Jun, an Oncological Foe or Friend?
Abstract
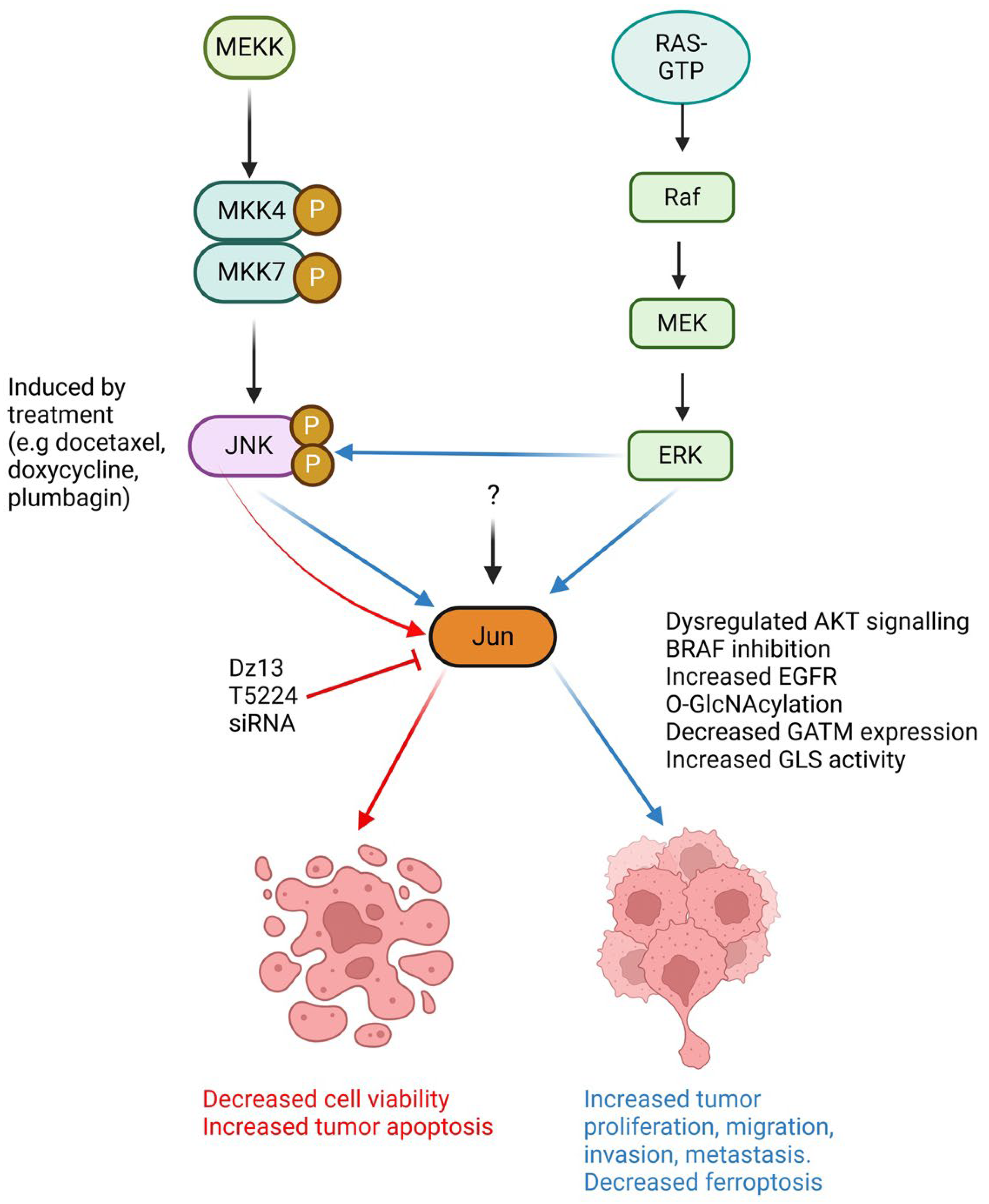
1. Introduction
2. Is Jun a Foe in Cancer?
2.1. Pro-Tumorigenic Role of Jun in the Tumor Microenvironment
2.2. Cancer-Associated Fibroblasts (CAFs)
2.3. Tumor-Associated Macrophages (TAMs)
2.4. Myeloid-Derived Suppressor Cells
2.5. Angiogenesis
3. Jun: Favourable in Cancer?
4. New Opportunities in Immunotherapy
4.1. T Cell Activation
4.2. T Cell Exhaustion
4.3. Jun Overexpression in T Cells
4.4. Roles of Jun in Other Immune Cells
4.5. Jun—A Double-Edged Sword in Immunotherapy?
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kurachi, M.; Barnitz, R.A.; Yosef, N.; Odorizzi, P.M.; DiIorio, M.A.; Lemieux, M.E.; Yates, K.; Godec, J.; Klatt, M.G.; Regev, A.; et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat. Immunol. 2014, 15, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Dunn, C.; Wiltshire, C.; MacLaren, A.; Gillespie, D.A.F. Molecular mechanism and biological functions of c-Jun N-terminal kinase signalling via the c-Jun transcription factor. Cell. Signal. 2002, 14, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Brennan, A.; Leech, J.T.; Kad, N.M.; Mason, J.M. Selective antagonism of cJun for cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 184. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.; Davis, R.J.; McLaren, A.; Cohen, P. A reinvestigation of the multisite phosphorylation of the transcription factor c-Jun. Embo J. 2003, 22, 3876–3886. [Google Scholar] [CrossRef]
- Lopez-Bergami, P.; Huang, C.; Goydos, J.S.; Yip, D.; Bar-Eli, M.; Herlyn, M.; Smalley, K.S.; Mahale, A.; Eroshkin, A.; Aaronson, S.; et al. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell 2007, 11, 447–460. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Berthenet, K.; Castillo Ferrer, C.; Fanfone, D.; Popgeorgiev, N.; Neves, D.; Bertolino, P.; Gibert, B.; Hernandez-Vargas, H.; Ichim, G. Failed Apoptosis Enhances Melanoma Cancer Cell Aggressiveness. Cell Rep. 2020, 31, 107731. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wu, Y.; Tang, X.; Xia, Y.; He, G.; Min, Z.; Li, C.; Xiong, S.; Shi, Z.; Lu, Y.; et al. HDAC inhibitors suppress c-Jun/Fra-1-mediated proliferation through transcriptionally downregulating MKK7 and Raf1 in neuroblastoma cells. Oncotarget 2016, 7, 6727–6747. [Google Scholar] [CrossRef]
- Zhong, S.; Wu, B.; Li, J.; Wang, X.; Jiang, S.; Hu, F.; Dou, G.; Zhang, Y.; Sheng, C.; Zhao, G.; et al. T5224, RSPO2 and AZD5363 are novel drugs against functional pituitary adenoma. Aging 2019, 11, 9043–9059. [Google Scholar] [CrossRef]
- Han, Y.; Katayama, S.; Futakuchi, M.; Nakamichi, K.; Wakabayashi, Y.; Sakamoto, M.; Nakayama, J.; Semba, K. Targeting c-Jun Is a Potential Therapy for Luminal Breast Cancer Bone Metastasis. Mol. Cancer Res. 2023, 21, 908–921. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.A.; Moloney, F.J.; Cai, H.; Au-Yeung, A.; China, C.; Scolyer, R.A.; Yosufi, B.; Raftery, M.J.; Deng, J.Z.; Morton, S.W.; et al. Safety and tolerability of an intratumorally injected DNAzyme, Dz13, in patients with nodular basal-cell carcinoma: A phase 1 first-in-human trial (DISCOVER). Lancet 2013, 381, 1835–1843. [Google Scholar] [CrossRef]
