Methyl Jasmonate Was Involved in Hydrogen Sulfide-Alleviated Cadmium Stress in Cucumber Plants Through ROS Homeostasis and Chlorophyll Metabolism
Abstract
1. Introduction
2. Results
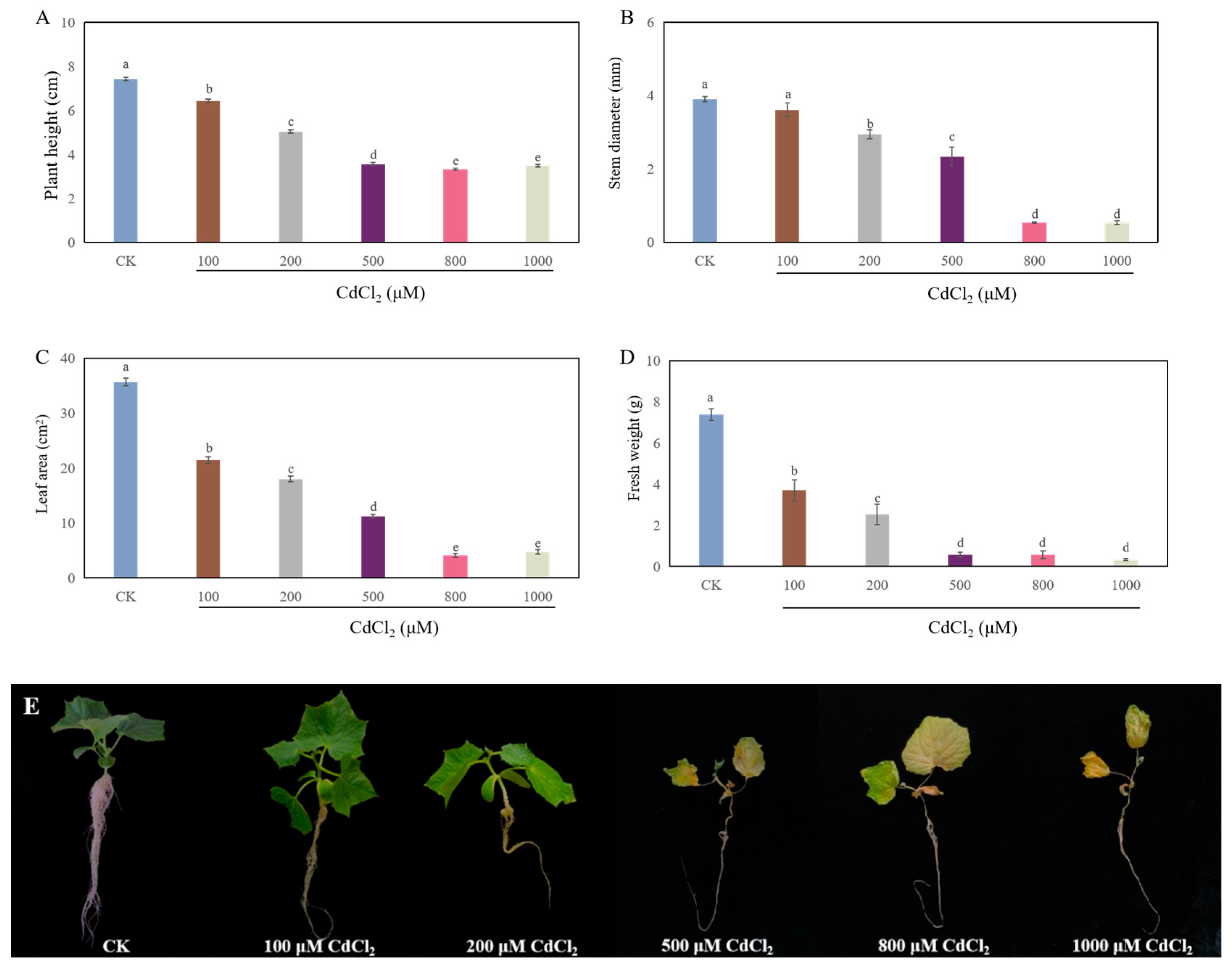
2.1. Cd Stress Inhibited the Growth of Cucumber Seedlings
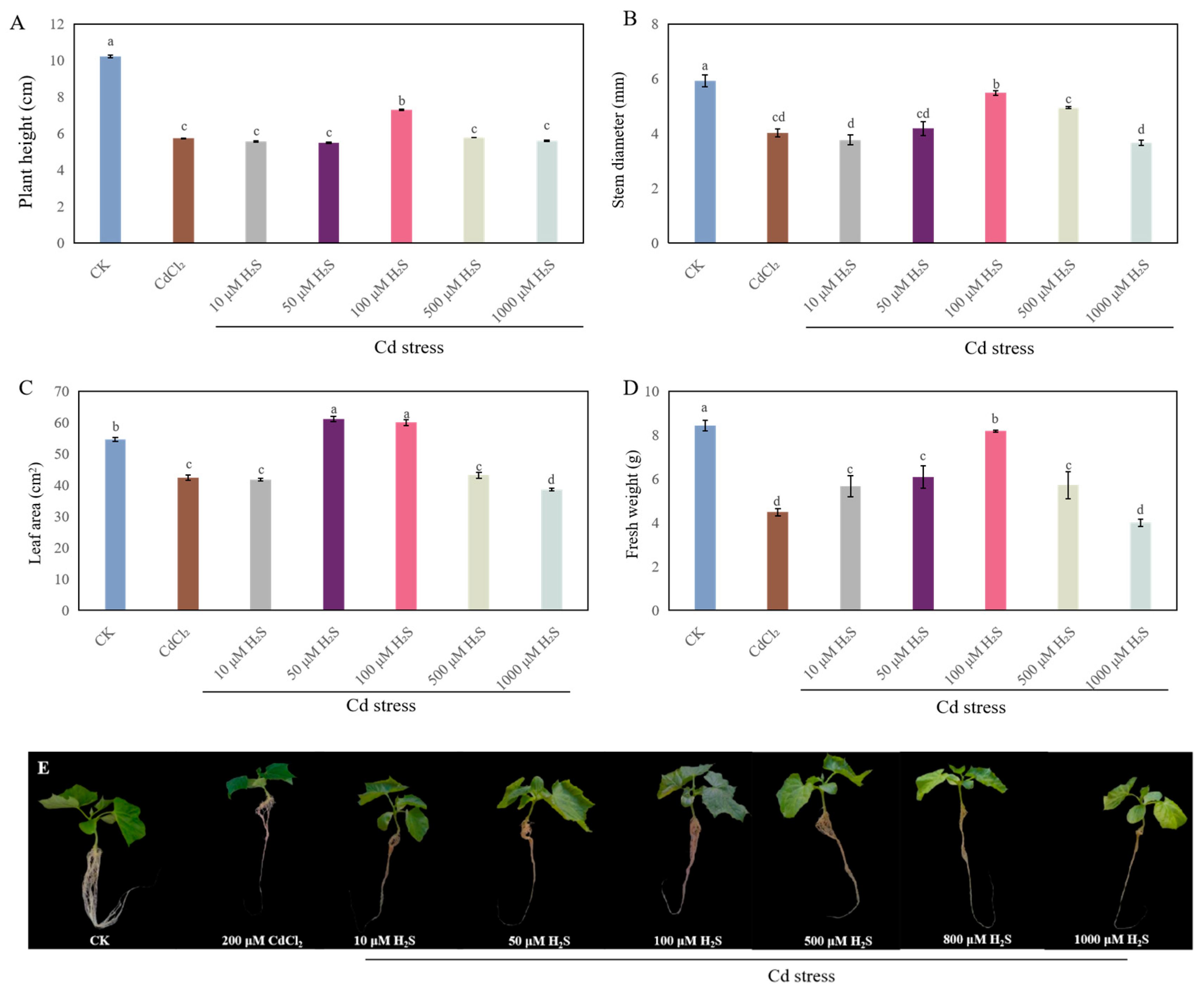
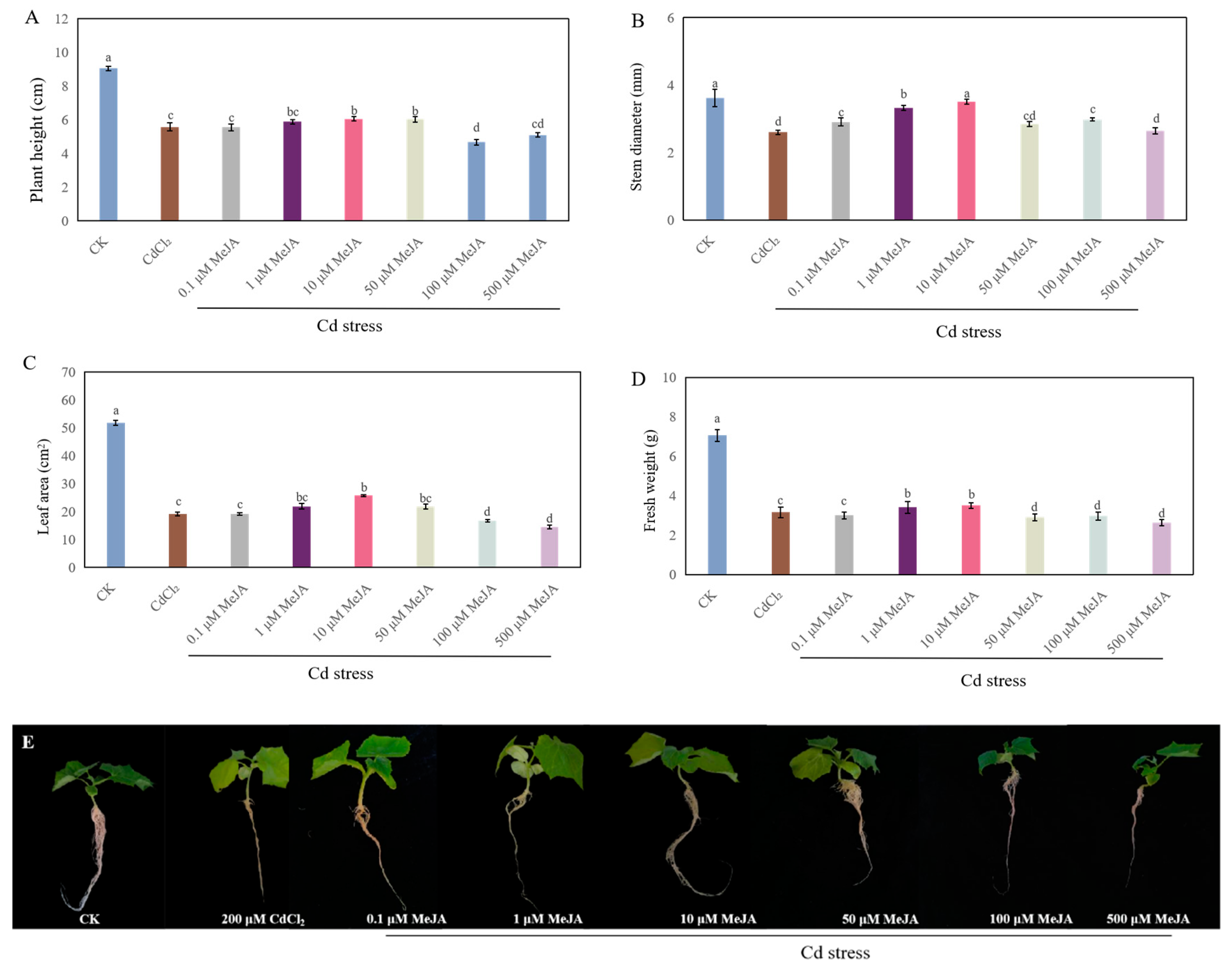
2.2. Appropriate Concentrations of H2S and MeJA Improved Seedling Growth Under Cd Stress
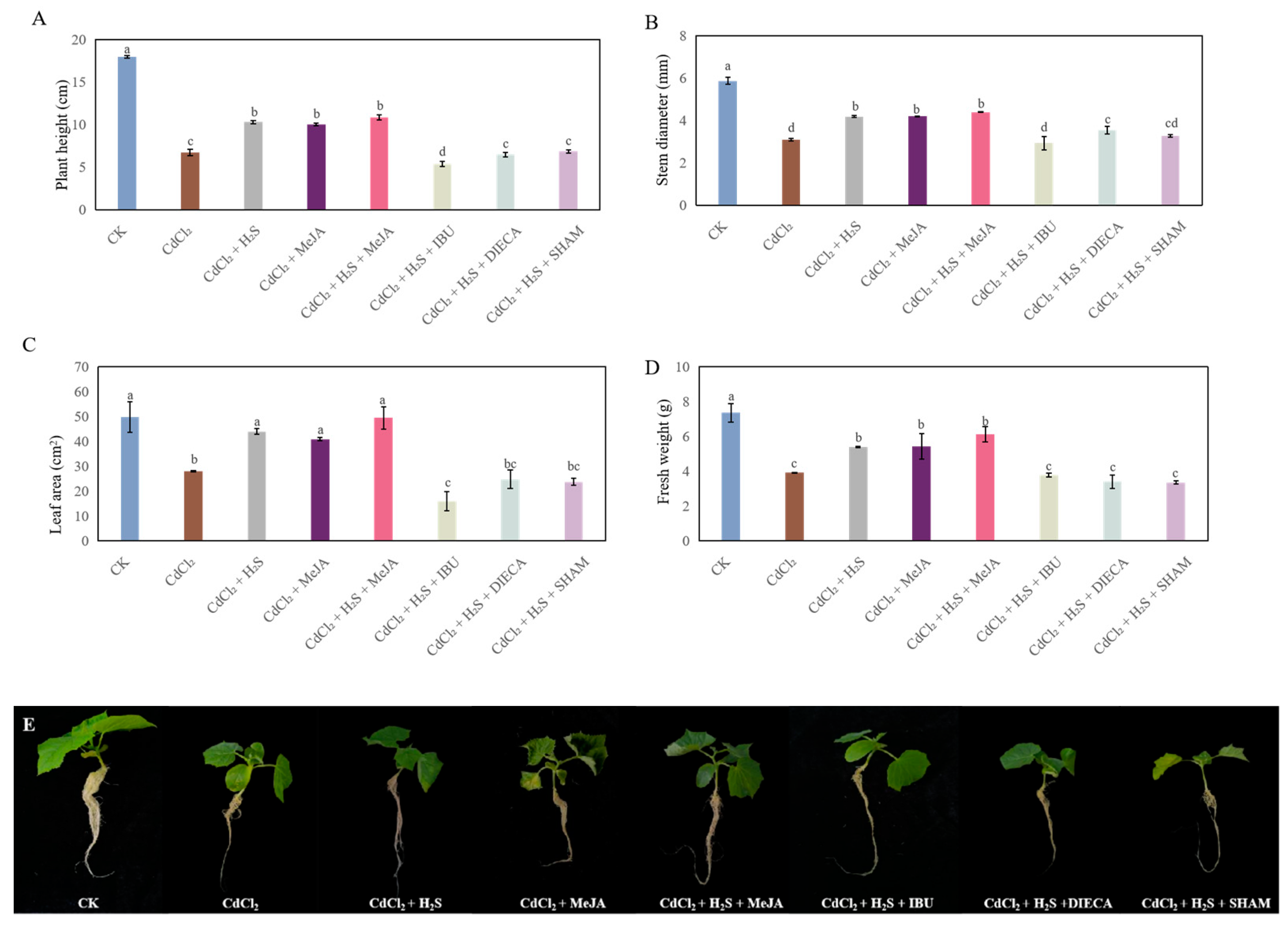
2.3. Effects of MeJA Biosynthesis Inhibitors on the Growth of Cucumber Under Cd Stress
2.4. Endogenous Hydrogen Peroxide (H2O2) and Superoxide Radical (O2·−) Level Under Different Treatments
2.5. The Ratio of AsA/DHA and GSH/GSSG in Cucumber Seedlings Under Different Treatments
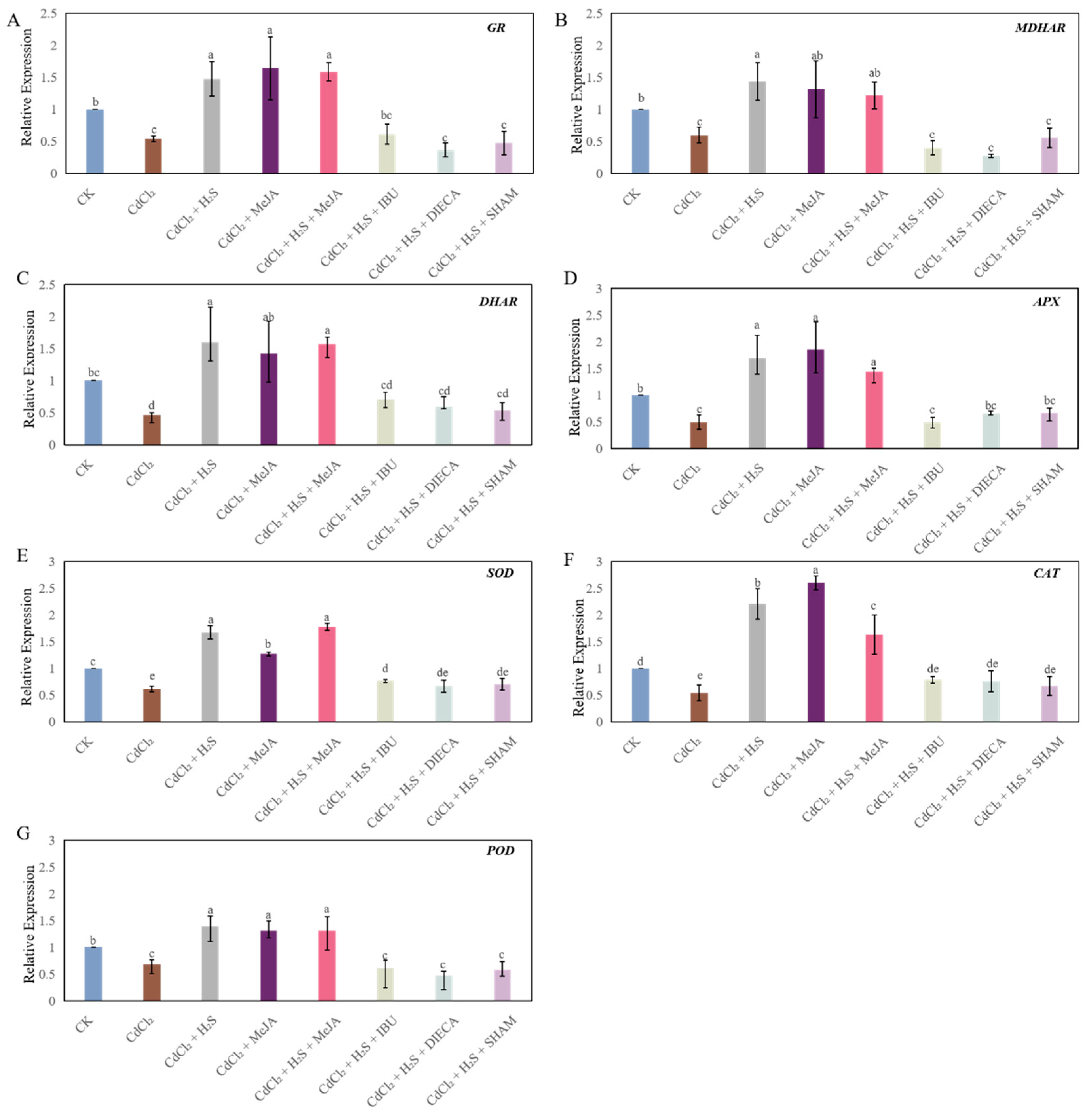
2.6. The Expression Level of the ROS Scavenge Genes in Cucumber Seedlings Under Different Treatments
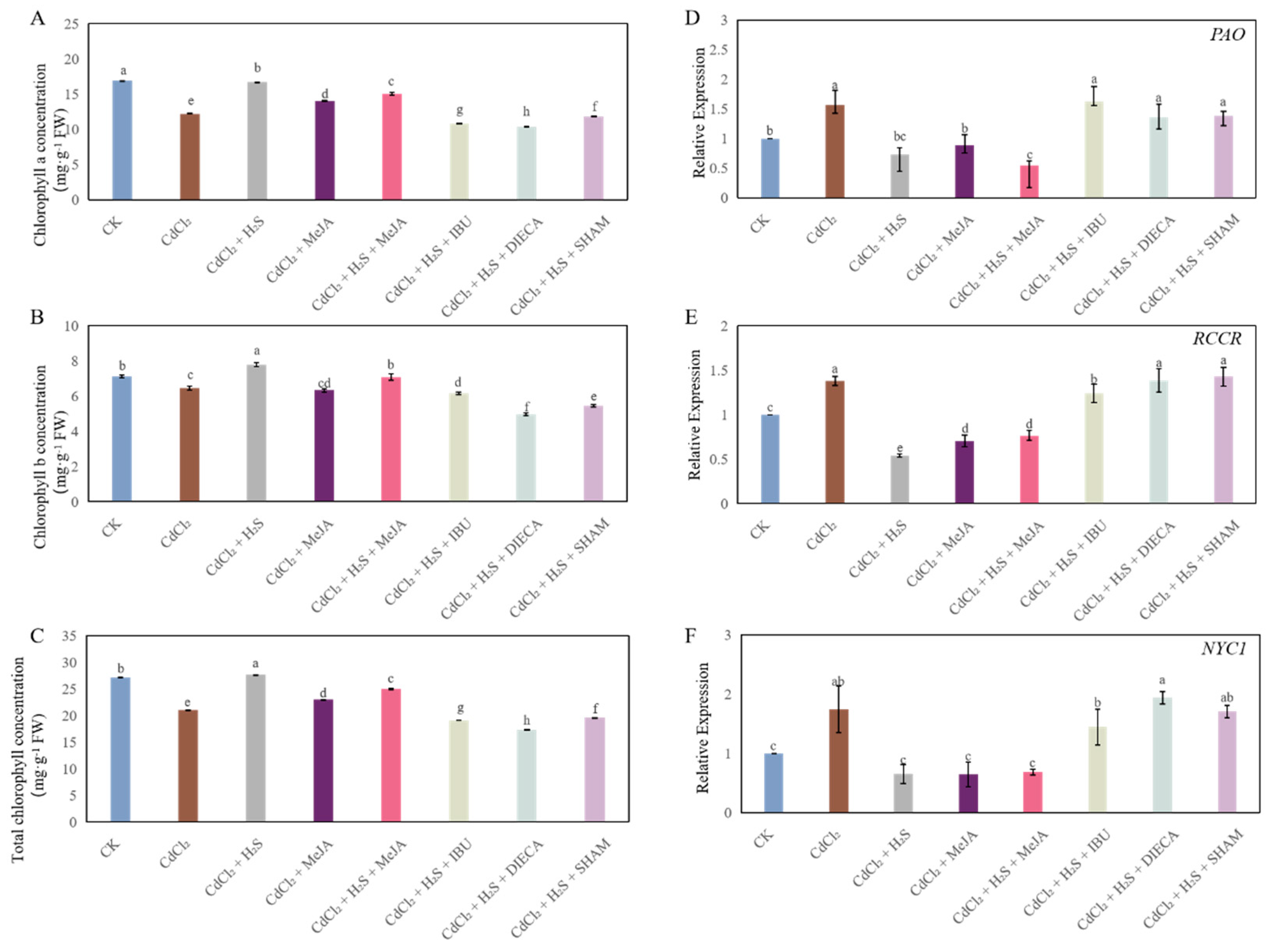
2.7. Chlorophyll Metabolism in Cucumber Seedlings Under Different Treatments
2.8. Change in Chlorophyll Fluorescence in Cucumber Seedlings Under Different Treatments
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Condition
4.2. Experiment Design
4.3. ROS Measurement
4.4. Measurement of the Content of AsA, DHA, GSH, GSSG
4.5. Chlorophyll Content and Chlorophyll Fluorescence Measurement
4.6. Quantative Real-Time PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, H.M.; Xiao, T.Y.; Zhou, H.; Xie, Y.J.; Shen, W.B. Hydrogen sulfide: A versatile regulator of environmental stress in plants. Acta Physiol. Plant. 2016, 38, 16. [Google Scholar] [CrossRef]
- Jin, Z.P.; Xue, S.W.; Luo, Y.N.; Tian, B.H.; Fang, H.H.; Li, H.; Pei, Y.X. Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol. Bioch. 2013, 62, 41–46. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, Y.; Ye, X.Y.; Li, Z.G. Signaling molecule hydrogen sulfide improves seed germination and seedling growth of maize (Zea mays L.) under high temperature by inducing antioxidant system and osmolyte biosynthesis. Front. Plant Sci. 2018, 9, 1288. [Google Scholar] [CrossRef]
