Diverse Members of the Phylum Armatimonadota Promote the Growth of Aquatic Plants, Duckweeds
Abstract
1. Introduction
2. Results
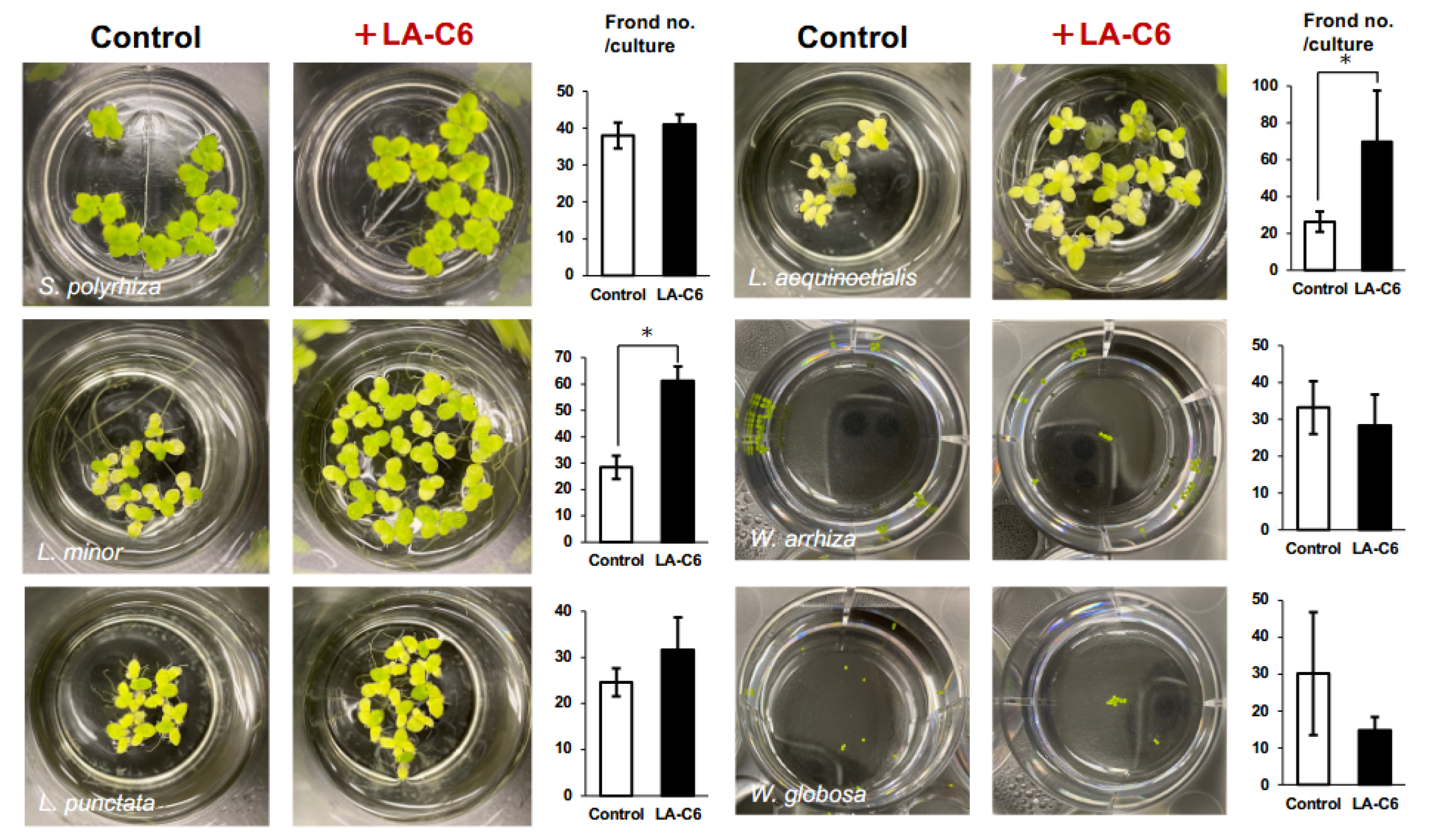
2.1. Plant Growth-Promoting Effect on Duckweeds by the Novel Armatimonadota Strain LA-C6
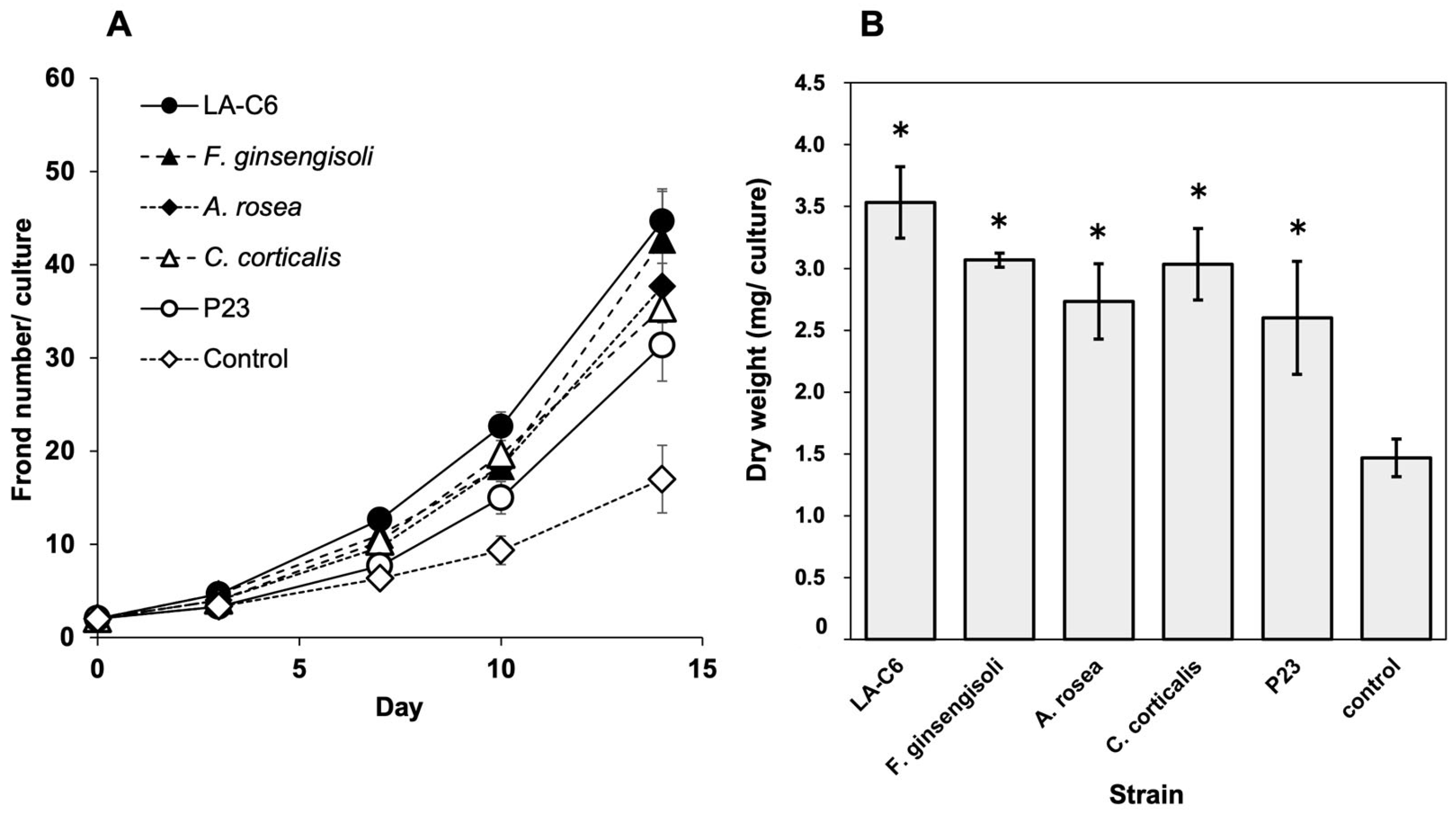
2.2. Plant Growth-Promoting Effect of Various Armatimonadota Species
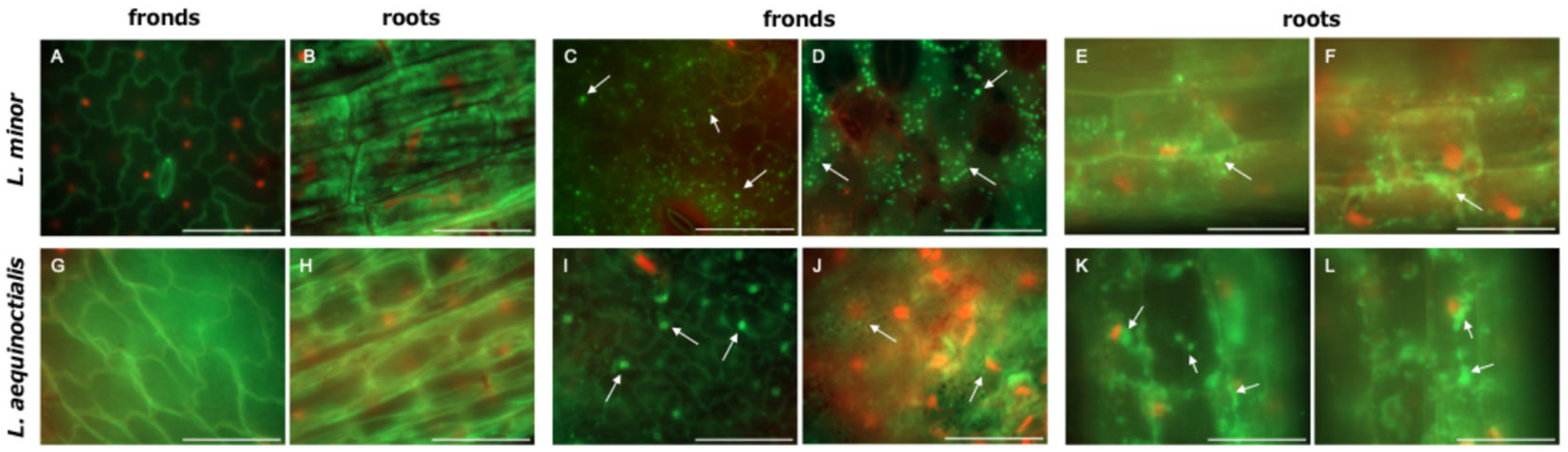
2.3. Colonization of Duckweed Surfaces by Strain LA-C6
2.4. Assays for Bacterial Plant Growth-Promoting Traits
2.5. PGP-Associated Genes in the Genome of Strain LA-C6
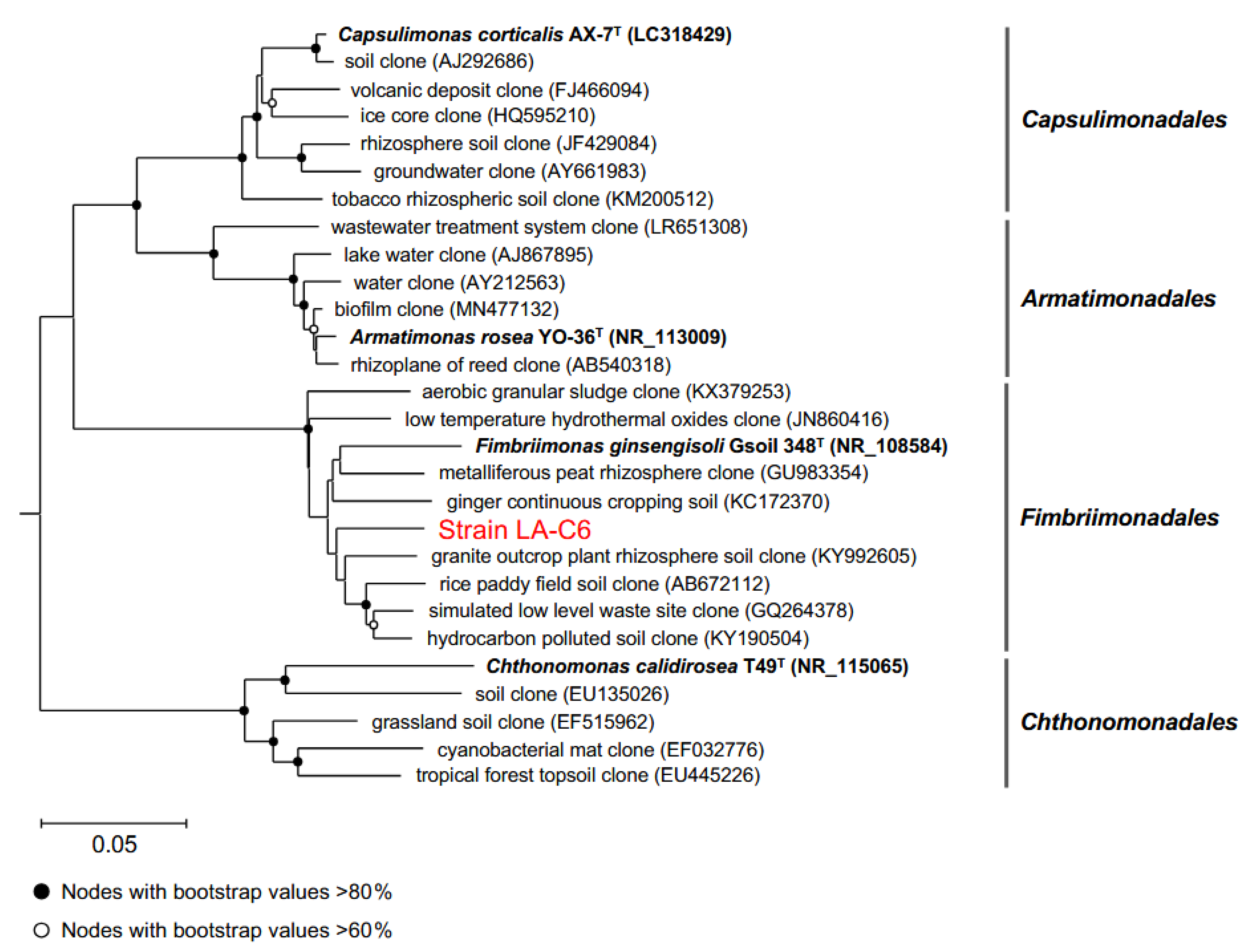
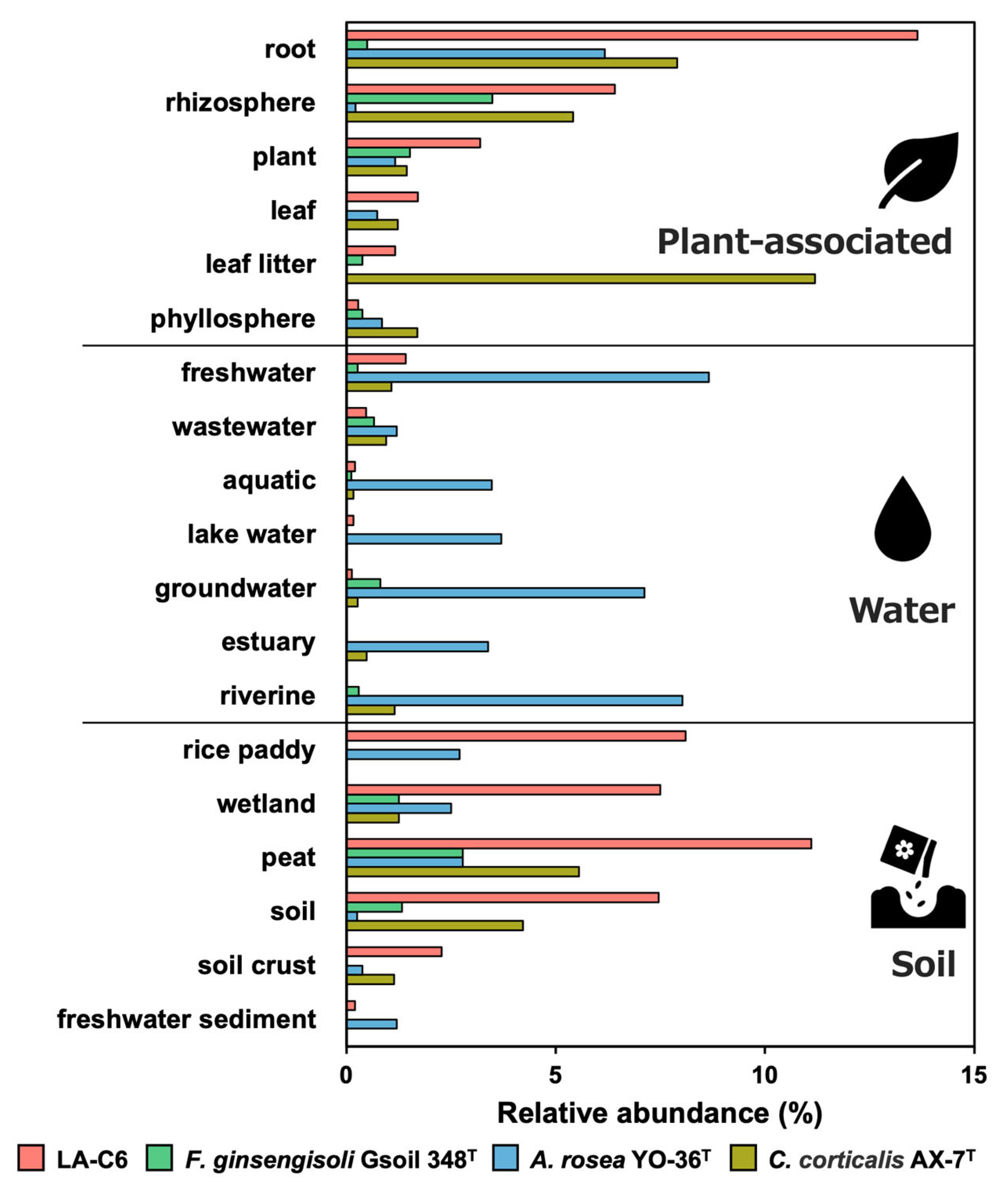
2.6. Environmental Distribution of Strain LA-C6 and Other Armatimonadota Strains
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Plant Materials and Growth Conditions
4.3. Preparation of Bacterial Strains
4.4. Evaluation of the PGP Effect of Strain LA-C6 on Duckweeds Cultivated in Bacterial Suspension
4.5. Evaluation of the PGP Effect of Strain LA-C6 on Bacteria-Attached Duckweeds in Sterile Medium
4.6. PGP Effect of Armatimonadota Strains on Duckweeds
4.7. Bacterial Colonization on Duckweed Surfaces
4.8. DNA Extraction and qPCR Analysis
4.9. Microscopic Observations
4.10. Assays for Plant Growth-Promoting Traits
4.11. Genome Analysis of a Taxonomically Novel Armatimonadota Strain LA-C6
4.12. IMNGS-Based 16S rRNA Amplicon Survey in Natural Environments
4.13. PGP Effect of Strain LA-C6 on Terrestrial Plants
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bashan, Y.; Holguin, G. Proposal for the Division of Plant Growth-Promoting Rhizobacteria into Two Classifications: Biocontrol-PGPB (Plant Growth-Promoting Bacteria) and PGPB. Soil Biol. Biochem. 1998, 30, 1225–1228. [Google Scholar] [CrossRef]
- Glick, B.R. Plant Growth-Promoting Bacteria: Mechanisms and Applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed]
- de-Bashan, L.E.; Hernandez, J.-P.; Bashan, Y. The Potential Contribution of Plant Growth-Promoting Bacteria to Reduce Environmental Degradation—A Comprehensive Evaluation. Appl. Soil Ecol. 2012, 61, 171–189. [Google Scholar] [CrossRef]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The Plant Endosphere World—Bacterial Life within Plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef] [PubMed]
