Ecometabolomics of Loggerhead Sea Turtles (Caretta caretta): The Impact of Age on Metabolomic Profiles
Abstract
1. Introduction
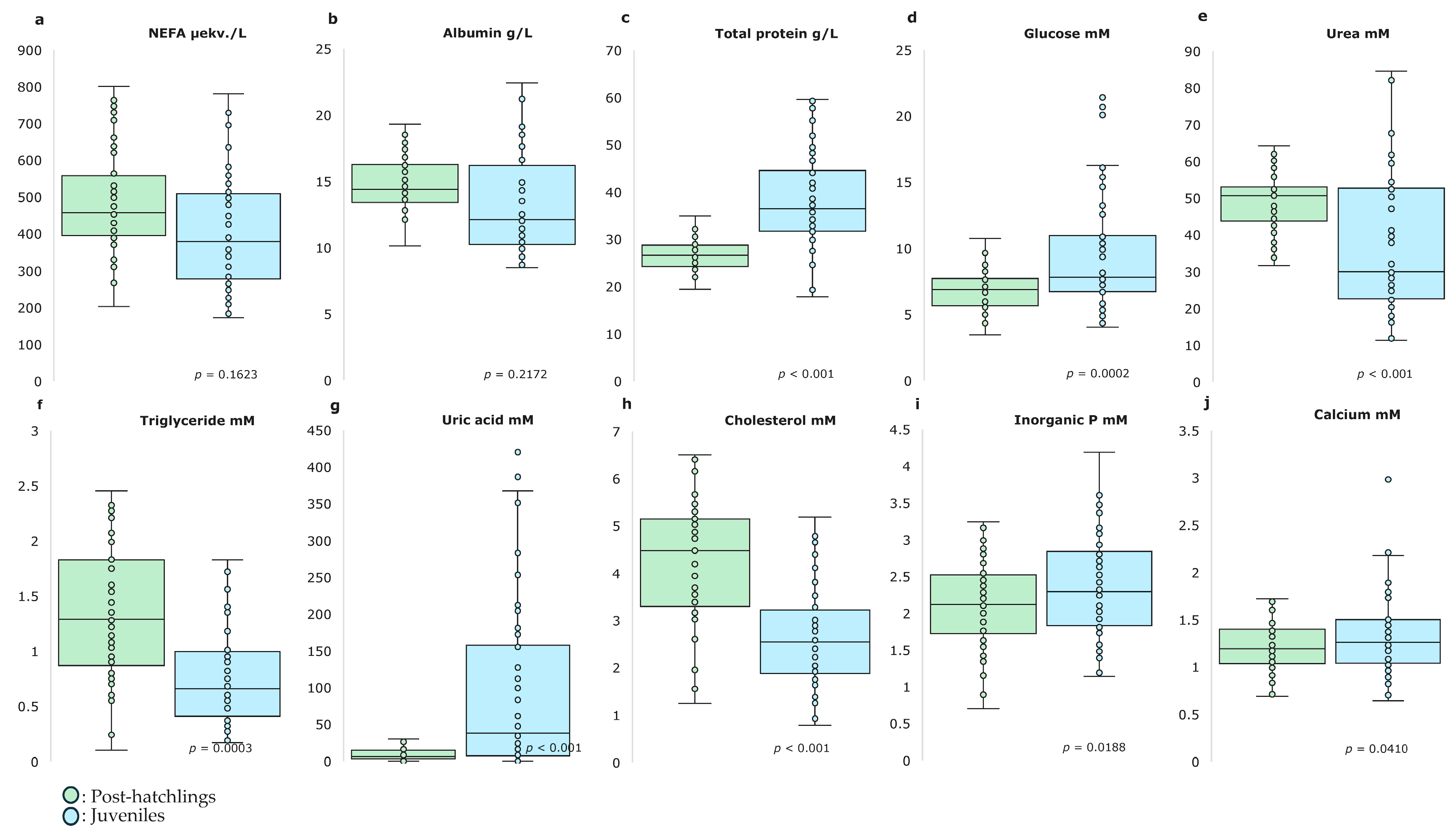
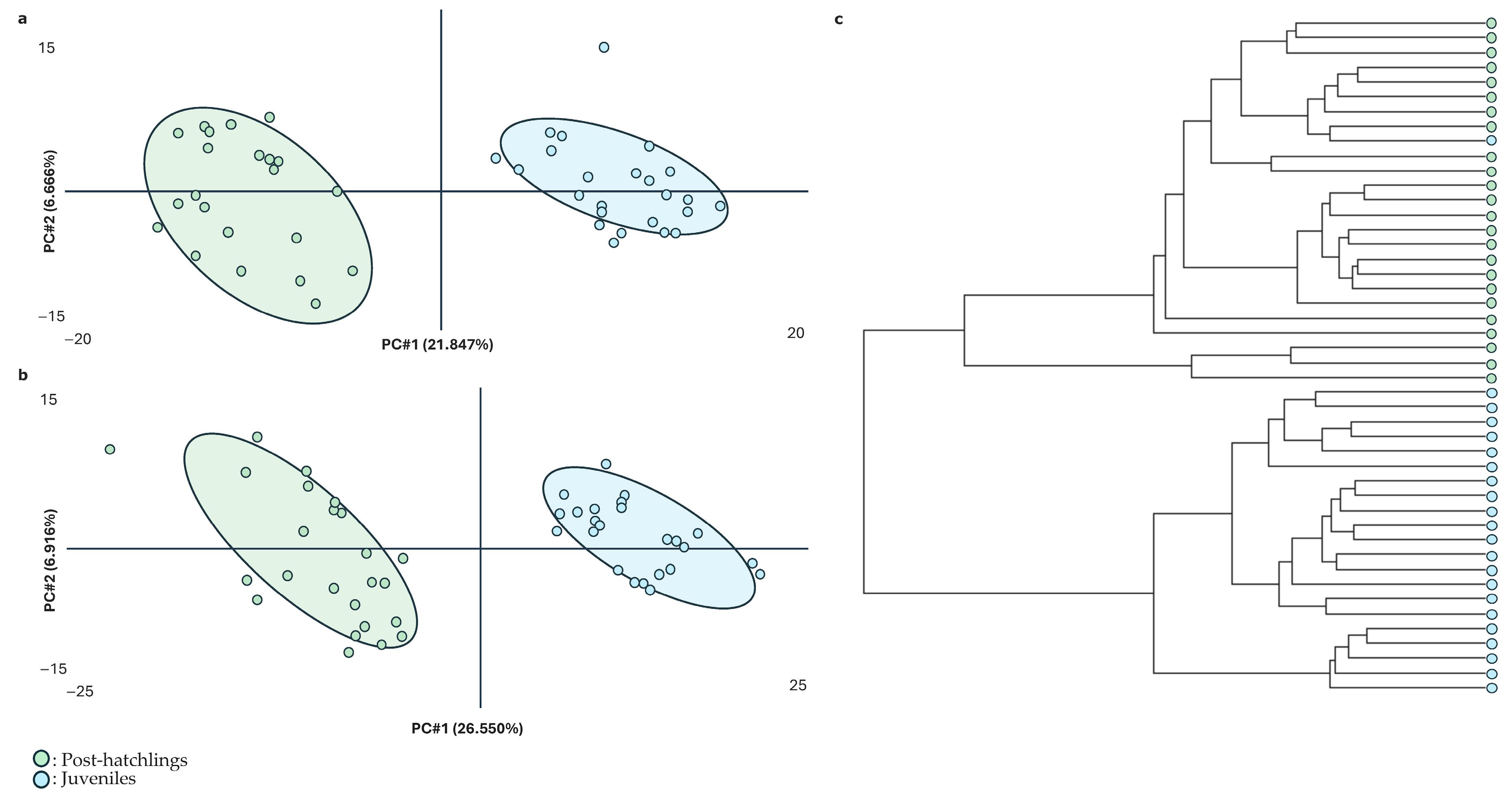
2. Results
3. Discussion
4. Materials and Methods
4.1. Animal Ethics Statement
4.2. Animals and Sampling
4.3. Targeted Metabolomic Analysis
4.4. Untargeted Metabolomic Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Peñuelas, J.; Sardans, J. Ecological Metabolomics. Chem. Ecol. 2009, 25, 305–309. [Google Scholar] [CrossRef]
- Kuzina, V.; Ekstrøm, C.T.; Andersen, S.B.; Nielsen, J.K.; Olsen, C.E.; Bak, S. Identification of Defense Compounds in Barbarea vulgaris against the Herbivore Phyllotreta nemorum by an Ecometabolomic Approach. Plant Physiol. 2009, 151, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J.; Rivas-Ubach, A. Ecological Metabolomics: Overview of Current Developments and Future Challenges. Chemoecology 2011, 21, 191–225. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Peñuelas, J.; Hódar, J.A.; Oravec, M.; Paša-Tolić, L.; Urban, O.; Sardans, J. We Are What We Eat: A Stoichiometric and Ecometabolomic Study of Caterpillars Feeding on Two Pine Subspecies of Pinus Sylvestris. Int. J. Mol. Sci. 2018, 20, 59. [Google Scholar] [CrossRef]
