholinesterase, Antiglucosidase, Antioxidant, and Neuroprotective Effects. BioMed Res. Int. 2017, 2017, 5140506. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, J.D.; Bueno, M.; Alvarez-Rivera, G.; Tudela, J.; Ibañez, E.; Cifuentes, A. In Vitro Neuroprotective Potential of Terpenes from Industrial Orange Juice by-Products. Food Funct. 2021, 12, 302–314. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for Extraction of Bioactive Compounds from Plant Materials: A Review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical Fluid Extraction of Essential Oils. TrAC Trends Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Wrona, O.; Rafińska, K.; Możeński, C.; Buszewski, B. Supercritical Fluid Extraction of Bioactive Compounds from Plant Materials. J. AOAC Int. 2017, 100, 1624–1635. [Google Scholar] [CrossRef]
- Knez, Ž.; Škerget, M.; KnezHrnčič, M. Principles of Supercritical Fluid Extraction and Applications in the Food, Beverage and Nutraceutical Industries. In Separation, Extraction and Concentration Processes in the Food, Beverage and Nutraceutical Industries; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3–38. ISBN 978-1-84569-645-0. [Google Scholar]
- Domingues, R.M.A.; Oliveira, E.L.G.; Freire, C.S.R.; Couto, R.M.; Simões, P.C.; Neto, C.P.; Silvestre, A.J.D.; Silva, C.M. Supercritical Fluid Extraction of Eucalyptus Globulus Bark—A Promising Approach for Triterpenoid Production. Int. J. Mol. Sci. 2012, 13, 7648–7662. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Oliveira, E.L.G.; Silvestre, A.J.D.; Silva, C.M. Supercritical Fluid Extraction of Triterpenic Acids from Eucalyptus Globulus Bark. J. Supercrit. Fluids 2012, 70, 137–145. [Google Scholar] [CrossRef]
- Jokić, S.; Molnar, M.; Jakovljević, M.; Aladić, K.; Jerković, I. Optimization of Supercritical CO2 Extraction of Salvia officinalis L. Leaves Targeted on Oxygenated Monoterpenes, α-Humulene, Viridiflorol and Manool. J. Supercrit. Fluids 2018, 133, 253–262. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Allawzi, M.; Al-Otoom, A.; Allaboun, H.; Al-Zoubi, A. Supercritical Fluid Extraction of Useful Compounds from Sage. Nat. Sci. 2012, 4, 544–551. [Google Scholar] [CrossRef]
- Kraujalis, P.; Kraujalienė, V.; Kazernavičiūtė, R.; Venskutonis, P.R. Supercritical Carbon Dioxide and Pressurized Liquid Extraction of Valuable Ingredients from Viburnum Opulus Pomace and Berries and Evaluation of Product Characteristics. J. Supercrit. Fluids 2017, 122, 99–108. [Google Scholar] [CrossRef]
- Bendif, H.; Adouni, K.; Miara, M.D.; Baranauskienė, R.; Kraujalis, P.; Venskutonis, P.R.; Nabavi, S.M.; Maggi, F. Essential Oils (EOs), Pressurized Liquid Extracts (PLE) and Carbon Dioxide Supercritical Fluid Extracts (SFE-CO2) from Algerian Thymus Munbyanus as Valuable Sources of Antioxidants to Be Used on an Industrial Level. Food Chem. 2018, 260, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Shala, A.Y.; Gururani, M.A. Phytochemical Properties and Diverse Beneficial Roles of Eucalyptus globulus Labill.: A Review. Horticulturae 2021, 7, 450. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological Properties of Salvia Officinalis and Its Components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Uddin, N.; Afrin, R.; Uddin, J.; Uddin, J.; Alam, A.H.M.K.; Rahman, A.A.; Sadik, G. Vanda Roxburghii Chloroform Extract as a Potential Source of Polyphenols with Antioxidant and Cholinesterase Inhibitory Activities: Identification of a Strong Phenolic Antioxidant. BMC Complement. Altern. Med. 2015, 15, 195. [Google Scholar] [CrossRef]
- Osorio-Tobón, J.F. Recent Advances and Comparisons of Conventional and Alternative Extraction Techniques of Phenolic Compounds. J. Food Sci. Technol. 2020, 57, 4299–4315. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Ramadon, D.; Jantan, I.; Mun’im, A. Natural Deep Eutectic Solvents (NADES): Phytochemical Extraction Performance Enhancer for Pharmaceutical and Nutraceutical Product Development. Plants 2021, 10, 2091. [Google Scholar] [CrossRef]
- de Oliveira, I.L.; Domínguez-Rodríguez, G.; Montero, L.; Viganó, J.; Cifuentes, A.; Rostagno, M.A.; Ibáñez, E. Advanced Extraction Techniques Combined with Natural Deep Eutectic Solvents for Extracting Phenolic Compounds from Pomegranate (Punica granatum L.) Peels. Int. J. Mol. Sci. 2024, 25, 9992. [Google Scholar] [CrossRef]
