Combined Transcriptomics and Metabolomics Uncover the Potential Mechanism of Plant Growth-Promoting Rhizobacteria on the Regrowth of Leymus chinensis After Mowing
Abstract
1. Introduction
2. Results
2.1. Isolation and Identification of B13
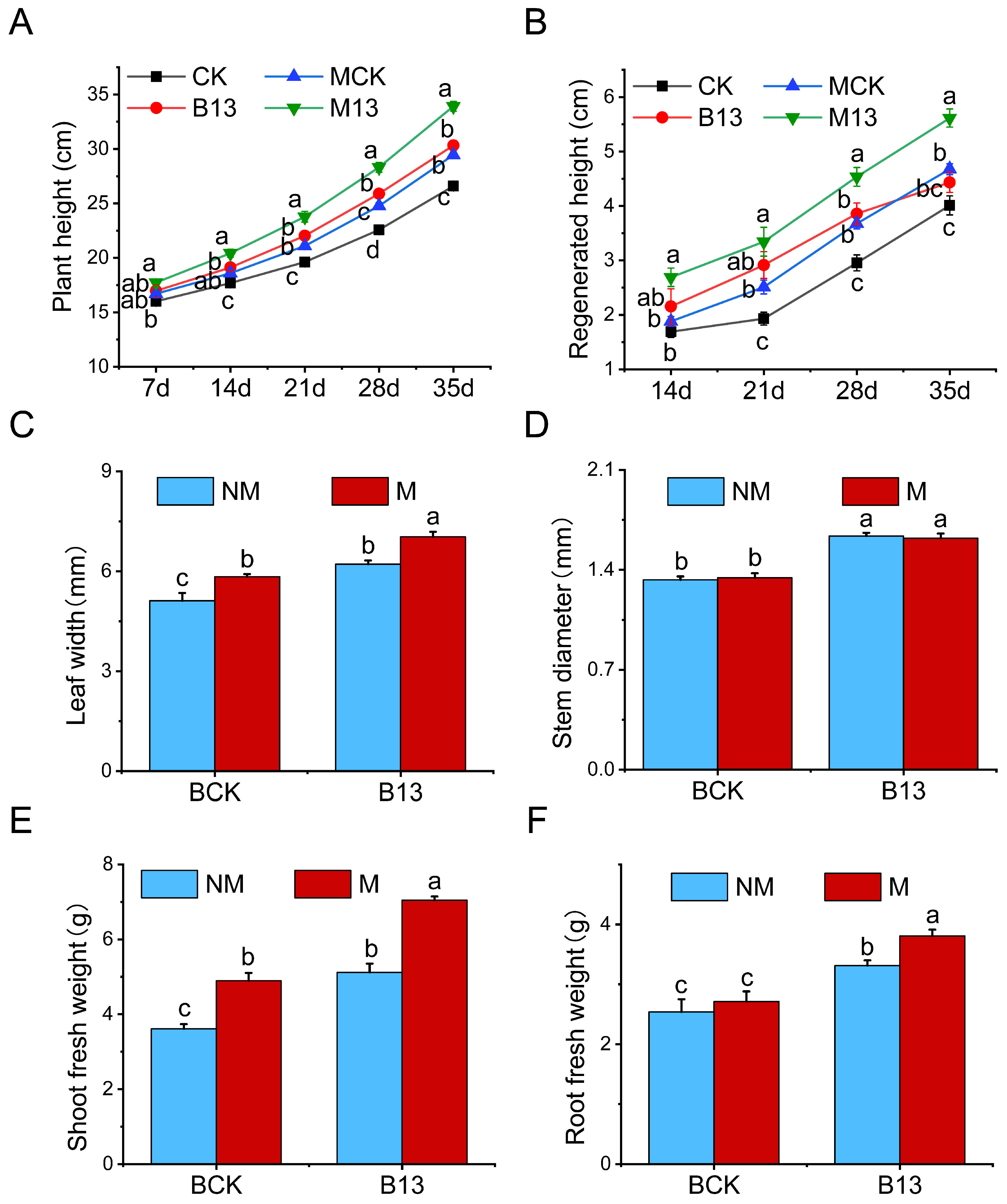
2.2. Effects of B13 Inoculation on Growth of L. chinensis
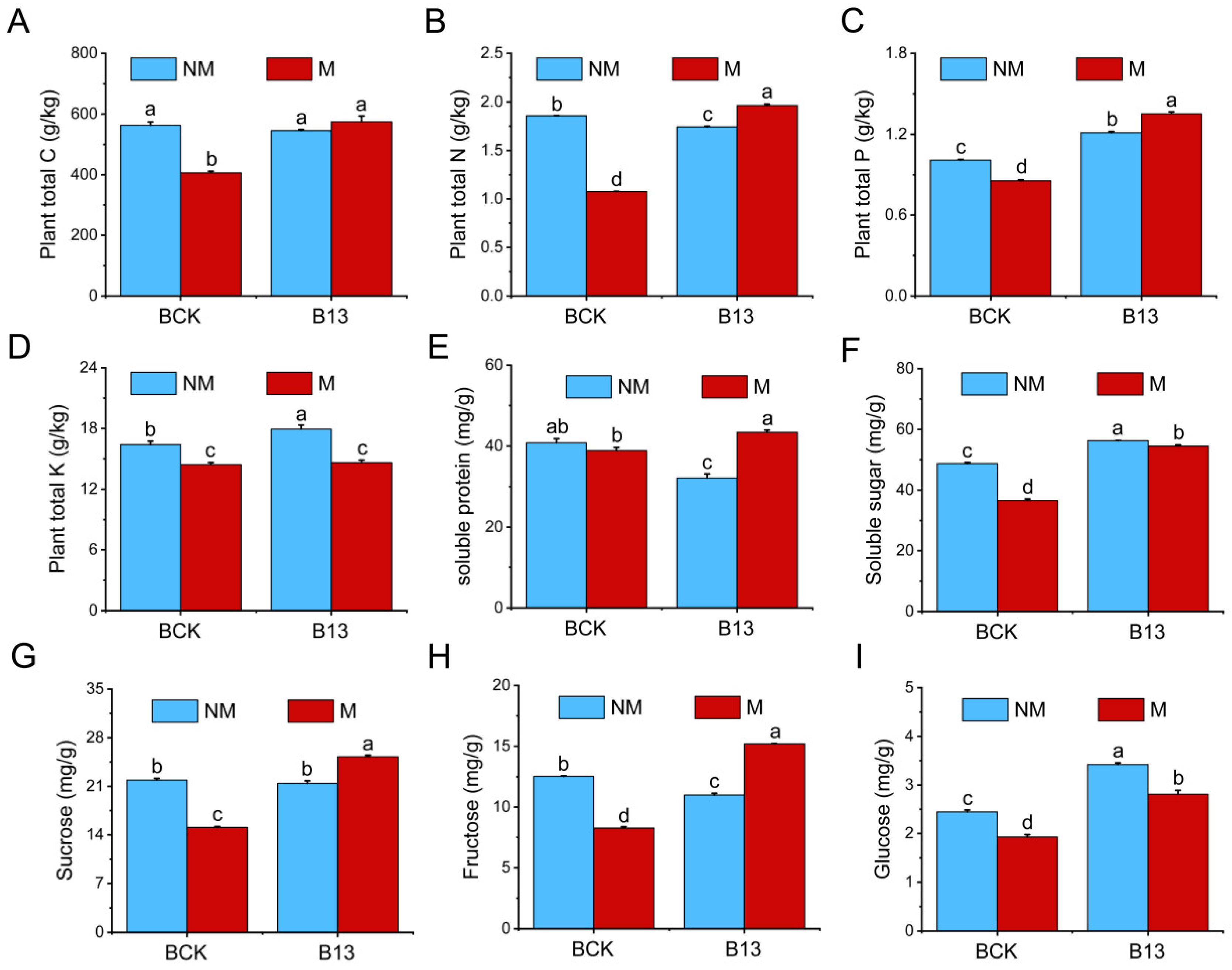
2.3. Effects of B13 Inoculation on the Accumulation of Nutrients and Sugars by L. chinensis Root
2.4. Effects of B13 Inoculation on the Phytohormones and Antioxidant Enzymes in L. chinensis
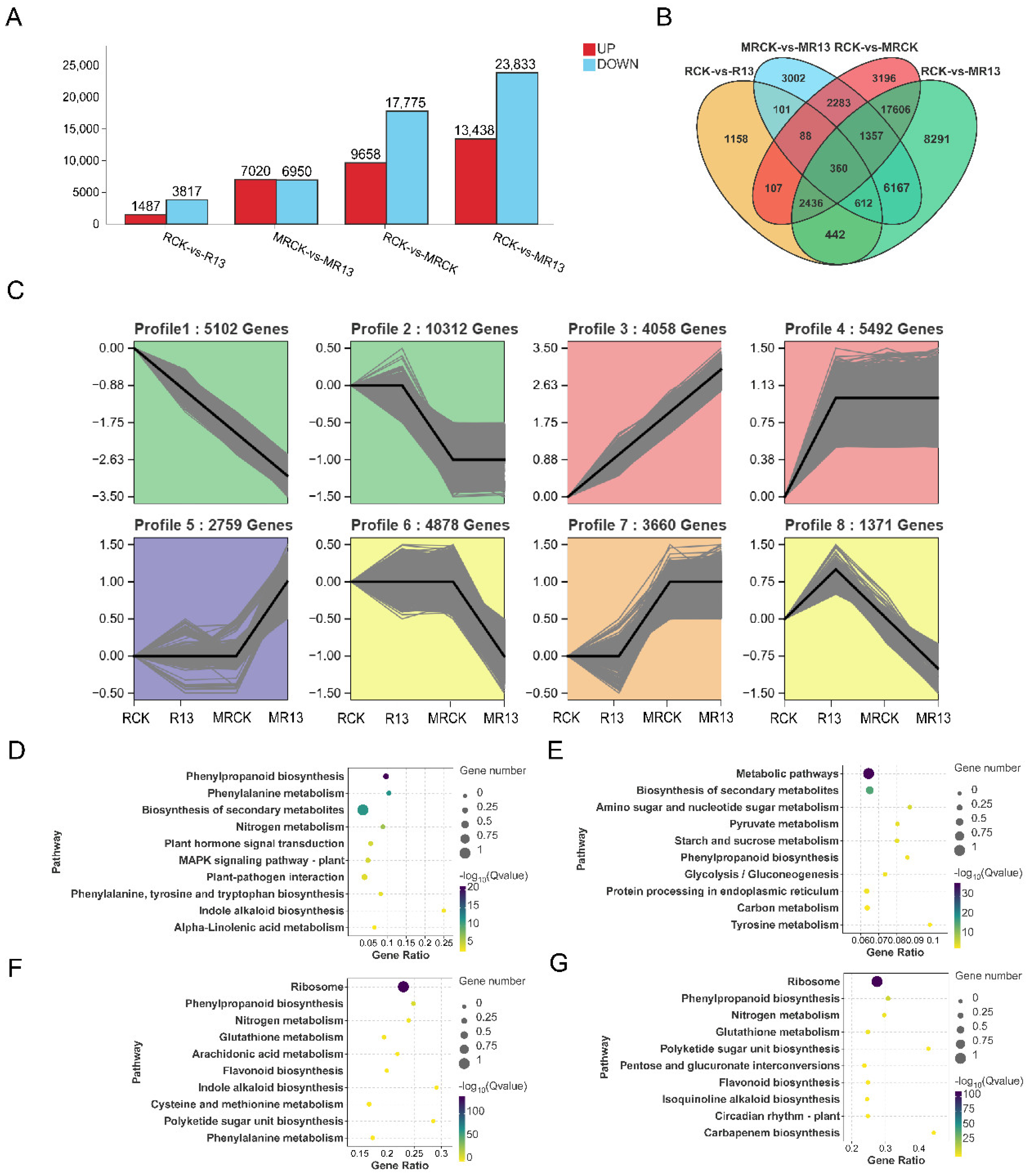
2.5. Transcriptomic Analysis
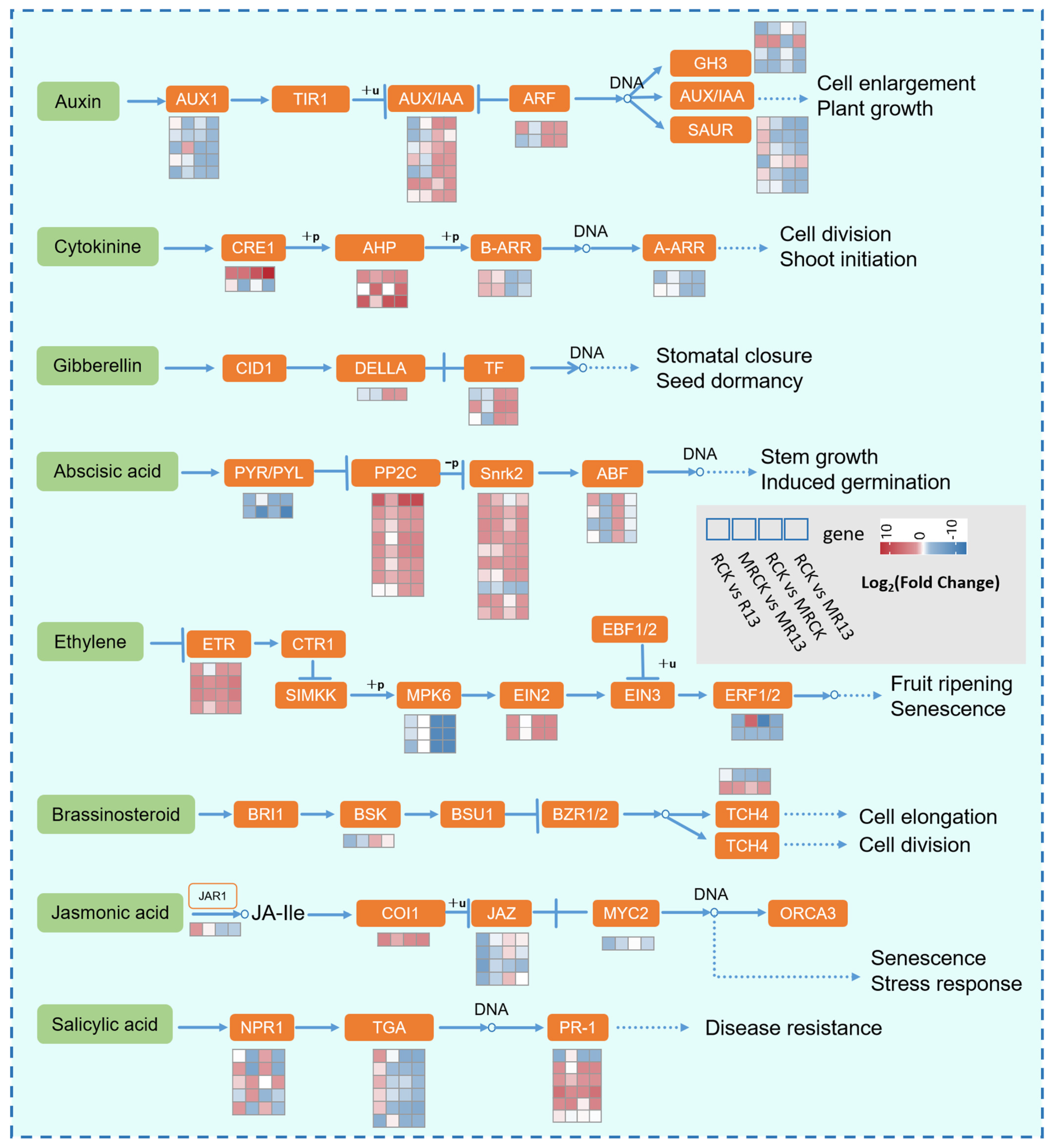
2.6. B13 Inoculation Affected Plant Hormone Signal Transduction Pathway
2.7. B13 Inoculation Affected Genes Related to Nutrient and Sugar Transport
2.8. Characterization of Plant Root Metabolome in Response to B13 Inoculation
2.9. Integrated Metabolomic and Transcriptomic Analysis
2.10. qRT-PCR Analysis
3. Discussion
3.1. B13 Inoculation Promotes Regrowth of L. chinensis After Mowing by Enhancing the Absorption and Transport of Nutrients
3.2. B13 Inoculation Responds to Mowing L. chinensis by Regulating Plant Hormone Signaling Pathways
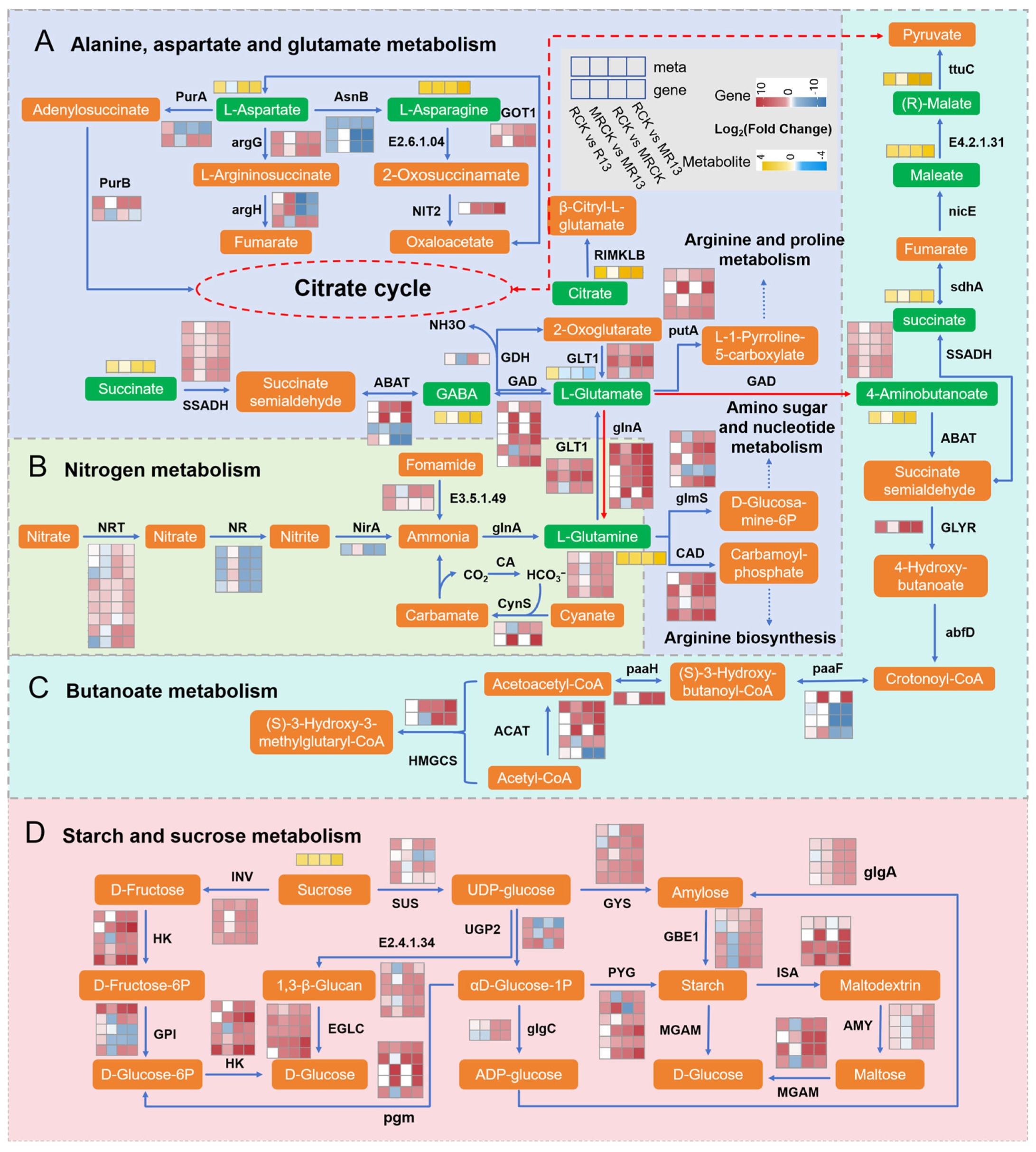
3.3. B13 Inoculation Promotes Root Growth and Regrowth of L. chinensis After Mowing Through Amino Acid Metabolism
3.4. B13 Inoculation Altered Carbohydrate Metabolism and Energy Metabolism of L. chinensis Root After Mowing
4. Materials and Methods
4.1. Identification and Growth-Promoting Properties of B13
4.2. Plant Materials and Treatment
4.3. Determination of Physiological Indices
4.4. Transcriptome Sequencing and Quantitative PCR Analyses
4.5. Metabolomic Profiling of L. chinensis Roots
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, Y.; Di, Y.; Qiu, Y.; Ji, Z.; Zhou, T.; Shen, S.; Du, N.; Zhang, T.; Dong, X.; et al. Plant growth-promoting rhizobacteria Pseudomonas aeruginosa HG28-5 improves salt tolerance by regulating Na+/K+ homeostasis and ABA signaling pathway in tomato. Microbiol. Res. 2024, 283, 127707. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, H.; Rashid, U.; Hassan, M.N.; Nosheen, A.; Naz, R.; Ilyas, N.; Sajjad, M.; Azmat, A.; Alyemeni, M.N. Volatile organic compounds produced by Pseudomonas pseudoalcaligenes alleviated drought stress by modulating defense system in maize (Zea mays L.). Physiol. Plant. 2021, 172, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Lamont, J.R.; Wilkins, O.; Bywater-Ekegärd, M.; Smith, D.L. From yogurt to yield: Potential applications of lactic acid bacteria in plant production. Soil Biol. Biochem. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Usharani, G.; Saranraj, P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar]