- Li, H.; Chen, H.Y.; Chen, L.L.; Wang, C.Y. The role of hydrogen sulfide in plant roots during development and in response to abiotic stress. Int. J. Mol. Sci. 2022, 23, 1024. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Zhang, L.P.; Pei, Z.Y.; Zhang, L.L.; Liu, Z.Q.; Liu, D.M.; Hao, X.F.; Jin, Z.P.; Pei, Y.X. Hydrogen sulfide promotes flowering in heading Chinese cabbage by S-sulfhydration of BraFLCs. Hortic. Res. 2021, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ni, Z.J.; Thakur, K.; Cao, S.Q.; Wei, Z.J. Recent update on the mechanism of hydrogen sulfide improving the preservation of postharvest fruits and vegetables. Curr. Opin. Food Sci. 2022, 47, 100906. [Google Scholar] [CrossRef]
- Scuffi, D.; Álvarez, C.; Laspina, N.; Gotor, C.; Lamattina, L.; García-Mata, C. Hydrogen sulfide generated by L-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol. 2014, 166, 2065–2076. [Google Scholar] [CrossRef]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Elbasan, F.; Kucukoduk, M.; Turkan, I. Hydrogen sulfide (H2S) and nitric oxide (NO) alleviate cobalt toxicity in wheat (Triticum aestivum L.) by modulating photosynthesis, chloroplastic redox and antioxidant capacity. J. Hazard. Mater. 2020, 388, 122061. [Google Scholar] [CrossRef]
- Thakur, M.; Anand, A. Hydrogen sulfide: An emerging signaling molecule regulating drought stress response in plants. Physiol. Plantarum. 2021, 172, 1227–1243. [Google Scholar] [CrossRef] [PubMed]
- Du, X.Z.; Jin, Z.P.; Liu, D.M.; Yang, G.D.; Pei, Y.X. Hydrogen sulfide alleviates the cold stress through MPK4 in Arabidopsis thaliana. Plant Physiol. Bioch. 2017, 120, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.L.; Tian, Y.; Li, L.; Yu, M.; Hou, R.P.; Ren, X.M. H2S alleviates salinity stress in cucumber by maintaining the Na+/K+ balance and regulating H2S metabolism and oxidative stress response. Front. Plant Sci. 2019, 10, 678. [Google Scholar] [CrossRef]
- Arif, M.S.; Yasmeen, T.; Abbas, Z.; Ali, S.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S.; Abdel-Daim, M.M. Role of exogenous and endogenous hydrogen sulfide (H2S) on functional traits of plants under heavy metal stresses: A recent perspective. Front. Plant Sci. 2021, 11, 545453. [Google Scholar] [CrossRef] [PubMed]
