Potentiation of Gelonin Cytotoxicity by Pulsed Electric Fields
Abstract
1. Introduction
2. Results
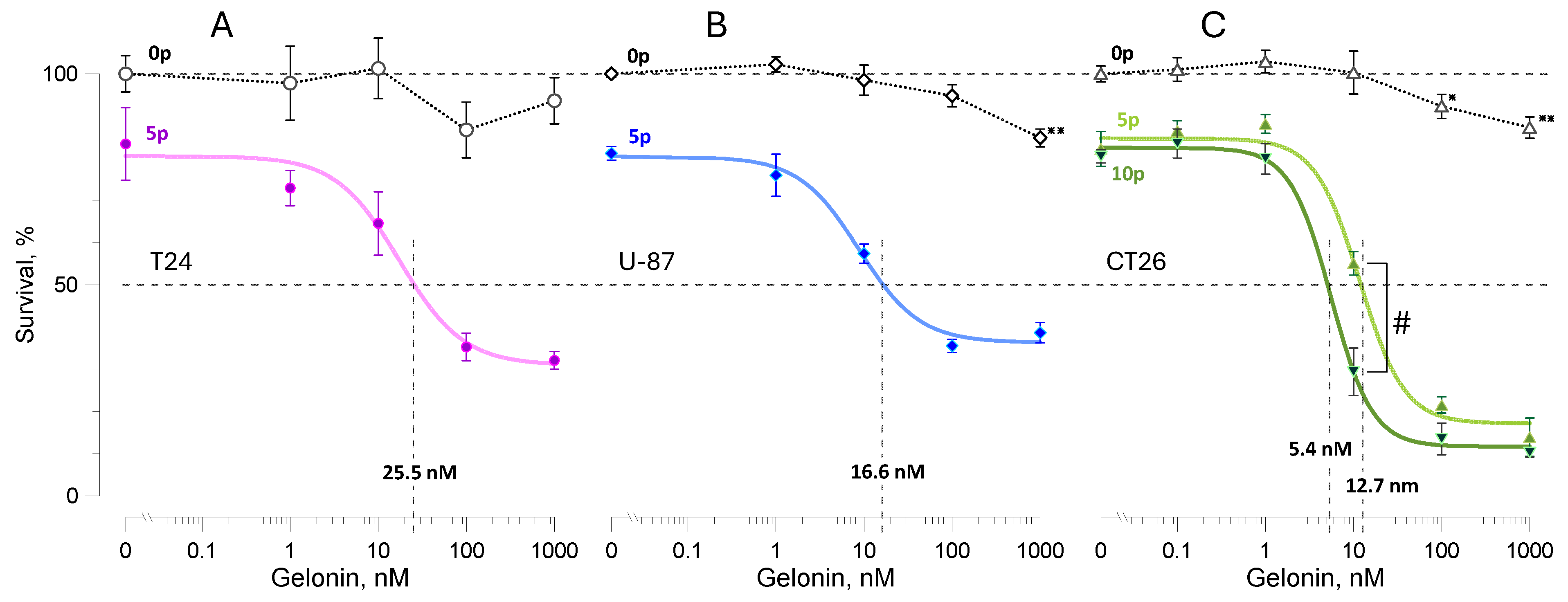
2.1. Electroporation Assists Cell Killing by Gelonin
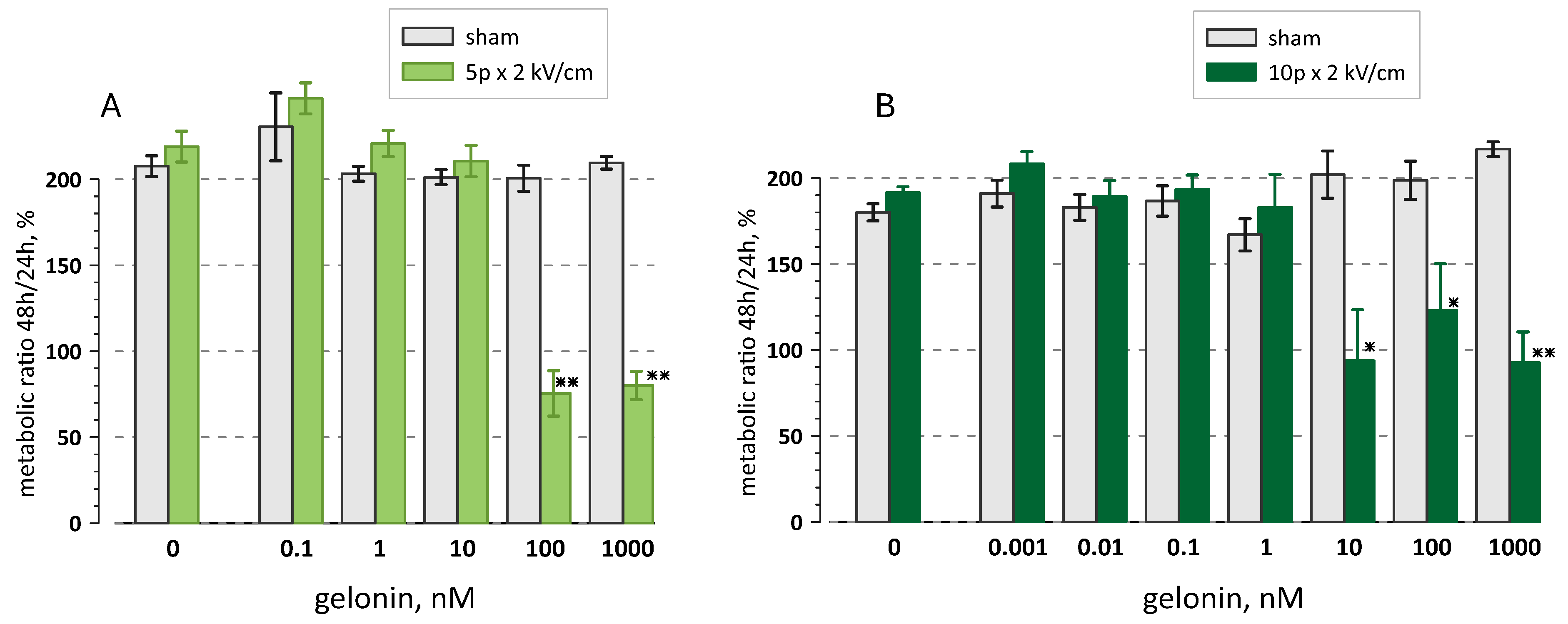
2.2. Longer Pulses Are More Efficient in Potentiating Gelonin Cytotoxicity
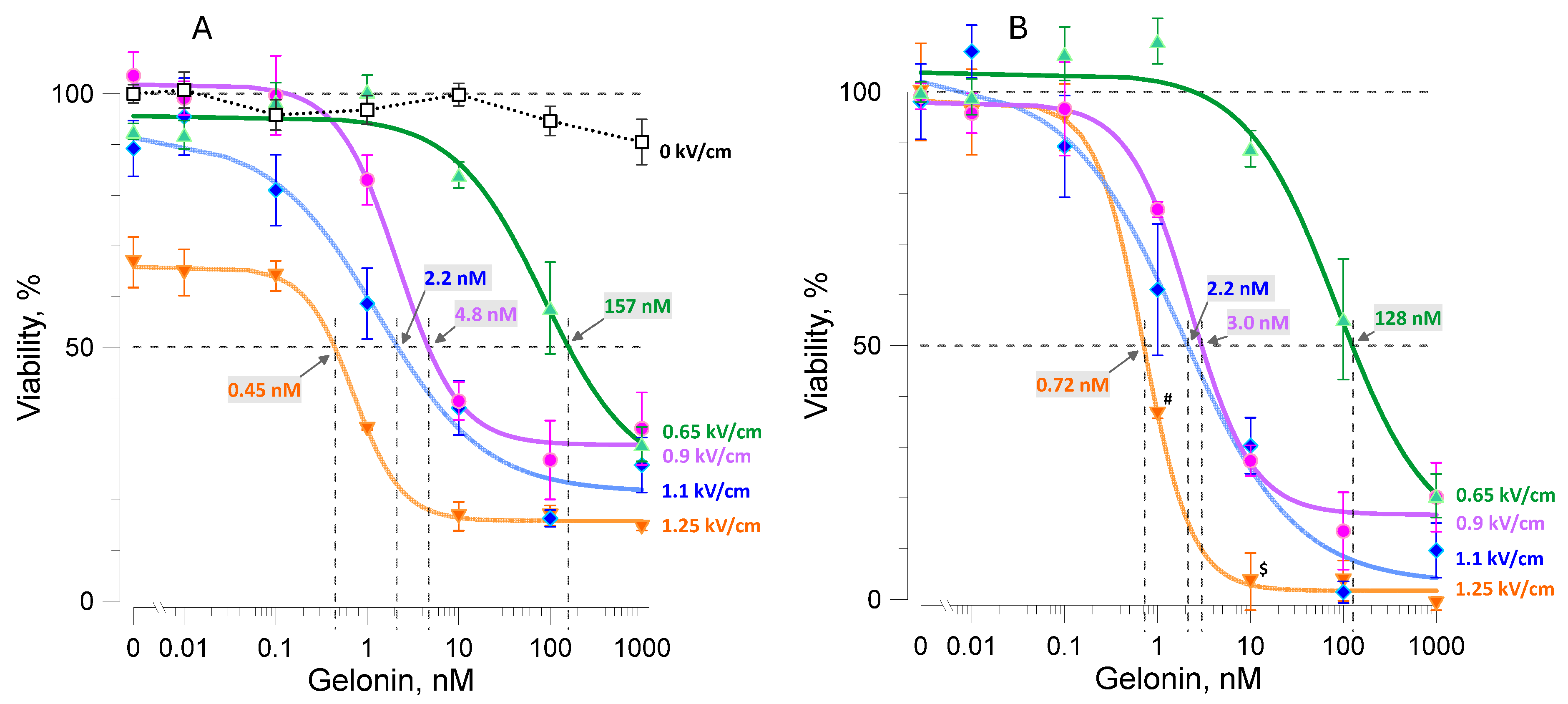
2.3. Synergistic Effect of the Electric Field Strength and Gelonin
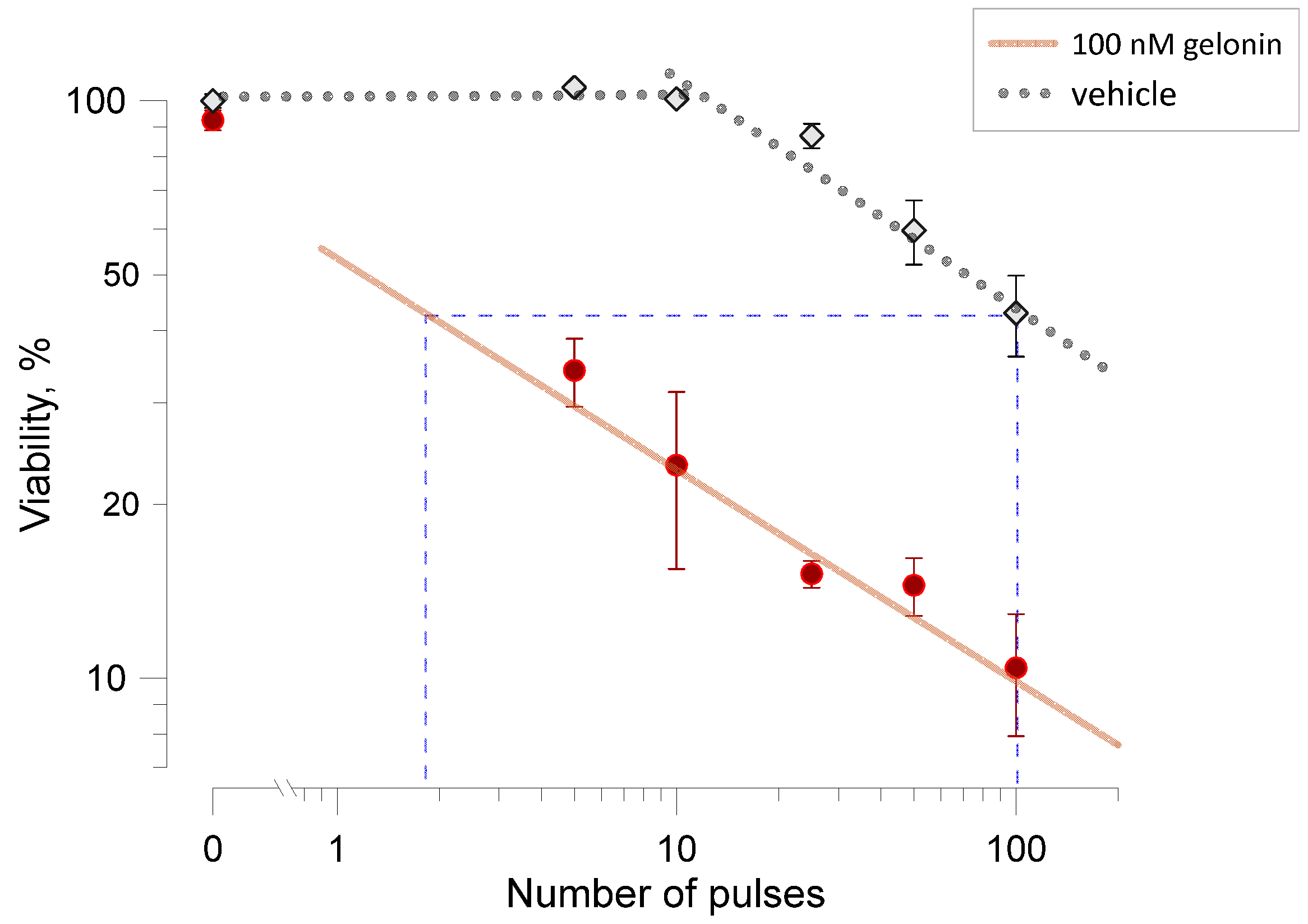
2.4. Gelonin Potentiates Cell Inactivation by Pulsed Electric Fields
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Growth Media
4.2. Preparation of Samples and Experiment Protocols
4.3. Pulsed Electric Field Treatments
4.4. Viability Measurements
4.5. Data Analysis and Statistics
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flavell, D.J.; Flavell, S.U. Plant-Derived Type I Ribosome Inactivating Protein-Based Targeted Toxins: A Review of the Clinical Experience. Toxins 2022, 14, 563. [Google Scholar] [CrossRef]
- Walsh, M.J.; Dodd, J.E.; Hautbergue, G.M. Ribosome-inactivating proteins: Potent poisons and molecular tools. Virulence 2013, 4, 774–784. [Google Scholar] [CrossRef]
- Stirpe, F. Ribosome-inactivating proteins: From toxins to useful proteins. Toxicon 2013, 67, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Bortolotti, M.; Maiello, S.; Battelli, M.G.; Polito, L. Ribosome-Inactivating Proteins from Plants: A Historical Overview. Molecules 2016, 21, 1627. [Google Scholar] [CrossRef]