- Plaza, M.; Domínguez-Rodríguez, G.; Sahelices, C.; Marina, M.L. A Sustainable Approach for Extracting Non-Extractable Phenolic Compounds from Mangosteen Peel Using Ultrasound-Assisted Extraction and Natural Deep Eutectic Solvents. Appl. Sci. 2021, 11, 5625. [Google Scholar] [CrossRef]
- Bragagnolo, F.S.; Socas-Rodríguez, B.; Mendiola, J.A.; Cifuentes, A.; Funari, C.S.; Ibáñez, E. Pressurized Natural Deep Eutectic Solvents: An Alternative Approach to Agro-Soy by-Products. Front. Nutr. 2022, 9, 953169. [Google Scholar] [CrossRef] [PubMed]
- Grisales-Mejía, J.F.; Cedeño-Fierro, V.; Ortega, J.P.; Torres-Castañeda, H.G.; Andrade-Mahecha, M.M.; Martínez-Correa, H.A.; Álvarez-Rivera, G.; Mendiola, J.A.; Cifuentes, A.; Ibañez, E. Advanced NADES-Based Extraction Processes for the Recovery of Phenolic Compounds from Hass Avocado Residues: A Sustainable Valorization Strategy. Sep. Purif. Technol. 2024, 351, 128104. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Amador-Luna, V.M.; Benešová, K.; Pernica, M.; Parada-Alfonso, F.; Ibáñez, E. Biorefinery Approach with Green Solvents for the Valorization of Citrus Reticulata Leaves to Obtain Antioxidant and Anticholinergic Extracts. Food Chem. 2024, 456, 140034. [Google Scholar] [CrossRef]
- Krauß, S.; Vetter, W. Phytol and Phytyl Fatty Acid Esters: Occurrence, Concentrations, and Relevance. Eur. J. Lipid Sci. Technol. 2018, 120, 1700387. [Google Scholar] [CrossRef]
- Swantara, I.M.D.; Bawa, I.G.A.G.; Suprapta, D.N.; Agustina, K.K.; Temaja, I.G.R.M. Identification Michelia Alba Barks Extract Using Gas Chromatography-Mass Spectrometry (GC-MS) and Its Antifungal Properties to Inhibit Microbial Growth. Biodivers. J. Biol. Divers. 2020, 21, 1541–1550. [Google Scholar] [CrossRef]
- Tang, C.; Tao, G.; Wang, Y.; Liu, Y.; Li, J. Identification of α-Tocopherol and Its Oxidation Products by Ultra-Performance Liquid Chromatography Coupled with Quadrupole Time-of-Flight Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 669–677. [Google Scholar] [CrossRef]
- Rangra, N.; Samanta, S.; Pradhan, K. Evaluation of Acacia auriculiformis Benth. Leaves for Wound Healing Activity in Type 2 Diabetic Rats. Pharmacogn. Mag. 2021, 17, 129. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Villaverde, J.J.; Freire, C.S.R.; Domingues, M.R.M.; Neto, C.P.; Silvestre, A.J.D. Phenolic Composition and Antioxidant Activity of Eucalyptus grandis, E. urograndis (E. grandis×E. urophylla) and E. maidenii Bark Extracts. Ind. Crops Prod. 2012, 39, 120–127. [Google Scholar] [CrossRef]
- Boulekbache-Makhlouf, L.; Meudec, E.; Mazauric, J.; Madani, K.; Cheynier, V. Qualitative and Semi-quantitative Analysis of Phenolics in Eucalyptus globulus Leaves by High-performance Liquid Chromatography Coupled with Diode Array Detection and Electrospray Ionisation Mass Spectrometry. Phytochem. Anal. 2013, 24, 162–170. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Vilela, C.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Ultra-High Performance Liquid Chromatography Coupled to Mass Spectrometry Applied to the Identification of Valuable Phenolic Compounds from Eucalyptus Wood. J. Chromatogr. B 2013, 938, 65–74. [Google Scholar] [CrossRef]
- Serrano, C.A.; Villena, G.K.; Rodríguez, E.F.; Calsino, B.; Ludeña, M.A.; Ccana-Ccapatinta, G.V. Phytochemical Analysis for Ten Peruvian mentheae (Lamiaceae) by Liquid Chromatography Associated with High Resolution Mass Spectrometry. Sci. Rep. 2023, 13, 10714. [Google Scholar] [CrossRef] [PubMed]
- Ożarowski, M.; Piasecka, A.; Gryszczyńska, A.; Sawikowska, A.; Pietrowiak, A.; Opala, B.; Mikołajczak, P.Ł.; Kujawski, R.; Kachlicki, P.; Buchwald, W.; et al. Determination of Phenolic Compounds and Diterpenes in Roots of Salvia Miltiorrhiza and Salvia Przewalskii by Two LC–MS Tools: Multi-Stage and High Resolution Tandem Mass Spectrometry with Assessment of Antioxidant Capacity. Phytochem. Lett. 2017, 20, 331–338. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Quispe, C.; Llaique, H.; Villalobos, M.; Smeriglio, A.; Trombetta, D.; Ezzat, S.M.; Salem, M.A.; Zayed, A.; et al. Insights into Eucalyptus Genus Chemical Constituents, Biological Activities and Health-Promoting Effects. Trends Food Sci. Technol. 2019, 91, 609–624. [Google Scholar] [CrossRef]