- Javad, S.; Shah, A.A.; Ramzan, M.; Sardar, R.; Javed, T.; Al-Huqail, A.A.; Ali, H.M.; Chaudhry, O.; Yasin, N.A.; Ahmed, S.; et al. Hydrogen sulphide alleviates cadmium stress in Trigonella foenum-graecum by modulating antioxidant enzymes and polyamine content. Plant Biol. 2022, 24, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ling, L.; Wang, X.Q.; Cheng, T.; Wang, H.Y.; Ruan, Y.Y. Exogenous hydrogen sulfide and methylglyoxal alleviate cadmium-induced oxidative stress in Salix matsudana Koidz by regulating glutathione metabolism. Bmc Plant Biol. 2023, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, L.; Gong, X.; Xu, J.F.; Li, M.H. Functions of jasmonic acid in plant regulation and response to abiotic stress. Int. J. Mol. Sci. 2020, 21, 1446. [Google Scholar] [CrossRef]
- Tang, H.; Hu, J.Y.; Zhao, M.; Cao, L.P.; Chen, Y.H. Comparative study of the physiological responses, secondary metabolites and gene expression of medicinal plant Prunella vulgaris L. treated with exogenous methyl jasmonate and salicylic acid. Acta Physiol. Plant. 2023, 45, 20. [Google Scholar] [CrossRef]
- Wang, F.B.; Wan, C.Z.; Wu, W.Y.; Zhang, Y.N.; Pan, Y.X.; Chen, X.M.; Li, C.; Pi, J.L.; Wang, Z.X.; Ye, Y.X.; et al. Methyl jasmonate (MeJA) enhances salt tolerance of okra (Abelmoschus esculentus L.) plants by regulating ABA signaling, osmotic adjustment substances, photosynthesis and ROS metabolism. Sci. Hortic. 2023, 319, 112145. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Gong, D.; Gao, Y.; Pan, R.; Hu, J.; Guan, Y. Priming with methyl jasmonate alleviates polyethylene glycol-induced osmotic stress in rice seeds by regulating the seed metabolic profile. Environ. Exp. Bot. 2018, 153, 236–248. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Masood, A.; Fatma, M. Methyl jasmonate alleviates cadmium-induced photosynthetic damages through increased S-assimilation and glutathione production in mustard. Front. Plant Sci. 2016, 7, 1933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Z.; Wang, H.R.; Zhang, Y.B.; Su, Z.; Hu, T.Z.; Liu, J.J.; Ding, Q.; Niu, N.; Ma, L.J. Methyl jasmonate enhances the safe production ability of Cd-stressed wheat by regulating the antioxidant capacity, Cd absorption and distribution in wheat. Plant Physiol. Bioch. 2024, 212, 108788. [Google Scholar] [CrossRef]
- Wang, F.B.; Wan, C.Z.; Wu, W.Y.; Pan, Y.X.; Cheng, X.M.; Li, C.; Pi, J.L.; Chen, X.H. Exogenous methyl jasmonate (MeJA) enhances the tolerance to cadmium (Cd) stress of okra (Abelmoschus esculentus L.) plants. Plant Cell Tiss. Org. 2023, 155, 907–922. [Google Scholar] [CrossRef]