- Schlaak, L.; Weise, C.; Kuropka, B.; Weng, A. Mutational Analysis of RIP Type I Dianthin-30 Suggests a Role for Arg24 in Endocytosis. Toxins 2024, 16, 219. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Polito, L.; Scicchitano, V.; Orrico, C.; Pasquinelli, G.; Musiani, S.; Santi, S.; Riccio, M.; Bortolotti, M.; Battelli, M.G. Endocytosis and intracellular localisation of type 1 ribosome-inactivating protein saporin-s6. J. Biol. Regul. Homeost. Agents 2012, 26, 97–109. [Google Scholar]
- Fuchs, H.; Bachran, C.; Flavell, D.J. Diving through Membranes: Molecular Cunning to Enforce the Endosomal Escape of Antibody-Targeted Anti-Tumor Toxins. Antibodies 2013, 2, 209–235. [Google Scholar] [CrossRef]
- Ham, S.H.; Min, K.A.; Shin, M.C. Molecular tumor targeting of gelonin by fusion with F3 peptide. Acta Pharmacol. Sin. 2017, 38, 897–906. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, X.; Lin, X.; Manorek, G.; Howell, S.B. Challenges associated with the targeted delivery of gelonin to claudin-expressing cancer cells with the use of activatable cell penetrating peptides to enhance potency. BMC Cancer 2011, 11, 61. [Google Scholar] [CrossRef]
- Pirie, C.M.; Hackel, B.J.; Rosenblum, M.G.; Wittrup, K.D. Convergent potency of internalized gelonin immunotoxins across varied cell lines, antigens, and targeting moieties. J. Biol. Chem. 2011, 286, 4165–4172. [Google Scholar] [CrossRef]
- Yu, L.; Gu, F.; Zhang, C.; Xie, S.; Guo, Y. Targeted diagnosis and treatment of superficial bladder cancer with monoclonal antibody BDI-1. Chin. Med J. 1998, 111, 404–407. [Google Scholar] [PubMed]
- Pham, D.D.; Pham, T.H.; Bui, T.H.; Britikova, E.V.; Britikov, V.V.; Bocharov, E.V.; Usanov, S.A.; Phan, V.C.; Le, T.B.T. In vitro and in vivo anti-tumor effect of Trichobakin fused with urokinase-type plasminogen activator ATF-TBK. Mol. Biol. Rep. 2024, 51, 130. [Google Scholar] [CrossRef] [PubMed]