- Sumayo, M.; Ghim, S.Y. Plant growth-promoting rhizobacteria for plant immunity. In Bacteria in Agrobiology: Crop Productivity; Maheshwari, D., Saraf, M., Aeron, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 329–349. [Google Scholar]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant Growth Promoting Bacteria in Agriculture: Two Sides of a Coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil. Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Figueiredo, M.V.B.; Seldin, L.; de Araujo, F.F.; Mariano, R.L.R. Plant growth promoting rhizobacteria: Fundamentals and applications. In Plant Growth and Health Promoting Bacteria. Microbiology Monographs; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 18, pp. 21–43. [Google Scholar]
- Appenroth, K.-J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional Value of Duckweeds (Lemnaceae) as Human Food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef]
- Stomp, A.M. The duckweeds: A valuable plant for biomanufacturing. Biotechnol. Annu. Rev. 2005, 11, 69–99. [Google Scholar]
- Xu, J.; Cui, W.; Cheng, J.J.; Stomp, A.-M. Production of High-Starch Duckweed and Its Conversion to Bioethanol. Biosyst. Eng. 2011, 110, 67–72. [Google Scholar] [CrossRef]
- Xu, J.; Dolan, M.C.; Medrano, G.; Cramer, C.L.; Weathers, P.J. Green Factory: Plants as Bioproduction Platforms for Recombinant Proteins. Biotechnol. Adv. 2012, 30, 1171–1184. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, W.; Sugawara, M.; Miwa, K.; Morikawa, M. Plant Growth-Promoting Bacterium Acinetobacter Calcoaceticus P23 Increases the Chlorophyll Content of the Monocot Lemna minor (Duckweed) and the Dicot Lactuca sativa (Lettuce). J. Biosci. Bioeng. 2014, 118, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, H.; Kuroda, M.; Morikawa, M.; Ike, M. Evaluation of Environmental Bacterial Communities as a Factor Affecting the Growth of Duckweed Lemna minor. Biotechnol. Biofuels 2017, 10, 62. [Google Scholar] [CrossRef]
- Yamakawa, Y.; Jog, R.; Morikawa, M. Effects of Co-Inoculation of Two Different Plant Growth-Promoting Bacteria on Duckweed. Plant Growth Regul. 2018, 86, 287–296. [Google Scholar] [CrossRef]
- Iwashita, T.; Tanaka, Y.; Tamaki, H.; Nakai, R.; Yoneda, Y.; Makino, A.; Toyama, T.; Kamagata, Y.; Morikawa, M.; Mori, K. Isolation and Characterization of Novel Plant Growth-Promoting Bacteria from the Fronds of Duckweed. Jpn. J. Water Treat. Biol. 2021, 57, 1–9. [Google Scholar] [CrossRef]
- Toyama, T.; Mori, K.; Tanaka, Y.; Ike, M.; Morikawa, M. Growth Promotion of Giant Duckweed Spirodela polyrhiza (Lemnaceae) by Ensifer Sp. SP4 Through Enhancement of Nitrogen Metabolism and Photosynthesis. Mol. Plant-Microbe Interact. 2022, 35, 28–38. [Google Scholar] [CrossRef]