- Gupta, A.; Mishra, R.; Rai, S.; Bano, A.; Pathak, N.; Fujita, M.; Kumar, M.; Hasanuzzaman, M. Mechanistic insights of plant growth promoting bacteria mediated drought and salt stress tolerance in plants for sustainable agriculture. Int. J. Mol. Sci. 2022, 23, 3741. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Li, M.; Zhang, K.; Ma, W.; Zheng, L.; Xu, H.; Cui, B.; Liu, R.; Yang, Y.; et al. Functional assembly of root-associated microbial consortia improves nutrient efficiency and yield in soybean. J. Integr. Plant Biol. 2021, 63, 1021–1035. [Google Scholar] [CrossRef]
- Zhong, Y.; Yang, Y.; Liu, P.; Xu, R.; Rensing, C.; Fu, X.; Liao, H. Genotype and rhizobium inoculation modulate the assembly of soybean rhizobacterial communities. Plant Cell Environ. 2019, 42, 2028–2044. [Google Scholar] [CrossRef]
- Ahmad Ansari, F.A.; Ahmad, I.; Pichtel, J. Synergistic effects of biofilm-producing PGPR strains on wheat plant colonization, growth and soil resilience under drought stress. Saudi J. Biol. Sci. 2023, 30, 103664. [Google Scholar] [CrossRef]
- Khandagale, P.P.; Kansara, S.S.; Padsala, J.; Patel, P.R. Plant growth promoting rhizobacteria for sustainable production of sugarcane and rice. Int. J. Plant Soil Sci. 2024, 36, 298–305. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023, 21, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms underlying legume–rhizobium symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, J.; Baoyin, T.; Zhang, L.; Yuan, T. The effects of different grazing periods on the functional traits of Leymus chinensis (Trin.) Tzvelev in a typical Inner Mongolia steppe. Agronomy 2024, 14, 2370. [Google Scholar] [CrossRef]
- Sasaki, T.; Lu, X.; Hirota, M.; Bai, Y. Species asynchrony and response diversity determine multifunctional stability of natural grasslands. J. Ecol. 2019, 107, 1862–1875. [Google Scholar] [CrossRef]
- Zhao, T.; Suo, R.; Alemu, A.W.; Zheng, J.; Zhang, F.; Iwaasa, A.D.; Guo, J.; Zhao, M.; Zhang, B. Mowing increased community stability in semiarid grasslands more than either fencing or grazing. Ecol. Appl. 2024, 34, e2985. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K. Growth regulators and mowing heights enhance the morphological and physiological performance of Seaspray turfgrass during drought conditions. Acta Physiol. Plant. 2015, 37, 232. [Google Scholar] [CrossRef]
- Robson, T.M.; Lavorel, S.; Clement, J.-C.; Le Roux, X. Neglect of mowing and manuring leads to slower nitrogen cycling in subalpine grasslands. Soil Biol. Biochem. 2007, 39, 930–941. [Google Scholar] [CrossRef]
- Ye, J.; Wu, S.; Mo, Y.; Yang, S.; Zhao, Y.; Zhang, J.; Lü, X.; Yang, G.; Han, X.; Liang, C.; et al. Non-linear response of plant caloric value to N addition and mowing treatments in a meadow steppe. Ecol. Processes 2024, 13, 67. [Google Scholar] [CrossRef]
- Schrama, M.J.; Cordlandwehr, V.; Visser, E.J.; Elzenga, T.M.; de Vries, Y.; Bakker, J.P. Grassland cutting regimes affect soil properties, and consequently vegetation composition and belowground plant traits. Plant Soil 2013, 366, 401–413. [Google Scholar] [CrossRef]