- Entwistle, J.; Kowalski, M.; Brown, J.; Cizeau, J.; MacDonald, G.C. The Preclinical and Clinical Evaluation of VB6-845: An Immunotoxin with a De-Immunized Payload for the Systemic Treatment of Solid Tumors. In Antibody-Drug Conjugates and Immunotoxins: From Pre-Clinical Development to Therapeutic Applications; Phillips, G.L., Ed.; Springer: New York, NY, USA, 2013; pp. 349–367. [Google Scholar]
- Lansigan, F.; Stearns, D.M.; Foss, F. Role of denileukin diftitox in the treatment of persistent or recurrent cutaneous T-cell lymphoma. Cancer Manag. Res. 2010, 2, 53–59. [Google Scholar] [CrossRef]
- Kreitman, R.J.; Stetler-Stevenson, M.; Margulies, I.; Noel, P.; Fitzgerald, D.J.; Wilson, W.H.; Pastan, I. Phase II trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J. Clin. Oncol. 2009, 27, 2983–2990. [Google Scholar] [CrossRef]
- Borthakur, G.; Rosenblum, M.G.; Talpaz, M.; Daver, N.; Ravandi, F.; Faderl, S.; Freireich, E.J.; Kadia, T.; Garcia-Manero, G.; Kantarjian, H.; et al. Phase 1 study of an anti-CD33 immunotoxin, humanized monoclonal antibody M195 conjugated to recombinant gelonin (HUM-195/rGEL), in patients with advanced myeloid malignancies. Haematologica 2013, 98, 217–221. [Google Scholar] [CrossRef]
- Pagliaro, L.C.; Liu, B.; Munker, R.; Andreeff, M.; Freireich, E.J.; Scheinberg, D.A.; Rosenblum, M.G. Humanized M195 monoclonal antibody conjugated to recombinant gelonin: An anti-CD33 immunotoxin with antileukemic activity. Clin. Cancer Res. 1998, 4, 1971–1976. [Google Scholar]
- Belehradek, M.; Domenge, C.; Luboinski, B.; Orlowski, S.; Belehradek, J., Jr.; Mir, L.M. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer 1993, 72, 3694–3700. [Google Scholar] [CrossRef]