- Tan, M.L.; Choong, P.F.; Dass, C.R. Direct anti-metastatic efficacy by the DNA enzyme Dz13 and downregulated MMP-2, MMP-9 and MT1-MMP in tumours. Cancer Cell Int. 2010, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Dass, C.R.; Galloway, S.J.; Clark, J.C.M.; Khachigian, L.M.; Choong, P.F.M. Involvement of c-jun in human liposarcoma growth: Supporting data from clinical immunohistochemistry and DNAzyme efficacy. Cancer Biol. Ther. 2008, 7, 1297–1301. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, G.; Dass, C.R.; Sumithran, E.; Di Girolamo, N.; Sun, L.-Q.; Khachigian, L.M. Effect of Deoxyribozymes Targeting c-Jun on Solid Tumor Growth and Angiogenesis in Rodents. JNCI J. Natl. Cancer Inst. 2004, 96, 683–696. [Google Scholar] [CrossRef]
- Cai, H.; Santiago, F.S.; Prado-Lourenco, L.; Wang, B.; Patrikakis, M.; Davenport, M.P.; Maghzal, G.J.; Stocker, R.; Parish, C.R.; Chong, B.H.; et al. DNAzyme Targeting c-jun Suppresses Skin Cancer Growth. Sci. Transl. Med. 2012, 4, ra82–ra139. [Google Scholar] [CrossRef]
- Cai, H.; Cho, E.A.; Li, Y.; Sockler, J.; Parish, C.R.; Chong, B.H.; Edwards, J.; Dodds, T.J.; Ferguson, P.M.; Wilmott, J.S.; et al. Melanoma protective antitumor immunity activated by catalytic DNA. Oncogene 2018, 37, 5115–5126. [Google Scholar] [CrossRef]
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Vara, J.Á.F.; Casado, E.; de Castro, J.; Cejas, P.; Belda-Iniesta, C.; González-Barón, M. PI3K/Akt signalling pathway and cancer. Cancer Treat. Rev. 2004, 30, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Kappelmann-Fenzl, M.; Gebhard, C.; Matthies, A.O.; Kuphal, S.; Rehli, M.; Bosserhoff, A.K. C-Jun drives melanoma progression in PTEN wild type melanoma cells. Cell Death Dis. 2019, 10, 584. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Investig. Dermatol. 2021, 141, 23–31. [Google Scholar] [CrossRef]
- Robert, C.; Grob Jean, J.; Stroyakovskiy, D.; Karaszewska, B.; Hauschild, A.; Levchenko, E.; Chiarion Sileni, V.; Schachter, J.; Garbe, C.; Bondarenko, I.; et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019, 381, 626–636. [Google Scholar] [CrossRef]
- Ramsdale, R.; Jorissen, R.N.; Li, F.Z.; Al-Obaidi, S.; Ward, T.; Sheppard, K.E.; Bukczynska, P.E.; Young, R.J.; Boyle, S.E.; Shackleton, M.; et al. The transcription cofactor c-JUN mediates phenotype switching and BRAF inhibitor resistance in melanoma. Sci. Signal. 2015, 8, ra82. [Google Scholar] [CrossRef] [PubMed]
- Delmas, A.; Cherier, J.; Pohorecka, M.; Medale-Giamarchi, C.; Meyer, N.; Casanova, A.; Sordet, O.; Lamant, L.; Savina, A.; Pradines, A.; et al. The c-Jun/RHOB/AKT pathway confers resistance of BRAF-mutant melanoma cells to MAPK inhibitors. Oncotarget 2015, 6, 15250–15264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, C.; Xing, Z.; Lou, C.; Fang, J.; Wang, Z.; Li, M.; He, H.; Bai, H. Fibronectin 1 derived from tumor-associated macrophages and fibroblasts promotes metastasis through the JUN pathway in hepatocellular carcinoma. Int. Immunopharmacol. 