- Allevato, D.M.; Kiyota, E.; Mazzafera, P.; Nixon, K.C. Ecometabolomic Analysis of Wild Populations of Pilocarpus pennatifolius (Rutaceae) Using Unimodal Analyses. Front. Plant Sci. 2019, 10, 258. [Google Scholar] [CrossRef]
- Sardans, J.; Gargallo-Garriga, A.; Urban, O.; Klem, K.; Walker, T.W.N.; Holub, P.; Janssens, I.A.; Peñuelas, J. Ecometabolomics for a Better Understanding of Plant Responses and Acclimation to Abiotic Factors Linked to Global Change. Metabolites 2020, 10, 239. [Google Scholar] [CrossRef]
- González Montoya, M.C. Eco-Metabolomics Approach for Understanding the Chemodiversity of Cocktails Found on Poison Frogs; Universidad de los Andes: Bogota, Colombia, 2021. [Google Scholar] [CrossRef]
- Marín-García, P.J.; Rouco, C.; Llobat, L.; Larsen, T.; Hedemann, M.S. Targeted and Untargeted Metabolomic Profiles in Wild Rabbit Does (Oryctolagus Cuniculus) of Different Breeding States (Pregnant and Lactating). J. Exp. Zool. Part A Ecol. Integr. Physiol. 2024, 341, 743–752. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, C.-M.; Diao, X. The Metabolic Responses of Aquatic Animal Exposed to POPs. In Environmental Metabolomics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 121–161. ISBN 978-0-12-818196-6. [Google Scholar]
