Metformin Induces Changes in Sphingosine-1-Phosphate-Related Signaling in Diabetic Mice Brain
Abstract
1. Introduction
2. Results
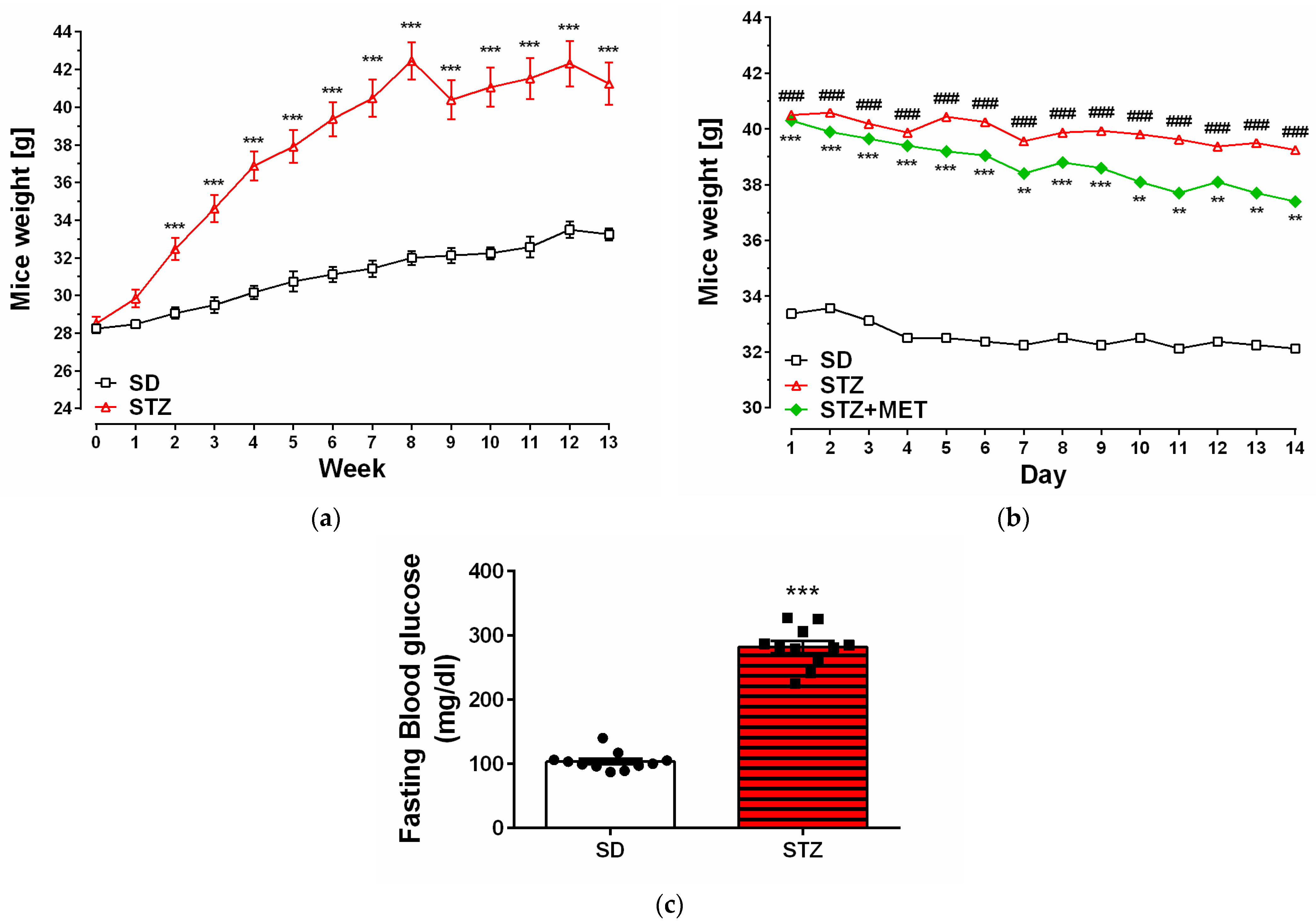
2.1. Model of Diabetes
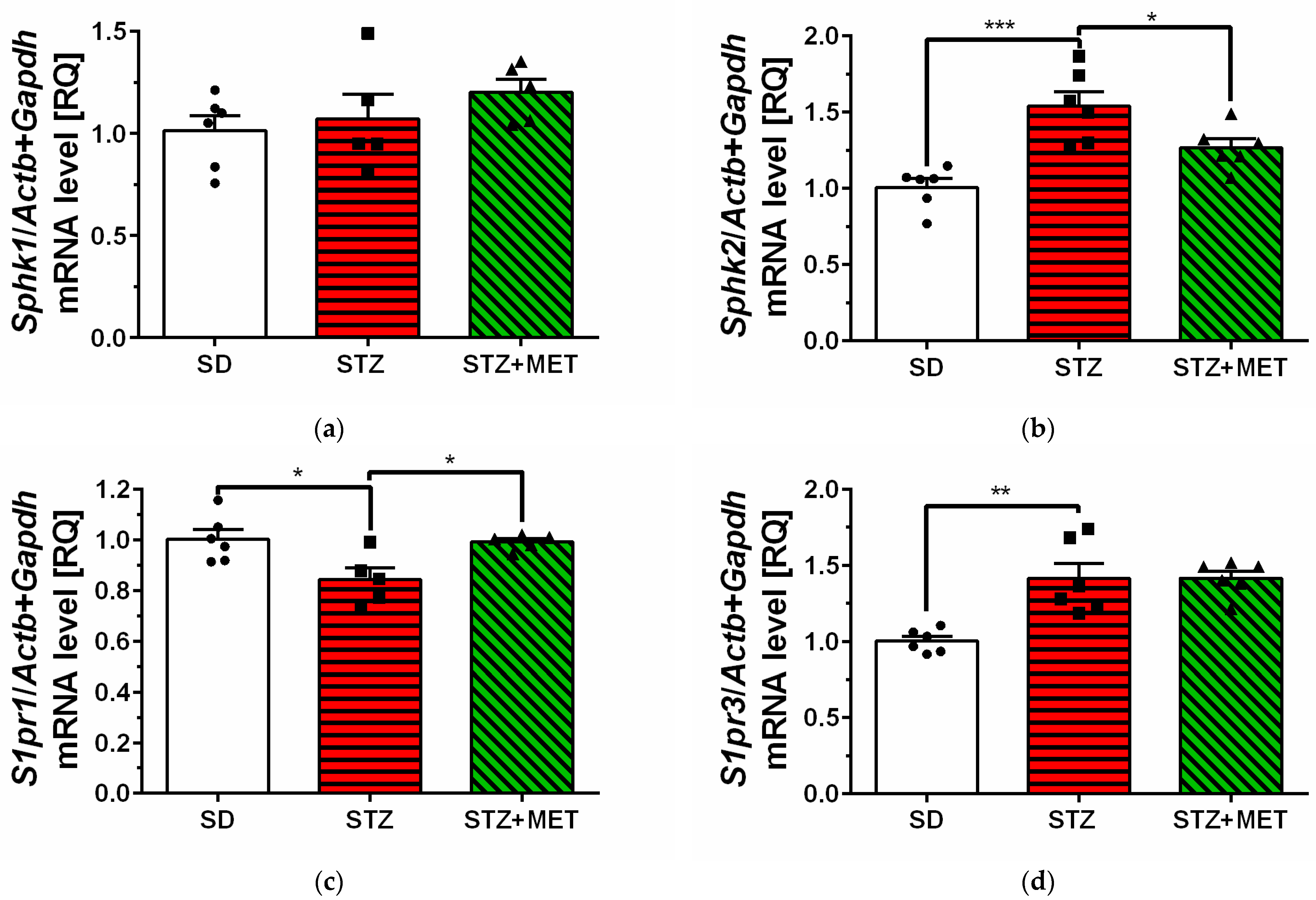
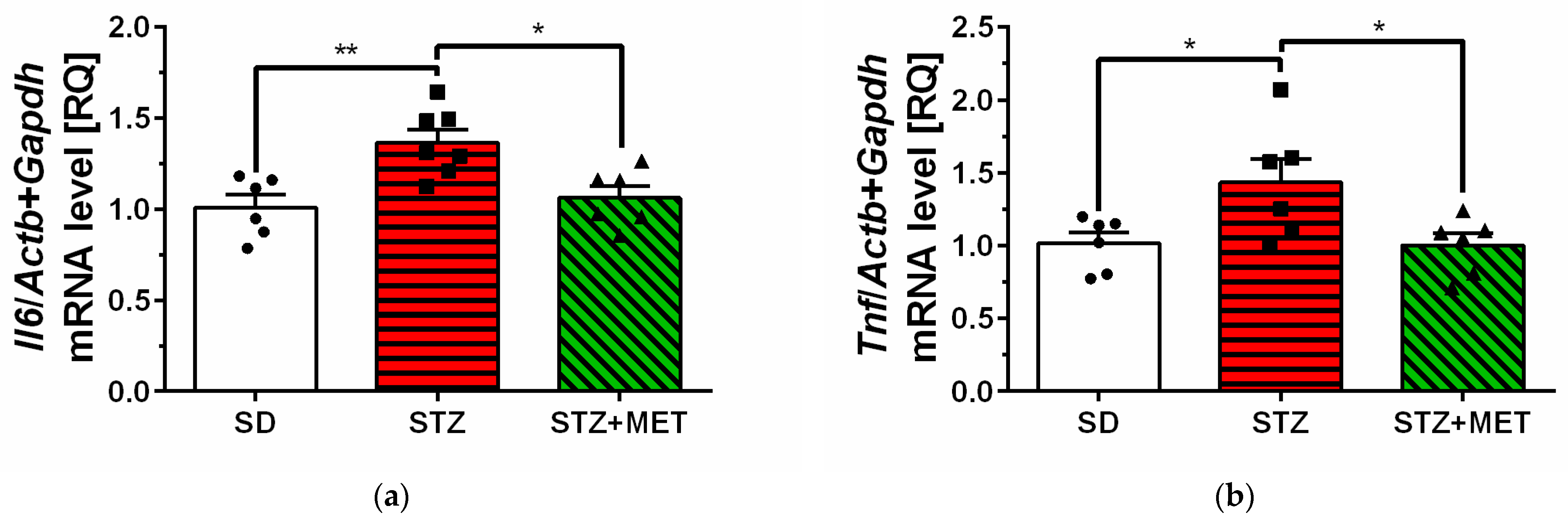
2.2. Sphingosine Kinases, S1P Receptors and Pro-Inflammatory Cytokine Expression in Hippocampus of Diabetic Mice
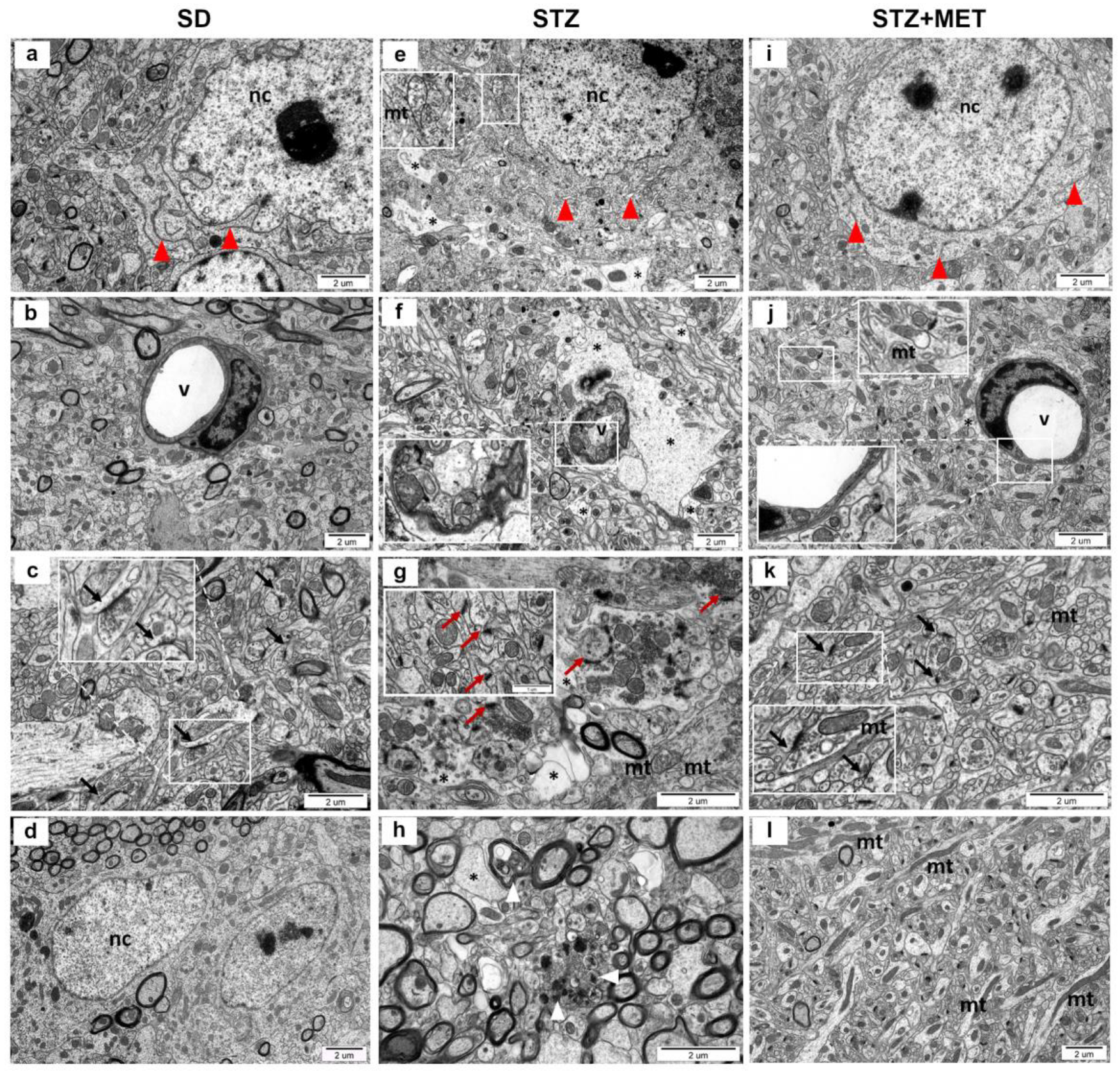
2.3. Ultrastructure of Diabetic Mice Hippocampus
3. Discussion
4. Materials and Methods
4.1. Glucose Test
4.2. Gene Expression Analysis
4.3. Immunochemical Determination of Protein Levels (Western Blot Analysis)
4.4. Ultrastructural Analysis of Hippocampus by TEM
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BS | bioactive sphingolipids |
| CNS | central nervous system |
| HFD | high-fat diet |
| MET | metformin |
| S1P | sphingosine-1-phosphate |
| S1PRs | sphingosine-1-phosphate receptors |
| SD | standard chow diet |
| Sph | sphingosine |
| SPHKs | sphingosine kinases |
| STZ | streptozotocin |
| T2DM | Type 2 diabetes mellitus |
References
- Emerging Risk Factors Collaboration. Life expectancy associated with different ages at diagnosis of type 2 diabetes in high-income countries: 23 million person-years of observation. Lancet Diabetes Endocrinol. 2023, 11, 731–742. [Google Scholar] [CrossRef]
- Cheng, G.; Huang, C.; Deng, H.; Wang, H. Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Intern. Med. J. 2012, 42, 484–491. [Google Scholar] [CrossRef]
- Hamed, S.A. Brain injury with diabetes mellitus: Evidence, mechanisms and treatment implications. Expert. Rev. Clin. Pharmacol. 2017, 10, 409–428. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Arumugam, T.V.; Cutler, R.G.; Lee, K.; Egan, J.M.; Mattson, M.P. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008, 11, 309–317. [Google Scholar] [CrossRef]
- Li, X.L.; Aou, S.; Oomura, Y.; Hori, N.; Fukunaga, K.; Hori, T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 2002, 113, 607–615. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, J.J.; Ortiz, O.; Jimenez-Palomares, M.; Kay, K.R.; Berrocoso, E.; Murillo-Carretero, M.I.; Perdomo, G.; Spires-Jones, T.; Cozar-Castellano, I.; Lechuga-Sancho, A.M.; et al. Differential central pathology and cognitive impairment in pre-diabetic and diabetic mice. Psychoneuroendocrinology 2013, 38, 2462–2475. [Google Scholar] [CrossRef]