- Yang, G.-J.; Lü, X.-T.; Stevens, C.J.; Zhang, G.-M.; Wang, H.-Y.; Wang, Z.-W.; Zhang, Z.-J.; Liu, Z.-Y.; Han, X.-G. Mowing mitigates the negative impacts of N addition on plant species diversity. Oecologia 2019, 189, 769–779. [Google Scholar] [CrossRef]
- Avice, J.-C.; Dily, F.L.; Goulas, E.; Noquet, C.; Meuriot, F.; Volenec, J.J.; Cunningham, S.M.; Sors, T.G.; Dhont, C.; Castonguay, Y.; et al. Vegetative storage proteins in overwintering storage organs of forage legumes: Roles and regulation. Can. J. Bot. 2003, 81, 1198–1212. [Google Scholar] [CrossRef]
- Klimešová, J.; Martínková, J.; Ottaviani, G. Belowground plant functional ecology: Towards an integrated perspective. Funct. Ecol. 2018, 32, 2115–2126. [Google Scholar] [CrossRef]
- Kobiela, B.; Biondini, M.; Sedivec, K. Comparing root and shoot responses to nutrient additions and mowing in a restored semi-arid grassland. Plant Ecol. 2016, 217, 303–314. [Google Scholar] [CrossRef]
- Chen, L.; Baoyin, T.; Minggagud, H. Effects of mowing regimes on above-and belowground biota in semi-arid grassland of northern China. J. Environ. Manag. 2021, 277, 111441. [Google Scholar] [CrossRef] [PubMed]
- Thorne, M.A.; Frank, D.A. The effects of clipping and soil moisture on leaf and root morphology and root respiration in two temperate and two tropical grasses. Plant Ecol. 2009, 200, 205–215. [Google Scholar] [CrossRef]
- Volf, M.; Redmond, C.; Albert, Á.J.; Le Bagousse-Pinguet, Y.; Biella, P.; Götzenberger, L.; Hrázský, Z.; Janeček, Š.; Klimešová, J.; Lepš, J.; et al. Effects of long-and short-term management on the functional structure of meadows through species turnover and intraspecific trait variability. Oecologia 2016, 180, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Minggagud, H.; Baoyin, T.; Li, F.Y. Plant production decreases whereas nutrients concentration increases in response to the decrease of mowing stubble height. J. Environ. Manag. 2020, 253, 109745. [Google Scholar] [CrossRef]
- Hou, D.-J.; Guo, K. Dynamic response of plant nutrients to grazing intensity in the growing season in typical steppe. Acta Agrestia Sin. 2021, 29, 141. [Google Scholar]
- Fernandez, O.; Ishihara, H.; George, G.M.; Mengin, V.; Flis, A.; Sumner, D.; Arrivault, S.; Feil, R.; Lunn, J.E.; Zeeman, S.C.; et al. Leaf starch turnover occurs in long days and in falling light at the end of the day. Plant Physiol. 2017, 174, 2199–2212. [Google Scholar] [CrossRef] [PubMed]
- Pommerrenig, B.; Ludewig, F.; Cvetkovic, J.; Trentmann, O.; Klemens, P.A.; Neuhaus, H.E. In concert: Orchestrated changes in carbohydrate homeostasis are critical for plant abiotic stress tolerance. Plant Cell Physiol. 2018, 59, 1290–1299. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Sueyoshi, K.; Ohyama, T. Recent advances in carbon and nitrogen metabolism in C3 plants. Int. J. Mol. Sci. 2020, 22, 318. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, W.; De Smet, I.; Talboys, P.; Loya, R.; Hassan, A.; Rong, H.; Jürgens, G.; Paul Knox, J.; Wang, M.H. ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. Plant J. 2010, 64, 764–774. [Google Scholar] [CrossRef]