- Makino, A.; Nakai, R.; Yoneda, Y.; Toyama, T.; Tanaka, Y.; Meng, X.-Y.; Mori, K.; Ike, M.; Morikawa, M.; Kamagata, Y.; et al. Isolation of Aquatic Plant Growth-Promoting Bacteria for the Floating Plant Duckweed (Lemna minor). Microorganisms 2022, 10, 1564. [Google Scholar] [CrossRef]
- Yoneda, Y.; Yamamoto, K.; Makino, A.; Tanaka, Y.; Meng, X.-Y.; Hashimoto, J.; Shin-ya, K.; Satoh, N.; Fujie, M.; Toyama, T.; et al. Novel Plant-Associated Acidobacteria Promotes Growth of Common Floating Aquatic Plants, Duckweeds. Microorganisms 2021, 9, 1133. [Google Scholar] [CrossRef]
- Iwashita, T.; Tanaka, Y.; Tamaki, H.; Yoneda, Y.; Makino, A.; Tateno, Y.; Li, Y.; Toyama, T.; Kamagata, Y.; Mori, K. Comparative Analysis of Microbial Communities in Fronds and Roots of Three Duckweed Species: Spirodela polyrhiza, Lemna minor, and Lemna aequinoctialis. Microb. Environ. 2020, 35, ME20081. [Google Scholar] [CrossRef]
- Dunbar, J.; Barns Susan, M.; Ticknor Lawrence, O.; Kuske Cheryl, R. Empirical and Theoretical Bacterial Diversity in Four Arizona Soils. Appl. Environ. Microbiol. 2002, 68, 3035–3045. [Google Scholar] [CrossRef]
- Lipson, D.A.; Schmidt, S.K. Seasonal Changes in an Alpine Soil Bacterial Community in the Colorado Rocky Mountains. Appl. Environ. Microbiol. 2004, 70, 2867–2879. [Google Scholar] [CrossRef]
- Crump, B.C.; Hobbie, J.E. Synchrony and Seasonality in Bacterioplankton Communities of Two Temperate Rivers. Limnol. Oceanogr. 2005, 50, 1718–1729. [Google Scholar] [CrossRef]
- Wu, X.; Xi, W.; Ye, W.; Yang, H. Bacterial Community Composition of a Shallow Hypertrophic Freshwater Lake in China, Revealed by 16S rRNA Gene Sequences: Bacterial Community of a Hypertrophic Freshwater Lake. FEMS Microbiol. Ecol. 2007, 61, 85–96. [Google Scholar] [CrossRef]
- Vourlitis, G.L.; Berry, P.; Cabuco, J.M.; Estrada, A.; García, K.H.; Hunter, B.T.; Mastaglio, L.; Murguia, E.; Nacauili, N.; Ponce, M.; et al. Composition and Activity of Soil Microbial Communities in Native and Non-Native Vegetation of Southern California. Appl. Soil Ecol. 2024, 193, 105164. [Google Scholar] [CrossRef]