2022, 113, 109420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pu, X.; Shi, M.; Chen, L.; Qian, L.; Song, Y.; Yuan, G.; Zhang, H.; Yu, M.; Hu, M.; et al. c-Jun, a crucial molecule in metastasis of breast cancer and potential target for biotherapy. Oncol. Rep. 2007, 18, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pu, X.; Shi, M.; Chen, L.; Song, Y.; Qian, L.; Yuan, G.; Zhang, H.; Yu, M.; Hu, M.; et al. Critical role of c-Jun overexpression in liver metastasis of human breast cancer xenograft model. BMC Cancer 2007, 7, 145. [Google Scholar] [CrossRef] [PubMed]
- Ebelt, N.D.; Cantrell, M.A.; Van Den Berg, C.L. c-Jun N-Terminal Kinases Mediate a Wide Range of Targets in the Metastatic Cascade. Genes Cancer 2013, 4, 378–387. [Google Scholar] [CrossRef]
- Anju, M.S.; Chandramohan, K.; Bhargavan, R.V.; Somanathan, T.; Subhadradevi, L. An overview on liposarcoma subtypes: Genetic alterations and recent advances in therapeutic strategies. J. Mol. Histol. 2024, 55, 227–240. [Google Scholar]
- Sioletic, S.; Czaplinski, J.; Hu, L.; Fletcher, J.A.; Fletcher, C.D.; Wagner, A.J.; Loda, M.; Demetri, G.D.; Sicinska, E.T.; Snyder, E.L. c-Jun promotes cell migration and drives expression of the motility factor ENPP2 in soft tissue sarcomas. J. Pathol. 2014, 234, 190–202. [Google Scholar] [CrossRef]
- Zhou, J.; Ji, Q.; Li, Q. Resistance to anti-EGFR therapies in metastatic colorectal cancer: Underlying mechanisms and reversal strategies. J. Exp. Clin. Cancer Res. 2021, 40, 328. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Zhang, M.; Zhao, Y.; Li, X.; Yao, R.; Wu, F.; Huang, R.; Li, K.; Miao, S.; Ma, C.; et al. RHBDD1 upregulates EGFR via the AP-1 pathway in colorectal cancer. Oncotarget 2017, 8, 25251. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, K.; Lin, G.; Wan, F.; Chen, L.; Zhu, X. Silencing c-Jun inhibits autophagy and abrogates radioresistance in nasopharyngeal carcinoma by activating the PI3K/AKT/mTOR pathway. Ann. Transl. Med. 2021, 9, 1085. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.-l.; Huang, Z.-j.; Lin, Z.-t.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Fardini, Y.; Dehennaut, V.; Lefebvre, T.; Issad, T. O-GlcNAcylation: A New Cancer Hallmark? Front. Endocrinol. 2013, 4, 99. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, G.; Liu, Y.; Wu, Q.; Zhang, X.; Bian, Z.; Zhang, Y.; Pan, Q.; Sun, F. O-GlcNAcylated c-Jun antagonizes ferroptosis via inhibiting GSH synthesis in liver cancer. Cell Signal 2019, 63, 109384. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gan, W.; Xiong, J.; Li, J. A novel biomarker GATM suppresses proliferation and malignancy of cholangiocarcinoma cells by modulating the JNK/c-Jun signalling pathways. Heliyon 2024, 10, e37344. [Google Scholar] [CrossRef] [PubMed]
- Lukey, M.J.; Greene, K.S.; Erickson, J.W.; Wilson, K.F.; Cerione, R.A. The oncogenic transcription factor c-Jun regulates glutaminase expression and sensitizes cells to glutaminase-targeted therapy. Nat. Commun. 2016, 7, 11321. [Google Scholar] [CrossRef]
