Therapeutic Potential of Bioactive Peptides Derived from Natural Products of Tortoiseshell and Antler in Alleviating Osteoporosis and Osteoarthritis
Abstract
1. Introduction
2. Results
2.1. Antioxidant Capacity of Tortoiseshell, Antler, and the Combination of Tortoiseshell and Antler
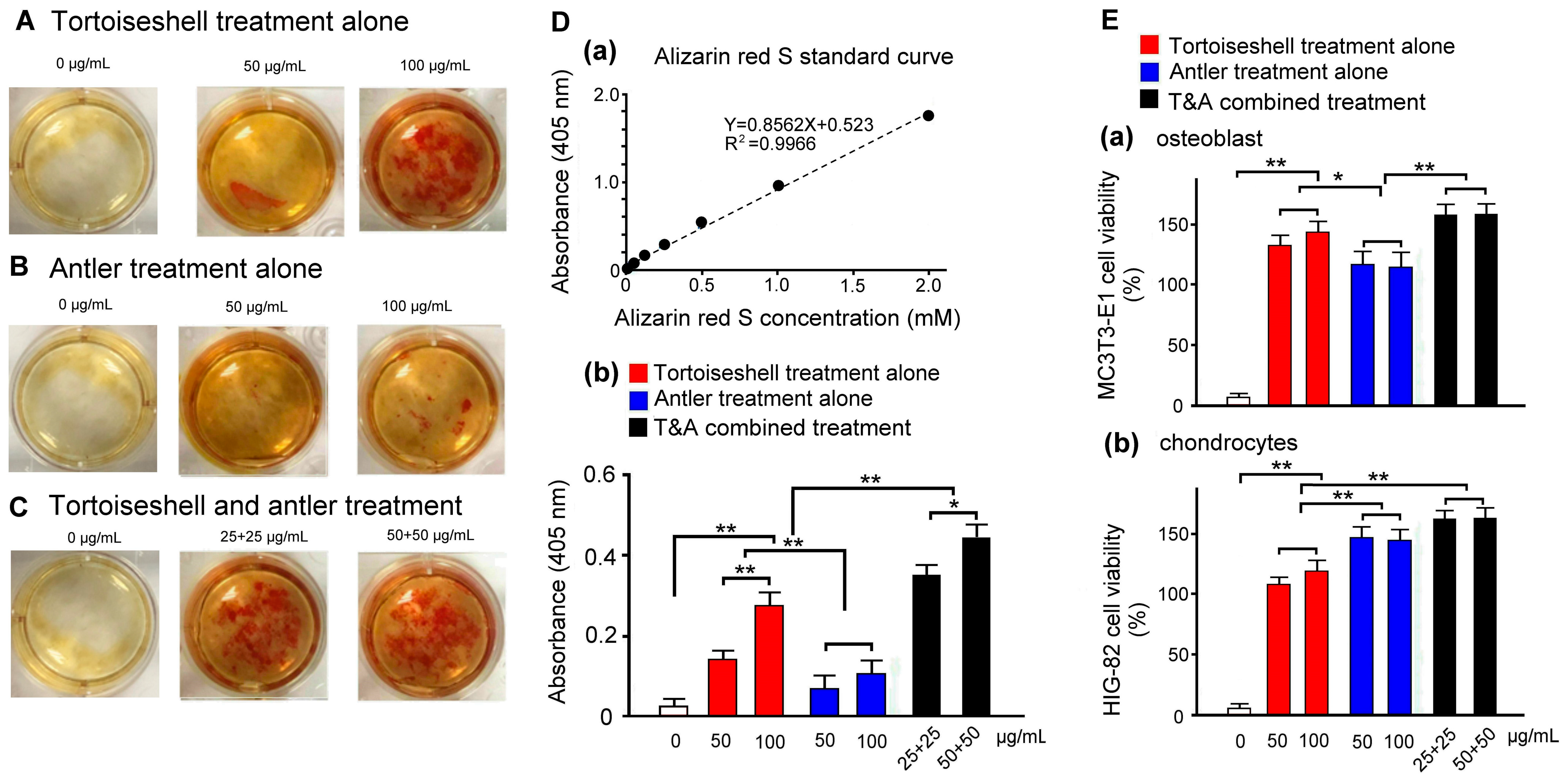
2.2. Effects of Tortoiseshell, Antler, and Their Combination on Mineralization in MC3T3-E1 Osteoblasts
2.3. Evaluation of Effects of Tortoiseshell, Antler, and Their Combination on the Cell Viability of MC3T3-E1 Osteoblasts and HIG-82 Chondrocytes
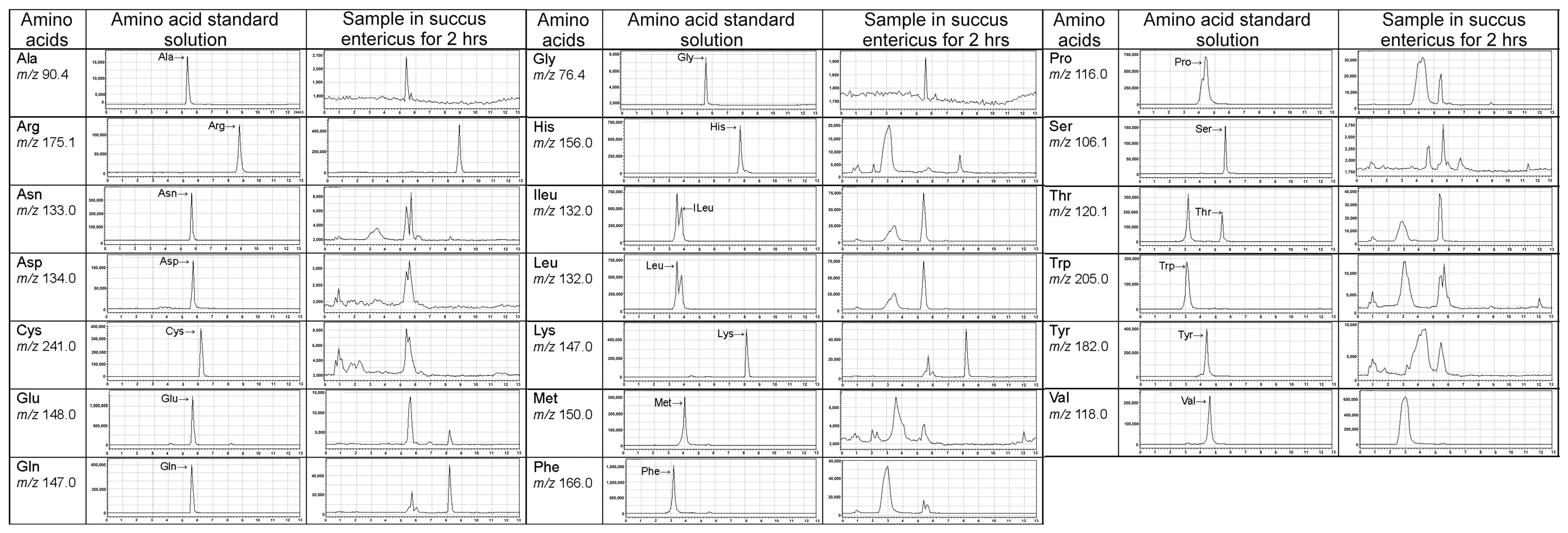
2.4. LC/MS Fingerprint of Amino Acids from Tortoiseshell, Antler, and Their Combination
2.5. Antioxidant Capacity of Bioactive Peptides from Tortoiseshell, Antler, and the Combination of Tortoiseshell and Antler
2.6. Effects of Bioactive Peptides from Tortoiseshell, Antler, and Their Combination on the Cell Viability of MC3T3-E1 Osteoblasts and HIG-82 Chondrocytes
3. Discussion
4. Materials and Methods
4.1. Fingerprint Analysis and Preparation of Soluble Peptides
- -
- Controller: BM-20A.
- -
- Pressurization system (pump): LC-20AD.
- -
- Degassing system (degasser): DGU-20A.
- -
- Sampling system (autosampler): SIL-20AC.
- -
- Column oven: CTO-20A.
- -
- Photodiode array detector: SPD-M20A.
- -
- Mass detector: LCMS-2020.
- -
- Pre-column: LichrospherRP-18 endcapped (5 μm, 4.0 × 10 mm, Merck).
- -
- Analytical column: Intrada Amino Acid (3 μm, 3 × 50 mm, Imtakt).
- -
- Column temperature: 35 °C.
- -
- Injection volume: 10 μL.
- -
- Moving phase: 100 mM HCOONH4; 0.1% HCOOHinCH3CN(1→1000).
- -
- Flow rate: 0.6 mL/min.
- -
- Analysis time: 13 min.