- Beauquis, J.; Homo-Delarche, F.; Giroix, M.H.; Ehses, J.; Coulaud, J.; Roig, P.; Portha, B.; De Nicola, A.F.; Saravia, F. Hippocampal neurovascular and hypothalamic-pituitary-adrenal axis alterations in spontaneously type 2 diabetic GK rats. Exp. Neurol. 2010, 222, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Buckman, L.B.; Hasty, A.H.; Flaherty, D.K.; Buckman, C.T.; Thompson, M.M.; Matlock, B.K.; Weller, K.; Ellacott, K.L. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav. Immun. 2014, 35, 33–42. [Google Scholar] [CrossRef]
- Alò, R.; Fazzari, G.; Zizza, M.; Avolio, E.; Di Vito, A.; Olvito, I.; Bruno, R.; Canonaco, M.; Facciolo, R.M. Emotional and Spontaneous Locomotor Behaviors Related to cerebellar Daidzein-dependent TrkB Expression Changes in Obese Hamsters. Cerebellum 2023, 22, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Raj, D.D.; Schaafsma, W.; van der Heijden, R.A.; Kooistra, S.M.; Reijne, A.C.; Zhang, X.; Moser, J.; Brouwer, N.; Heeringa, P.; et al. Low-Fat Diet With Caloric Restriction Reduces White Matter Microglia Activation During Aging. Front. Mol. Neurosci. 2018, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Wencel, P.L.; Blecharz-Klin, K.; Piechal, A.; Pyrzanowska, J.; Mirowska-Guzel, D.; Strosznajder, R.P. Fingolimod Modulates the Gene Expression of Proteins Engaged in Inflammation and Amyloid-Beta Metabolism and Improves Exploratory and Anxiety-Like Behavior in Obese Mice. Neurotherapeutics 2023, 20, 1388–1404. [Google Scholar] [CrossRef]
- González Olmo, B.M.; Bettes, M.N.; DeMarsh, J.W.; Zhao, F.; Askwith, C.; Barrientos, R.M. Short-term high-fat diet consumption impairs synaptic plasticity in the aged hippocampus via IL-1 signaling. NPJ Sci. Food 2023, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Boitard, C.; Etchamendy, N.; Sauvant, J.; Aubert, A.; Tronel, S.; Marighetto, A.; Layé, S.; Ferreira, G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 2012, 22, 2095–2100. [Google Scholar] [CrossRef]
- de Paula, G.C.; Brunetta, H.S.; Engel, D.F.; Gaspar, J.M.; Velloso, L.A.; Engblom, D.; de Oliveira, J.; de Bem, A.F. Hippocampal Function Is Impaired by a Short-Term High-Fat Diet in Mice: Increased Blood-Brain Barrier Permeability and Neuroinflammation as Triggering Events. Front. Neurosci. 2021, 15, 734158. [Google Scholar] [CrossRef]