- Hanna, E.; Quick, J.; Libutti, S.K. The tumour microenvironment: A novel target for cancer therapy. Oral Dis. 2009, 15, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Sporn, M.B. The tumour microenvironment as a target for chemoprevention. Nat. Rev. Cancer 2007, 7, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Goliwas, K.F.; Deshane, J.S.; Elmets, C.A.; Athar, M. Moving immune therapy forward targeting tme. Physiol. Rev. 2020, 101, 417–425. [Google Scholar] [CrossRef]
- Hu, M.; Huang, L. Strategies targeting tumor immune and stromal microenvironment and their clinical relevance. Adv. Drug Deliv. Rev. 2022, 183, 114137. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Sanford-Crane, H.; Abrego, J.; Sherman, M.H. Fibroblasts as Modulators of Local and Systemic Cancer Metabolism. Cancers 2019, 11, 619. [Google Scholar] [CrossRef] [PubMed]
- Nihashi, Y.; Song, X.; Yamamoto, M.; Setoyama, D.; Kida, Y.S. Decoding Metabolic Symbiosis between Pancreatic Cancer Cells and Cancer-Associated Fibroblasts Using Cultured Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 11015. [Google Scholar] [CrossRef]
- Li, Q.; Lv, X.; Han, C.; Kong, Y.; Dai, Z.; Huo, D.; Li, T.; Li, D.; Li, W.; Wang, X.; et al. Enhancer reprogramming promotes the activation of cancer-associated fibroblasts and breast cancer metastasis. Theranostics 2022, 12, 7491–7508. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Cai, H.; Zhu, X.-D.; Ao, J.-Y.; Ye, B.-G.; Zhang, Y.-Y.; Chai, Z.-T.; Wang, C.-H.; Shi, W.-K.; Cao, M.-Q.; Li, X.-L.; et al. Colony-stimulating factor-1-induced AIF1 expression in tumor-associated macrophages enhances the progression of hepatocellular carcinoma. OncoImmunology 2017, 6, e1333213. [Google Scholar] [CrossRef]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Song, J.; Lee, J.; Kim, J.; Jo, S.; Kim, Y.J.; Baek, J.E.; Kwon, E.S.; Lee, K.P.; Yang, S.; Kwon, K.S.; et al. Pancreatic adenocarcinoma up-regulated factor (PAUF) enhances the accumulation and functional activity of myeloid-derived suppressor cells (MDSCs) in pancreatic cancer. Oncotarget 2016, 7, 51840–51853. [Google Scholar] [CrossRef]
- Lee, H.J.; Oh, J.Y. Mesenchymal Stem/Stromal Cells Induce Myeloid-Derived Suppressor Cells in the Bone Marrow via the Activation of the c-Jun N-Terminal Kinase Signaling Pathway. Int. J. Mol. Sci. 2024, 25, 1119. [Google Scholar] [CrossRef]
- Karin, M. The Regulation of AP-1 Activity by Mitogen-activated Protein Kinases *. J. Biol. Chem. 1995, 270, 16483–16486. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- Zhang, A.B.; Mozaffari, K.; Aguirre, B.; Li, V.; Kubba, R.; Desai, N.C.; Wei, D.; Yang, I.; Wadehra, M. Exploring the Past, Present, and Future of Anti-Angiogenic Therapy in Glioblastoma. Cancers 2023, 15, 830. [Google Scholar] [CrossRef] [PubMed]
- Vleugel, M.M.; Greijer, A.E.; Bos, R.; van der Wall, E.; van Diest, P.J. c-Jun activation is associated with proliferation and angiogenesis in invasive breast cancer. Hum. Pathol. 2006, 37, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Folkman, J. Angiogenesis and c-Jun. J. Natl. Cancer Inst. 2004, 96, 644. [Google Scholar] [CrossRef] [PubMed]
- Podar, K.; Raab, M.S.; Tonon, G.; Sattler, M.; Barilà, D.; Zhang, J.; Tai, Y.-T.; Yasui, H.; Raje, N.; DePinho, R.A.; et al. Up-Regulation of c-Jun Inhibits Proliferation and Induces Apoptosis via Caspase-Triggered c-Abl Cleavage in Human Multiple Myeloma. Cancer Res. 2007, 67, 1680–1688. [Google Scholar] [CrossRef] [PubMed]