- Zhang, Z.; Gong, J.; Wang, B.; Li, X.; Ding, Y.; Yang, B.; Zhu, C.; Liu, M.; Zhang, W. Regrowth strategies of Leymus chinensis in response to different grazing intensities. Ecol. Appl. 2020, 30, e02113. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Ren, W.; Wang, Z.; Fry, E.L.; Tang, S.; Yin, J.; Zhang, J.; Jia, Z. How does the pattern of root metabolites regulating beneficial microorganisms change with different grazing pressures? Front. Plant Sci. 2023, 14, 1180576. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Silva, E.F.L.; Casals, P.; Sodek, L.; Delitti, W.B.C.; Vallejo, V.R. Post-fire nitrogen uptake and allocation by two resprouting herbaceous species with contrasting belowground traits. Environ. Exp. Bot. 2019, 159, 157–167. [Google Scholar] [CrossRef]
- Zhang, T.; Li, F.Y.; Wang, H.; Wu, L.; Shi, C.; Li, Y.; Hu, J. Effects of defoliation timing on plant nutrient resorption and hay production in a semi-arid steppe. J. Plant Ecol. 2021, 14, 44–57. [Google Scholar] [CrossRef]
- Wang, R.; Cresswell, T.; Johansen, M.P.; Harrison, J.; Jiang, Y.; Keitel, C.; Cavagnaro, T.R.; Dijkstra, F.A. Reallocation of nitrogen and phosphorus from roots drives regrowth of grasses and sedges after defoliation under deficit irrigation and nitrogen enrichment. J. Ecol. 2021, 109, 4071–4080. [Google Scholar] [CrossRef]
- Abdelkefi, N.; Louati, I.; Mechichi, H.-Z.; Sayahi, N.; El-Sayed, W.S.; Nayal, A.E.; Ismail, W.; Hanin, M.; Mechichi, T. Enhanced salt stress tolerance in tomato plants following inoculation with newly isolated plant growth-promoting rhizobacteria. Sci. Hortic. 2024, 328, 112921. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, X.; Xing, Y.; Dao, J.; Zhao, D.; Li, Y.; Li, W.; Wang, Z. A meta-analysis on morphological, physiological and biochemical responses of plants with PGPR inoculation under drought stress. Plant Cell Environ. 2023, 46, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Bano, A.; Ullah, A.; Shahid, M.A.; Khan, N. A comparative study of plant growth promoting rhizobacteria (PGPR) and sowing methods on nutrient availability in wheat and rhizosphere soil under salinity stress. Rhizosphere 2022, 23, 100571. [Google Scholar] [CrossRef]
- Garrido-Oter, R.; Nakano, R.T.; Dombrowski, N.; Ma, K.-W.; AgBiome Team; McHardy, A.C.; Schulze-Lefert, P. Modular traits of the Rhizobiales root microbiota and their evolutionary relationship with symbiotic rhizobia. Cell Host Microbe 2018, 24, 155-167.e5. [Google Scholar] [CrossRef] [PubMed]
- Mantelin, S.; Desbrosses, G.; Larcher, M.; Tranbarger, T.J.; Cleyet-Marel, J.-C.; Touraine, B. Nitrate-dependent control of root architecture and N nutrition are altered by a plant growth-promoting Phyllobacterium sp. Planta 2006, 223, 591–603. [Google Scholar] [CrossRef]