- Deng, G.B.; Zhou, L.J.; Wang, Y.Y.; Zhang, G.S.; Chen, X.L. Hydrogen sulfide acts downstream of jasmonic acid to inhibit stomatal development in Arabidopsis. Planta 2020, 251, 42. [Google Scholar] [CrossRef]
- Su, Z.Z.; Lan, Z.X.; Gao, Y.F.; Liu, Y.F.; Shen, J.; Guo, Y.L.; Ma, J.X.; Zhang, Y.; Luan, F.S.; Zhang, X.; et al. Methyl jasmonate-dependent hydrogen sulfide mediates melatonin-induced aphid resistance of watermelon. Food Energy Secur. 2023, 12, e485. [Google Scholar] [CrossRef]
- Huang, T.H.; Duan, B.; Zuo, X.X.; Du, H.Y.; Wang, J.; Cai, Z.P.; Shen, Y.G.; Zhang, W.; Chen, J.Y.; Zhu, L.Q.; et al. Hydrogen sulfide enhances kiwifruit resistance to soft rot by regulating jasmonic acid signaling pathway. Plant Physiol. Bioch. 2024, 214, 108880. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.M.; Chen, Y.; Zhu, Q.F.; Wu, X.P.; Jiang, S.; Wei, Y.Y.; Ye, J.F.; Xu, F.; Shao, X. Hydrogen sulfide mediated methyl jasmonate-induced cold resistance in peach fruit. Postharvest Biol. Technol. 2024, 210, 112779. [Google Scholar] [CrossRef]
- Tian, B.H.; Zhang, Y.J.; Jin, Z.P.; Liu, Z.Q.; Pei, Y.X. Role of hydrogen sulfide in the methyl jasmonate response to cadmium stress in foxtail millet. Front. Biosci. 2017, 22, 530–538. [Google Scholar]
- Ou, C.; Cheng, W.H.; Wang, Z.L.; Yao, X.M.; Yang, S.M. Exogenous melatonin enhances Cd stress tolerance in Platycladus orientalis seedlings by improving mineral nutrient uptake and oxidative stress. Ecotox Environ. Safe 2023, 252, 114619. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, J.C.; Yu, Y.; Cheng, X.; Du, Y.L.; Zhao, Q.; Du, J.D. Interactive effects of drought and cadmium stress on adzuki bean seedling growth, DNA damage repair and Cd accumulation. Sci. Hortic. 2024, 324, 112624. [Google Scholar] [CrossRef]
- Zhang, H.H.; Li, X.; Xu, Z.S.; Wang, Y.W.; Teng, Z.Y.; An, M.J.; Zhang, Y.H.; Zhu, W.X.; Xu, N.B.; Sun, G.Y. Toxic effects of heavy metals Pb and Cd on mulberry (Morus alba L.) seedling leaves: Photosynthetic function and reactive oxygen species (ROS) metabolism responses. Ecotox Environ. Safe 2020, 195, 110469. [Google Scholar]
- Chen, Z.; Chen, M.S.; Jiang, M. Hydrogen sulfide alleviates mercury toxicity by sequestering it in roots or regulating reactive oxygen species productions in rice seedlings. Plant Physiol. Bioch. 2017, 111, 179–192. [Google Scholar] [CrossRef]