- Bertram, H.C.; Jakobsen, L.M.A. Nutrimetabolomics: Integrating Metabolomics in Nutrition to Disentangle Intake of Animal-Based Foods. Metabolomics 2018, 14, 34. [Google Scholar] [CrossRef]
- Marín-García, P.J.; Llobat, L.; Cambra-López, M.; Blas, E.; Larsen, T.; Pascual, J.J.; Hedemann, M.S. Biomarkers for Ideal Protein: Rabbit Diet Metabolomics Varying Key Amino Acids. Commun. Biol. 2024, 7, 712. [Google Scholar] [CrossRef]
- Dove, A.D.M. Metabolomics Has Great Potential for Clinical and Nutritional Care and Research with Exotic Animals. Zoo Biol. 2013, 32, 246–250. [Google Scholar] [CrossRef]
- Casale, P.; Abbate, G.; Freggi, D.; Conte, N.; Oliverio, M.; Argano, R. Foraging Ecology of Loggerhead Sea Turtles Caretta caretta in the Central Mediterranean Sea: Evidence for a Relaxed Life History Model. Mar. Ecol. Prog. Ser. 2008, 372, 265–276. [Google Scholar] [CrossRef]
- Van De Vijver, B.; Robert, K.; Majewska, R.; Frankovich, T.A.; Panagopoulou, A.; Bosak, S. Geographical Variation in the Diatom Communities Associated with Loggerhead Sea Turtles (Caretta caretta). PLoS ONE 2020, 15, e0236513. [Google Scholar] [CrossRef] [PubMed]
- Casale, P.; Tucker, A.D. The IUCN Red List of Threatened Species 2017 2015. Available online: https://www.iucnredlist.org/species/3897/119333622 (accessed on 12 May 2024).