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.; Tang, X.; Zhang, F. P for two, sharing a scarce resource: Soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol. 2011, 156, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Dong, L.; Lü, W.; Lü, J.; Meng, Q.; Liu, P. Transcriptome analysis of maize seedling roots in response to nitrogen-, phosphorus-, and potassium deficiency. Plant Soil 2020, 447, 637–658. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Ye, W.; Yao, Y.; Sun, F.; Zhang, C.; Liu, S.; Xi, Y. Effect of mowing on wheat growth at seeding stage. Int. J. Mol. Sci. 2023, 24, 15353. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhao, M.; Zhang, S.; Wang, Z.; Meng, M.; Sun, F.; Zhang, C.; Xi, Y. MicroRNA and regulation of auxin and cytokinin signalling during post-mowing regeneration of winter wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2020, 155, 769–779. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, M.; Tan, H.; Wang, Z.; Meng, M.; Sun, F.; Zhang, C. RNA sequencing reveals dynamic carbohydrate metabolism and phytohormone signaling accompanying post-mowing regeneration of forage winter wheat (Triticum aestivum L.). Front. Plant Sci. 2021, 12, 664933. [Google Scholar] [CrossRef]
- Wang, D.; Sun, C.X.; Cui, M.; Shen, X.; Zhang, Y.; Xiao, J.; Liu, P.; Zhang, Y.; Xie, H. An integrated analysis of transcriptome and metabolome provides insights into the responses of maize (Zea mays L.) roots to different straw and fertilizer conditions. Environ. Exp. Bot. 2022, 194, 104732. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, G.E. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Sabri, A.N.; Hasnain, S. Rhizobacterial potential to alter auxin content and growth of Vigna radiata (L.). World J. Microbiol. Biotechnol. 2010, 26, 1379–1384. [Google Scholar] [CrossRef]
- Campos, M.L.; Yoshida, Y.; Major, I.T.; de Oliveira Ferreira, D.; Weraduwage, S.M.; Froehlich, J.E.; Johnson, B.F.; Kramer, D.M.; Jander, G.; Sharkey, T.D.; et al. Rewiring of jasmonate and phytochrome B signalling uncouples plant growth-defense tradeoffs. Nat. Commun. 2016, 7, 12570. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Song, S. Jasmonates: Biosynthesis, metabolism, and signaling by proteins activating and repressing transcription. J. Exp. Bot. 2017, 68, 1303–1321. [Google Scholar] [CrossRef]
- Hickman, R.; Van Verk, M.C.; Van Dijken, A.J.; Mendes, M.P.; Vroegop-Vos, I.A.; Caarls, L.; Steenbergen, M.; Van der Nagel, I.; Wesselink, G.J.; Jironkin, A.; et al. Architecture and dynamics of the jasmonic acid gene regulatory network. Plant Cell 2017, 29, 2086–2105. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [PubMed]
- Hongna, C.; Leyuan, T.; Junmei, S.; Xiaori, H.; Xianguo, C. Exogenous salicylic acid signal reveals an osmotic regulatory role in priming the seed germination of Leymus chinensis under salt-alkali stress. Environ. Exp. Bot. 2021, 188, 104498. [Google Scholar] [CrossRef]