- Qi, Q.; Guo, Z.L.; Liang, Y.L.; Li, K.Z.; Xu, H.N. Hydrogen sulfide alleviates oxidative damage under excess nitrate stress through MAPK/NO signaling in cucumber. Plant Physiol. Bioch. 2019, 135, 1–8. [Google Scholar] [CrossRef]
- Sehar, Z.; Fatma, M.; Khan, S.; Mir, I.R.; Abdi, G.; Khan, N.A. Melatonin influences methyl jasmonate-induced protection of photosynthetic activity in wheat plants against heat stress by regulating ethylene-synthesis genes and antioxidant metabolism. Sci. Rep. 2023, 13, 7468. [Google Scholar] [CrossRef]
- Avashthi, H.; Pathak, R.K.; Pandey, N.; Arora, S.; Mishra, A.K.; Gupta, V.K.; Ramteke, P.W.; Kumar, A. Transcriptome-wide identification of genes involved in Ascorbate–Glutathione cycle (Halliwell–Asada pathway) and related pathway for elucidating its role in antioxidative potential in finger millet (Eleusine coracana L.). 3 Biotech 2018, 8, 499. [Google Scholar] [CrossRef]
- Asada, K. Production and action of active oxygen species in photosynthetic tissue. Causes of photooxidative stress and amelioration of defense system in plants. CRC Press. 2019, 77–104. [Google Scholar]
- Chao, Y.Y.; Hong, C.Y.; Kao, C.H. The decline in ascorbic acid content is associated with cadmium toxicity of rice seedlings. Plant Physiol. Bioch. 2010, 48, 374–381. [Google Scholar] [CrossRef]
- Semane, B.; Cuypers, A.; Smeets, K.; Van Belleghem, F.; Horemans, N.; Schat, H.; Vangronsveld, J. Cadmium responses in Arabidopsis thaliana: Glutathione metabolism and antioxidative defence system. Physiol. Plantarum. 2007, 129, 519–528. [Google Scholar] [CrossRef]
- Shan, C.J.; Wang, B.S.; Sun, H.L.; Gao, S.; Li, H. H2S induces NO in the regulation of AsA-GSH cycle in wheat seedlings by water stress. Protoplasma 2020, 257, 1487–1493. [Google Scholar] [CrossRef]
- Kaya, C.; Ugurlar, F.; Ashraf, M.; Alam, P.; Ahmad, P. Nitric oxide and hydrogen sulfide work together to improve tolerance to salinity stress in wheat plants by upraising the AsA-GSH cycle. Plant Physiol. Bioch. 2023, 194, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Prasad, S.M. Modulation of AsA-GSH cycle by exogenous nitric oxide and hydrogen peroxide to minimize the Cd-induced damages in photosynthetic cyanobacteria. Plant Stress 2023, 10, 100269. [Google Scholar] [CrossRef]