- Rebelos, E.; Hirvonen, J.; Bucci, M.; Pekkarinen, L.; Nyman, M.; Hannukainen, J.C.; Iozzo, P.; Salminen, P.; Nummenmaa, L.; Ferrannini, E.; et al. Brain free fatty acid uptake is elevated in morbid obesity, and is irreversible 6 months after bariatric surgery: A positron emission tomography study. Diabetes Obes. Metab. 2020, 22, 1074–1082. [Google Scholar] [CrossRef]
- Chaurasia, B.; Summers, S.A. Ceramides—Lipotoxic Inducers of Metabolic Disorders. Trends Endocrinol. Metab. 2015, 26, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Summers, S.A. Sphingolipids, Insulin Resistance, and Metabolic Disease: New Insights from in Vivo Manipulation of Sphingolipid Metabolism. Endocr. Rev. 2008, 29, 381–402. [Google Scholar] [CrossRef]
- Arsenault, E.J.; McGill, C.M.; Barth, B.M. Sphingolipids as Regulators of Neuro-Inflammation and NADPH Oxidase 2. Neuromolecular Med. 2021, 23, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Mizugishi, K.; Yamashita, T.; Olivera, A.; Miller, G.F.; Spiegel, S.; Proia, R.L. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell. Biol. 2005, 25, 11113–11121. [Google Scholar] [CrossRef]
- Ding, G.; Sonoda, H.; Yu, H.; Kajimoto, T.; Goparaju, S.K.; Jahangeer, S.; Okada, T.; Nakamura, S.I. Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J. Biol. Chem. 2007, 282, 27493–27502. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, W.; Song, Z.; Aji, G.; Liu, X.T.; Xia, P. Role of Sphingosine Kinase in Type 2 Diabetes Mellitus. Front. Endocrinol. 2020, 11, 627076. [Google Scholar] [CrossRef]
- Song, Z.; Wang, W.; Li, N.; Yan, S.; Rong, K.; Lan, T.; Xia, P. Sphingosine kinase 2 promotes lipotoxicity in pancreatic β-cells and the progression of diabetes. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 3636–3646. [Google Scholar] [CrossRef]