- Perry, N.S.L.; Bollen, C.; Perry, E.K.; Ballard, C. Salvia for Dementia Therapy: Review of Pharmacological Activity and Pilot Tolerability Clinical Trial. Pharmacol. Biochem. Behav. 2003, 75, 651–659. [Google Scholar] [CrossRef]
- Imanshahidi, M.; Hosseinzadeh, H. The Pharmacological Effects of Salvia Species on the Central Nervous System. Phytother. Res. 2006, 20, 427–437. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; De Melo, M.M.R.; Portugal, I.; Silva, C.M. Supercritical Fluid Extraction of Eucalyptus Globulus Leaves. Experimental and Modelling Studies of the Influence of Operating Conditions and Biomass Pretreatment upon Yields and Kinetics. Sep. Purif. Technol. 2018, 191, 173–181. [Google Scholar] [CrossRef]
- Pavić, V.; Jakovljević, M.; Molnar, M.; Jokić, S. Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity. Plants 2019, 8, 16. [Google Scholar] [CrossRef]
- Menaker, A.; Kravets, M.; Koel, M.; Orav, A. Identification and Characterization of Supercritical Fluid Extracts from Herbs. Comptes Rendus Chim. 2004, 7, 629–633. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; De Melo, M.M.R.; Portugal, I.; Silva, C.M. Extraction of Eucalyptus Leaves Using Solvents of Distinct Polarity. Cluster Analysis and Extracts Characterization. J. Supercrit. Fluids 2018, 135, 263–274. [Google Scholar] [CrossRef]
- Pavlić, B.; Bera, O.; Vidović, S.; Ilić, L.; Zeković, Z. Extraction Kinetics and ANN Simulation of Supercritical Fluid Extraction of Sage Herbal Dust. J. Supercrit. Fluids 2017, 130, 327–336. [Google Scholar] [CrossRef]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant and Antiacetylcholinesterase Activities of Some Commercial Essential Oils and Their Major Compounds. Molecules 2011, 16, 7672–7690. [Google Scholar] [CrossRef] [PubMed]
- Nur, N.H.M.; Zaiton, M.S.S.; Jamshed, M.S.; Zaidul, I.S.M.; Mokhlesur, M.R.; Juahir, H.; Tengku, M.A.; Wan, N.W.B.; Nurul, I.M.S. The Superiority of Supercritical Fluid Extraction Over Steam Distillation and Solvent Extraction Methods for the Extraction of Aroma from Salacca Zalacca (Gaertn.) Voss. Orient. J. Chem. 2019, 35, 1669–1677. [Google Scholar] [CrossRef]
- Chen, W.N.; Chin, K.W.; Tang, K.S.; Agatonovic-Kustrin, S.; Yeong, K.Y. Neuroprotective, Neurite Enhancing, and Cholinesterase Inhibitory Effects of Lamiaceae Family Essential Oils in Alzheimer’s Disease Model. J. Herb. Med. 2023, 41, 100696. [Google Scholar] [CrossRef]
- Singh, A.; Ahmad, A.; Bushra, R. Supercritical Carbon Dioxide Extraction of Essential Oils from Leaves of Eucalyptus globulus L., Their Analysis and Application. Anal. Methods 2016, 8, 1339–1350. [Google Scholar] [CrossRef]
- Zardi-Bergaoui, A.; Znati, M.; Harzallah-Skhiri, F.; Jannet, H.B. Caryophyllene Sesquiterpenes from Pulicaria Vulgaris Gaertn.: Isolation, Structure Determination, Bioactivity and Structure−Activity Relationship. Chem. Biodivers. 2019, 16, e1800483. [Google Scholar] [CrossRef]
- Manjima, R.B.; Ramya, S.; Kavithaa, K.; Paulpandi, M.; Saranya, T.; Winster, S.B.H.; Balachandar, V.; Arul, N. Spathulenol Attenuates 6-Hydroxydopamine Induced Neurotoxicity in SH-SY5Y Neuroblastoma Cells. Gene Rep. 2021, 25, 101396. [Google Scholar] [CrossRef]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J.; Streicher, C.; Stimson, J. Identification and Characterization of Polyphenols and Volatile Terpenoid Compounds in Different Extracts of Garden Sage (Salvia officinalis L.). Pharmacogn. Res. 2020, 12, 149. [Google Scholar] [CrossRef]
- Aleksovski, S.A.; Sovová, H. Supercritical CO2 Extraction of Salvia officinalis L. J. Supercrit. Fluids 2007, 40, 239–245. [Google Scholar] [CrossRef]
- Radulescu, V.; Chiliment, S.; Oprea, E. Capillary Gas Chromatography—Mass Spectrometry of Volatile and Semi-Volatile Compounds of Salvia officinalis. J. Chromatogr. A 2004, 1027, 121–126. [Google Scholar] [CrossRef]
