Immunotherapy in Oncogene-Addicted NSCLC: Evidence and Therapeutic Approaches
Abstract
1. Introduction
2. Intrinsic Mechanisms of Resistance to ICIs in Oncogene-Addicted NSCLC
3. Efficacy of Immunotherapy in NSCLC with Oncogene Drivers
3.1. EGFR
3.2. EGFR Ex20Ins
3.3. ERBB2
3.4. ALK and ROS1 Rearrangement
3.5. RET Rearrangement
3.6. KRAS Mutations
3.7. BRAF Mutations
3.8. MET Alterations
3.9. Perioperative Immunotherapy for NSCLC
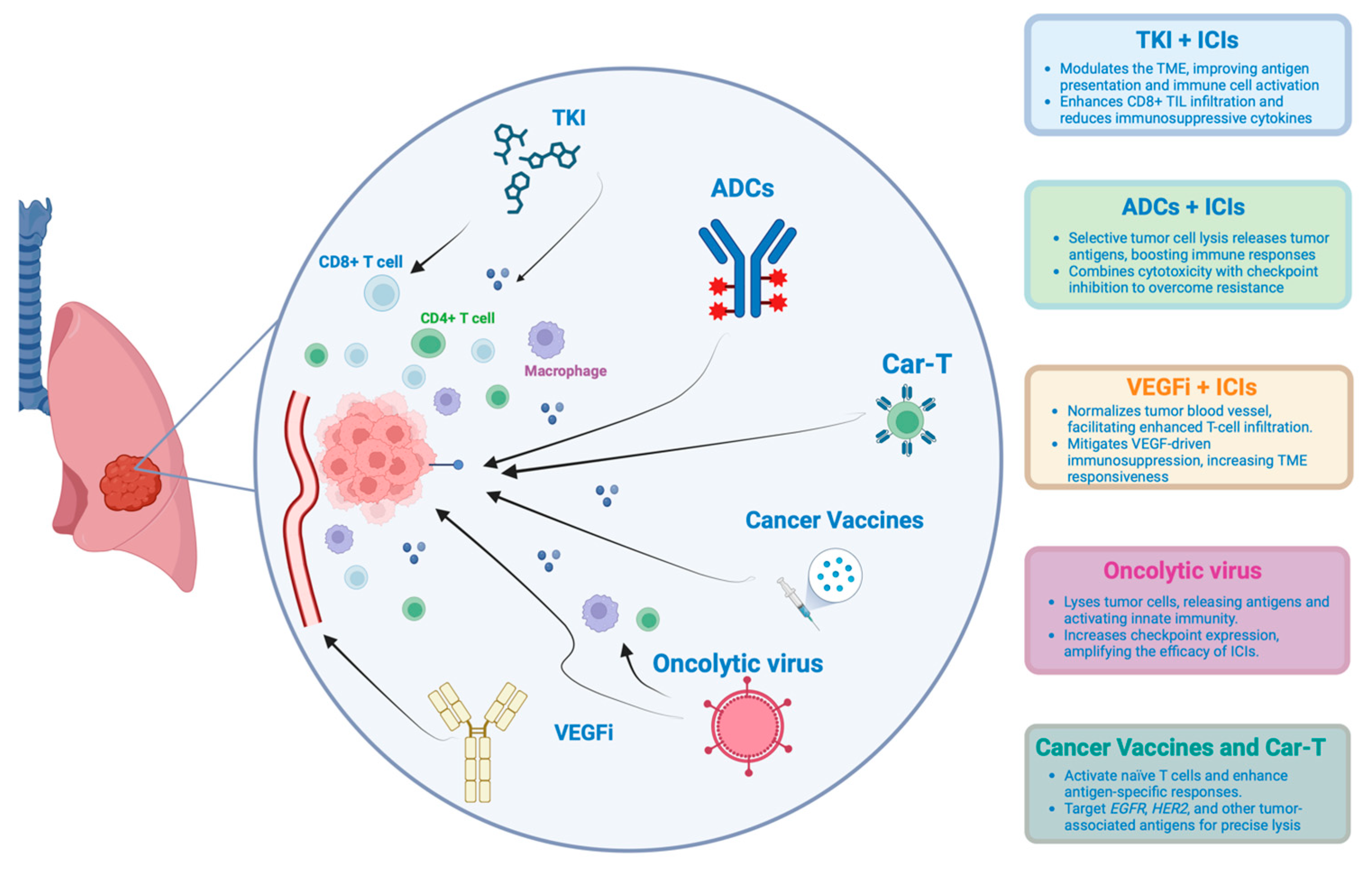
3.10. New Therapeutic Strategies
3.11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Cancer Facts and Statistics 2015|Research. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2015.html (accessed on 19 October 2024).
- Pao, W.; Miller, V.; Zakowski, M.; Doherty, J.; Politi, K.; Sarkaria, I.; Singh, B.; Heelan, R.; Rusch, V.; Fulton, L.; et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc. Natl. Acad. Sci. USA 2004, 101, 13306–13311. [Google Scholar] [CrossRef]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Rolfo, C.; Mack, P.; Scagliotti, G.V.; Aggarwal, C.; Arcila, M.E.; Barlesi, F.; Bivona, T.; Diehn, M.; Dive, C.; Dziadziuszko, R.; et al. Liquid Biopsy for Advanced NSCLC: A Consensus Statement From the International Association for the Study of Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 1647–1662. [Google Scholar] [CrossRef]
- Tang, S.; Qin, C.; Hu, H.; Liu, T.; He, Y.; Guo, H.; Yan, H.; Zhang, J.; Tang, S.; Zhou, H. Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer: Progress, Challenges, and Prospects. Cells 2022, 11, 320. [Google Scholar] [CrossRef]
- Mountzios, G.; Remon, J.; Hendriks, L.E.L.; García-Campelo, R.; Rolfo, C.; Van Schil, P.; Forde, P.M.; Besse, B.; Subbiah, V.; Reck, M.; et al. Immune-checkpoint inhibition for resectable non-small-cell lung cancer—Opportunities and challenges. Nat. Rev. Clin. Oncol. 2023, 20, 664–677. [Google Scholar] [CrossRef] [PubMed]
- Hastings, K.; Yu, H.A.; Wei, W.; Sanchez-Vega, F.; DeVeaux, M.; Choi, J.; Rizvi, H.; Lisberg, A.; Truini, A.; Lydon, C.A.; et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non-small-cell lung cancer. Ann. Oncol. 2019, 30, 1311–1320. [Google Scholar] [CrossRef]
- Lisberg, A.; Cummings, A.; Goldman, J.W.; Bornazyan, K.; Reese, N.; Wang, T.; Coluzzi, P.; Ledezma, B.; Mendenhall, M.; Hunt, J.; et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naïve Patients With Advanced NSCLC. J. Thorac. Oncol. 2018, 13, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-Y.; Zhang, J.-T.; Liu, S.-Y.; Su, J.; Zhang, C.; Xie, Z.; Zhou, Q.; Tu, H.-Y.; Xu, C.-R.; Yan, L.-X.; et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology 2017, 6, e1356145. [Google Scholar] [CrossRef]
- Sugiyama, E.; Togashi, Y.; Takeuchi, Y.; Shinya, S.; Tada, Y.; Kataoka, K.; Tane, K.; Sato, E.; Ishii, G.; Goto, K.; et al. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR-mutated non-small cell lung cancer. Sci. Immunol. 2020, 5, eaav3937. [Google Scholar] [CrossRef] [PubMed]
- Lan, B.; Ma, C.; Zhang, C.; Chai, S.; Wang, P.; Ding, L.; Wang, K. Association between PD-L1 expression and driver gene status in non-small-cell lung cancer: A meta-analysis. Oncotarget 2018, 9, 7684–7699. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.A.; de Carné Trécesson, S.; Rana, S.; Zecchin, D.; Moore, C.; Molina-Arcas, M.; East, P.; Spencer-Dene, B.; Nye, E.; Barnouin, K.; et al. Oncogenic RAS Signaling Promotes Tumor Immunoresistance by Stabilizing PD-L1 mRNA. Immunity 2017, 47, 1083–1099.e6. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Rizvi, H.; Bandlamudi, C.; Sauter, J.L.; Travis, W.D.; Rekhtman, N.; Plodkowski, A.J.; Perez-Johnston, R.; Sawan, P.; Beras, A.; et al. Clinical and molecular correlates of PD-L1 expression in patients with lung adenocarcinomas. Ann. Oncol. 2020, 31, 599–608. [Google Scholar] [CrossRef]
- Ricciuti, B.; Wang, X.; Alessi, J.V.; Rizvi, H.; Mahadevan, N.R.; Li, Y.Y.; Polio, A.; Lindsay, J.; Umeton, R.; Sinha, R.; et al. Association of High Tumor Mutation Burden in Non–Small Cell Lung Cancers With Increased Immune Infiltration and Improved Clinical Outcomes of PD-L1 Blockade Across PD-L1 Expression Levels. JAMA Oncol. 2022, 8, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Bravaccini, S.; Bronte, G.; Ulivi, P. TMB in NSCLC: A Broken Dream? Int. J. Mol. Sci. 2021, 22, 6536. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Offin, M.; Rizvi, H.; Tenet, M.; Ni, A.; Sanchez-Vega, F.; Li, B.T.; Drilon, A.; Kris, M.G.; Rudin, C.M.; Schultz, N.; et al. Tumor Mutation Burden and Efficacy of EGFR-Tyrosine Kinase Inhibitors in Patients with EGFR-Mutant Lung Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 1063–1069. [Google Scholar] [CrossRef]
- Negrao, M.V.; Skoulidis, F.; Montesion, M.; Schulze, K.; Bara, I.; Shen, V.; Xu, H.; Hu, S.; Sui, D.; Elamin, Y.Y.; et al. Oncogene-specific differences in tumor mutational burden, PD-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer. J. Immunother. Cancer 2021, 9, e002891. [Google Scholar] [CrossRef]
- Calles, A.; Liao, X.; Sholl, L.M.; Rodig, S.J.; Freeman, G.J.; Butaney, M.; Lydon, C.; Dahlberg, S.E.; Hodi, F.S.; Oxnard, G.R.; et al. Expression of PD-1 and Its Ligands, PD-L1 and PD-L2, in Smokers and Never Smokers with KRAS-Mutant Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2015, 10, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Margolis, C.A.; Vokes, N.I.; Liu, D.; Taylor-Weiner, A.; Wankowicz, S.M.; Adeegbe, D.; Keliher, D.; Schilling, B.; Tracy, A.; et al. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat. Genet. 2018, 50, 1271–1281. [Google Scholar] [CrossRef]