- López-Mendilaharsu, M.; Giffoni, B.; Monteiro, D.; Prosdocimi, L.; Vélez-Rubio, G.; Fallabrino, A.; Estrades, A.; Santos, A.; Lara, P.; Pires, T.; et al. Multiple-Threats Analysis for Loggerhead Sea Turtles in the Southwest Atlantic Ocean. Endanger. Species Res. 2020, 41, 183–196. [Google Scholar] [CrossRef]
- Wallace, B.P.; Avens, L.; Braun-McNeill, J.; McClellan, C.M. The Diet Composition of Immature Loggerheads: Insights on Trophic Niche, Growth Rates, and Fisheries Interactions. J. Exp. Mar. Biol. Ecol. 2009, 373, 50–57. [Google Scholar] [CrossRef]
- Tomas, J.; Aznar, F.J.; Raga, J.A. Feeding Ecology of the Loggerhead Turtle Caretta caretta in the Western Mediterranean. J. Zool. 2001, 255, 525–532. [Google Scholar] [CrossRef]
- Plotkin, P.T.; Wicksten, M.K.; Amos, A.F. Feeding Ecology of the Loggerhead Sea Turtle Caretta caretta in the Northwestern Gulf of Mexico. Mar. Biol. 1993, 115, 1–5. [Google Scholar] [CrossRef]
- Allen, M.E.; Ullrey, D.E. Relationships among Nutrition and Reproduction and Relevance for Wild Animals. Zoo Biol. 2004, 23, 475–487. [Google Scholar] [CrossRef]
- Perrault, J.R.; Stacy, N.I. Note on the Unique Physiologic State of Loggerhead Sea Turtles (Caretta caretta) during Nesting Season as Evidenced by a Suite of Health Variables. Mar. Biol. 2018, 165, 71. [Google Scholar] [CrossRef]
- Souza, N.L.N.; Carneiro, M.T.W.D.; Pimentel, E.F.; Frossard, A.; Freire, J.B.; Endringer, D.C.; Ferreira Júnior, P.D. Trace Elements Influence the Hatching Success and Emergence of Caretta caretta and Chelonia mydas. J. Trace Elem. Med. Biol. 2018, 50, 117–122. [Google Scholar] [CrossRef]
- Casale, P.; d’Astore, P.P.; Argona, R. Age at Size and Growth Rates of Early Juvenile Loggerhead Sea Turtles (Caretta caretta) in the Mediterranean Based on Length Frequency Analysis. Herpetol. J. 2009, 19, 29–35. [Google Scholar]
- Casale, P.; Mazaris, A.D.; Freggi, D.; Vallini, C.; Argano, R. Growth Rates and Age at Adult Size of Loggerhead Sea Turtles (Caretta caretta) in the Mediterranean Sea, Estimated through Capture-Mark-Recapture Records. Sci. Mar. 2009, 73, 589–595. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-Independent MS/MS Deconvolution for Comprehensive Metabolome Analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Dührkop, K.; Fleischauer, M.; Ludwig, M.; Aksenov, A.A.; Melnik, A.V.; Meusel, M.; Dorrestein, P.C.; Rousu, J.; Böcker, S. SIRIUS 4: A Rapid Tool for Turning Tandem Mass Spectra into Metabolite Structure Information. Nat. Methods 2019, 16, 299–302. [Google Scholar] [CrossRef]