- Morley, J.; Grocott, P.; Purssell, E.; Murrells, T. Electrochemotherapy for the palliative management of cutaneous metastases: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, N.; Friebe, M. Electrochemotherapy: A Review of Current Status, Alternative IGP Approaches, and Future Perspectives. J. Healthc. Eng. 2019, 2019, 2784516. [Google Scholar] [CrossRef] [PubMed]
- Probst, U.; Fuhrmann, I.; Beyer, L.; Wiggermann, P. Electrochemotherapy as a New Modality in Interventional Oncology: A Review. Technol. Cancer Res. Treat. 2018, 17, 1533033818785329. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Ralli, M.; Longo, L.; Mancini, P.; Attanasio, G.; Atturo, F.; De Vincentiis, M.; Greco, A. Electrochemotherapy in head and neck cancer: A review of an emerging cancer treatment. Oncol. Lett. 2018, 16, 3415–3423. [Google Scholar] [CrossRef]
- Lenzi, R.; Muscatello, L.; Saibene, A.M.; Felisati, G.; Pipolo, C. The controversial role of electrochemotherapy in head and neck cancer: A systematic review of the literature. Eur. Arch. Otorhinolaryngol. 2017, 274, 2389–2394. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, S.K.; Hansen, H.F.; Gehl, J. New Drugs for Electrochemotherapy with Emphasis on Calcium Electroporation. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1637–1650. [Google Scholar]
- Miklavcic, D.; Mali, B.; Kos, B.; Heller, R.; Sersa, G. Electrochemotherapy: From the drawing board into medical practice. Biomed. Eng. Online 2014, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Serša, G.; Bosnjak, M.; Čemažar, M.; Heller, R. Preclinical Studies on Electrochemotherapy. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1511–1525. [Google Scholar]