- Kumar, M.S.; Hancock, D.C.; Molina-Arcas, M.; Steckel, M.; East, P.; Diefenbacher, M.; Armenteros-Monterroso, E.; Lassailly, F.; Matthews, N.; Nye, E.; et al. The GATA2 transcriptional network is requisite for RAS oncogene-driven non-small cell lung cancer. Cell 2012, 149, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.D.; McLeod, L.; Alhayyani, S.; Miller, A.; Russell, P.A.; Ferlin, W.; Rose-John, S.; Ruwanpura, S.; Jenkins, B.J. IL6 Trans-signaling Promotes KRAS-Driven Lung Carcinogenesis. Cancer Res. 2016, 76, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; He, Y.-T.; Dong, S.; Wei, X.-W.; Chen, Z.-H.; Zhang, B.; Chen, W.-D.; Yang, X.-R.; Wang, F.; Shang, X.-M.; et al. Single-cell transcriptome analysis revealed a suppressive tumor immune microenvironment in EGFR mutant lung adenocarcinoma. J. Immunother. Cancer 2022, 10, e003534. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Rosenthal, R.; Hiley, C.T.; Rowan, A.J.; Watkins, T.B.K.; Wilson, G.A.; Birkbak, N.J.; Veeriah, S.; Van Loo, P.; Herrero, J.; et al. Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell 2017, 171, 1259–1271.e11. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Moran, T.; Queralt, C.; Porta, R.; Cardenal, F.; Camps, C.; Majem, M.; Lopez-Vivanco, G.; Isla, D.; Provencio, M.; et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009, 361, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.-H.; Boggon, T.J.; Li, Y.; Woo, M.S.; Greulich, H.; Meyerson, M.; Eck, M.J. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: Mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell 2007, 11, 217–227. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Y.-L.; Chen, G.; Feng, J.; Liu, X.-Q.; Wang, C.; Zhang, S.; Wang, J.; Zhou, S.; Ren, S.; et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011, 12, 735–742. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Thongprasert, S.; Yang, C.-H.; Chu, D.-T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Yang, J.C.-H.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.-M.; Boyer, M.; et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Fang, W.; Zhan, J.; Hong, S.; Tang, Y.; Kang, S.; Zhang, Y.; He, X.; Zhou, T.; Qin, T.; et al. Upregulation of PD-L1 by EGFR Activation Mediates the Immune Escape in EGFR-Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J. Thorac. Oncol. 2015, 10, 910–923. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Cheng, J.; Yang, T.; Li, Y.; Zhu, B. EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through inhibiting NF-κB. Biochem. Biophys. Res. Commun. 2015, 463, 95–101. [Google Scholar] [CrossRef]
- Toki, M.I.; Mani, N.; Smithy, J.W.; Liu, Y.; Altan, M.; Wasserman, B.; Tuktamyshov, R.; Schalper, K.; Syrigos, K.N.; Rimm, D.L. Immune Marker Profiling and Programmed Death Ligand 1 Expression Across NSCLC Mutations. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Pretelli, G.; Spagnolo, C.C.; Ciappina, G.; Santarpia, M.; Pasello, G. Overview on Therapeutic Options in Uncommon EGFR Mutant Non-Small Cell Lung Cancer (NSCLC): New Lights for an Unmet Medical Need. Int. J. Mol. Sci. 2023, 24, 8878. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Rizvi, N.A.; Goldman, J.W.; Gettinger, S.N.; Borghaei, H.; Brahmer, J.R.; Ready, N.E.; Gerber, D.E.; Chow, L.Q.; Juergens, R.A.; et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): Results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017, 18, 31–41. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet Lond. Engl. 2017, 389, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Gettinger, S.; Johnson, M.L.; Jänne, P.A.; Garassino, M.C.; Christoph, D.; Toh, C.K.; Rizvi, N.A.; Chaft, J.E.; Carcereny Costa, E.; et al. Phase II Trial of Atezolizumab As First-Line or Subsequent Therapy for Patients With Programmed Death-Ligand 1-Selected Advanced Non-Small-Cell Lung Cancer (BIRCH). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Cho, B.-C.; Kim, J.-H.; Mazières, J.; Vansteenkiste, J.; Lena, H.; Jaime, J.C.; Gray, J.E.; Powderly, J.; Chouaid, C.; et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): An open-label, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef]
- Gainor, J.F.; Shaw, A.T.; Sequist, L.V.; Fu, X.; Azzoli, C.G.; Piotrowska, Z.; Huynh, T.G.; Zhao, L.; Fulton, L.; Schultz, K.R.; et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 4585–4593. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, W.; Chen, M.; Cai, Z.; Zhan, P.; Liu, X.; Zhu, S.; Ye, M.; Lv, T.; Lv, J.; et al. Efficacy of immunotherapy in patients with oncogene-driven non-small-cell lung cancer: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2024, 16, 17588359231225036. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Brahmer, J.R.; Juergens, R.A.; Borghaei, H.; Gettinger, S.; Chow, L.Q.; Gerber, D.E.; Laurie, S.A.; Goldman, J.W.; et al. Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 2969. [Google Scholar] [CrossRef]
- Hong, L.; Lewis, W.E.; Nilsson, M.; Patel, S.; Varghese, S.; Rivera, M.J.; Du, R.R.; Chen, P.; Kemp, H.N.; Rinsurongkawong, W.; et al. Limited Benefit from the Addition of Immunotherapy to Chemotherapy in TKI-Refractory EGFR-Mutant Lung Adenocarcinoma. Cancers 2022, 14, 3473. [Google Scholar] [CrossRef] [PubMed]
- White, M.N.; Piper-Vallillo, A.J.; Gardner, R.M.; Cunanan, K.; Neal, J.W.; Das, M.; Padda, S.K.; Ramchandran, K.; Chen, T.T.; Sequist, L.V.; et al. Chemotherapy Plus Immunotherapy Versus Chemotherapy Plus Bevacizumab Versus Chemotherapy Alone in EGFR-Mutant NSCLC After Progression on Osimertinib. Clin. Lung Cancer 2022, 23, e210–e221. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Wang, P.; Zhang, J.; Zhao, Y.; Zhou, J.; Fan, Y.; Shu, Y.; Liu, X.; Zhang, H.; He, J.; et al. Toripalimab plus chemotherapy as second-line treatment in previously EGFR-TKI treated patients with EGFR-mutant-advanced NSCLC: A multicenter phase-II trial. Signal Transduct. Target. Ther. 2021, 6, 355. [Google Scholar] [CrossRef]