- Tundis, R.; Leporini, M.; Bonesi, M.; Rovito, S.; Passalacqua, N.G. Salvia officinalis L. from Italy: A Comparative Chemical and Biological Study of Its Essential Oil in the Mediterranean Context. Molecules 2020, 25, 5826. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, Z.-W.; Zhang, Y.; Wang, W.-H.; Wu, X.-Y.; Liu, S.-Z.; Bin, Y.-L.; Cai, B.-P.; Huang, S.-Y.; Fang, M.-J.; et al. Hinokione: An Abietene Diterpene with Pancreatic β Cells Regeneration and Hypoglycemic Activity, and Other Derivatives with Novel Structures from the Woods of Agathis Dammara. J. Nat. Med. 2024, 78, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, S.A. Blood—Brain Barrier Permeability Considerations for CNS-Targeted Compound Library Design. Curr. Opin. Chem. Biol. 2008, 12, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Brand-Rubalcava, P.A.; Tejeda-Martínez, A.R.; González-Reynoso, O.; Nápoles-Medina, A.Y.; Chaparro-Huerta, V.; Flores-Soto, M.E. β-Caryophyllene Decreases Neuroinflammation and Exerts Neuroprotection of Dopaminergic Neurons in a Model of Hemiparkinsonism Through Inhibition of the NLRP3 Inflammasome. Park. Relat. Disord. 2023, 117, 105906. [Google Scholar] [CrossRef]
- Yang, M.; Lv, Y.; Tian, X.; Lou, J.; An, R.; Zhang, Q.; Li, M.; Xu, L.; Dong, Z. Neuroprotective Effect of β-Caryophyllene on Cerebral Ischemia-Reperfusion Injury via Regulation of Necroptotic Neuronal Death and Inflammation: In Vivo and in Vitro. Front. Neurosci. 2017, 11, 583. [Google Scholar] [CrossRef]
- Rashed, A.A.; Rahman, A.Z.A.; Rathi, D.N.G. Essential Oils as a Potential Neuroprotective Remedy for Age-Related Neurodegenerative Diseases: A Review. Molecules 2021, 26, 1107. [Google Scholar] [CrossRef]
- Jäger, A.K.; Almqvist, J.P.; Vangsøe, S.A.K.; Stafford, G.I.; Adsersen, A.; Van Staden, J. Compounds from Mentha Aquatica with Affinity to the GABA-Benzodiazepine Receptor. S. Afr. J. Bot. 2007, 73, 518–521. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, X.; Chang, S.; Wang, Y.; Xu, Y.; Ran, S.; Huang, Z.; Li, P.; Li, J.; Zhang, L.; et al. Totarol Prevents Neuronal Injury in Vitro and Ameliorates Brain Ischemic Stroke: Potential Roles of Akt Activation and HO-1 Induction. Toxicol. Appl. Pharmacol. 2015, 289, 142–154. [Google Scholar] [CrossRef]
- Jamshaid, S.; Ahmed, D. Optimization of Ultrasound-Assisted Extraction of Valuable Compounds from Fruit of Melia Azedarach with Glycerol-Choline Chloride Deep Eutectic Solvent. Sustain. Chem. Pharm. 2022, 29, 100827. [Google Scholar] [CrossRef]
- Zannou, O.; Pashazadeh, H.; Ghellam, M.; Ibrahim, S.A.; Koca, I. Extraction of Anthocyanins from Borage (Echium amoenum) Flowers Using Choline Chloride and a Glycerol-Based, Deep Eutectic Solvent: Optimization, Antioxidant Activity, and In Vitro Bioavailability. Molecules 2021, 27, 134. [Google Scholar] [CrossRef]
- Domínguez-Rodríguez, G.; Amador-Luna, V.M.; Mendiola, J.A.; Parada-Alfonso, F.; Ibáñez, E. Development of a Sustainable Extraction and Storage Stability of Antioxidant and Anticholinergic Pressurized Natural Deep Eutectic Solvent Extracts from Citrus Reticulata Leaves. Food Bioprod. Process. 2025, 149, 70–81. [Google Scholar] [CrossRef]
- Farhat, M.B.; Landoulsi, A.; Chaouch-Hamada, R.; Sotomayor, J.A.; Jordán, M.J. Characterization and Quantification of Phenolic Compounds and Antioxidant Properties of Salvia Species Growing in Different Habitats. Ind. Crops Prod. 2013, 49, 904–914. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Poliwoda, A.; Petecka, M.; Buslovych, O.; Shlyapnikov, V.A.; Wieczorek, P.P. Antioxidant Phenolic Compounds in Salvia officinalis L. and Salvia sclarea L. Ecol. Chem. Eng. S 2018, 25, 133–142. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M. Value-Added Compound Recovery from Invasive Forest for Biofunctional Applications: Eucalyptus Species as a Case Study. Molecules 2020, 25, 4227. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Muñiz-Mouro, A.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Green Approaches for the Extraction of Antioxidants from Eucalyptus Leaves. Ind. Crops Prod. 2019, 138, 111473. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.Y.; Son, Y.G.; Kang, S.D.; Lee, S.W.; Kim, K.D.; Kim, J.Y. Characterization of Chemical Composition and Antioxidant Activity of Eucalyptus Globulus Leaves Under Different Extraction Conditions. Appl. Sci. 2023, 13, 9984. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Freire, C.S.R.; Domingues, M.R.M.; Silvestre, A.J.D.; Neto, C.P. Characterization of Phenolic Components in Polar Extracts of Eucalyptus globulus Labill. Bark by High-Performance Liquid Chromatography–Mass Spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef]