- Ulhassan, Z.; Gill, R.A.; Huang, H.F.; Ali, S.; Mwamba, T.M.; Ali, B.; Huang, Q.; Hamid, Y.; Khan, A.R.; Wang, J.; et al. Selenium mitigates the chromium toxicity in Brassicca napus L. by ameliorating nutrients uptake, amino acids metabolism and antioxidant defense system. Plant Physiol. Bioch. 2019, 145, 142–152. [Google Scholar] [CrossRef]
- Qiu, X.; Xu, Y.H.; Xiong, B.; Dai, L.; Huang, S.J.; Dong, T.T.; Sun, G.C.; Liao, L.; Deng, Q.X.; Wang, X.; et al. Effects of exogenous methyl jasmonate on the synthesis of endogenous jasmonates and the regulation of photosynthesis in citrus. Physiol. Plantarum. 2020, 170, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.L.; Liu, D.D.; Chen, H.S.; Qiu, T.Y.; Zhao, S.L.; Duan, C.J.; Cui, Y.X.; Zhu, X.Z.; Chao, H.R.; Wang, Y.H.; et al. Synergistic interplay between Azospirillum brasilense and exogenous signaling molecule H2S promotes Cd stress resistance and growth in pak choi (Brassica chinensis L.). J. Hazard. Mater. 2023, 444, 130425. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Egert, A.; Süssenbacher, I.; Kräutler, B.; Bartels, D.; Peters, S.; Hörtensteiner, S. Water deficit induces chlorophyll degradation via the ‘PAO/phyllobilin’ pathway in leaves of homoio—(Craterostigma pumilum) and poikilochlorophyllous (Xerophyta viscosa) resurrection plants. Plant Cell Environ. 2014, 37, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.N.; Shu, S.; Guo, S.R.; Sun, J.; Wu, J.Q. The positive roles of exogenous putrescine on chlorophyll metabolism and xanthophyll cycle in salt-stressed cucumber seedlings. Photosynthetica 2018, 56, 557–566. [Google Scholar] [CrossRef]
- Zhu, Z.P.; Yu, J.X.; Qiao, X.H.; Yu, Z.F.; Xiong, A.S.; Sun, M. Hydrogen sulfide delays yellowing and softening, inhibits nutrient loss in postharvest celery. Sci. Hortic. 2023, 315, 111991. [Google Scholar] [CrossRef]
- Lv, J.Y.; Zhang, Y.Z.; Tang, W.J.; Chen, J.X.; Ge, Y.H.; Li, J.R. Concentration-dependent impacts of exogenous methyl jasmonate (MeJA) on chlorophyll degradation of apple fruit during ripening. Postharvest Biol. Technol. 2023, 203, 112398. [Google Scholar] [CrossRef]
- Ma, Y.L.; Wang, H.F.; Wang, P.; Yu, C.G.; Luo, S.Q.; Zhang, Y.F.; Xie, Y.F. Effects of cadmium stress on the antioxidant system and chlorophyll fluorescence characteristics of two Taxodium clones. Plant Cell Rep. 2018, 37, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Liu, Y.H.; Liu, Q.; Zong, B.; Yuan, X.P.; Sun, H.; Wang, J.; Zang, L.; Xu, Y.; Ma, Z.Z.; et al. Nitric oxide is involved in abscisic acid-induced photosynthesis and antioxidant system of tall fescue seedlings response to low-light stress. Environ. Exp. Bot. 2018, 155, 226–238. [Google Scholar] [CrossRef]