- Mok, T.; Nakagawa, K.; Park, K.; Ohe, Y.; Girard, N.; Kim, H.R.; Wu, Y.-L.; Gainor, J.; Lee, S.-H.; Chiu, C.-H.; et al. Nivolumab Plus Chemotherapy in Epidermal Growth Factor Receptor-Mutated Metastatic Non-Small-Cell Lung Cancer After Disease Progression on Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors: Final Results of CheckMate 722. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2024, 42, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.-H.; Lee, D.H.; Lee, J.-S.; Fan, Y.; De Marinis, F.; Iwama, E.; Inoue, T.; Rodríguez-Cid, J.; Zhang, L.; Yang, C.-T.; et al. Phase III KEYNOTE-789 Study of Pemetrexed and Platinum With or Without Pembrolizumab for Tyrosine Kinase Inhibitor–Resistant, EGFR –Mutant, Metastatic Nonsquamous Non–Small Cell Lung Cancer. J. Clin. Oncol. 2024, 42, 4029–4039. [Google Scholar] [CrossRef] [PubMed]
- Oxnard, G.R.; Yang, J.C.-H.; Yu, H.; Kim, S.-W.; Saka, H.; Horn, L.; Goto, K.; Ohe, Y.; Mann, H.; Thress, K.S.; et al. TATTON: A multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann. Oncol. 2020, 31, 507–516. [Google Scholar] [CrossRef]
- Ma, B.B.Y.; Rudin, C.M.; Cervantes, A.; Dowlati, A.; Costa, D.; Schmid, P.; Heist, R.; Villaflor, V.M.; Sarkar, I.; Huseni, M.A.; et al. 441O Preliminary safety and clinical activity of erlotinib plus atezolizumab from a Phase Ib study in advanced NSCLC. Ann. Oncol. 2016, 27, ix141. [Google Scholar] [CrossRef]
- Yang, J.C.-H.; Gadgeel, S.M.; Sequist, L.V.; Wu, C.-L.; Papadimitrakopoulou, V.A.; Su, W.-C.; Fiore, J.; Saraf, S.; Raftopoulos, H.; Patnaik, A. Pembrolizumab in Combination With Erlotinib or Gefitinib as First-Line Therapy for Advanced NSCLC With Sensitizing EGFR Mutation. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Gettinger, S.; Hellmann, M.D.; Chow, L.Q.M.; Borghaei, H.; Antonia, S.; Brahmer, J.R.; Goldman, J.W.; Gerber, D.E.; Juergens, R.A.; Shepherd, F.A.; et al. Nivolumab Plus Erlotinib in Patients With EGFR-Mutant Advanced NSCLC. J. Thorac. Oncol. 2018, 13, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.-H.; Shepherd, F.A.; Kim, D.-W.; Lee, G.-W.; Lee, J.S.; Chang, G.-C.; Lee, S.S.; Wei, Y.-F.; Lee, Y.G.; Laus, G.; et al. Osimertinib Plus Durvalumab versus Osimertinib Monotherapy in EGFR T790M-Positive NSCLC following Previous EGFR TKI Therapy: CAURAL Brief Report. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Tanimoto, T.; Yuji, K.; Tojo, A. EGFR-TKI-Associated Interstitial Pneumonitis in Nivolumab-Treated Patients With Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 1112–1115. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Arbour, K.C.; Rizvi, H.; Iqbal, A.N.; Gadgeel, S.M.; Girshman, J.; Kris, M.G.; Riely, G.J.; Yu, H.A.; Hellmann, M.D. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann. Oncol. 2019, 30, 839–844. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, S.; Jiang, T.; Li, X.; Zhao, C.; Liu, Y.; Han, R.; Qiao, M.; Liu, S.; Su, C.; et al. Impact of EGFR-TKIs combined with PD-L1 antibody on the lung tissue of EGFR-driven tumor-bearing mice. Lung Cancer Amst. Neth. 2019, 137, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Yu, L.; Chen, S.; Liu, N.; Tang, J.; Yang, N. The safety profile of EGFR/ALK-TKIs administered immediately before or after ICIs in advanced NSCLC. Int. Immunopharmacol. 2023, 116, 109787. [Google Scholar] [CrossRef]
- Zhou, C.; Ramalingam, S.S.; Kim, T.M.; Kim, S.-W.; Yang, J.C.-H.; Riely, G.J.; Mekhail, T.; Nguyen, D.; Garcia Campelo, M.R.; Felip, E.; et al. Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients With EGFR Exon 20 Insertion–Positive Metastatic Non–Small Cell Lung Cancer: A Phase 1/2 Open-label Nonrandomized Clinical Trial. JAMA Oncol. 2021, 7, e214761. [Google Scholar] [CrossRef]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.-Y.; Kim, S.-W.; Lee, C.K.; et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Hirai, S.; Katayama, Y.; Yoshimura, A.; Shiotsu, S.; Watanabe, S.; Kikuchi, T.; Hirose, K.; Kubota, Y.; Chihara, Y.; et al. Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR-mutated non-small cell lung cancer. Cancer Med. 2019, 8, 1521–1529. [Google Scholar] [CrossRef]
- Lau, S.C.M.; Fares, A.F.; Le, L.W.; Mackay, K.M.; Soberano, S.; Chan, S.W.; Smith, E.; Ryan, M.; Tsao, M.S.; Bradbury, P.A.; et al. Subtypes of EGFR- and HER2-Mutant Metastatic NSCLC Influence Response to Immune Checkpoint Inhibitors. Clin. Lung Cancer 2021, 22, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, M.; Zhao, R.; Qiang, H.; Chang, Q.; Qian, J.; Lu, H.; Shen, Y.; Han, Y.; Su, C.; et al. Anti-angiogenic therapy or immunotherapy? A real-world study of patients with advanced non-small cell lung cancer with EGFR/HER2 exon 20 insertion mutations. Front. Oncol. 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, Q.; Yu, M.; Xue, J.; Huang, M.; Lu, Y.; Zhang, Y. Immunotherapy for non-small cell lung cancer with EGFR or HER2 exon 20 insertion mutations: A real-world analysis. Transl. Lung Cancer Res. 2023, 12, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Girard, N.; Minchom, A.; Ou, S.-H.I.; Gadgeel, S.M.; Trigo, J.; Viteri, S.; Bauml, J.M.; Londhe, A.; Mahadevia, P.; Bazhenova, L. Comparative Clinical Outcomes Between EGFR Ex20ins and Wildtype NSCLC Treated with Immune Checkpoint Inhibitors. Clin. Lung Cancer 2022, 23, 571–577. [Google Scholar] [CrossRef]
- Behera, M.; Jiang, R.; Huang, Z.; Bunn, B.; Wynes, M.W.; Switchenko, J.; Scagliotti, G.V.; Belani, C.P.; Ramalingam, S.S. Natural History and Real-World Treatment Outcomes for Patients With NSCLC Having EGFR Exon 20 Insertion Mutation: An International Association for the Study of Lung Cancer–American Society of Clinical Oncology CancerLinQ Study. JTO Clin. Res. Rep. 2024, 5, 100592. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, S.; Hai, J.; Wang, X.; Chen, T.; Quinn, M.M.; Gao, P.; Zhang, Y.; Ji, H.; Cross, D.A.E.; et al. Targeting HER2 Aberrations in Non-Small Cell Lung Cancer with Osimertinib. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 2594–2604. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.; Wang, Q.; Gao, G.; Zhang, Y.; Chen, J.; Shu, Y.; Hu, Y.; Fan, Y.; Fang, J.; et al. Pyrotinib in HER2-Mutant Advanced Lung Adenocarcinoma After Platinum-Based Chemotherapy: A Multicenter, Open-Label, Single-Arm, Phase II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2753–2761. [Google Scholar] [CrossRef]