- Nguyen, H.D. Neurotherapeutic Effects of Quercetin and Its Metabolite Compounds on Cognitive Impairment and Parkinson’s Disease: An In Silico Study. Eur. J. Drug Metab. Pharmacokinet. 2023, 48, 151–169. [Google Scholar] [CrossRef]
- Sabarathinam, S. Unraveling the Therapeutic Potential of Quercetin and Quercetin-3-O-Glucuronide in Alzheimer’s Disease through Network Pharmacology, Molecular Docking, and Dynamic Simulations. Sci. Rep. 2024, 14, 14852. [Google Scholar] [CrossRef]
- Hossain, M.B.; Brunton, N.P.; Martin-Diana, A.B.; Barry-Ryan, C. Application of Response Surface Methodology to Optimize Pressurized Liquid Extraction of Antioxidant Compounds from Sage (Salvia officinalis L.), Basil (Ocimum basilicum L.) and Thyme (Thymus vulgaris L.). Food Funct. 2010, 1, 269–277. [Google Scholar] [CrossRef]
- Ollanketo, M.; Peltoketo, A.; Hartonen, K.; Hiltunen, R.; Riekkola, M.-L. Extraction of Sage (Salvia officinalis L.) by Pressurized Hot Water and Conventional Methods: Antioxidant Activity of the Extracts. Eur. Food Res. Technol. 2002, 215, 158–163. [Google Scholar] [CrossRef]
- Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Phytochemical Composition of Fractions Isolated from Ten Salvia Species by Supercritical Carbon Dioxide and Pressurized Liquid Extraction Methods. Food Chem. 2017, 224, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gomes, P.C.; Seabra, R.M.; Andrade, P.B.; Fernandes-Ferreira, M. Phenolic Antioxidant Compounds Produced by in Vitro Shoots of Sage (Salvia officinalis L.). Plant Sci. 2002, 162, 981–987. [Google Scholar] [CrossRef]
- Spréa, R.M.; Caleja, C.; Pinela, J.; Finimundy, T.C.; Calhelha, R.C.; Kostić, M.; Sokovic, M.; Prieto, M.A.; Pereira, E.; Amaral, J.S.; et al. Comparative Study on the Phenolic Composition and In Vitro Bioactivity of Medicinal and Aromatic Plants from the Lamiaceae Family. Food Res. Int. 2022, 161, 111875. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, I.M.; George, M.Y.; Menze, E.T.; Mahmoud, M.; Botros, M.; Essam, M.; Ashmawy, I.; Shendi, P.; Hany, A.; Galal, M.; et al. Insights into the Neuroprotective Effects of Salvia officinalis L. and Salvia microphylla Kunth in the Memory Impairment Rat Model. Food Funct. 2022, 13, 2253–2268. [Google Scholar] [CrossRef]
- Faridzadeh, A.; Salimi, Y.; Ghasemirad, H.; Kargar, M.; Rashtchian, A.; Mahmoudvand, G.; Karimi, M.A.; Zerangian, N.; Jahani, N.; Masoudi, A.; et al. Neuroprotective Potential of Aromatic Herbs: Rosemary, Sage, and Lavender. Front. Neurosci. 2022, 16, 909833. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Plaza, M.; Marina, M.L.; García, M.C. Sustainable Extraction of Proteins and Bioactive Substances from Pomegranate Peel (Punica granatum L.) Using Pressurized Liquids and Deep Eutectic Solvents. Innov. Food Sci. Emerg. Technol. 2020, 60, 102314. [Google Scholar] [CrossRef]
- Da Silva, D.T.; Pauletto, R.; da Silva Cavalheiro, S.; Bochi, V.C.; Rodrigues, E.; Weber, J.; da Silva, C.d.B.; Morisso, F.D.P.; Barcia, M.T.; Emanuelli, T. Natural Deep Eutectic Solvents as a Biocompatible Tool for the Extraction of Blueberry Anthocyanins. J. Food Compos. Anal. 2020, 89, 103470. [Google Scholar] [CrossRef]
- Koşar, M.; Dorman, H.J.D.; Hiltunen, R. Effect of an Acid Treatment on the Phytochemical and Antioxidant Characteristics of Extracts from Selected Lamiaceae Species. Food Chem. 2005, 91, 525–533. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Sánchez-Martínez, J.D.; Valdés, A.; Gallego, R.; Suárez-Montenegro, Z.J.; Alarcón, M.; Ibañez, E.; Alvarez-Rivera, G.; Cifuentes, A. Blood–Brain Barrier Permeability Study of Potential Neuroprotective Compounds Recovered from Plants and Agri-Food by-Products. Front. Nutr. 2022, 9, 924596. [Google Scholar] [CrossRef]
- Könczöl, Á.; Rendes, K.; Dékány, M.; Müller, J.; Riethmüller, E.; Balogh, G.T. Blood-Brain Barrier Specific Permeability Assay Reveals N -Methylated Tyramine Derivatives in Standardised Leaf Extracts and Herbal Products of Ginkgo Biloba. J. Pharm. Biomed. Anal. 2016, 131, 167–174. [Google Scholar] [CrossRef]