- Cantrell Stanford, J.; Morris, A.J.; Sunkara, M.; Popa, G.J.; Larson, K.L.; Ozcan, S. Sphingosine 1-phosphate (S1P) regulates glucose-stimulated insulin secretion in pancreatic beta cells. J. Biol. Chem. 2012, 287, 13457–13464. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Tong, M.; Nguyen, V.; Setshedi, M.; Longato, L.; Wands, J.R. Ceramide-Mediated Insulin Resistance and Impairment of Cognitive-Motor Functions. J. Alzheimers Dis. JAD 2010, 21, 967–984. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metabolism. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Fu, M.; Wang, C.; Quon, M.J.; Yang, P. Cellular Stress, Excessive Apoptosis, and the Effect of Metformin in a Mouse Model of Type 2 Diabetic Embryopathy. Diabetes 2015, 64, 2526–2536. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 569–589. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.M.; Mechanic-Hamilton, D.; Xie, S.X.; Combs, M.F.; Cappola, A.R.; Xie, L.; Detre, J.A.; Wolk, D.A.; Arnold, S.E. Effects of the Insulin Sensitizer Metformin in Alzheimer Disease: Pilot Data From a Randomized Placebo-controlled Crossover Study. Alzheimer Dis. Assoc. Disord. 2017, 31, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Mandwie, M.; Karunia, J.; Niaz, A.; Keay, K.A.; Musumeci, G.; Rennie, C.; McGrath, K.; Al-Badri, G.; Castorina, A. Metformin Treatment Attenuates Brain Inflammation and Rescues PACAP/VIP Neuropeptide Alterations in Mice Fed a High-Fat Diet. Int. J. Mol. Sci. 2021, 22, 13660. [Google Scholar] [CrossRef]
- Mastrocola, R.; Dal Bello, F.; Cento, A.S.; Gaens, K.; Collotta, D.; Aragno, M.; Medana, C.; Collino, M.; Wouters, K.; Schalkwijk, C.G. Altered hepatic sphingolipid metabolism in insulin resistant mice: Role of advanced glycation endproducts. Free. Radic. Biol. Med. 2021, 169, 425–435. [Google Scholar] [CrossRef]
- Holm, L.J.; Krogvold, L.; Hasselby, J.P.; Kaur, S.; Claessens, L.A.; Russell, M.A.; Mathews, C.E.; Hanssen, K.F.; Morgan, N.G.; Koeleman, B.P.C.; et al. Abnormal islet sphingolipid metabolism in type 1 diabetes. Diabetologia 2018, 61, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Finlin, B.S.; Kern, P.A.; Özcan, S. Sphk2(-/-) mice are protected from obesity and insulin resistance. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 570–576. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, W.; Xia, M.F.; Lu, Y.L.; Bian, H.; Yu, C.; Li, X.Y.; Vadas, M.A.; Gao, X.; Lin, H.D.; et al. Identification of circulating sphingosine kinase-related metabolites for prediction of type 2 diabetes. J. Transl. Med. 2021, 19, 393. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Thangada, S.; Dasgupta, O.; Khanna, K.M.; Yamase, H.T.; Kashgarian, M.; Hla, T.; Shapiro, L.H.; Ferrer, F.A. Cell-intrinsic sphingosine kinase 2 promotes macrophage polarization and renal inflammation in response to unilateral ureteral obstruction. PLoS ONE 2018, 13, e0194053. [Google Scholar] [CrossRef]