- Peters, S.; Curioni-Fontecedro, A.; Nechushtan, H.; Shih, J.-Y.; Liao, W.-Y.; Gautschi, O.; Spataro, V.; Unk, M.; Yang, J.C.-H.; Lorence, R.M.; et al. Activity of Afatinib in Heavily Pretreated Patients With ERBB2 Mutation-Positive Advanced NSCLC: Findings From a Global Named Patient Use Program. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1897–1905. [Google Scholar] [CrossRef]
- Smit, E.F.; Felip, E.; Uprety, D.; Nagasaka, M.; Nakagawa, K.; Rodríguez, L.P.-A.; Pacheco, J.M.; Li, B.T.; Planchard, D.; Baik, C.; et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): Primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 2024, 25, 439–454. [Google Scholar] [CrossRef]
- Goto, K.; Goto, Y.; Kubo, T.; Ninomiya, K.; Kim, S.-W.; Planchard, D.; Ahn, M.-J.; Smit, E.F.; De Langen, A.J.; Pérol, M.; et al. Trastuzumab Deruxtecan in Patients With HER2 -Mutant Metastatic Non–Small-Cell Lung Cancer: Primary Results From the Randomized, Phase II DESTINY-Lung02 Trial. J. Clin. Oncol. 2023, 41, 4852–4863. [Google Scholar] [CrossRef]
- Lai, W.-C.V.; Feldman, D.L.; Buonocore, D.J.; Brzostowski, E.B.; Rizvi, H.; Plodkowski, A.J.; Ni, A.; Sabari, J.K.; Offin, M.D.; Kris, M.G.; et al. PD-L1 expression, tumor mutation burden and response to immune checkpoint blockade in patients with HER2-mutant lung cancers. J. Clin. Oncol. 2018, 36, 9060. [Google Scholar] [CrossRef]
- Dantoing, E.; Piton, N.; Salaün, M.; Thiberville, L.; Guisier, F. Anti-PD1/PD-L1 Immunotherapy for Non-Small Cell Lung Cancer with Actionable Oncogenic Driver Mutations. Int. J. Mol. Sci. 2021, 22, 6288. [Google Scholar] [CrossRef] [PubMed]
- Guisier, F.; Dubos-Arvis, C.; Viñas, F.; Doubre, H.; Ricordel, C.; Ropert, S.; Janicot, H.; Bernardi, M.; Fournel, P.; Lamy, R.; et al. Efficacy and Safety of Anti-PD-1 Immunotherapy in Patients With Advanced NSCLC With BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 628–636. [Google Scholar] [CrossRef]
- Guaitoli, G.; Tiseo, M.; Di Maio, M.; Friboulet, L.; Facchinetti, F. Immune checkpoint inhibitors in oncogene-addicted non-small cell lung cancer: A systematic review and meta-analysis. Transl. Lung Cancer Res. 2021, 10, 2890–2916. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Oskar, S.; Arunachalam, A.; Zu, K.; Kao, Y.-H.; Chen, C.; Meng, W.; Pietanza, M.C.; Zhao, B.; Aggarwal, H. Real-World Treatment Patterns and Outcomes of First-Line Immunotherapy Among Patients With Advanced Nonsquamous NSCLC Harboring BRAF, MET, or HER2 Alterations. JTO Clin. Res. Rep. 2023, 4, 100568. [Google Scholar] [CrossRef]
- Abu Rous, F.; Gutta, R.; Li, P.; Halmos, B.; Gadgeel, S. Pembrolizumab in Combination with Chemotherapy in Patients with ERBB2-Mutated Non-Small Cell Lung Cancer. Target. Oncol. 2022, 17, 187–192. [Google Scholar] [CrossRef]
- Zhang, J.; Han, W.; Guo, J.; Zhang, C.; Cao, L.; Peng, L.; Han, X.; Wang, Z. Efficacy of immunotherapy in HER2-mutated non-small cell lung cancer: A single-arm meta-analysis. J. Cancer Res. Clin. Oncol. 2024, 150, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Xian, X.; Tian, P.; Li, W.; Wang, K.; Li, Y. Efficacy of Combination Chemo-Immunotherapy as a First-Line Treatment for Advanced Non-Small-Cell Lung Cancer Patients With HER2 Alterations: A Case Series. Front. Oncol. 2021, 11, 633522. [Google Scholar] [CrossRef]
- Yang, G.; Yang, Y.; Liu, R.; Li, W.; Xu, H.; Hao, X.; Li, J.; Xing, P.; Zhang, S.; Ai, X.; et al. First-line immunotherapy or angiogenesis inhibitor plus chemotherapy for HER2-altered NSCLC: A retrospective real-world POLISH study. Ther. Adv. Med. Oncol. 2022, 14, 17588359221082339. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Fu, Y.; Chen, Y.; Li, Q.; Liu, T.; Ding, Z. Poor Efficacy of Immune Checkpoint Inhibitors Plus Chemotherapy in Lung Cancer Patients with EGFR/ERBB2 Exon 20 Insertion. Curr. Oncol. Tor. Ont 2023, 30, 9929–9939. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.N.; Ishii, C.; Ishida, S.; Ogitani, Y.; Wada, T.; Agatsuma, T. A HER2-Targeting Antibody-Drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), Enhances Antitumor Immunity in a Mouse Model. Mol. Cancer Ther. 2018, 17, 1494–1503. [Google Scholar] [CrossRef]
- Sankyo, D. A Phase 1b, Multicenter, Two-Part, Open-Label Study of Trastuzumab Deruxtecan (DS-8201a), An Anti-Human Epidermal Growth Factor Receptor-2 (HER2)-Antibody Drug Conjugate (ADC). In Combination with Pembrolizumab, an Anti-PD-1 Antibody, for Subjects with Locally Advanced/Metastatic Breast or Non-Small Cell Lung Cancer (NSCLC); ClinicalTrials.gov: Bethesda, MD, USA, 2024. [Google Scholar]
- Planchard, D.; Brahmer, J.R.; Yang, J.C.-H.; Kim, H.R.; Li, R.K.; Han, J.-Y.; Cortinovis, D.L.; Runglodvatana, Y.; Nakajima, E.; Ragone, A.; et al. 1507TiP Phase Ib multicenter study of trastuzumab deruxtecan (T-DXd) and immunotherapy with or without chemotherapy in first-line treatment of patients (pts) with advanced or metastatic nonsquamous non-small cell lung cancer (NSCLC) and HER2 overexpression (OE): DESTINY-Lung03. Ann. Oncol. 2023, 34, S848–S849. [Google Scholar] [CrossRef]
- Jiangsu Hengrui Medicine Co., Ltd. Phase IB/II Clinical Study of the Safety, Tolerability, Pharmacoki-Netics, and Efficacy of Injectable SHR-A1811 in Combination with Pyrotinib or SHR-1316 in Subjects With Advanced Non-Small Cell Lung Cancer with HER2; ClinicalTrials.gov: Bethesda, MD, USA, 2023.