- Khoshbakht, D.; Asghari, M.R.; Haghighi, M. Effects of foliar applications of nitric oxide and spermidine on chlorophyll fluorescence, photosynthesis and antioxidant enzyme activities of citrus seedlings under salinity stress. Photosynthetica 2018, 56, 1313–1325. [Google Scholar] [CrossRef]
- Chen, P.; Yang, W.X.; Wen, M.X.; Jin, S.H.; Liu, Y. Hydrogen sulfide alleviates salinity stress in Cyclocarya paliurus by maintaining chlorophyll fluorescence and regulating nitric oxide level and antioxidant capacity. Plant Physiol. Bioch. 2021, 167, 738–747. [Google Scholar] [CrossRef]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl jasmonate protects the PS II system by maintaining the stability of chloroplast D1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef] [PubMed]
- Nandagopal, J.T.; Harinarayanan, U.D.; Raghavan, S.; Girija, S. Foliar selenium application mitigates low-temperature stress in chilli (Capsicum annuum L.) seedlings. Energy Nexus 2022, 6, 100079. [Google Scholar] [CrossRef]
- Jiang, M.Y.; Zhang, J.H. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Hodges, D.M.; Andrews, C.J.; Johnson, D.A.; Hamilton, R.I. Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. Physiol. Plantarum. 1996, 98, 685–692. [Google Scholar] [CrossRef]
- Mohammadi, H.; Kazemi, Z.; Aghaee, A.; Hazrati, S.; Golzari Dehno, R.; Ghorbanpour, M. Unraveling the influence of TiO2 nanoparticles on growth, physiological and phytochemical characteristics of Mentha piperita L. in cadmium-contaminated soil. Sci. Rep. 2023, 13, 22280. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Bba-Gen. Subjects 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Wang, B.L.; Zhang, J.L.; Xia, X.Z.; Zhang, W.H. Ameliorative effect of brassinosteroid and ethylene on germination of cucumber seeds in the presence of sodium chloride. Plant Growth Regul. 2011, 65, 407–413. [Google Scholar] [CrossRef]


| Treatments | The Maximum Quantum Yield of PSII (Fv/Fm) | Effective Quantum Yield of PSII (φPSII) | Photochemical Quenching (qP) | Non-Photochemical Quenching (NPQ) |
|---|---|---|---|---|
| CK | 0.72 a | 0.74 a | 0.78 a | 0.24 c |