- Górska, E.B.; Stępień, W.; Hewelke, E.; Lata, J.-C.; Gworek, B.; Gozdowski, D.; Sas-Paszt, L.; Bazot, S.; Lisek, A.; Gradowski, M.; et al. Response of Soil Microbiota to Various Soil Management Practices in 100-Year-Old Agriculture Field and Identification of Potential Bacterial Ecological Indicator. Ecol. Indic. 2024, 158, 11154. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tamaki, H.; Tanaka, K.; Tozawa, E.; Matsuzawa, H.; Toyama, T.; Kamagata, Y.; Mori, K. “Duckweed-Microbe Co-Cultivation Method” for Isolating a Wide Variety of Microbes Including Taxonomically Novel Microbes. Microb. Environ. 2018, 33, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Fraga, R.; Gonzalez, T.; Bashan, Y. Genetics of Phosphate Solubilization and Its Potential Applications for Improving Plant Growth-Promoting Bacteria. Plant Soil 2006, 287, 15–21. [Google Scholar] [CrossRef]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial Features of Plant Growth-Promoting Rhizobacteria for Improving Plant Growth and Health in Challenging Conditions: A Methodical Review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Koirala, A.; Brözel, V.S. Phylogeny of Nitrogenase Structural and Assembly Components Reveals New Insights into the Origin and Distribution of Nitrogen Fixation across Bacteria and Archaea. Microorganisms 2021, 9, 1662. [Google Scholar] [CrossRef]
- Kumar, P.; Rani, S.; Dahiya, P.; Kumar, A.; Dang, A.S.; Suneja, P. Whole Genome Analysis for Plant Growth Promotion Profiling of Pantoea Agglomerans CPHN2, a Non-Rhizobial Nodule Endophyte. Front. Microbiol. 2022, 13, 998821. [Google Scholar] [CrossRef]
- Taghavi, S.; Van Der Lelie, D.; Hoffman, A.; Zhang, Y.-B.; Walla, M.D.; Vangronsveld, J.; Newman, L.; Monchy, S. Genome Sequence of the Plant Growth Promoting Endophytic Bacterium Enterobacter Sp. 638. PLoS Genet. 2010, 6, e1000943. [Google Scholar] [CrossRef]
- Pothier, J.F.; Prigent-Combaret, C.; Haurat, J.; Moënne-Loccoz, Y.; Wisniewski-Dyé, F. Duplication of Plasmid-Borne Nitrite Reductase Gene nirK in the Wheat-Associated Plant Growth–Promoting Rhizobacterium Azospirillum Brasilense Sp245. Mol. Plant-Microbe Interact. 2008, 21, 831–842. [Google Scholar] [CrossRef] [PubMed]
- Onofre-Lemus, J.; Hernández-Lucas, I.; Girard, L.; Caballero-Mellado, J. ACC (1-Aminocyclopropane-1-Carboxylate) Deaminase Activity, a Widespread Trait in Burkholderia Species, and Its Growth-Promoting Effect on Tomato Plants. Appl. Environ. Microbiol. 2009, 75, 6581–6590. [Google Scholar] [CrossRef] [PubMed]