- Bossy-Wetzel, E.; Bakiri, L.; Yaniv, M. Induction of apoptosis by the transcription factor c-Jun. Embo J. 1997, 16, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Mhaidat, N.M.; Zhang, X.D.; Jiang, C.C.; Hersey, P. Docetaxel-Induced Apoptosis of Human Melanoma Is Mediated by Activation of c-Jun NH2-Terminal Kinase and Inhibited by the Mitogen-Activated Protein Kinase Extracellular Signal-Regulated Kinase 1/2 Pathway. Clin. Cancer Res. 2007, 13, 1308–1314. [Google Scholar] [CrossRef] [PubMed]
- Shieh, J.-M.; Huang, T.-F.; Hung, C.-F.; Chou, K.-H.; Tsai, Y.-J.; Wu, W.-B. Activation of c-Jun N-terminal kinase is essential for mitochondrial membrane potential change and apoptosis induced by doxycycline in melanoma cells. Br. J. Pharmacol. 2010, 160, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Fernando, P.; Zhen, A.X.; Piao, M.J.; Herath, H.; Kang, K.A.; Yoon, S.P.; Boo, H.J.; Hyun, C.L.; Hyun, J.W. Naringenin Induces Cellular Apoptosis in Melanoma Cells via Intracellular ROS Generation. Anticancer Res. 2024, 44, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- El-Khattouti, A.; Sheehan, N.T.; Monico, J.; Drummond, H.A.; Haikel, Y.; Brodell, R.T.; Megahed, M.; Hassan, M. CD133(+) melanoma subpopulation acquired resistance to caffeic acid phenethyl ester-induced apoptosis is attributed to the elevated expression of ABCB5: Significance for melanoma treatment. Cancer Lett. 2015, 357, 83–104. [Google Scholar] [CrossRef]
- Shieu, M.K.; Chuang, Y.C.; Ho, H.Y.; Lin, C.C.; Lo, Y.S.; Hsieh, M.J. Hellebrigenin induces apoptosis by triggering cellular inhibitor of apoptosis 1 and Jun N-terminal kinase pathway in melanoma cells. Dermatol. Sin. 2024, 42, 19–30. [Google Scholar] [CrossRef]
- Wang, C.C.; Chiang, Y.M.; Sung, S.C.; Hsu, Y.L.; Chang, J.K.; Kuo, P.L. Plumbagin induces cell cycle arrest and apoptosis through reactive oxygen species/c-Jun N-terminal kinase pathways in human melanoma A375.S2 cells. Cancer Lett. 2008, 259, 82–98. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Liang, A.; Liu, Y.; Deng, B.; Wang, H. Immune-related chemotactic factors were found in acute coronary syndromes by bioinformatics. Mol. Biol. Rep. 2014, 41, 4389–4395. [Google Scholar] [CrossRef]
- Connolly, R.M.; Rudek, M.A.; Piekarz, R. Entinostat: A promising treatment option for patients with advanced breast cancer. Future Oncol. 2017, 13, 1137–1148. [Google Scholar] [CrossRef]
- Tanioka, M.; Mott, K.R.; Hollern, D.P.; Fan, C.; Darr, D.B.; Perou, C.M. Identification of Jun loss promotes resistance to histone deacetylase inhibitor entinostat through Myc signaling in luminal breast cancer. Genome Med. 2018, 10, 86. [Google Scholar] [CrossRef]
- Dang, C.V. MYC on the path to cancer. Cell 2012, 149, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.J.; Lan, L.; Diefenbacher, M.E.; Riising, E.M.; Da Costa, C.; Chakraborty, A.; Hoeck, J.D.; Spencer-Dene, B.; Kelly, G.; David, J.-P.; et al. JunD, not c-Jun, is the AP-1 transcription factor required for Ras-induced lung cancer. JCI Insight 2021, 6, e124985. [Google Scholar] [CrossRef] [PubMed]