- Jakšić, Ž.; Mrljak, V.; Horvatić, A.; Gelemanović, A.; Mičić, M. Loggerhead Sea Turtle Caretta caretta Plasma Biochemistry and Proteome Profile Modulation during Recovery. J. Proteom. 2022, 252, 104433. [Google Scholar] [CrossRef]
- Molter, C.M.; Norton, T.M.; Hoopes, L.A.; Nelson, S.E.; Kaylor, M.; Hupp, A.; Thomas, R.; Kemler, E.; Kass, P.H.; Arendt, M.D.; et al. Health and Nutrition of Loggerhead Sea Turtles (Caretta caretta) in the Southeastern United States. Anim. Physiol. Nutr. 2022, 106, 205–219. [Google Scholar] [CrossRef]
- Kunze, P.E.; Perrault, J.R.; Chang, Y.-M.; Manire, C.A.; Clark, S.; Stacy, N.I. Pre-/Analytical Factors Affecting Whole Blood and Plasma Glucose Concentrations in Loggerhead Sea Turtles (Caretta caretta). PLoS ONE 2020, 15, e0229800. [Google Scholar] [CrossRef]
- Da Fonseca, L.A.; Fagundes, V.; Girardi, F.M.; Dornelas, L.R.S. Blood Values of Cortisol, Glucose, and Lactate in Healthy Green Turtle (Chelonia mydas) and Affected by Fibropapillomatosis. Comp. Clin. Pathol. 2020, 29, 1099–1105. [Google Scholar] [CrossRef]
- Pinya, S.; Renga, E.; Fernández, G.; Mateu-Vicens, G.; Tejada, S.; Capó, X.; Sureda, A. Physiological Biomarkers in Loggerhead Turtles (Caretta caretta) as a Tool for Monitoring Sanitary Evolution in Marine Recovery Centres. Sci. Total Environ. 2021, 757, 143930. [Google Scholar] [CrossRef]
- Omedes, S.; Crespo-Picazo, J.L.; García-Párraga, D.; Sole, M. B-Esterase Measurements and Other Blood Related Biomarkers in Loggerhead Sea Turtles (Caretta caretta) as Indicators of Health Status. Sci. Total Environ. 2023, 879, 163040. [Google Scholar] [CrossRef]
- McNally, K.L.; Innis, C.J. Plasma Biochemistry and Hematologic Values of Cold-Stunned Loggerhead Sea Turtles (Caretta caretta). J. Herpetol. Med. Surg. 2020, 30, 88. [Google Scholar] [CrossRef]