- Drilon, A.; Jenkins, C.; Iyer, S.; Schoenfeld, A.; Keddy, C.; Davare, M.A. ROS1-dependent cancers - biology, diagnostics and therapeutics. Nat. Rev. Clin. Oncol. 2021, 18, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Reynolds, C.; Waterhouse, D.; Garon, E.B.; Chandler, J.; Babu, S.; Thurmes, P.; Spira, A.; Jotte, R.; Zhu, J.; et al. Phase 1/2 Study of the Safety and Tolerability of Nivolumab Plus Crizotinib for the First-Line Treatment of Anaplastic Lymphoma Kinase Translocation - Positive Advanced Non-Small Cell Lung Cancer (CheckMate 370). J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Lee, S.-H.; Ramalingam, S.S.; Bauer, T.M.; Boyer, M.J.; Carcereny Costa, E.; Felip, E.; Han, J.-Y.; Hida, T.; Hughes, B.G.M.; et al. Avelumab (anti–PD-L1) in combination with crizotinib or lorlatinib in patients with previously treated advanced NSCLC: Phase 1b results from JAVELIN Lung 101. J. Clin. Oncol. 2018, 36, 9008. [Google Scholar] [CrossRef]
- Felip, E.; de Braud, F.G.; Maur, M.; Loong, H.H.; Shaw, A.T.; Vansteenkiste, J.F.; John, T.; Liu, G.; Lolkema, M.P.; Selvaggi, G.; et al. Ceritinib plus Nivolumab in Patients with Advanced ALK-Rearranged Non-Small Cell Lung Cancer: Results of an Open-Label, Multicenter, Phase 1B Study. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 392–403. [Google Scholar] [CrossRef]
- Kim, D.-W.; Gadgeel, S.; Gettinger, S.N.; Riely, G.J.; Oxnard, G.R.; Mekhail, T.; Schmid, P.; Dowlati, A.; Heist, R.S.; Wozniak, A.J.; et al. Brief Report: Safety and Antitumor Activity of Alectinib Plus Atezolizumab From a Phase 1b Study in Advanced ALK-Positive NSCLC. JTO Clin. Res. Rep. 2022, 3, 100367. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Choudhury, N.J.; Schneider, J.L.; Patil, T.; Zhu, V.W.; Goldman, D.A.; Yang, S.-R.; Falcon, C.J.; Do, A.; Nie, Y.; Plodkowski, A.J.; et al. Response to Immune Checkpoint Inhibition as Monotherapy or in Combination With Chemotherapy in Metastatic ROS1-Rearranged Lung Cancers. JTO Clin. Res. Rep. 2021, 2, 100187. [Google Scholar] [CrossRef]
- Yoh, K.; Matsumoto, S.; Kunimasa, K.; Kodani, M.; Nishi, K.; Nakagawa, T.; Sugawara, S.; Kato, T.; Sakakibara-Konishi, J.; Hayashi, Y.; et al. The efficacy of immune checkpoint inhibitors and PD-L1 status in patients with advanced non-small cell lung cancer harboring oncogenic driver alterations: Immuno-oncology biomarker study in LC-SCRUM-Japan. J. Clin. Oncol. 2019, 37, 9046. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, H.; Wang, Y.; Yang, L.; Zhou, C.; Yin, W.; Wang, G.; Mao, X.; Xiang, J.; Li, B.; et al. Clinical Characteristics and Molecular Patterns of RET-Rearranged Lung Cancer in Chinese Patients. Oncol. Res. 2019, 27, 575–582. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.; Loong, H.H.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Curigliano, G.; Kim, D.-W.; Lee, D.H.; Besse, B.; Baik, C.S.; Doebele, R.C.; Cassier, P.A.; Lopes, G.; Tan, D.S.W.; et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021, 22, 959–969. [Google Scholar] [CrossRef]
- Offin, M.; Guo, R.; Wu, S.L.; Sabari, J.; Land, J.D.; Ni, A.; Montecalvo, J.; Halpenny, D.F.; Buie, L.W.; Pak, T.; et al. Immunophenotype and Response to Immunotherapy of RET-Rearranged Lung Cancers. JCO Precis. Oncol. 2019, 3, PO.18.00386. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Zhang, H.; Shen, S.; Guo, S.; Li, X. Response to immune checkpoint inhibitor combination therapy in metastatic RET-mutated lung cancer from real-world retrospective data. BMC Cancer 2024, 24, 178. [Google Scholar] [CrossRef]
- Hegde, A.; Andreev-Drakhlin, A.Y.; Roszik, J.; Huang, L.; Liu, S.; Hess, K.; Cabanillas, M.; Hu, M.I.; Busaidy, N.L.; Sherman, S.I.; et al. Responsiveness to immune checkpoint inhibitors versus other systemic therapies in RET-aberrant malignancies. ESMO Open 2020, 5, e000799. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, S.; Jin, R.; Wang, X.; Wang, F.; Zang, R.; Xu, H.; Lu, Z.; Huang, J.; Lei, Y.; et al. The superior efficacy of anti-PD-1/PD-L1 immunotherapy in KRAS-mutant non-small cell lung cancer that correlates with an inflammatory phenotype and increased immunogenicity. Cancer Lett. 2020, 470, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS mutation: From undruggable to druggable in cancer. Signal Transduct. Target. Ther. 2021, 6, 1–20. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef] [PubMed]
- De Langen, A.J.; Johnson, M.L.; Mazieres, J.; Dingemans, A.-M.C.; Mountzios, G.; Pless, M.; Wolf, J.; Schuler, M.; Lena, H.; Skoulidis, F.; et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRASG12C mutation: A randomised, open-label, phase 3 trial. Lancet 2023, 401, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Yaeger, R.; Spira, A.I.; Pelster, M.; Sabari, J.K.; Hafez, N.; Barve, M.A.; Velastegui, K.; Yan, X.; Der-Torossian, H.; et al. KRYSTAL-1: Activity and safety of adagrasib (MRTX849) in patients with advanced solid tumors harboring a KRASG12C mutation. J. Clin. Oncol. 2023, 41, 425082. [Google Scholar] [CrossRef]
- Herbst, R.S.; Lopes, G.; Kowalski, D.M.; Kasahara, K.; Wu, Y.-L.; Castro, G.D.; Cho, B.C.; Turna, H.Z.; Cristescu, R.; Aurora-Garg, D.; et al. LBA4 Association of KRAS mutational status with response to pembrolizumab monotherapy given as first-line therapy for PD-L1-positive advanced non-squamous NSCLC in Keynote-042. Ann. Oncol. 2019, 30, xi63–xi64. [Google Scholar] [CrossRef]
- Cefalì, M.; Epistolio, S.; Ramelli, G.; Mangan, D.; Molinari, F.; Martin, V.; Freguia, S.; Mazzucchelli, L.; Froesch, P.; Frattini, M.; et al. Correlation of KRAS G12C Mutation and High PD-L1 Expression with Clinical Outcome in NSCLC Patients Treated with Anti-PD1 Immunotherapy. J. Clin. Med. 2022, 11, 1627. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Nakajima, E.C.; Ren, Y.; Vallejo, J.J.; Akinboro, O.; Mishra-Kalyani, P.S.; Larkins, E.A.; Drezner, N.L.; Tang, S.; Pazdur, R.; Beaver, J.A.; et al. Outcomes of first-line immune checkpoint inhibitors with or without chemotherapy according to KRAS mutational status and PD-L1 expression in patients with advanced NSCLC: FDA pooled analysis. J. Clin. Oncol. 2022, 40, 9001. [Google Scholar] [CrossRef]
- Ferrer, I.; Zugazagoitia, J.; Herbertz, S.; John, W.; Paz-Ares, L.; Schmid-Bindert, G. KRAS-Mutant non-small cell lung cancer: From biology to therapy. Lung Cancer Amst. Neth. 2018, 124, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Byers, L.A.; Diao, L.; Papadimitrakopoulou, V.A.; Tong, P.; Izzo, J.; Behrens, C.; Kadara, H.; Parra, E.R.; Canales, J.R.; et al. Co-occurring genomic alterations define major subsets of KRAS-mutant lung adenocarcinoma with distinct biology, immune profiles, and therapeutic vulnerabilities. Cancer Discov. 2015, 5, 860–877. [Google Scholar] [CrossRef]