- Redmer, T.; Raigel, M.; Sternberg, C.; Ziegler, R.; Probst, C.; Lindner, D.; Aufinger, A.; Limberger, T.; Trachtova, K.; Kodajova, P.; et al. JUN mediates the senescence associated secretory phenotype and immune cell recruitment to prevent prostate cancer progression. Mol. Cancer 2024, 23, 114. [Google Scholar] [CrossRef]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Grob, J.-J.; Aamdal, S.; Bondarenko, I.; Robert, C.; Thomas, L.; Garbe, C.; Chiarion-Sileni, V.; Testori, A.; Chen, T.-T.; et al. Five-Year Survival Rates for Treatment-Naive Patients with Advanced Melanoma Who Received Ipilimumab Plus Dacarbazine in a Phase III Trial. J. Clin. Oncol. 2015, 33, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Johnson, D.B.; Nebhan, C.A.; Moslehi, J.J.; Balko, J.M. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022, 19, 254–267. [Google Scholar] [CrossRef]
- Dobosz, P.; Stępień, M.; Golke, A.; Dzieciątkowski, T. Challenges of the Immunotherapy: Perspectives and Limitations of the Immune Checkpoint Inhibitor Treatment. Int. J. Mol. Sci. 2022, 23, 2847. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long Georgina, V.; Arance, A.; Grob Jean, J.; Mortier, L.; Daud, A.; Carlino Matteo, S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Atsaves, V.; Leventaki, V.; Rassidakis, G.Z.; Claret, F.X. AP-1 Transcription Factors as Regulators of Immune Responses in Cancer. Cancers 2019, 11, 1037. [Google Scholar] [CrossRef]
- Müller, M.R.; Rao, A. NFAT, immunity and cancer: A transcription factor comes of age. Nat. Rev. Immunol. 2010, 10, 645–656. [Google Scholar] [CrossRef]
- Esensten, J.H.; Helou, Y.A.; Chopra, G.; Weiss, A.; Bluestone, J.A. CD28 Costimulation: From Mechanism to Therapy. Immunity 2016, 44, 973–988. [Google Scholar] [CrossRef]
- Mognol, G.P.; González-Avalos, E.; Ghosh, S.; Spreafico, R.; Gudlur, A.; Rao, A.; Damoiseaux, R.; Hogan, P.G. Targeting the NFAT:AP-1 transcriptional complex on DNA with a small-molecule inhibitor. Proc. Natl. Acad. Sci. USA 2019, 116, 9959–9968. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-O.; Kim, H.W.; Baek, K.-M.; Kang, C.-Y. NF-κB and AP-1 regulate activation-dependent CD137 (4-1BB) expression in T cells. FEBS Lett. 2003, 541, 163–170. [Google Scholar] [CrossRef]
- Yukawa, M.; Jagannathan, S.; Vallabh, S.; Kartashov, A.V.; Chen, X.; Weirauch, M.T.; Barski, A. AP-1 activity induced by co-stimulation is required for chromatin opening during T cell activation. J. Exp. Med. 2020, 217, e20182009. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining ‘T cell exhaustion’. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef]
- Wherry, E.J. T cell exhaustion. Nat. Immunol. 2011, 12, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Somasundaram, A.; Manne, S.; Gocher, A.M.; Szymczak-Workman, A.L.; Vignali, K.M.; Scott, E.N.; Normolle, D.P.; John Wherry, E.; Lipson, E.J.; et al. Neuropilin-1 is a T cell memory checkpoint limiting long-term antitumor immunity. Nat. Immunol. 2020, 21, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Wherry, E.J.; Ha, S.-J.; Kaech, S.M.; Haining, W.N.; Sarkar, S.; Kalia, V.; Subramaniam, S.; Blattman, J.N.; Barber, D.L.; Ahmed, R. Molecular Signature of CD8+ T Cell Exhaustion during Chronic Viral Infection. Immunity 2007, 27, 670–684. [Google Scholar] [CrossRef]