- Tang, D.; Quan, C.; Lin, Y.; Wei, K.; Qin, S.; Liang, Y.; Wei, F.; Miao, J. Physio-morphological, biochemical and transcriptomic analyses provide insights into drought stress responses in Mesona chinensis Benth. Front. Plant Sci. 2022, 13, 809723. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Liu, X.; Feng, W.; Li, Q.; Long, M.; Cui, Y.; He, S.; Yang, P.; Hu, T.; et al. Integrated transcriptomic and metabolomic analyses reveals the molecular bases of alfalfa regrowth processes of new shoots after cutting under different water and nitrogen availability. Ind. Crops Prod. 2024, 213, 118476. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, X.; Yang, R.; Wang, Q.; Bai, L.; Gong, D.; Han, Y.; Prusky, D.; Bi, Y. UV-C radiation promoted flavonoid synthesis during early healing in potato tuber wounds with the possible involvement of ABA and related transcription factor regulation. Postharvest Biol. Technol. 2024, 209, 112683. [Google Scholar] [CrossRef]
- Qu, K.; Cheng, Y.; Gao, K.; Ren, W.; Fry, E.L.; Yin, J.; Liu, Y. Growth-defense trade-offs induced by long-term overgrazing could act as a stress memory. Front. Plant Sci. 2022, 13, 917354. [Google Scholar] [CrossRef]
- Ito, S.; Watanabe, A.; Osanai, T. Regulation of l-aspartate oxidase contributes to NADP+ biosynthesis in Synechocystis sp. PCC 6803. Plant Physiol. 2024, 194, 945–957. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, W.; Li, D.; Wang, H.; Yang, Y.; You, J.; Liu, H.; Ai, L.; Zhang, M. Combined transcriptome and metabolome analyses reveal the effects of selenium on the growth and quality of Lilium lancifolium. Front. Plant Sci. 2024, 15, 1399152. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, Ł.P.; Signorelli, S.; Höfte, M. γ-aminobutyric acid and related amino acids in plant immune responses: Emerging mechanisms of action. Plant Cell Environ. 2020, 43, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Isayenkov, S.V. The role of the γ-aminobutyric acid (GABA) in plant salt stress tolerance. Horticulturae 2023, 9, 230. [Google Scholar] [CrossRef]
- Hasan, M.M.; Alabdallah, N.M.; Alharbi, B.M.; Waseem, M.; Yao, G.; Liu, X.D.; Abd El-Gawad, H.G.; El-Yazied, A.A.; Ibrahim, M.F.M.; Jahan, M.S.; et al. GABA: A key player in drought stress resistance in plants. Int. J. Mol. Sci. 2021, 22, 10136. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Ainalidou, A.; Mellidou, I.; Karamanoli, K. Metabolome and transcriptome reprogramming underlying tomato drought resistance triggered by a Pseudomonas strain. Plant Physiol. Biochem. 2023, 203, 108080. [Google Scholar] [CrossRef]
- Ramesh, S.A.; Tyerman, S.D.; Xu, B.; Bose, J.; Kaur, S.; Conn, V.; Domingos, P.; Ullah, S.; Wege, S.; Shabala, S.; et al. GABA signalling modulates plant growth by directly regulating the activity of plant-specific anion transporters. Nat. Commun. 2015, 6, 7879. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Zhang, S.; Li, B.; Li, J.; Wang, X.; Zhang, J.; Guan, C.; Ji, J. The combined use of a plant growth promoting Bacillus sp. strain and GABA promotes the growth of rice under salt stress by regulating antioxidant enzyme system, enhancing photosynthesis and improving soil enzyme activities. Microbiol. Res. 2023, 266, 127225. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jia, Q.; Ji, S.; Gong, B.; Li, J.; Lü, G.; Gao, H. Gamma-aminobutyric acid (GABA) alleviates salt damage in tomato by modulating Na+ uptake, the GAD gene, amino acid synthesis and reactive oxygen species metabolism. BMC Plant Biol. 2020, 20, 465. [Google Scholar] [CrossRef]