- Canzanella, S.; Danese, A.; Mandato, M.; Lucifora, G.; Riverso, C.; Federico, G.; Gallo, P.; Esposito, M. Concentrations of Trace Elements in Tissues of Loggerhead Turtles (Caretta caretta) from the Tyrrhenian and the Ionian Coastlines (Calabria, Italy). Environ. Sci. Pollut. Res. 2021, 28, 26545–26557. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, P.J.; Llobat, L.; López-Lujan, M.C.; Cambra-López, M.; Blas, E.; Pascual, J.J. Urea Nitrogen Metabolite Can Contribute to Implementing the Ideal Protein Concept in Monogastric Animals. Animals 2022, 12, 2344. [Google Scholar] [CrossRef] [PubMed]
- Yalçın Özdilek, Ş.; Sönmez, B.; Mestav, B. Body Size-Related Polymorphic Foraging Strategy in Adult Green Turtles. Ocean. Coast. Manag. 2023, 237, 106538. [Google Scholar] [CrossRef]
- March, D.; Ariel, E.; Blyde, D.; Christidis, L.; Kelaher, B.P. Influence of Exercise and Fasting on Blood Parameters in Juvenile Green Turtles (Chelonia mydas): Implications for Health Assessments. Comp. Exerc. Physiol. 2021, 17, 181–187. [Google Scholar] [CrossRef]
- Moon, D.-Y.; Owens, D.W.; MacKenzie, D.S. The Effects of Fasting and Increased Feeding on Plasma Thyroid Hormones, Glucose, and Total Protein in Sea Turtles. Zool. Sci. 1999, 16, 579–586. [Google Scholar] [CrossRef]
- Makide, K.; Uwamizu, A.; Shinjo, Y.; Ishiguro, J.; Okutani, M.; Inoue, A.; Aoki, J. Novel Lysophosphoplipid Receptors: Their Structure and Function. J. Lipid Res. 2014, 55, 1986–1995. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, Z.F.; Park, J.C.; Kim, I.H. Evaluation of Houttuynia Cordata and Taraxacum Officinale on Growth Performance, Nutrient Digestibility, Blood Characteristics, and Fecal Microbial Shedding in Diet for Weaning Pigs. Asian-Australas. J. Anim. Sci. 2012, 25, 1439–1444. [Google Scholar] [CrossRef]
- Ding, L.; Liu, Y.; Kang, M.; Wei, X.; Geng, C.; Liu, W.; Han, L.; Yuan, F.; Wang, P.; Wang, B.; et al. UPLC-QTOF/MS Metabolomics and Biochemical Assays Reveal Changes in Hepatic Nutrition and Energy Metabolism during Sexual Maturation in Female Rainbow Trout (Oncorhynchus mykiss). Biology 2022, 11, 1679. [Google Scholar] [CrossRef]
- Lutkewitte, A.J.; Finck, B.N. Regulation of Signaling and Metabolism by Lipin-Mediated Phosphatidic Acid Phosphohydrolase Activity. Biomolecules 2020, 10, 1386. [Google Scholar] [CrossRef]