- Hu, G.; Chen, J. A genome-wide regulatory network identifies key transcription factors for memory CD8⁺ T-cell development. Nat. Commun. 2013, 4, 2830. [Google Scholar] [CrossRef] [PubMed]
- Mizukoshi, E.; Nakagawa, H.; Tamai, T.; Kitahara, M.; Fushimi, K.; Nio, K.; Terashima, T.; Iida, N.; Arai, K.; Yamashita, T.; et al. Peptide vaccine-treated, long-term surviving cancer patients harbor self-renewing tumor-specific CD8+ T cells. Nat. Commun. 2022, 13, 3123. [Google Scholar] [CrossRef] [PubMed]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Lynn, R.C.; Weber, E.W.; Sotillo, E.; Gennert, D.; Xu, P.; Good, Z.; Anbunathan, H.; Lattin, J.; Jones, R.; Tieu, V.; et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature 2019, 576, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.S.; Li, Q.; Mao, R.; Peng, Y.; He, Y. TCR T cells overexpressing c-Jun have better functionality with improved tumor infiltration and persistence in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1114770. [Google Scholar] [CrossRef]
- Heitzeneder, S.; Bosse, K.R.; Zhu, Z.; Zhelev, D.; Majzner, R.G.; Radosevich, M.T.; Dhingra, S.; Sotillo, E.; Buongervino, S.; Pascual-Pasto, G.; et al. GPC2-CAR T cells tuned for low antigen density mediate potent activity against neuroblastoma without toxicity. Cancer Cell 2022, 40, 53–69.e9. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, C.; Chen, X.; Bai, J.; Wang, E.; Sun, M. Coexpression of C-Jun in Multiple-Chain DAP-CAR-engineered T-Cells for Solid Tumor Therapy. Immunotherapy 2022, 14, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Zuo, S.; Li, C.; Sun, X.; Deng, B.; Zhang, Y.; Han, Y.; Ling, Z.; Xu, J.; Duan, J.; Wang, Z.; et al. C-JUN overexpressing CAR-T cells in acute myeloid leukemia: Preclinical characterization and phase I trial. Nat. Commun. 2024, 15, 6155. [Google Scholar] [CrossRef]
- Novoszel, P.; Drobits, B.; Holcmann, M.; Fernandes, C.D.S.; Tschismarov, R.; Derdak, S.; Decker, T.; Wagner, E.F.; Sibilia, M. The AP-1 transcription factors c-Jun and JunB are essential for CD8α conventional dendritic cell identity. Cell Death Differ. 2021, 28, 2404–2420. [Google Scholar] [CrossRef]
- Bayerl, F.; Meiser, P.; Donakonda, S.; Hirschberger, A.; Lacher, S.B.; Pedde, A.-M.; Hermann, C.D.; Elewaut, A.; Knolle, M.; Ramsauer, L.; et al. Tumor-derived prostaglandin E2 programs cDC1 dysfunction to impair intratumoral orchestration of anti-cancer T cell responses. Immunity 2023, 56, 1341–1358.e11. [Google Scholar] [CrossRef]
- Rebuffet, L.; Melsen, J.E.; Escalière, B.; Basurto-Lozada, D.; Bhandoola, A.; Björkström, N.K.; Bryceson, Y.T.; Castriconi, R.; Cichocki, F.; Colonna, M.; et al. High-dimensional single-cell analysis of human natural killer cell heterogeneity. Nat. Immunol. 2024, 25, 1474–1488. [Google Scholar] [CrossRef]