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.K.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S.; et al. Return of the Lemnaceae: Duckweed as a Model Plant System in the Genomics and Postgenomics Era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, H.; Tada, M.; Kuroda, M.; Inoue, D.; Ike, M. Performance of Plant Growth-Promoting Bacterium of Duckweed under Different Kinds of Abiotic Stress Factors. Biocatal. Agric. Biotechnol. 2019, 19, 101146. [Google Scholar] [CrossRef]
- Lam, E.; Appenroth, K.J.; Michael, T.; Mori, K.; Fakhoorian, T. Duckweed in Bloom: The 2nd International Conference on Duckweed Research and Applications Heralds the Return of a Plant Model for Plant Biology. Plant Mol. Biol. 2014, 84, 737–742. [Google Scholar] [CrossRef]
- Toyama, T.; Kuroda, M.; Ogata, Y.; Hachiya, Y.; Quach, A.; Tokura, K.; Tanaka, Y.; Mori, K.; Morikawa, M.; Ike, M. Enhanced Biomass Production of Duckweeds by Inoculating a Plant Growth-Promoting Bacterium, Acinetobacter calcoaceticus P23, in Sterile Medium and Non-Sterile Environmental Waters. Water Sci. Technol. 2017, 76, 1418–1428. [Google Scholar] [CrossRef]
- Tamaki, H.; Tanaka, Y.; Matsuzawa, H.; Muramatsu, M.; Meng, X.-Y.; Hanada, S.; Mori, K.; Kamagata, Y. Armatimonas rosea Gen. Nov., Sp. Nov., of a Novel Bacterial Phylum, Armatimonadetes Phyl. Nov., Formally Called the Candidate Phylum OP10. Int. J. Syst. Evol. Microbiol. 2011, 61, 1442–1447. [Google Scholar] [CrossRef]
- Im, W.-T.; Hu, Z.-Y.; Kim, K.-H.; Rhee, S.-K.; Meng, H.; Lee, S.-T.; Quan, Z.-X. Description of Fimbriimonas ginsengisoli Gen. Nov., Sp. Nov. within the Fimbriimonadia Class Nov., of the Phylum Armatimonadetes. Antonie Leeuwenhoek 2012, 102, 307–317. [Google Scholar] [CrossRef]
- Li, J.; Kudo, C.; Tonouchi, A. Capsulimonas corticalis Gen. Nov., Sp. Nov., an Aerobic Capsulated Bacterium, of a Novel Bacterial Order, Capsulimonadales Ord. Nov., of the Class Armatimonadia of the Phylum Armatimonadetes. Int. J. Syst. Evol. Microbiol. 2019, 69, 220–226. [Google Scholar] [CrossRef]
- Popržen, T.; Jevremović, S.; Milošević, S.; Đurić, M.; Uzelac, B.; Stanković, S.; Radulović, O. Antioxidative Response of Duckweed (Lemna minor L.) to Rhizosphere-Associated Pseudomonas Strains and Exogenous Indole-3-Acetic Acid. Horticulturae 2024, 10, 562. [Google Scholar] [CrossRef]
- Utami, D.; Kawahata, A.; Sugawara, M.; Jog, R.N.; Miwa, K.; Morikawa, M. Effect of Exogenous General Plant Growth Regulators on the Growth of the Duckweed Lemna Minor. Front. Chem. 2018, 6, 251. [Google Scholar] [CrossRef]