- Jin, B.-Y.; Kim, H.-J.; Oh, M.-J.; Ha, N.-H.; Jeong, Y.T.; Choi, S.-H.; Lee, J.-S.; Kim, N.H.; Kim, D.-H. Metformin acts as a dual glucose regulator in mouse brain. Front. Pharmacol. 2023, 14, 1108660. [Google Scholar] [CrossRef]
- Guo, W.-R.; Liu, J.; Cheng, L.-D.; Liu, Z.-Y.; Zheng, X.-B.; Liang, H.; Xu, F. Metformin Alleviates Steatohepatitis in Diet-Induced Obese Mice in a SIRT1-Dependent Way. Front. Pharmacol. 2021, 12, 704112. [Google Scholar] [CrossRef] [PubMed]
- Horakova, O.; Kroupova, P.; Bardova, K.; Buresova, J.; Janovska, P.; Kopecky, J.; Rossmeisl, M. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci. Rep. 2019, 9, 6156. [Google Scholar] [CrossRef]
- Sharma, A.; Krick, B.; Li, Y.; Summers, S.A.; Playdon, M.C.; Welt, C. The Use of Ceramides to Predict Metabolic Response to Metformin in Women With PCOS. J. Endocr. Soc. 2022, 6, bvac131. [Google Scholar] [CrossRef]
- Xing, L.; Wu, S.; Shi, Y.; Wei, L.; Yue, F.; Lam, S.M.; Shui, G.; Russell, R.; Zhang, D. Metformin alleviates sphingolipids dysregulation and improves obesity-related kidney disease in high-fat diet rats. J. Pharmacol. Exp. Ther. 2025, 392, 103388. [Google Scholar] [CrossRef]
- Dahabiyeh, L.A.; Mujammami, M.; AlMalki, R.H.; Arafat, T.; Benabdelkamel, H.; Alfadda, A.A.; Abdel Rahman, A.M. Lipids Alterations Associated with Metformin in Healthy Subjects: An Investigation Using Mass Spectrometry Shotgun Approach. Int. J. Mol. Sci. 2022, 23, 11478. [Google Scholar] [CrossRef] [PubMed]
- El-Mahdy, N.A.; El-Sayed, E.-S.M.; Hassan, E.-K.A.; and Abu-Risha, S.E.L.S. Metformin alleviates inflammation in oxazolone induced ulcerative colitis in rats: Plausible role of sphingosine kinase 1/sphingosine 1 phosphate signaling pathway. Immunopharmacol. Immunotoxicol. 2021, 43, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Guarnaccia, L.; Navone, S.E.; Masseroli, M.M.; Balsamo, M.; Caroli, M.; Valtorta, S.; Moresco, R.M.; Campanella, R.; Schisano, L.; Fiore, G.; et al. Effects of Metformin as Add-On Therapy against Glioblastoma: An Old Medicine for Novel Oncology Therapeutics. Cancers 2022, 14, 1412. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.C.; Chiyoda, T.; Liu, X.; Weigert, M.; Curtis, M.; Chiang, C.Y.; Loth, R.; Lastra, R.; McGregor, S.M.; Locasale, J.W.; et al. SPHK1 Is a Novel Target of Metformin in Ovarian Cancer. Mol. Cancer Res. 2019, 17, 870–881. [Google Scholar] [CrossRef]
- Oo, M.L.; Chang, S.H.; Thangada, S.; Wu, M.T.; Rezaul, K.; Blaho, V.; Hwang, S.I.; Han, D.K.; Hla, T. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J. Clin. Investig. 2011, 121, 2290–2300. [Google Scholar] [CrossRef]
- Galvani, S.; Sanson, M.; Blaho, V.A.; Swendeman, S.L.; Obinata, H.; Conger, H.; Dahlbäck, B.; Kono, M.; Proia, R.L.; Smith, J.D.; et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal 2015, 8, ra79. [Google Scholar] [CrossRef]
- Cartier, A.; Hla, T. Sphingosine 1-phosphate: Lipid signaling in pathology and therapy. Science 2019, 366, eaar5551. [Google Scholar] [CrossRef]
- Skoug, C.; Erdogan, H.; Vanherle, L.; Vieira, J.P.P.; Matthes, F.; Eliasson, L.; Meissner, A.; Duarte, J.M.N. Density of Sphingosine-1-Phosphate Receptors Is Altered in Cortical Nerve-Terminals of Insulin-Resistant Goto-Kakizaki Rats and Diet-Induced Obese Mice. Neurochem. Res. 2024, 49, 338–347. [Google Scholar] [CrossRef]
- Silva, V.R.; Micheletti, T.O.; Pimentel, G.D.; Katashima, C.K.; Lenhare, L.; Morari, J.; Mendes, M.C.; Razolli, D.S.; Rocha, G.Z.; de Souza, C.T.; et al. Hypothalamic S1P/S1PR1 axis controls energy homeostasis. Nat. Commun. 2014, 5, 4859. [Google Scholar] [CrossRef]
- Yue, H.; Hu, B.; Luo, Z.; Liu, M. Metformin protects against sevoflurane-induced neuronal apoptosis through the S1P1 and ERK signaling pathways. Exp. Ther. Med. 2019, 17, 1463–1469. [Google Scholar] [CrossRef]
- Gaire, B.P.; Song, M.-R.; Choi, J.W. Sphingosine 1-phosphate receptor subtype 3 (S1P3) contributes to brain injury after transient focal cerebral ischemia via modulating microglial activation and their M1 polarization. J. Neuroinflammation 2018, 15, 284. [Google Scholar] [CrossRef] [PubMed]