- Campbell, J.W.; Vorhaben, J.E.; Smith, D.D. Uricoteley: Its Nature and Origin during the Evolution of Tetrapod Vertebrates. J. Exp. Zool. 1987, 243, 349–363. [Google Scholar] [CrossRef]
- Langner, J.; Bergström, R.; Foltescu, V. Impact of Climate Change on Surface Ozone and Deposition of Sulphur and Nitrogen in Europe. Atmos. Environ. 2005, 39, 1129–1141. [Google Scholar] [CrossRef]
- Van Milgen, J.; Dourmad, J.-Y. Concept and Application of Ideal Protein for Pigs. J. Anim. Sci. Biotechnol. 2015, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Hashimoto, M.; Kawaguchi, N.; Shibata, H.; Fukami, H.; Tanaka, T.; Higuchi, N. Synthesis and Inhibitory Activity of Acyl-Peptidyl-Pyrrolidine Derivatives Toward Post-Proline Cleaving Enzyme; A Study of Subsite Specificity. J. Enzym. Inhib. 1991, 5, 51–75. [Google Scholar] [CrossRef]
- Abraham, G.N.; Podell, D.N. Pyroglutamic Acid: Non-Metabolic Formation, Function in Proteins and Peptides, and Characteristics of the Enzymes Effecting Its Removal. Mol. Cell. Biochem. 1981, 38, 181–190. [Google Scholar] [CrossRef]
- Wood, J.R.; Wood, F.E. Growth and Digestibility for the Green Turtle (Chelonia mydas) Fed Diets Containing Varying Protein Levels. Aquaculture 1981, 25, 269–274. [Google Scholar] [CrossRef]
- Bjorndal, K.A.; Bolten, A.B. Body Size and Digestive Efficiency in a Herbivorous Freshwater Turtle: Advantages of Small Bite Size. Physiol. Zool. 1992, 65, 1028–1039. [Google Scholar] [CrossRef]
- Johnstone, C.P.; Reina, R.D.; Lill, A. Interpreting Indices of Physiological Stress in Free-Living Vertebrates. J. Comp. Physiol. B 2012, 182, 861–879. [Google Scholar] [CrossRef]
- O’Shea-Stone, G.; Lambert, R.; Tripet, B.; Berardinelli, J.; Thomson, J.; Copié, V.; Garrott, R. 1H NMR Based Metabolic Profiling Distinguishes the Differential Impact of Capture Techniques on Wild Bighorn Sheep. Sci. Rep. 2021, 11, 11308. [Google Scholar] [CrossRef]
- Marín-García, P.J.; García-Párraga, D.; Crespo-Picazo, J.L. Stacy, N.I.; Llobat, L.; Cambra-López, M.C.; Pascual, J.J.; Larsen, T.; Hedemann, M.S. Decoding sex-specific metabolomic biomarkers in the loggerhead sea turtle (Caretta caretta). Commun. Biol. 2024, 7, 1329. [Google Scholar] [CrossRef]
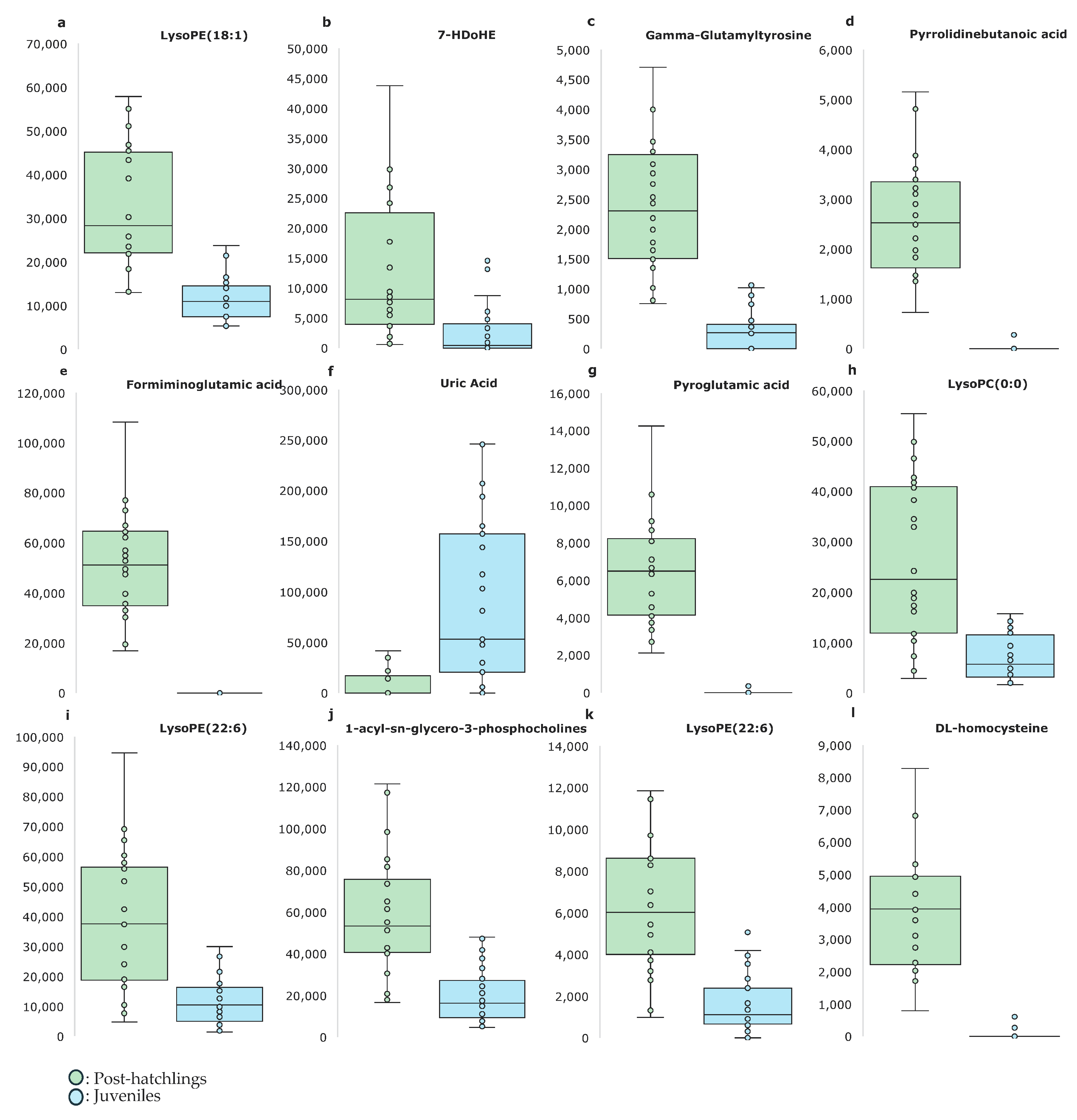

| m/z-RT | ION | Metabolite | p-Value |
|---|---|---|---|