- Cemazar, M.; Sersa, G. Recent Advances in Electrochemotherapy. Bioelectricity 2019, 1, 204–213. [Google Scholar] [CrossRef]
- Nesin, O.M.; Pakhomova, O.N.; Xiao, S.; Pakhomov, A.G. Manipulation of cell volume and membrane pore comparison following single cell permeabilization with 60- and 600-ns electric pulses. Biochim. Biophys. Acta 2011, 1808, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Faurie, C.; Rebersek, M.; Golzio, M.; Kanduser, M.; Escoffre, J.M.; Pavlin, M.; Teissie, J.; Miklavcic, D.; Rols, M.P. Electro-mediated gene transfer and expression are controlled by the life-time of DNA/membrane complex formation. J. Gene Med. 2010, 12, 117–125. [Google Scholar] [CrossRef]
- Golzio, M.; Teissie, J.; Rols, M.P. Direct visualization at the single-cell level of electrically mediated gene delivery. Proc. Natl. Acad. Sci. USA 2002, 99, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Rols, M.P.; Teissie, J. Electropermeabilization of mammalian cells to macromolecules: Control by pulse duration. Biophys. J. 1998, 75, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy—An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Baldi, A. Electrochemotherapy in Veterinary Oncology: State-of-the-Art and Perspectives. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 967–979. [Google Scholar] [CrossRef] [PubMed]