| ID | Proposed Compound | RT (min) | Molecular Formula | Measured Mass | Main Fragment Ions (m/z) | SC-CO2 Extract * | Permeable BBB Fraction * | Non-Permeable BBB Fraction * |
|---|---|---|---|---|---|---|---|---|
| 1 | Cryptone | 9.58 | C9H14O | 138 | 138, 97, 96, 95, 81, 67, 43 | 743 ± 145 | - | 293 ± 37 |
| 2 | Cineole | 10.13 | C10H18O | 154 | 126, 111, 58, 43 | 86 ± 8 | - | 68 ± 6 |
| 3 | Limonene dioxide | 10.45 | C10H16O2 | 168 | 153, 107, 55, 43 | 271 ± 75 | - | 154 ± 8 |
| 4 | Camphene | 11.06 | C10H16 | 152 | 136, 121, 107, 93 | 415 ± 59 | - | 149 ± 6 |
| 5 | Terpineol | 12.08 | C10H18O | 154 | 153, 139, 121, 83, 93, 69, 55, 43 | 573 ± 53 | - | 57 ± 6 |
| 6 | Linalool | 12.27 | C10H18O | 154 | 122, 121, 93, 83, 69, 67, 55, 43 | 385 ± 52 | - | 12 ± 1 |
| 7 | Terpineol isomer | 12.44 | C10H18O | 154 | 111, 93, 71, 69, 57, 55, 43 | 548 ± 87 | - | 208 ± 19 |
| 8 | Piperitone oxide | 13.08 | C10H16O2 | 168 | 139, 125, 97, 69, 55, 43, 41 | 678 ± 3 | - | 196 ± 14 |
| 9 | Spathulenol | 14.92 | C15H24O | 220 | 205, 159, 119, 91, 43 | 9754 ± 731 | - | 56 ± 3 |
| 10 | Caryophyllene oxide | 16.61 | C15H24O | 220 | 161, 109, 107, 93, 79, 43 | 3195 ± 268 | 233 ± 6 | 880 ± 131 |
| 11 | Globulol | 16.66 | C15H26O | 222 | 204, 161, 109, 93, 69, 55, 43 | 2182 ± 90 | 103 ± 6 | 549 ± 62 |
| 12 | Aromadendrene epoxide | 16.84 | C15H24O | 220 | 149, 147, 121, 107, 105, 95, 91, 55, 43 | 1482 ± 42 | 83 ± 6 | 280 ± 36 |
| 13 | Acetoxy-kauranal | 16.92 | C22H34O3 | 346 | 159, 147, 135, 131, 109, 107, 55, 43 | 1242 ± 37 | 124 ± 6 | 421 ± 48 |
| 14 | Patchoulane | 17.12 | C15H26 | 206 | 149, 107, 105, 91, 79, 67, 55, 41 | 526 ± 8 | - | - |
| 15 | Viridiflorol | 17.80 | C15H26O | 222 | 204, 189, 149, 135, 109, 95, 93, 81, 71, 59, 43 | 844 ± 12 | 125 ± 16 | 565 ± 70 |
| 16 | Andrographolide | 17.85 | C20H30O5 | 350 | 187, 159, 145, 133, 107, 105, 91, 79, 77, 67, 55, 43. 41 | 1171 ± 122 | - | 116 ± 18 |
| 17 | Guaiol | 18.08 | C15H26O | 222 | 162, 161, 147, 133, 119, 107, 105, 91, 81, 67, 59, 43 | 792 ± 58 | 69 ± 18 | 378 ± 62 |
| 18 | Bisabolene epoxide | 18.96 | C15H24O | 220 | 149, 135, 121, 109, 105, 93, 91, 79, 67, 57, 55, 43 | 542 ± 9 | - | 210 ± 38 |
| 19 | Phytol | 21.62 | C20H40O | 296 | 278, 197, 137, 123, 111, 95, 81, 71, 55, 43 | 14,900 ± 4320 | - | 3031 ± 956 |
| 20 | α-Tocopherol | 36.08 | C29H50O2 | 430 | 430, 205, 165, 121, 57, 43 | 5129 ± 431 | - | 361 ± 151 |
| 21 | β-Sitosterol | 38.80 | C29H50O | 414 | 414, 396, 329, 303, 255, 231, 163, 145, 119, 105, 81, 55, 43 | 4736 ± 796 | - | 706 ± 12 |
| ID | Proposed Compound | RT (min) | Molecular Formula | Measured Mass | Main Fragment Ions (m/z) | SC-CO2 Extract * | Permeable BBB Fraction * | Non-Permeable BBB Fraction * |
|---|---|---|---|---|---|---|---|---|
| 1 | Pinene | 5.58 | C10H16 | 136 | 136, 121, 105, 93, 91, 79,77, 67, 53, 41 | 245 ± 4 | - | 207 ± 26 |
| 2 | Borneol | 9.28 | C10H18O | 154 | 139, 121, 110, 95, 79, 67, 55, 43, 41 | 92 ± 54 | - | - |
| 3 | Citronellol | 11.29 | C10H20O | 156 | 138, 123, 109, 96, 95, 81, 69, 55, 44, 41 | 29 ± 12 | - | - |
| 4 | Camphene | 11.80 | C10H16 | 152 | 136, 121, 107, 93 | 2976 ± 477 | 158 ± 1 | 219 ± 57 |
| 5 | Artemiseole | 12.85 | C10H16O | 152 | 137, 109, 95, 91, 79, 77, 67, 57, 55, 43, 41 | 93 ± 5 | - | - |
| 6 | Aromadendrene | 13.19 | C15H24 | 204 | 204, 189, 162, 161, 147, 133, 122, 119, 105, 93, 91, 79, 67, 55, 43, 41 | 106 ± 43 | - | 27 ± 7 |
| 7 | Myrtenol | 13.45 | C10H16O | 152 | 121, 119, 108, 96, 91, 82, 79, 77, 67, 55, 53, 43, 41 | 164 ± 18 | - | 161 ± 24 |
| 8 | α-Humulene | 13.90 | C15H24 | 204 | 204, 161, 121, 119, 115, 107, 105, 103, 95, 93, 91, 81, 79, 77, 67, 65, 55, 53, 43, 40 | 80 ± 13 | - | - |
| 9 | Palustrol | 14.83 | C15H26O | 222 | 204, 189, 161, 147, 133, 122, 111, 107, 95, 93, 81, 79, 69, 67, 55, 53, 41 | 1250 ± 150 | - | - |
| 10 | Spathulenol | 14.92 | C15H24O | 220 | 205, 202, 159, 147, 131, 119, 105, 93, 91, 79, 67, 55, 43, 41 | 407 ± 46 | . | - |
| 11 | Patchulane | 15.01 | C15H26 | 206 | 135, 121, 109, 108, 107, 93, 91, 79, 77, 69, 67, 55, 43, 41 | 277 ± 31 | - | - |
| 12 | Viridiflorol | 15.12 | C15H26O | 222 | 204, 189, 161, 147, 133, 121, 109, 107, 105, 93, 81, 69, 67, 55, 43 | 5508 ± 397 | - | - |