- Mgrditchian, T.; Arakelian, T.; Paggetti, J.; Noman, M.Z.; Viry, E.; Moussay, E.; Van Moer, K.; Kreis, S.; Guerin, C.; Buart, S.; et al. Targeting autophagy inhibits melanoma growth by enhancing NK cells infiltration in a CCL5-dependent manner. Proc. Natl. Acad. Sci. USA 2017, 114, E9271–E9279. [Google Scholar] [CrossRef]
- Yu, P.; Wei, H.; Li, K.; Zhu, S.; Li, J.; Chen, C.; Zhang, D.; Li, Y.; Zhu, L.; Yi, X.; et al. The traditional chinese medicine monomer Ailanthone improves the therapeutic efficacy of anti-PD-L1 in melanoma cells by targeting c-Jun. J. Exp. Clin. Cancer Res. 2022, 41, 346. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Koizumi, S.-i.; Sasaki, D.; Hsieh, T.-H.; Taira, N.; Arakaki, N.; Yamasaki, S.; Wang, K.; Sarkar, S.; Shirahata, H.; Miyagi, M.; et al. JunB regulates homeostasis and suppressive functions of effector regulatory T cells. Nat. Commun. 2018, 9, 5344. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Sun, P.; Zheng, J.; Wu, M.; Yang, C.; Cheng, M.; Yin, M.; Cui, C.; Wang, G.; Yuan, L.; et al. JNK Signaling Promotes Bladder Cancer Immune Escape by Regulating METTL3-Mediated m6A Modification of PD-L1 mRNA. Cancer Res. 2022, 82, 1789–1802. [Google Scholar] [CrossRef]
- Ma, X.-M.; Luo, Y.-F.; Zeng, F.-F.; Su, C.; Liu, X.; Li, X.-P.; Lu, J. TGF-β1-Mediated PD-L1 Glycosylation Contributes to Immune Escape via c-Jun/STT3A Pathway in Nasopharyngeal Carcinoma. Front. Oncol. 2022, 12, 815437. [Google Scholar] [CrossRef] [PubMed]
- Geels, S.N.; Moshensky, A.; Sousa, R.S.; Murat, C.; Bustos, M.A.; Walker, B.L.; Singh, R.; Harbour, S.N.; Gutierrez, G.; Hwang, M.; et al. Interruption of the intratumor CD8(+) T cell:Treg crosstalk improves the efficacy of PD-1 immunotherapy. Cancer Cell 2024, 42, 1051–1066.e7. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, D.R.; Tonon, G.; Huang, Y.; Zhang, Y.; Sinha, R.; Feng, B.; Stewart, J.P.; Zhan, F.; Khatry, D.; Protopopova, M.; et al. High-resolution genomic profiles define distinct clinico-pathogenetic subgroups of multiple myeloma patients. Cancer Cell 2006, 9, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ran, T.; Li, Y.; Tian, L.; Yang, L.; Liu, Z.; Yao, B. Identification of JUN gene and cellular microenvironment in response to PD-1 blockade treatment in lung cancer patients via single-cell RNA sequencing. Aging 2024, 16, 10348–10365. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafri, Z.; Li, Y.; Zhang, J.; O’Meara, C.H.; Khachigian, L.M. Jun, an Oncological Foe or Friend? Int. J. Mol. Sci. 2025, 26, 555. https://doi.org/10.3390/ijms26020555
Jafri Z, Li Y, Zhang J, O’Meara CH, Khachigian LM. Jun, an Oncological Foe or Friend? International Journal of Molecular Sciences. 2025; 26(2):555. https://doi.org/10.3390/ijms26020555
Chicago/Turabian StyleJafri, Zuhayr, Yue Li, Jingwen Zhang, Connor H. O’Meara, and Levon M. Khachigian. 2025. "Jun, an Oncological Foe or Friend?" International Journal of Molecular Sciences 26, no. 2: 555. https://doi.org/10.3390/ijms26020555
APA StyleJafri, Z., Li, Y., Zhang, J., O’Meara, C. H., & Khachigian, L. M. (2025). Jun, an Oncological Foe or Friend? International Journal of Molecular Sciences, 26(2), 555. https://doi.org/10.3390/ijms26020555