- Dusaban, S.S.; Chun, J.; Rosen, H.; Purcell, N.H.; Brown, J.H. Sphingosine 1-phosphate receptor 3 and RhoA signaling mediate inflammatory gene expression in astrocytes. J. Neuroinflammation 2017, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- English, D.; Welch, Z.; Kovala, A.T.; Harvey, K.; Volpert, O.V.; Brindley, D.N.; Garcia, J.G.N. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 2000, 14, 2255–2265. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Bui, Q.; Badeanlou, L.; Hester, K.; Chun, J.; Ruf, W.; Ciaraldi, T.P.; Samad, F. S1P/S1PR3 signalling axis protects against obesity-induced metabolic dysfunction. Adipocyte 2022, 11, 69–83. [Google Scholar] [CrossRef]
- Mauricio, D.; Gratacòs, M.; Franch-Nadal, J. Diabetic microvascular disease in non-classical beds: The hidden impact beyond the retina, the kidney, and the peripheral nerves. Cardiovasc. Diabetol. 2023, 22, 314. [Google Scholar] [CrossRef]
- van Sloten, T.T.; Sedaghat, S.; Carnethon, M.R.; Launer, L.J.; Stehouwer, C.D.A. Cerebral microvascular complications of type 2 diabetes: Stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 2020, 8, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A.; Maarouf, A.; Dawood, A.F.; Bayoumy, N.M.; Alqahtani, Y.A.; Eid, R.A.; Alqahtani, S.M.; Abd Ellatif, M.; Al-Ani, B.; Albawardi, A. Lower Extremity Arterial Disease in Type 2 Diabetes Mellitus: Metformin Inhibits Femoral Artery Ultrastructural Alterations as well as Vascular Tissue Levels of AGEs/ET-1 Axis-Mediated Inflammation and Modulation of Vascular iNOS and eNOS Expression. Biomedicines 2023, 11, 361. [Google Scholar] [CrossRef]
- Yin, Z.; Fan, L.; Wei, L.; Gao, H.; Zhang, R.; Tao, L.; Cao, F.; Wang, H. FTY720 Protects Cardiac Microvessels of Diabetes: A Critical Role of S1P1/3 in Diabetic Heart Disease. PLoS ONE 2012, 7, e42900. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Q.; Cai, Z.; Ni, C.; Wang, H.; Liu, H.; Zhao, Y.; Wang, J.; Liu, S.; Wang, X. Knockdown of PDPN in astrocytes reduces hippocampal inflammation in T2DM mice. Front. Immunol. 2025, 16, 1503807. [Google Scholar] [CrossRef]
- Cameron, A.R.; Morrison, V.L.; Levin, D.; Mohan, M.; Forteath, C.; Beall, C.; McNeilly, A.D.; Balfour, D.J.K.; Savinko, T.; Wong, A.K.F.; et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ. Res. 2016, 119, 652–665. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, L.; Zhang, J.; Li, P.; Lv, Z. Metformin improves cognitive impairment in diabetic mice induced by a combination of streptozotocin and isoflurane anesthesia. Bioengineered 2021, 12, 10982–10993. [Google Scholar] [CrossRef]