- Elhaj Baddar, Z.; Bier, R.; Spencer, B.; Xu, X. Microbial Community Changes across Time and Space in a Constructed Wetland. ACS Environ. Au 2024, 4, 307–316. [Google Scholar] [CrossRef]
- He, D.; Ren, L.; Wu, Q.L. Contrasting Diversity of Epibiotic Bacteria and Surrounding Bacterioplankton of a Common Submerged Macrophyte, Potamogeton Crispus, in Freshwater Lakes. FEMS Microbiol. Ecol. 2014, 90, 551–562. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tamaki, H.; Matsuzawa, H.; Nigaya, M.; Mori, K.; Kamagata, Y. Microbial Community Analysis in the Roots of Aquatic Plants and Isolation of Novel Microbes Including an Organism of the Candidate Phylum OP10. Microb. Environ. 2012, 27, 149–157. [Google Scholar] [CrossRef]
- Sarria-Guzmán, Y.; Chávez-Romero, Y.; Gómez-Acata, S.; Montes-Molina, J.A.; Morales-Salazar, E.; Dendooven, L.; Navarro-Noya, Y.E. Bacterial Communities Associated with Different Anthurium Andraeanum L. Plant Tissues. Microb. Environ. 2016, 31, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.-W.; Yoou, M.-H.; Hong, W.-J.; Kim, Y.-J.; Lee, E.-J.; Jung, K.-H. Re-Analysis of 16S Amplicon Sequencing Data Reveals Soil Microbial Population Shifts in Rice Fields under Drought Condition. Rice 2020, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Meepagala, K.M.; Anderson, C.M.; Techen, N.; Duke, S.O. Pantoea Ananatis, a Plant Growth Stimulating Bacterium, and Its Metabolites Isolated from Hydrocotyle Umbellata (Dollarweed). Plant Signal. Behav. 2024, 19, 2331894. [Google Scholar] [CrossRef] [PubMed]
- Stott, M.B.; Crowe, M.A.; Mountain, B.W.; Smirnova, A.V.; Hou, S.; Alam, M.; Dunfield, P.F. Isolation of Novel Bacteria, Including a Candidate Division, from Geothermal Soils in New Zealand. Environ. Microbiol. 2008, 10, 2030–2041. [Google Scholar] [CrossRef]
- Lee, K.C.-Y.; Dunfield, P.F.; Morgan, X.C.; Crowe, M.A.; Houghton, K.M.; Vyssotski, M.; Ryan, J.L.J.; Lagutin, K.; McDonald, I.R.; Stott, M.B. Chthonomonas calidirosea Gen. Nov., Sp. Nov., an Aerobic, Pigmented, Thermophilic Micro-Organism of a Novel Bacterial Class, Chthonomonadetes Classis Nov., of the Newly Described Phylum Armatimonadetes Originally Designated Candidate Division OP10. Int. J. Syst. Evol. Microbiol. 2011, 61, 2482–2490. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Jiang, B.; Feng, Y.; Zhu, T.; Tao, H.; Tang, X.; Liu, S. Genome-Centered Metagenomics Analysis Reveals the Symbiotic Organisms Possessing Ability to Cross-Feed with Anammox Bacteria in Anammox Consortia. Environ. Sci. Technol. 2018, 52, 11285–11296. [Google Scholar] [CrossRef]