| CdCl2 | 0.68 c | 0.55 c | 0.51 c | 0.52 b |
| CdCl2 + H2S | 0.71 a | 0.65 b | 0.68 b | 0.26 c |
| CdCl2 + MeJA | 0.71 b | 0.63 b | 0.66 b | 0.26 c |
| CdCl2 + H2S + MeJA | 0.72 a | 0.64 b | 0.70 b | 0.36 c |
| CdCl2 + H2S + IBU | 0.69 c | 0.56 c | 0.49 c | 0.53 b |
| CdCl2 + H2S + DIECA | 0.68 c | 0.44 d | 0.47 c | 0.74 a |
| CdCl2 + H2S + SHAM | 0.69 c | 0.56 c | 0.51 c | 0.50 b |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Actin | TTGAATCCCAAGGCGAATAG | TGCGACCACTGGCATAAAG |
| GR | TGCGAAGTGTTACAAGGCGA | AGAAACTTTGACACATCGAGACG |
| MDHAR | ACAGCCTTCTTCTGTTGCCTTCAG | CTCTATTGTCGTTGGCGAAATCCG |
| DHAR | ATGTCGGGCTCCAGA ATCCAACCA | AAAGCGAGGAATTGGAAGGAAGGT |
| APX | TCACACATTGGGTAGGGCAC | TGCCTTGTCTGATGCCAACT |
| SOD | GCTGATGGAGTAGCAGAGGC | CCAATCTTCCACCCGCATTG |
| CAT | ACTTTA AGGAGCCCGGAGAGAG | CGGATAAATCGTTCCTGCCTGTC |
| POD | TTGTGATGGGTCGGTGCTAC | TGTCCTGATGCCAAGGTGAC |
| PAO | GGGCATTGAAAACTGGAAGA | TTACTTGGCGATCAAAAATGG |
| RCCR | TTCGAGTATGGGTAGACGAA | ATCTTGGCAAACTAGAACCC |
| NYC1 | TGATGATATGTTGCCGAGAG | AGTTCTGCCTGTAACGACTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, L.; Zhao, H.; Tang, Y.; Zhu, B.; Zhao, Y.; Wang, Q.; Yu, J. Methyl Jasmonate Was Involved in Hydrogen Sulfide-Alleviated Cadmium Stress in Cucumber Plants Through ROS Homeostasis and Chlorophyll Metabolism. Int. J. Mol. Sci. 2025, 26, 475. https://doi.org/10.3390/ijms26020475
Niu L, Zhao H, Tang Y, Zhu B, Zhao Y, Wang Q, Yu J. Methyl Jasmonate Was Involved in Hydrogen Sulfide-Alleviated Cadmium Stress in Cucumber Plants Through ROS Homeostasis and Chlorophyll Metabolism. International Journal of Molecular Sciences. 2025; 26(2):475. https://doi.org/10.3390/ijms26020475
Chicago/Turabian StyleNiu, Lijuan, Haixia Zhao, Yunlai Tang, Bo Zhu, Yanshuo Zhao, Qian Wang, and Jian Yu. 2025. "Methyl Jasmonate Was Involved in Hydrogen Sulfide-Alleviated Cadmium Stress in Cucumber Plants Through ROS Homeostasis and Chlorophyll Metabolism" International Journal of Molecular Sciences 26, no. 2: 475. https://doi.org/10.3390/ijms26020475
APA StyleNiu, L., Zhao, H., Tang, Y., Zhu, B., Zhao, Y., Wang, Q., & Yu, J. (2025). Methyl Jasmonate Was Involved in Hydrogen Sulfide-Alleviated Cadmium Stress in Cucumber Plants Through ROS Homeostasis and Chlorophyll Metabolism. International Journal of Molecular Sciences, 26(2), 475. https://doi.org/10.3390/ijms26020475