- Li, J.; Yin, M.; Wang, Z.; Xiong, Y.; Fang, X.; Fang, H. Fingolimod alleviates type 2 diabetes associated cognitive decline by regulating autophagy and neuronal apoptosis via AMPK/mTOR pathway. Brain Res. 2025, 1846, 149241. [Google Scholar] [CrossRef] [PubMed]
- Natrus, L.; Klys, Y.; Osadchuk, Y.; Anft, M.; Westhoff, T.; Babel, N. Combined Administration of Metformin and Propionate Reduces the Degree of Oxidative/Nitrosative Damage of Hypothalamic Neurons in Rat Model of Type 2 Diabetes Mellitus. Mol. Neurobiol. 2025, 62, 4338–4354. [Google Scholar] [CrossRef] [PubMed]
- Natrus, L.V.; Osadchuk, Y.S.; Lisakovska, O.O.; Labudzinskyi, D.O.; Klys, Y.G.; Chaikovsky, Y.B. Effect of Propionic Acid on Diabetes-Induced Impairment of Unfolded Protein Response Signaling and Astrocyte/Microglia Crosstalk in Rat Ventromedial Nucleus of the Hypothalamus. Neural Plast. 2022, 2022, 6404964. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wencel, P.L.; Czubowicz, K.; Gewartowska, M.; Frontczak-Baniewicz, M.; Strosznajder, R.P. Metformin Induces Changes in Sphingosine-1-Phosphate-Related Signaling in Diabetic Mice Brain. Int. J. Mol. Sci. 2025, 26, 9832. https://doi.org/10.3390/ijms26199832
Wencel PL, Czubowicz K, Gewartowska M, Frontczak-Baniewicz M, Strosznajder RP. Metformin Induces Changes in Sphingosine-1-Phosphate-Related Signaling in Diabetic Mice Brain. International Journal of Molecular Sciences. 2025; 26(19):9832. https://doi.org/10.3390/ijms26199832
Chicago/Turabian StyleWencel, Przemysław Leonard, Kinga Czubowicz, Magdalena Gewartowska, Małgorzata Frontczak-Baniewicz, and Robert Piotr Strosznajder. 2025. "Metformin Induces Changes in Sphingosine-1-Phosphate-Related Signaling in Diabetic Mice Brain" International Journal of Molecular Sciences 26, no. 19: 9832. https://doi.org/10.3390/ijms26199832
APA StyleWencel, P. L., Czubowicz, K., Gewartowska, M., Frontczak-Baniewicz, M., & Strosznajder, R. P. (2025). Metformin Induces Changes in Sphingosine-1-Phosphate-Related Signaling in Diabetic Mice Brain. International Journal of Molecular Sciences, 26(19), 9832. https://doi.org/10.3390/ijms26199832