| 478.2916RT10.224 | [M-H]− | C23H46NO7P—LysoPE(18:1/0:0) | <0.001 |
| 343.2258RT10.837 | [M-H]− | C22H32O3—7-HDoHE | 0.0121 |
| 309.10709RT2.661 | [M-H]− | Gamma-Glutamyltyrosine | <0.001 |
| 229.0813RT1.003 | [M-H]− | Pyrrolidinebutanoic acid 1 | <0.001 |
| 173.0555RT0.757 | [M-H]− | Formiminoglutamic acid | <0.001 |
| 167.01961RT1.227 | [M-H]− | Uric acid | <0.001 |
| 128.034RT0.752 | [M-H]− | Pyroglutamic acid | <0.001 |
| 568.33643RT9.411 | [M+H]+ | C28H50NO7P—LysoPC(0:0/20:4) | <0.001 |
| 526.28967RT9.367 | [M+H]+ | C27H44NO7P—LysoPE(22:6/0:0) | <0.001 |
| 522.35211RT10.295 | [M+H]+ | C26H52NO7P—1-acyl-sn-glycero-3-phosphocholine | <0.001 |
| 500.27371RT8.816 | [M+H]+ | C27H44NO7P—LysoPE(22:6/0:0) | <0.001 |
| 291.04099RT0.828 | [M+Na]+ | DL-homocysteine | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín-García, P.J.; García-Párraga, D.; Crespo-Picazo, J.L.; Llobat, L.; Cambra-López, M.; Bordignon, F.; Pascual, J.J.; Larsen, T.; Hedemann, M.S. Ecometabolomics of Loggerhead Sea Turtles (Caretta caretta): The Impact of Age on Metabolomic Profiles. Int. J. Mol. Sci. 2025, 26, 545. https://doi.org/10.3390/ijms26020545
Marín-García PJ, García-Párraga D, Crespo-Picazo JL, Llobat L, Cambra-López M, Bordignon F, Pascual JJ, Larsen T, Hedemann MS. Ecometabolomics of Loggerhead Sea Turtles (Caretta caretta): The Impact of Age on Metabolomic Profiles. International Journal of Molecular Sciences. 2025; 26(2):545. https://doi.org/10.3390/ijms26020545
Chicago/Turabian StyleMarín-García, Pablo Jesús, Daniel García-Párraga, Jose Luis Crespo-Picazo, Lola Llobat, María Cambra-López, Francesco Bordignon, Juan José Pascual, Torben Larsen, and Mette Skou Hedemann. 2025. "Ecometabolomics of Loggerhead Sea Turtles (Caretta caretta): The Impact of Age on Metabolomic Profiles" International Journal of Molecular Sciences 26, no. 2: 545. https://doi.org/10.3390/ijms26020545
APA StyleMarín-García, P. J., García-Párraga, D., Crespo-Picazo, J. L., Llobat, L., Cambra-López, M., Bordignon, F., Pascual, J. J., Larsen, T., & Hedemann, M. S. (2025). Ecometabolomics of Loggerhead Sea Turtles (Caretta caretta): The Impact of Age on Metabolomic Profiles. International Journal of Molecular Sciences, 26(2), 545. https://doi.org/10.3390/ijms26020545