- Kato, S.; Masuda, S.; Shibata, A.; Shirasu, K.; Ohkuma, M. Insights into Ecological Roles of Uncultivated Bacteria in Katase Hot Spring Sediment from Long-Read Metagenomics. Front. Microbiol. 2022, 13, 1045931. [Google Scholar] [CrossRef]
- Carlton, J.D.; Langwig, M.V.; Gong, X.; Aguilar-Pine, E.J.; Vázquez-Rosas-Landa, M.; Seitz, K.W.; Baker, B.J.; De Anda, V. Expansion of Armatimonadota through Marine Sediment Sequencing Describes Two Classes with Unique Ecological Roles. ISME Commun. 2023, 3, 64. [Google Scholar] [CrossRef]
- Trinh, H.P.; Lee, S.-H.; Kim, N.-K.; Nguyen, T.V.; Park, H.-D. Fimbriimonadales Performed Dissimilatory Nitrate Reduction to Ammonium (DNRA) in an Anammox Reactor. Water Res. 2025, 268, 122575. [Google Scholar] [CrossRef] [PubMed]
- Tsurumaru, H.; Okubo, T.; Okazaki, K.; Hashimoto, M.; Kakizaki, K.; Hanzawa, E.; Takahashi, H.; Asanome, N.; Tanaka, F.; Sekiyama, Y.; et al. Metagenomic Analysis of the Bacterial Community Associated with the Taproot of Sugar Beet. Microb. Environ. 2015, 30, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, O.S.; Creevey, C.J.; Santana, M.F. Designing a Synthetic Microbial Community through Genome Metabolic Modeling to Enhance Plant–Microbe Interaction. Environ. Microbiomes 2023, 18, 81. [Google Scholar] [CrossRef] [PubMed]
- Funnicelli, M.I.G.; De Carvalho, L.A.L.; Teheran-Sierra, L.G.; Dibelli, S.C.; Lemos, E.G.D.M.; Pinheiro, D.G. Unveiling Genomic Features Linked to Traits of Plant Growth-Promoting Bacterial Communities from Sugarcane. Sci. Total Environ. 2024, 947, 174577. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S Ribosomal DNA Amplification for Phylogenetic Study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef]
- Gürtler, V.; Stanisich, V.A. New Approaches to Typing and Identification of Bacteria Using the 16S-23S rDNA Spacer Region. Microbiology 1996, 142, 3–16. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Giacomazzi, S.; Leroi, F.; Joffraud, J.-J. Comparison of Three Methods of DNA Extraction from Cold-Smoked Salmon and Impact of Physical Treatments. J. Appl. Microbiol. 2005, 98, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, R.G.; Tanaka, Y.; Malla, B.; Tandukar, S.; Bhandari, D.; Inoue, D.; Sei, K.; Sherchand, J.B.; Haramoto, E. Development of a Quantitative PCR Assay for Arcobacter Spp. and Its Application to Environmental Water Samples. Microb. Environ. 2018, 33, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal Chemical Assay for the Detection and Determination of Siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Adesh, K.; Shambhoo, P.; Sushil, K.S. Screening of Free Living Rhizobacteria Associated with Wheat Rhizosphere for Plant Growth Promoting Traits. Afr. J. Agric. Res. 2014, 9, 1094–1100. [Google Scholar] [CrossRef]