- -
- Residence time: Ala: ~5.4 min, Glu: ~5.6 min, Leu: ~3.5 min, Ser: ~5.7 min, Arg: ~8.8 min, Gln: ~5.6 min, Lys: ~8.2 min, Thr: ~5.5 min, Asn: ~5.7 min, Gly: ~5.6 min, Met: ~4.0 min, Trp: ~3.2 min, Asp: ~5.7 min, His: ~7.8 min, Phe: ~3.2 min, Tyr: ~4.4 min, Cys: ~6.2 min, Ileu: ~3.8 min, Pro: ~4.4 min, Val: ~4.6 min.
- -
- Ion source: electrospray ionization (ESI).
- -
- Ion source interface voltage (interface voltage): select Tuning file.
- -
- Nebulizing gas flow: 1.5 L/min.
- -
- Drying gas flow: 20.00 L/min.
- -
- Desolvation tube temperature (DL temp.): 300 °C.
- -
- Heating module temperature (Heat block temp.): 500 °C.
- -
- Scan mode: scan (+mode) m/z30-930.
- -
- Selected ion monitoring mode: SIM (+mode).
- -
- Amino acids and their m/z values: Ala: m/z 90.4, Glu: m/z 148, Leu: m/z 132, Ser: m/z 106.1, Arg: m/z 175.1, Gln: m/z 147, Lys: m/z 147, Thr: m/z 120.1, Asn: m/z 133, Gly: m/z 76.4, Met: m/z 150, Trp: m/z 205, Asp: m/z 134, His: m/z 156, Phe: m/z 166, Tyr: m/z 182, Cys: m/z 241, Ileu: m/z 132, Pro: m/z 116, Val: m/z 118.
4.2. DPPH Determination of Oxidative Free Radical Scavenging Ability
4.3. Cell Culture of MC3T3-E1 Osteoblasts and HIG-82 Chondrocytes
4.4. MTT Assay of Surviving MC3T3-E1 Osteoblasts and HIG-82 Chondrocytes
4.5. Mineralization Assay of Surviving MC3T3-E1 Osteoblasts by Alizarin Red S Staining
4.6. Statistics and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liao, J.A.; Yeh, Y.C.; Chang, Z.Y. The efficacy and safety of traditional Chinese medicine Guilu Erxian Jiao in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2022, 46, 101515. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H.; Lee, T.H.; Lee, H.P.; Li, T.M.; Lee, I.T.; Shieh, P.C.; Tang, C.H. Kuei-Lu-Er-Xian-Jiao extract enhances BMP-2 production in osteoblasts. BioMedicine 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.J.; Chuu, J.J.; Peng, Y.J.; Cheng, Y.H.; Chang, C.H.; Chang, C.M.; Liu, H.W. The potent anti-inflammatory effect of Guilu Erxian Glue extracts remedy joint pain and ameliorate the progression of osteoarthritis in mice. J. Orthop. Surg. Res. 2018, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Chou, Y.Y.; Chen, Y.M.; Tang, Y.J.; Ho, H.C.; Chen, D.Y. Effect of the herbal drug guilu erxian jiao on muscle strength, articular pain, and disability in elderly men with knee osteoarthritis. Evid.-Based Complement. Altern. Med. Ecam 2014, 2014, 297458. [Google Scholar] [CrossRef] [PubMed]
- Lien, C.Y.; Lu, C.W.; Lin, Y.H.; Wu, W.J.; Hsu, C.H.; Chuang, T.Y.; Lin, K.F.; Chuang, W.C.; Lee, M.C.; Wu, C.H. Chinese Herbal Medicine, Guilu Erxian Glue, as Alternative Medicine for Adverse Side Effects of Chemotherapy in Doxorubicin-Treated Cell and Mouse Models. Evid.-Based Complement. Altern. Med. Ecam 2021, 2021, 5548968. [Google Scholar] [CrossRef] [PubMed]
- Li, L.Q.; Baibado, J.T.; Shen, Q.; Cheung, H.Y. Determination of the authenticity of plastron-derived functional foods based on amino acid profiles analysed by MEKC. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1070, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Lu, X.; Jiang, G.; Deng, J.; Tang, S.; Li, S. Comparison of the nourishing-yin functions of different types of tortoise shell. Lishizhen Med. Mater. Med. Res. 2007, 18, 1417–1418. [Google Scholar]
- Li, X.; Xie, X.; Huang, C.; Zhong, Y.; Li, Y.; Zhou, J.; Chen, D. Repairing of oxidative damage to mesenchymal stem cell in rats and anti-lipid peroxidation by Plastrum testudinis ethanolic extract. Chin. Tradit. Herb. Drugs 2007, 38, 1043–1046. [Google Scholar]
- Xie, X.; Li, X.; Zhong, Y.; Huang, C.; Du, S.; Li, Y.; Chen, D. Study of antioxidant activities of Plastrum testudinis in Vitro. China Pharm. 2006, 17, 1368–1370. [Google Scholar]