- Otegui, N.; Houry, M.; Arozarena, I.; Serrano, D.; Redin, E.; Exposito, F.; Leon, S.; Valencia, K.; Montuenga, L.; Calvo, A. Cancer Cell-Intrinsic Alterations Associated with an Immunosuppressive Tumor Microenvironment and Resistance to Immunotherapy in Lung Cancer. Cancers 2023, 15, 3076. [Google Scholar] [CrossRef]
- Li, B.T.; Falchook, G.S.; Durm, G.A.; Burns, T.F.; Skoulidis, F.; Ramalingam, S.S.; Spira, A.; Bestvina, C.M.; Goldberg, S.B.; Veluswamy, R.; et al. OA03.06 CodeBreaK 100/101: First Report of Safety/Efficacy of Sotorasib in Combination with Pembrolizumab or Atezolizumab in Advanced KRAS p.G12C NSCLC. J. Thorac. Oncol. 2022, 17, S10–S11. [Google Scholar] [CrossRef]
- Wiesweg, M.; Preuß, C.; Roeper, J.; Metzenmacher, M.; Eberhardt, W.; Stropiep, U.; Wedeken, K.; Reis, H.; Herold, T.; Darwiche, K.; et al. BRAF mutations and BRAF mutation functional class have no negative impact on the clinical outcome of advanced NSCLC and associate with susceptibility to immunotherapy. Eur. J. Cancer Oxf. Engl. 2021, 149, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Guo, S.; Zhang, H.; Zhang, Z.; Shen, S.; Li, X. BRAF-Mutated Non-Small Cell Lung Cancer: Current Treatment Status and Future Perspective. Front. Oncol. 2022, 12, 863043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, C.; Lin, J.; Li, Z.; Wang, H. Patients With BRAF-Mutant NSCLC May Not Benefit From Immune Checkpoint Inhibitors: A Population-Based Study. JTO Clin. Res. Rep. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Rihawi, K.; Giannarelli, D.; Galetta, D.; Delmonte, A.; Giavarra, M.; Turci, D.; Garassino, M.; Tiseo, M.; Barbieri, F.; Panni, S.; et al. BRAF Mutant NSCLC and Immune Checkpoint Inhibitors: Results From a Real-World Experience. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, e57–e59. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Jin, B.; Liu, T.; Chen, J.; Li, G.; Dang, J. The effect of smoking status on efficacy of immune checkpoint inhibitors in metastatic non-small cell lung cancer: A systematic review and meta-analysis. eClinicalMedicine 2021, 38. [Google Scholar] [CrossRef]
- Corke, L.K.; Li, J.J.N.; Leighl, N.B.; Eng, L. Tobacco Use and Response to Immune Checkpoint Inhibitor Therapy in Non-Small Cell Lung Cancer. Curr. Oncol. 2022, 29, 6260. [Google Scholar] [CrossRef]
- Dudnik, E.; Peled, N.; Nechushtan, H.; Wollner, M.; Onn, A.; Agbarya, A.; Moskovitz, M.; Keren, S.; Popovits-Hadari, N.; Urban, D.; et al. BRAF Mutant Lung Cancer: Programmed Death Ligand 1 Expression, Tumor Mutational Burden, Microsatellite Instability Status, and Response to Immune Check-Point Inhibitors. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2018, 13, 1128–1137. [Google Scholar] [CrossRef]
- Murciano-Goroff, Y.R.; Pak, T.; Mondaca, S.; Flynn, J.R.; Montecalvo, J.; Rekhtman, N.; Halpenny, D.; Plodkowski, A.J.; Wu, S.L.; Kris, M.G.; et al. Immune biomarkers and response to checkpoint inhibition of BRAFV600 and BRAF non-V600 altered lung cancers. Br. J. Cancer 2022, 126, 889–898. [Google Scholar] [CrossRef]
- Offin, M.; Pak, T.; Mondaca, S.; Montecalvo, J.; Rekhtman, N.; Halpenny, D.; Wu, S.; Kris, M.; Paik, P.; Riely, G.; et al. P1.04-39 Molecular Characteristics, Immunophenotype, and Immune Checkpoint Inhibitor Response in BRAF Non-V600 Mutant Lung Cancers. J. Thorac. Oncol. 2019, 14, S455. [Google Scholar] [CrossRef]
- Remon, J.; Hendriks, L.E.L.; Mountzios, G.; García-Campelo, R.; Saw, S.P.L.; Uprety, D.; Recondo, G.; Villacampa, G.; Reck, M. MET alterations in NSCLC-Current Perspectives and Future Challenges. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2023, 18, 419–435. [Google Scholar] [CrossRef]
- Recondo, G.; Che, J.; Jänne, P.A.; Awad, M.M. Targeting MET Dysregulation in Cancer. Cancer Discov. 2020, 10, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Research C for DE and FDA Grants Accelerated Approval to Tepotinib for Metastatic Non-Small Cell Lung Cancer; FDA: Silver Spring, MD, USA, 2024.
- Research C for DE and FDA Grants Accelerated Approval to Capmatinib for Metastatic Non-Small Cell Lung Cancer; FDA: Silver Spring, MD, USA, 2024.
- Xu, Z.; Li, H.; Dong, Y.; Cheng, P.; Luo, F.; Fu, S.; Gao, M.; Kong, L.; Che, N. Incidence and PD-L1 Expression of MET 14 Skipping in Chinese Population: A Non-Selective NSCLC Cohort Study Using RNA-Based Sequencing. OncoTargets Ther. 2020, 13, 6245. [Google Scholar] [CrossRef] [PubMed]
- Kron, A.; Scheffler, M.; Heydt, C.; Ruge, L.; Schaepers, C.; Eisert, A.-K.; Merkelbach-Bruse, S.; Riedel, R.; Nogova, L.; Fischer, R.N.; et al. Genetic Heterogeneity of MET-Aberrant NSCLC and Its Impact on the Outcome of Immunotherapy. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 572–582. [Google Scholar] [CrossRef]
- Sabari, J.K.; Leonardi, G.C.; Shu, C.A.; Umeton, R.; Montecalvo, J.; Ni, A.; Chen, R.; Dienstag, J.; Mrad, C.; Bergagnini, I.; et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann. Oncol. 2018, 29, 2085–2091. [Google Scholar] [CrossRef]
- Guisier, F.; Descourt, R.; Babey, H.; Huchot, E.; Falchero, L.; Veillon, R.; Cortot, A.B.; Tissot, C.; Chouaid, C.; Decroisette, C. Brief Report: First-line Pembrolizumab in Metastatic Non-Small Cell Lung Cancer Habouring MET Exon 14 Skipping Mutation and PD-L1 ≥50% (GFPC 01-20 Study). Clin. Lung Cancer 2022, 23, e545–e549. [Google Scholar] [CrossRef] [PubMed]
- Blasi, M.; Kuon, J.; Lüders, H.; Misch, D.; Kauffmann-Guerrero, D.; Hilbrandt, M.; Kazdal, D.; Falkenstern-Ge, R.-F.; Hackanson, B.; Dintner, S.; et al. First-line immunotherapy for lung cancer with MET exon 14 skipping and the relevance of TP53 mutations. Eur. J. Cancer 2024, 199, 113556. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Oselin, K.; Shim, B.Y.; Okada, M.; Bryl, M.; Bonanno, L.; Demirag, G.; Colantonio, I.; Kimmich, M.; Janzic, U.; Vansteenkiste, J.F.; et al. Pembrolizumab vs placebo for early-stage non–small-cell lung cancer after resection and adjuvant therapy: Subgroup analysis of patients who received adjuvant chemotherapy in the phase 3 PEARLS/KEYNOTE-091 study. J. Clin. Oncol. 2023, 41, 8520. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Rothschild, S.I.; Zippelius, A.; Eboulet, E.I.; Savic Prince, S.; Betticher, D.; Bettini, A.; Früh, M.; Joerger, M.; Lardinois, D.; Gelpke, H.; et al. SAKK 16/14: Durvalumab in Addition to Neoadjuvant Chemotherapy in Patients With Stage IIIA(N2) Non-Small-Cell Lung Cancer-A Multicenter Single-Arm Phase II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 2872–2880. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; González-Larriba, J.L.; Martínez-Martí, A.; Bernabé, R.; Bosch-Barrera, J.; Casal-Rubio, J.; Calvo, V.; Insa, A.; Ponce, S.; et al. Perioperative Nivolumab and Chemotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 504–513. [Google Scholar] [CrossRef]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 1672–1684. [Google Scholar] [CrossRef] [PubMed]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Gao, S.; Chen, K.-N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative Pembrolizumab for Early-Stage Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar] [CrossRef]