- Ibey, B.L.; Pakhomov, A.G.; Gregory, B.W.; Khorokhorina, V.A.; Roth, C.C.; Rassokhin, M.A.; Bernhard, J.A.; Wilmink, G.J.; Pakhomova, O.N. Selective cytotoxicity of intense nanosecond-duration electric pulses in mammalian cells. Biochim. Biophys. Acta 2010, 1800, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Ibey, B.L.; Xiao, S.; Schoenbach, K.H.; Murphy, M.R.; Pakhomov, A.G. Plasma membrane permeabilization by 60- and 600-ns electric pulses is determined by the absorbed dose. Bioelectromagnetics 2009, 30, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.J.; Miklavcic, D.; Vlachos, K.; Bordignon, S.; Scherr, D.; Jais, P.; Schmidt, B. State-of-the-art pulsed field ablation for cardiac arrhythmias: Ongoing evolution and future perspective. Europace 2024, 26, euae134. [Google Scholar] [CrossRef]
- Di Monaco, A.; Vitulano, N.; Troisi, F.; Quadrini, F.; Romanazzi, I.; Calvi, V.; Grimaldi, M. Pulsed Field Ablation to Treat Atrial Fibrillation: A Review of the Literature. J. Cardiovasc. Dev. Dis. 2022, 9, 94. [Google Scholar] [CrossRef]
- Romeo, S.; Wu, Y.H.; Levine, Z.A.; Gundersen, M.A.; Vernier, P.T. Water influx and cell swelling after nanosecond electropermeabilization. Biochim. Biophys. Acta 2013, 1828, 1715–1722. [Google Scholar] [CrossRef]
- Son, R.S.; Smith, K.C.; Gowrishankar, T.R.; Vernier, P.T.; Weaver, J.C. Basic Features of a Cell Electroporation Model: Illustrative Behavior for Two Very Different Pulses. J. Membr. Biol. 2014, 247, 1209–1228. [Google Scholar] [CrossRef]
- Levine, Z.A.; Vernier, P.T. Life cycle of an electropore: Field-dependent and field-independent steps in pore creation and annihilation. J. Membr. Biol. 2010, 236, 27–36. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Gianulis, E.; Vernier, P.T.; Semenov, I.; Xiao, S.; Pakhomova, O.N. Multiple nanosecond electric pulses increase the number but not the size of long-lived nanopores in the cell membrane. Biochim. Biophys. Acta 2015, 1848, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavcic, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef]