- Figueiredo, M.d.V.B.; Seldin, L.; de Araujo, F.F.; Mariano, R.d.L.R. Plant growth promoting rhizobacteria: Fundamentals and applications. Plant Growth Health Promot. Bact. 2011, 21–43. [Google Scholar]
- Kumar, R.; Mukherjee, S.; Ayele, B.T. Molecular aspects of sucrose transport and its metabolism to starch during seed development in wheat: A comprehensive review. Biotechnol. Adv. 2018, 36, 954–967. [Google Scholar] [CrossRef]
- Jiang, D.; Li, Y.; Wang, J.; Lv, X.; Jiang, Z.; Cao, B.; Qu, J.; Ma, S.; Zhang, Y. Exogenous application of Bradyrhizobium japonicum AC20 enhances soybean tolerance to atrazine via regulating rhizosphere soil microbial community and amino acid, carbohydrate metabolism related genes expression. Plant Physiol. Biochem. 2023, 196, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-S.; Ryoo, N.; Hahn, T.-R.; Walia, H.; Nakamura, Y. Starch biosynthesis in cereal endosperm. Plant Physiol. Biochem. 2010, 48, 383–392. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.-H.; Tun, W.; Jeon, J.-S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2021, 302, 110703. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Jiao, C.; Wang, G.; Sun, Y.; Sun, Y. Effects of dopamine on growth, carbon metabolism, and nitrogen metabolism in cucumber under nitrate stress. Sci. Hortic. 2020, 260, 108790. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.; Chen, H.; Jin, J.; Zhang, P.; Shen, L.; Hu, S.; Liu, H. Metabolomic analysis reveals the impact of ketoprofen on carbon and nitrogen metabolism in rice (Oryza sativa L.) seedling leaves. Environ. Sci. Pollut. Res. Int. 2023, 30, 21825–21837. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.; Barutcular, C.; Koc, M.; Dizlek, H.; Hossain, A.; Islam, M.S.; Toptas, I.; Basdemir, F.; Albayrak, O.; Akinci, C.; et al. Assessment of the grain quality of wheat genotypes grown under multiple environments using GGE biplot analysis. Fresenius Environ. Bull. 2018, 27, 4830–4837. [Google Scholar]
- Kechid, M.; Desbrosses, G.; Rokhsi, W.; Varoquaux, F.; Djekoun, A.; Touraine, B. The NRT 2.5 and NRT 2.6 genes are involved in growth promotion of Arabidopsis by the plant growth-promoting rhizobacterium (PGPR) strain Phyllobacterium brassicacearum STM 196. New Phytol. 2013, 198, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, L.; Addo-Danso, S.D.; Ding, G.; Sun, M.; Wu, S.; Lin, S. Nitrogen supply enhances the physiological resistance of Chinese fir plantlets under polyethylene glycol (PEG)-induced drought stress. Sci. Rep. 2020, 10, 7509. [Google Scholar] [CrossRef]