- Tong, B.C.; Gu, L.; Wang, X.; Wigle, D.A.; Phillips, J.D.; Harpole, D.H.; Klapper, J.A.; Sporn, T.; Ready, N.E.; D’Amico, T.A. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2022, 163, 427–436. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, W.; Wu, L.; Wang, W.; Zhang, P.; Fang, W.; Neotorch Investigators. Perioperative Toripalimab Plus Chemotherapy for Patients With Resectable Non–Small Cell Lung Cancer: The Neotorch Randomized Clinical Trial. JAMA 2024, 331, 201–211. [Google Scholar] [CrossRef]
- Cascone, T.; Awad, M.M.; Spicer, J.D.; He, J.; Lu, S.; Sepesi, B.; Tanaka, F.; Taube, J.M.; Cornelissen, R.; Havel, L.; et al. Perioperative Nivolumab in Resectable Lung Cancer. N. Engl. J. Med. 2024, 390, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.; Paz-Ares, L.; Marreaud, S.; Dafni, U.; Oselin, K.; Havel, L.; Esteban, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB–IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): An interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022, 23, 1274–1286. [Google Scholar] [CrossRef]
- Tsuboi, M.; Herbst, R.S.; John, T.; Kato, T.; Majem, M.; Grohé, C.; Wang, J.; Goldman, J.W.; Lu, S.; Su, W.-C.; et al. Overall Survival with Osimertinib in Resected EGFR-Mutated NSCLC. N. Engl. J. Med. 2023, 389, 137–147. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Dziadziuszko, R.; Ahn, J.S.; Barlesi, F.; Nishio, M.; Lee, D.H.; Lee, J.-S.; Zhong, W.; Horinouchi, H.; Mao, W.; et al. Alectinib in Resected ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 390, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, M.; Weder, W.; Escriu, C.; Blakely, C.; He, J.; Dacic, S.; Yatabe, Y.; Zeng, L.; Walding, A.; Chaft, J. P03.02 Neoadjuvant Osimertinib with/without Chemotherapy vs Chemotherapy for EGFR Mutated Resectable NSCLC: NeoADAURA. J. Thorac. Oncol. 2021, 16, S258. [Google Scholar] [CrossRef]
- Lee, J.M.; Sepesi, B.; Toloza, E.M.; Lin, J.; Pass, H.I.; Johnson, B.E.; Heymach, J.V.; Johnson, M.L.; Ding, B.; Schulze, K.; et al. EP02.04-005 Phase II NAUTIKA1 Study of Targeted Therapies in Stage II-III NSCLC: Preliminary Data of Neoadjuvant Alectinib for ALK+ NSCLC. J. Thorac. Oncol. 2022, 17, S233–S234. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol.J Hematol Oncol 2021, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Ebert, P.J.R.; Cheung, J.; Yang, Y.; McNamara, E.; Hong, R.; Moskalenko, M.; Gould, S.E.; Maecker, H.; Irving, B.A.; Kim, J.M.; et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016, 44, 609–621. [Google Scholar] [CrossRef]
- Wilmott, J.S.; Long, G.V.; Howle, J.R.; Haydu, L.E.; Sharma, R.N.; Thompson, J.F.; Kefford, R.F.; Hersey, P.; Scolyer, R.A. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Pfizer Inc. A Phase 1b/2 Open Label Umbrella Study of Sasanlimab Combined With Anti-Cancer Therapies Targeting Multiple Molecular Mechanisms in Participants with Non-Small Cell Lung Cancer (NSCLC); ClinicalTrials.gov: Bethesda, MD, USA, 2024.
- Mugarza, E.; van Maldegem, F.; Boumelha, J.; Moore, C.; Rana, S.; Sopena, M.L.; East, P.; Ambler, R.; Anastasiou, P.; Romero-Clavijo, P.; et al. Therapeutic KRASG12C inhi-bition drives effective interferon-mediated anti-tumour immunity in immunogenic lung cancers. Sci Adv. 2021, 8, eabm8780. [Google Scholar] [CrossRef]
- Garassino, M.C.; Theelen, W.S.M.E.; Jotte, R.; Laskin, J.; de Marinis, F.; Aguado, C.; Badin, F.B.; Chmielewska, I.; Hochmair, M.J.; Lu, S.; et al. LBA65 KRYSTAL-7: Efficacy and safety of adagrasib with pembrolizumab in patients with treatment-naïve, advanced non-small cell lung cancer (NSCLC) harboring a KRASG12C mutation. Ann. Oncol. 2023, 34, S1309–S1310. [Google Scholar] [CrossRef]
- Nogami, N.; Barlesi, F.; Socinski, M.A.; Reck, M.; Thomas, C.A.; Cappuzzo, F.; Mok, T.S.K.; Finley, G.; Aerts, J.G.; Orlandi, F.; et al. IMpower150 Final Exploratory Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in Key NSCLC Patient Subgroups With EGFR Mutations or Metastases in the Liver or Brain. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 17, 309–323. [Google Scholar] [CrossRef]
- Goto, Y.; Su, W.-C.; Levy, B.P.; Rixe, O.; Yang, T.-Y.; Tolcher, A.W.; Lou, Y.; Zenke, Y.; Savvides, P.; Felip, E.; et al. TROPION-Lung02: Datopotamab deruxtecan (Dato-DXd) plus pembrolizumab (pembro) with or without platinum chemotherapy (Pt-CT) in advanced non-small cell lung cancer (aNSCLC). J. Clin. Oncol. 2023, 41, 9004. [Google Scholar] [CrossRef]
- Karanikas, V.; Colau, D.; Baurain, J.F.; Chiari, R.; Thonnard, J.; Gutierrez-Roelens, I.; Goffinet, C.; Van Schaftingen, E.V.; Weynants, P.; Boon, T.; et al. High frequency of cytolytic T lymphocytes directed against a tumor-specific mutated antigen detectable with HLA tetramers in the blood of a lung carcinoma patient with long survival. Cancer Res. 2001, 61, 3718–3724. [Google Scholar] [PubMed]
- De Giglio, A.; Di Federico, A.; Nuvola, G.; Deiana, C.; Gelsomino, F. The Landscape of Immunotherapy in Advanced NSCLC: Driving Beyond PD-1/PD-L1 Inhibitors (CTLA-4, LAG3, IDO, OX40, TIGIT, Vaccines). Curr. Oncol. Rep. 2021, 23, 126. [Google Scholar] [CrossRef] [PubMed]
- Tu, E.; McGlinchey, K.; Wang, J.; Martin, P.; Ching, S.L.; Floc’h, N.; Kurasawa, J.; Starrett, J.H.; Lazdun, Y.; Wetzel, L.; et al. Anti-PD-L1 and anti-CD73 combination therapy promotes T cell response to EGFR-mutated NSCLC. JCI Insight 2022, 7, e142843. [Google Scholar] [CrossRef]
- Stagg, J.; Divisekera, U.; Duret, H.; Sparwasser, T.; Teng, M.W.L.; Darcy, P.K.; Smyth, M.J. CD73-Deficient Mice Have Increased Antitumor Immunity and Are Resistant to Experimental Metastasis. Cancer Res. 2011, 71, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Stagg, J.; Beavis, P.A.; Divisekera, U.; Liu, M.C.P.; Möller, A.; Darcy, P.K.; Smyth, M.J. CD73-Deficient Mice Are Resistant to Carcinogenesis. Cancer Res. 2012, 72, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Vigano, S.; Alatzoglou, D.; Irving, M.; Ménétrier-Caux, C.; Caux, C.; Romero, P.; Coukos, G. Targeting Adenosine in Cancer Immunotherapy to Enhance T-Cell Function. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wainwright, D.A.; Wu, J.D.; Wan, Y.; Matei, D.E.; Zhang, Y.; Zhang, B. CD73: An emerging checkpoint for cancer immunotherapy. Immunotherapy 2019, 11, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Nilsson, M.B.; Yang, Y.; Le, X.; Tran, H.T.; Elamin, Y.Y.; Yu, X.; Zhang, F.; Poteete, A.; Ren, X.; et al. IL6 Mediates Suppression of T- and NK-cell Function in EMT-associated TKI-resistant EGFR-mutant NSCLC. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2023, 29, 1292–1304. [Google Scholar] [CrossRef]