- Gianulis, E.C.; Labib, C.; Saulis, G.; Novickij, V.; Pakhomova, O.N.; Pakhomov, A.G. Selective susceptibility to nanosecond pulsed electric field (nsPEF) across different human cell types. Cell Mol. Life Sci. 2017, 74, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Pakhomova, O.N.; Gregory, B.W.; Pakhomov, A.G. Facilitation of electroporative drug uptake and cell killing by electrosensitization. J. Cell. Mol. Med. 2013, 17, 154–159. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakhomova, O.N.; Zivla, E.; Silkuniene, G.; Silkunas, M.; Pakhomov, A.G. Potentiation of Gelonin Cytotoxicity by Pulsed Electric Fields. Int. J. Mol. Sci. 2025, 26, 458. https://doi.org/10.3390/ijms26020458
Pakhomova ON, Zivla E, Silkuniene G, Silkunas M, Pakhomov AG. Potentiation of Gelonin Cytotoxicity by Pulsed Electric Fields. International Journal of Molecular Sciences. 2025; 26(2):458. https://doi.org/10.3390/ijms26020458
Chicago/Turabian StylePakhomova, Olga N., Eleni Zivla, Giedre Silkuniene, Mantas Silkunas, and Andrei G. Pakhomov. 2025. "Potentiation of Gelonin Cytotoxicity by Pulsed Electric Fields" International Journal of Molecular Sciences 26, no. 2: 458. https://doi.org/10.3390/ijms26020458
APA StylePakhomova, O. N., Zivla, E., Silkuniene, G., Silkunas, M., & Pakhomov, A. G. (2025). Potentiation of Gelonin Cytotoxicity by Pulsed Electric Fields. International Journal of Molecular Sciences, 26(2), 458. https://doi.org/10.3390/ijms26020458