- Fortunato, S.; Nigro, D.; Lasorella, C.; Marcotuli, I.; Gadaleta, A.; de Pinto, M.C. The role of glutamine synthetase (GS) and glutamate synthase (GOGAT) in the Improvement of nitrogen use efficiency in cereals. Biomolecules 2023, 13, 1771. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Subramanian, S.; Smith, D.L. Soybean leaf proteomic profile influenced by rhizobacteria under optimal and salt stress conditions. Front. Plant Sci. 2022, 13, 809906. [Google Scholar] [CrossRef] [PubMed]
- Poly, F.; Monrozier, L.J.; Bally, R. Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res. Microbiol. 2001, 152, 95–103. [Google Scholar] [CrossRef] [PubMed]
- King, E.J. The colorimetric determination of phosphorus. Biochem. J. 1932, 26, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Chrastil, J. Colorimetric estimation of indole-3-acetic acid. Anal. Biochem. 1976, 72, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, Y.-W. Mutualistic symbiosis of fungi and nitrogen-fixing bacteria in halophilic aerobic granular sludge treating nitrogen-deficient hypersaline organic wastewater. Bioresour. Technol. 2024, 394, 130183. [Google Scholar] [CrossRef] [PubMed]
- Jia-Yi, Y.; Meng-Qiang, S.; Zhi-Liang, C.; Yu-Tang, X.; Hang, W.; Jian-Qiang, Z.; Ling, H.; Qi, Z. Effect of foliage applied chitosan-based silicon nanoparticles on arsenic uptake and translocation in rice (Oryza sativa L.). J. Hazard. Mater. 2022, 433, 128781. [Google Scholar] [CrossRef]
- Castro-Valdecantos, P.; Puértolas, J.; Albacete, A.; Dodd, I.C. Girdling changes root and shoot hormonal balance but does not alter drought-induced stomatal closure in soybean. Environ. Exp. Bot. 2021, 192, 104657. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, T.; Ren, W.; Zhang, J.; Mahmood, M.; Fry, E.L.; Meng, R. Combined Transcriptomics and Metabolomics Uncover the Potential Mechanism of Plant Growth-Promoting Rhizobacteria on the Regrowth of Leymus chinensis After Mowing. Int. J. Mol. Sci. 2025, 26, 565. https://doi.org/10.3390/ijms26020565
Yuan T, Ren W, Zhang J, Mahmood M, Fry EL, Meng R. Combined Transcriptomics and Metabolomics Uncover the Potential Mechanism of Plant Growth-Promoting Rhizobacteria on the Regrowth of Leymus chinensis After Mowing. International Journal of Molecular Sciences. 2025; 26(2):565. https://doi.org/10.3390/ijms26020565
Chicago/Turabian StyleYuan, Ting, Weibo Ren, Jiatao Zhang, Mohsin Mahmood, Ellen L. Fry, and Ru Meng. 2025. "Combined Transcriptomics and Metabolomics Uncover the Potential Mechanism of Plant Growth-Promoting Rhizobacteria on the Regrowth of Leymus chinensis After Mowing" International Journal of Molecular Sciences 26, no. 2: 565. https://doi.org/10.3390/ijms26020565
APA StyleYuan, T., Ren, W., Zhang, J., Mahmood, M., Fry, E. L., & Meng, R. (2025). Combined Transcriptomics and Metabolomics Uncover the Potential Mechanism of Plant Growth-Promoting Rhizobacteria on the Regrowth of Leymus chinensis After Mowing. International Journal of Molecular Sciences, 26(2), 565. https://doi.org/10.3390/ijms26020565