| 13 | Ledol | 15.25 | C15H26O | 222 | 204, 189, 161, 147, 133, 122, 109, 107, 95, 93, 81, 69, 55, 53, 43 | 1556 ± 149 | - | - |
| 14 | Pinene isomer | 16.57 | C10H16 | 136 | 137, 121, 119, 107, 93, 91, 79, 69, 55, 41 | 322.5 ± 0.4 | - | 25 ± 5 |
| 15 | Germacrene-D | 18.89 | C15H24 | 204 | 161, 133, 119, 105, 95, 91, 79, 67, 55, 41 | 508.0 ± 0.1 | - | 63 ± 13 |
| 16 | Manool | 22.40 | C20H34O | 290 | 272, 257, 204, 189, 177, 161, 148, 137, 121, 109, 107, 95, 81, 71, 69, 55, 43, 41 | 26,557 ± 11 | - | 2779 ± 466 |
| 17 | Phytol | 23.26 | C20H40O | 296 | 196, 137, 123, 111, 95, 83, 81, 72, 71, 57, 55, 43, 41 | 6641 ± 127 | - | 1792 ± 298 |
| 18 | Totarol | 30.46 | C20H28O2 | 300 | 300, 285, 257, 243, 229, 217, 217, 128, 115, 83, 69, 55, 43, 41 | 1824 ± 162 | 78 ± 7 | 435 ± 18 |
| 19 | Carnosol | 31.21 | C20H26O4 | 330 | 287, 286, 271, 243, 215, 204, 187, 143, 128, 115, 91, 77, 55, 43, 41 | 752 ± 82 | - | - |
| 20 | Hinokione | 42.16 | C20H28O2 | 300 | 300, 285, 243, 213, 187, 115, 91, 83, 69, 55, 43 | 21,080 ± 64 | 69 ± 9 | 1434 ± 145 |
| 21 | Ferruginol | 44.06 | C20H30O | 286 | 286, 271, 253, 229, 189, 147, 105, 69, 55 | 4334 ± 82 | - | 257 ± 35 |
| 22 | Farnesol | 44.51 | C15H26O | 222 | 161, 136, 121, 107, 95, 93, 81, 69, 55, 41 | 387 ± 8 | - | 262 ± 32 |
| 23 | α-Tocopherol | 53.87 | C29H50O2 | 430 | 430, 205, 165, 121, 57, 43, 41 | 2358 ± 77 | - | 439 ± 48 |
| 24 | β-Sitosterol | 59.00 | C29H50O | 414 | 414, 396, 329, 303, 255, 213, 173, 159, 145, 133, 131, 119, 109, 105, 95, 91, 81, 69, 67, 57, 55, 43, 41 | 2905 ± 27 | - | 2152 ± 267 |
| ID | RT (min) | Proposed Compound | m/z [M-H]− | Main Fragment Ions | PLE-NaDES * | Permeable Fraction * | Non-Permeable Fraction * |
|---|---|---|---|---|---|---|---|
| 1 | 1.568 | Gallic acid | 169.0151 | 125.0236, 108.0207, 79.0183, 69.0336, 51.0231 | 142 ± 9 | 85 ± 16 | - |
| 2 | 2.798 | HHDP galloylglucose | 633.0768 | 301.0002, 275.0217, 249.0374, 231.7478 | 15 ± 4 | 10 ± 1 | - |
| 3 | 2.836 | Protocatechuic acid | 153.0200 | 109.0285, 108.0213 | 14 ± 2 | 13 ± 1 | 3.3 ± 0.6 |
| 4 | 2.953 | Pedunculagin | 783.0736 | 481.0623, 300.9975, 275.0200 | 19 ± 2 | 1.1 ± 0.7 | - |
| 5 | 3.029 | Methyl gallate | 183.0307 | 168.0088, 156.0095, 138.9440, 124.0158, 101.6277, 87.2672, 78.0102 | 21 ± 2 | 19 ± 5 | - |
| 6 | 3.264 | Catechin | 289.0741 | 246.0853, 221.0821, 203.0697, 175.0747, 149.0233, 125.0238, 109.0301, 89.0242, 71.0126, 59.0145 | 105 ± 4 | 98 ± 13 | - |
| 7 | 3.338 | Digalloylglucose | 483.0815 | 331.0669, 313.0553, 271.0458 | 60 ± 6 | 25 ±11 | - |
| 8 | 3.409 | Digalloylglucose isomer | 483.0815 | 313.0531, 271.0477, 211.0242, 169.0139, 151.0027, 124.0151 | 54 ± 15 | 56 ± 4 | - |
| 9 | 3.459 | Chlorogenic acid | 353.0904 | 191.0565, 179.0358, 173.0455, 161.0615, 121.8473 | 17 ± 1 | - | - |
| 10 | 3.815 | Tellimagrandin | 785.0890 | 633.0647, 615.0651, 483.0839, 419.0577, 300.9995, 275.0198 | 45 ± 5 | - | - |
| 11 | 4.089 | Trigalloylglucose | 635.0933 | 483.0803, 465.0670, 313.0550 | 21 ± 4 | 22.3 ± 0.9 | - |
| 12 | 4.857 | Tetragalloylglucose | 787.1044 | 635.0847, 617.0757, 465.0603, 169.0132 | 14 ± 2 | 18 ± 1 | - |
| 13 | 4.981 | Methylphloroglucinol-digalloyl glucose | 605.1186 | 453.1073, 313.0541, 169.0140 | 30 ± 9 | 27 ±5 | - |
| 14 | 5.130 | Quercetin-galactoside-gallate | 615.1025 | 463.0895, 373.0714, 300.0281, 271.0260 | 148.2 ± 0.3 | 115 ± 4 | - |
| 15 | 5.222 | Isorhamnetin-hexoside | 477.0711 | 315.0149, 299.9903 | 41 ± 1 | 27 ± 6 | - |
| 16 | 5.346 | Quercetin-glucuronide | 477.0659 | 301.0346 | 407 ± 15 | 394 ± 12 | - |
| 17 | 5.941 | Methyl-ellagic acid-pentose | 447.0954 | 315.0156, 301.0369 | 44 ± 5 | 13 ± 6 | - |
| 18 | 5.992 | Quercetin-hexoside | 463.0917 | 301.0361, 258.0544, 179.9977, 151.0038, 107.0140 | 16 ± 1 | 19 ± 7 | - |
| 19 | 6.217 | Isorhamnetin-rhamnoside | 461.0763 | 315.0158, 301.0319 | 24 ± 4 | 15 ± 9 | - |
| 20 | 7.116 | Quercetin | 301.0376 | 178.9981, 151.0038, 121.0302, 107.0142 | 31 ± 2 | 48 ± 8 | - |
| 21 | 7.459 | Dimethyl-hesperetin | 329.0331 | 314.0068, 298.9847, 285.0033 | 7 ± 1 | 8 ± 3 | 0.6 ± 0.1 |
| 22 | 7.695 | Naringenin | 271.0628 | 151.0036, 119.0506 | 3.2 ± 0.7 | - | 1.4 ± 0.4 |
| 23 | 7.816 | Cypellocarpin C | 519.1903 | 335.0768, 233.0436 | 5.7 ± 1.5 | - | - |