- Kudling, T.V.; Clubb, J.H.A.; Pakola, S.; Quixabeira, D.C.A.; Lähdeniemi, I.A.K.; Heiniö, C.; Arias, V.; Havunen, R.; Cervera-Carrascon, V.; Santos, J.M.; et al. Effective intravenous delivery of adenovirus armed with TNFα and IL-2 improves anti-PD-1 checkpoint blockade in non-small cell lung cancer. Oncoimmunology 2023, 12, 2241710. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Wong, D.J.; Infante, J.R.; Korn, W.M.; Aljumaily, R.; Papadopoulos, K.P.; Autio, K.A.; Pant, S.; Bauer, T.M.; Drakaki, A.; et al. Pegilodecakin combined with pembrolizumab or nivolumab for patients with advanced solid tumours (IVY): A multicentre, multicohort, open-label, phase 1b trial. Lancet Oncol. 2019, 20, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Dummer, R.; Puzanov, I.; VanderWalde, A.; Andtbacka, R.H.I.; Michielin, O.; Olszanski, A.J.; Malvehy, J.; Cebon, J.; Fernandez, E.; et al. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell 2017, 170, 1109–1119.e10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, W.; Huang, K.; Zhang, Y.; Kupfer, G.; Zhao, Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: Lessons learned and strategies for moving forward. J. Hematol. Oncol.J Hematol Oncol 2018, 11, 22. [Google Scholar] [CrossRef]
- Chicaybam, L.; Bonamino, M.H.; Invitti, A.L.; Rozenchan, P.B.; Vieira, I.d.L.; Strauss, B.E. Overhauling CAR T Cells to Improve Efficacy, Safety and Cost. Cancers 2020, 12, 2360. [Google Scholar] [CrossRef]
- Lubiński, J.; Lener, M.R.; Marciniak, W.; Pietrzak, S.; Derkacz, R.; Cybulski, C.; Gronwald, J.; Dębniak, T.; Jakubowska, A.; Huzarski, T.; et al. Serum Essential Elements and Survival after Cancer Diagnosis. Nutrients 2023, 15, 2611. [Google Scholar] [CrossRef]
- Uchida, T.; Kaira, K.; Yamaguchi, O.; Mouri, A.; Shiono, A.; Miura, Y.; Hashimoto, K.; Nishihara, F.; Murayama, Y.; Kobayashi, K.; et al. Different incidence of interstitial lung disease according to different kinds of EGFR-tyrosine kinase inhibitors administered immediately before and/or after anti-PD-1 antibodies in lung cancer. Thorac. Cancer 2019, 10, 975–979. [Google Scholar] [CrossRef] [PubMed]
| TRIAL | Phase | Treatment | ORR (%) | Median PFS (Months) | PMID |
|---|---|---|---|---|---|
| EGFR | |||||
| ATLANTIC (NCT02087423) | II | Durvalumab | 11 | 1.9 | 29545095 |
| NCT02879994 | II | Pembrolizumab | 0 | 4 | 29874546 |
| OAK (NCT02008227) | II | Atezolizumab | NA | NA | 27979383 |
| BIRCH (NCT02031458) | II | Atezolizumab | Cohort 1: 23 Cohort 2: 0 Cohort 3: 7 | Cohort 1: 5.5 Cohort 2: 1.3 Cohort 3: 1.4 | 28609226 |
| CheckMate012 (NCT01454102) | Ib | Nivolumab + chemotherapy | Gemcitabine–cisplatin: 33 Pemetrexed–cisplatin: 47 Paclitaxel–carboplatin: 47 | Gemcitabine–cisplatin: 5.7 Pemetrexed–cisplatin: 6.8 Paclitaxel–carboplatin: 4.8 | 27932067 |
| NCT03924050 | II | Toripalimab + chemotherapy | 50 | 7.0 | 34650034 |
| CheckMate 722 (NCT02864251) | III | Nivolumab + chemotherapy | 31.3 | 5.6 | 38252907 |
| KEYNOTE-789 (NCT03515837) | III | Pembrolizumab + chemotherapy | 29 | 5.6 | 39173098 |
| ORIENT-31 (NCT03802240) | III | Sintilimab + IBI305 + chemotherapy | 44 | 6.9 | 37156249 |
| TATTON (NCT02143466) | Ib | Osimertinib + durvalumab | Part A: 43 Part B: 82 | Part A: NA Part B: 9.0 | 32139298 |
| Impower150 (NCT02366143) | III | Atezolizumab + bevacizumab + chemotherapy | 71 | 10 | 34311108 |
| Checkmate 012 (NCT02864251) | Ib | Erlotinib + nivolumab | 15 | 5 | 27932067 |
| Ma15.02 (NCT02013219) | Ib | Erlotinib + atezolizumab | 75 | 15 | 36871392 |
| KEYNOTE-021 (NCT02039674) | I/II | Pembrolizumab + erlotinib/gefitinib | Erlotinib: 41.7 Gefitinib: 14 | Erlotinib: 19 Gefitinib: 1 | 30529597 |
| KRAS | |||||
| CheckMate 057 (NCT01642004) | III | Nivolumab vs. docetaxel | KRAS and TP53 co-mutations: 57 KRAS and STK11 co-mutations: 0 KRAS and KEAP1 co-mutations 18 | NA | 33449799 |
| Impower150 (NCT02366143) | III | Atezolizumab + bevacizumab + chemotherapy | NA | 8 | 34311108 |
| CodeBreak100/101 (NCT03600883) | I | Sotorasib + pembrolizumab/atezolizumab | 29 | NA | // |
| ALK | |||||
| CheckMate 370 (NCT02393625) | I/II | Nivolumab + crizotinib | 38 | NA | 29518553 |
| JAVELIN Lung 101 (NCT02584634) | Ib | Avelumab + crizotinib or lorlatinib | 46 | NA | 39034968 |
| NCT02012219 | Ib | Alectinib + atezolizumab | 86 | NA | 35875467 |
| TRIAL | Phase | Target | Treatment |
|---|---|---|---|
| B-FAST (NCT03178552) | II/III | BRAF V600 | Atezolizumab + vemurafenib + cobimetinib |
| Landscape 1011 (NCT04585815) | Ib/II | BRAF V600 | Encorafenib + binimetinib + sasanlimab |
| SUNRAY-01 (NCT06119581) | 1/2 | KRAS G12C | LY3537982 + pembrolizumab |
| NCT044498v74 | II | KRAS G12C | GDC-6036 + atezolizumab |
| NCT06456138 | I/II | KRAS G12C | Tislelizumab + trametinib + anlotinib |
| DESTINY-Lung03 (NCT04686305) | Ib | HER2 | T-DXD with durvalumab with or without chemotherapy |
| HUDSON (NCT03334617) | II Umbrella | HER2 | T-DXD with durvalumab |
| TROPION-Lung02 (NCT04526691) | Ib | TROP2 | Datopotamab deruxtecan plus permbrolizumab with or without chemotherapy |
| TROPION-Lung04 (NCT04612751) | Ib | TROP2 | Datopotamab deruxtecan plus immunotherapy with or without chemotherapy |
| NCT04306900 | I/Ib | CD39 | TTX-030 + immunotherapy |
| NCT06507306 | I/1b | SOS1 | KQB198 + osimertinib |
| NCT05067283 | I | KRAS G12C | K-1084 + pembrolizumab |
| TACTI-004 | III | LAG-3 | Eftilagimod alfa plus pembrolizumab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foffano, L.; Bertoli, E.; Bortolot, M.; Torresan, S.; De Carlo, E.; Stanzione, B.; Del Conte, A.; Puglisi, F.; Spina, M.; Bearz, A. Immunotherapy in Oncogene-Addicted NSCLC: Evidence and Therapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 583. https://doi.org/10.3390/ijms26020583
Foffano L, Bertoli E, Bortolot M, Torresan S, De Carlo E, Stanzione B, Del Conte A, Puglisi F, Spina M, Bearz A. Immunotherapy in Oncogene-Addicted NSCLC: Evidence and Therapeutic Approaches. International Journal of Molecular Sciences. 2025; 26(2):583. https://doi.org/10.3390/ijms26020583
Chicago/Turabian StyleFoffano, Lorenzo, Elisa Bertoli, Martina Bortolot, Sara Torresan, Elisa De Carlo, Brigida Stanzione, Alessandro Del Conte, Fabio Puglisi, Michele Spina, and Alessandra Bearz. 2025. "Immunotherapy in Oncogene-Addicted NSCLC: Evidence and Therapeutic Approaches" International Journal of Molecular Sciences 26, no. 2: 583. https://doi.org/10.3390/ijms26020583
APA StyleFoffano, L., Bertoli, E., Bortolot, M., Torresan, S., De Carlo, E., Stanzione, B., Del Conte, A., Puglisi, F., Spina, M., & Bearz, A. (2025). Immunotherapy in Oncogene-Addicted NSCLC: Evidence and Therapeutic Approaches. International Journal of Molecular Sciences, 26(2), 583. https://doi.org/10.3390/ijms26020583