- Li, Y.; Cui, X.; Chen, D.; Du, S.; Li, H.; Zhou, J. Effect of tortoises shell on neural stem cell following injuried spinal cord. Chin. J. Neuroanat. 2003, 19, 321–324. [Google Scholar]
- Cheung, H.Y.; Cheung, C.S. Nutritive value of plastron extracts and its effects on the differentiation of cancer cell. Hong Kong Pharm. J. 1998, 7, 104–107. [Google Scholar]
- Luo, D.; Li, H.; Li, X.; Zhou, J.; Du, F.; Wang, L. Determination of collagen in tortoise shell using HPLC with phenyl isothiocyanate derivatization. Chin. Tradit. Herb. Drugs 2008, 39, 851–852. [Google Scholar]
- Wu, F.; Li, H.; Jin, L.; Li, X.; Ma, Y.; You, J.; Li, S.; Xu, Y. Deer antler base as a traditional Chinese medicine: A review of its traditional uses, chemistry and pharmacology. J. Ethnopharmacol. 2013, 145, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.J.; Lin, J.H.; Lin, S.Z.; Tsai, W.T.; Wu, J.R.; Chen, H.P. Isolation, Identification, and Characterization of Bioactive Peptides in Human Bone Cells from Tortoiseshell and Deer Antler Gelatin. Int. J. Mol. Sci. 2023, 24, 1759. [Google Scholar] [CrossRef] [PubMed]
- Riegger, J.; Schoppa, A.; Ruths, L.; Haffner-Luntzer, M.; Ignatius, A. Oxidative stress as a key modulator of cell fate decision in osteoarthritis and osteoporosis: A narrative review. Cell. Mol. Biol. Lett. 2023, 28, 76. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; O’Brien, C.A. Basic biology of skeletal aging: Role of stress response pathways. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Martin-Millan, M.; O’Brien, C.A.; Manolagas, S.C. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box O-mediated transcription. J. Biol. Chem. 2007, 282, 27298–27305. [Google Scholar] [CrossRef] [PubMed]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Chung, K.T.; Su, L.Y.; Wu, W.J.; Wang, P.H.; Lee, M.C.; Shen, S.C.; Wu, C.H. Chinese Herbal Medicines as Natural Alternative Products to Antibiotics in Weaned Piglets through Intestinal Microbiota Regulation. Int. J. Mol. Sci. 2024, 25, 11034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bernar, A.; Gebetsberger, J.V.; Bauer, M.; Streif, W.; Schirmer, M. Optimization of the Alizarin Red S Assay by Enhancing Mineralization of Osteoblasts. Int. J. Mol. Sci. 2022, 24, 723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, K.-T.; Wu, H.-M.; Lee, M.-C.; Chuang, W.-C.; Wu, C.-H. Therapeutic Potential of Bioactive Peptides Derived from Natural Products of Tortoiseshell and Antler in Alleviating Osteoporosis and Osteoarthritis. Int. J. Mol. Sci. 2025, 26, 581. https://doi.org/10.3390/ijms26020581
Chung K-T, Wu H-M, Lee M-C, Chuang W-C, Wu C-H. Therapeutic Potential of Bioactive Peptides Derived from Natural Products of Tortoiseshell and Antler in Alleviating Osteoporosis and Osteoarthritis. International Journal of Molecular Sciences. 2025; 26(2):581. https://doi.org/10.3390/ijms26020581
Chicago/Turabian StyleChung, Kou-Toung, Hsuan-Mei Wu, Ming-Chung Lee, Wu-Chang Chuang, and Chung-Hsin Wu. 2025. "Therapeutic Potential of Bioactive Peptides Derived from Natural Products of Tortoiseshell and Antler in Alleviating Osteoporosis and Osteoarthritis" International Journal of Molecular Sciences 26, no. 2: 581. https://doi.org/10.3390/ijms26020581
APA StyleChung, K.-T., Wu, H.-M., Lee, M.-C., Chuang, W.-C., & Wu, C.-H. (2025). Therapeutic Potential of Bioactive Peptides Derived from Natural Products of Tortoiseshell and Antler in Alleviating Osteoporosis and Osteoarthritis. International Journal of Molecular Sciences, 26(2), 581. https://doi.org/10.3390/ijms26020581