| ID | RT (min) | Proposed Compound | m/z [M-H]− | Main Fragment Ions | PLE-NaDES * | Permeable Fraction * | Non-Permeable Fraction * |
|---|---|---|---|---|---|---|---|
| 1 | 2.031 | 3,4-dihydroxyphenyl lactic acid “danshensu” | 197.0417 | 179.0388, 135.0447, 123.0464 | 17 ± 2 | 12 ± 6 | - |
| 2 | 3.121 | Chicoric acid | 473.1216 | 293.0871, 179.0353, 161.0241, 135.0449 | 59 ± 28 | 75 ± 12 | - |
| 3 | 3.298 | Cafeic acid O-hexoside | 341.0820 | 281.0664, 251.0569, 233.0462, 179.0352, 161.0235 | 65 ± 12 | 107 ± 12 | - |
| 4 | 3.406 | Quinic acid | 191.0526 | 126.6511 | 16 ± 2 | - | - |
| 5 | 3.441 | Cafeoylquinic acid | 353.0815 | 191.0563, 178.0511, 164.6262 | 88 ± 2 | - | - |
| 6 | 3.540 | Caffeic acid | 179.0320 | 164.9249, 135.0461, 118.0360, 117.0345, 107.0500, 89.0399, | 225 ± 10 | 23 ± 2 | 7 ± 1 |
| 7 | 3.768 | p-Coumaric acid | 163.0376 | 119.0388 | 19.8 ± 0.7 | 12 ± 2 | - |
| 8 | 3.959 | Medioresinol | 387.1586 | 207.1027, 163.1117 | 271 ± 11 | 306 ± 26 | - |
| 9 | 4.101 | Salvianic acid C | 377.0810 | 179.0352, 161.0245, 135.0455 | 117 ± 2 | 197 ± 14 | - |
| 10 | 4.725 | Lithospermic acid | 537.0933 | 493.1153, 357.0586, 313.0750, 295.0617, 179.0350, 135.0440 | 104 ± 4 | - | - |
| 11 | 4.915 | Quercetin-glucuronide | 477.0584 | 301.0358 | 53 ± 3 | 43 ± 18 | - |
| 12 | 5.248 | Rosmarinic acid glucoside | 521.1198 | 429.0422, 359.0976, 311.0569, 161.0247 | 8.6 ± 0.5 | - | - |
| 13 | 5.683 | Isorhamnetin-hexoside | 477.0951 | 462.0713, 323.0740, 315.0700, 300.0216 | 93 ± 7 | - | - |
| 14 | 5.870 | Apigenin-rutinoside | 577.1461 | 269.046 | 8.3 ± 0.6 | - | - |
| 15 | 5.905 | Sagerinic acid | 719.1470 | 359.0771, 197.0458, 179.0342, 161.0241 | 174 ± 94 | 171 ± 39 | - |
| 16 | 6.076 | Rosmarinic acid | 359.0710 | 197.5398, 179.0344, 161.0240, 151.0385, 133.0292 | 301 ± 35 | 312 ± 12 | 1.07 ± 0.03 |
| 17 | 7.091 | Ethyl caffeate | 207.0630 | 179.0345, 161.0238, 135.0449 | 120 ± 5 | 79 ± 3 | 0.75 ± 0.05 |
| 18 | 7.991 | Apigenin | 269.0410 | 151.0024, 117.0347 | 13 ± 4 | 16 ± 6 | 12 ± 9 |
| 19 | 8.098 | Dimethylrosmarinic acid | 387.1387 | 179.0341, 161.0253, 135.0460 | 15 ± 5 | 26 ± 3 | 4 ±1 |
| 20 | 8.521 | Epirosmanol | 345.1653 | 301.1816, 283.1712 | 198 ± 12 | 108 ± 16 | 26 ± 7 |
| 21 | 8.749 | Dimethylquercetin | 329.1348 | 314.1499, 301.1833, 287.2003, 179.0319, 161.0253, 151.0769, 133.0289, 119.0347 | 8 ± 1 | - | - |
| 22 | 9.222 | Rosmadial | 343.1497 | 315.1580, 299.1693, 285.1906, 256.1099, 243.1050, 227.1169 | 12 ± 1 | 7.7 ± 0.4 | 10 ± 3 |
| 23 | 9.318 | Carnosol | 329.1710 | 285.1876 | 1465 ± 51 | 309 ± 68 | 30 ± 6 |
| 24 | 9.623 | Carnosic acid | 331.1860 | 287.2031, 271.1711, 189.0928, 157.0673 | 1443 ± 247 | 12 ±1 | 62 ± 13 |
| 25 | 9.820 | Methyl carnosate | 345.2018 | 301.2168, 286.1940, 271.1709, 257.1541 | 1264 ± 22 | 20 ± 8 | 635 ± 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, E.; Domínguez-Rodríguez, G.; Mannina, L.; Cifuentes, A.; Ibáñez, E. Sequential Obtention of Blood–Brain Barrier-Permeable Non-Polar and Polar Compounds from Salvia officinalis L. and Eucalyptus globulus Labill. with Neuroprotective Purposes. Int. J. Mol. Sci. 2025, 26, 601. https://doi.org/10.3390/ijms26020601
Romano E, Domínguez-Rodríguez G, Mannina L, Cifuentes A, Ibáñez E. Sequential Obtention of Blood–Brain Barrier-Permeable Non-Polar and Polar Compounds from Salvia officinalis L. and Eucalyptus globulus Labill. with Neuroprotective Purposes. International Journal of Molecular Sciences. 2025; 26(2):601. https://doi.org/10.3390/ijms26020601
Chicago/Turabian StyleRomano, Enrico, Gloria Domínguez-Rodríguez, Luisa Mannina, Alejandro Cifuentes, and Elena Ibáñez. 2025. "Sequential Obtention of Blood–Brain Barrier-Permeable Non-Polar and Polar Compounds from Salvia officinalis L. and Eucalyptus globulus Labill. with Neuroprotective Purposes" International Journal of Molecular Sciences 26, no. 2: 601. https://doi.org/10.3390/ijms26020601
APA StyleRomano, E., Domínguez-Rodríguez, G., Mannina, L., Cifuentes, A., & Ibáñez, E. (2025). Sequential Obtention of Blood–Brain Barrier-Permeable Non-Polar and Polar Compounds from Salvia officinalis L. and Eucalyptus globulus Labill. with Neuroprotective Purposes. International Journal of Molecular Sciences, 26(2), 601. https://doi.org/10.3390/ijms26020601