- Bric, J.M.; Bostock, R.M.; Silverstone, S.E. Rapid In Situ Assay for Indoleacetic Acid Production by Bacteria Immobilized on a Nitrocellulose Membrane. Appl. Environ. Microbiol. 1991, 57, 535–538. [Google Scholar] [CrossRef]
- Morinaga, K.; Kusada, H.; Sakamoto, S.; Murakami, T.; Toyoda, A.; Mori, H.; Meng, X.-Y.; Takashino, M.; Murotomi, K.; Tamaki, H. Granulimonas faecalis Gen. Nov., Sp. Nov., and Leptogranulimonas caecicola Gen. Nov., Sp. Nov., Novel Lactate-Producing Atopobiaceae Bacteria Isolated from Mouse Intestines, and an Emended Description of the Family Atopobiaceae. Int. J. Syst. Evol. Microbiol. 2022, 72. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The Protein Families Database. Nucl. Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef]
- Lagkouvardos, I.; Joseph, D.; Kapfhammer, M.; Giritli, S.; Horn, M.; Haller, D.; Clavel, T. IMNGS: A Comprehensive Open Resource of Processed 16S rRNA Microbial Profiles for Ecology and Diversity Studies. Sci. Rep. 2016, 6, 33721. [Google Scholar] [CrossRef]
- Shulse, C.N.; Chovatia, M.; Agosto, C.; Wang, G.; Hamilton, M.; Deutsch, S.; Yoshikuni, Y.; Blow, M.J. Engineered Root Bacteria Release Plant-Available Phosphate from Phytate. Appl. Environ. Microbiol. 2019, 85, e01210–e01219. [Google Scholar] [CrossRef]


| Strain | Order | Isolation Source |
|---|---|---|
| A. rosea strain YO-36T | Armatimonadales | rhizoplanes of reeds |
| F. ginsengisoli strain Gsoil 348T | Fimbriimonadales | ginseng field soil |
| C. corticalis strain AX-7T | Capsulimonadales | trunk surface of Japanese beech |
| Strain LA-C6 | Fimbriimonadales | fronds of duckweeds |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwashita, T.; Makino, A.; Nakai, R.; Yoneda, Y.; Kamagata, Y.; Toyama, T.; Mori, K.; Tanaka, Y.; Tamaki, H. Diverse Members of the Phylum Armatimonadota Promote the Growth of Aquatic Plants, Duckweeds. Int. J. Mol. Sci. 2025, 26, 9824. https://doi.org/10.3390/ijms26199824
Iwashita T, Makino A, Nakai R, Yoneda Y, Kamagata Y, Toyama T, Mori K, Tanaka Y, Tamaki H. Diverse Members of the Phylum Armatimonadota Promote the Growth of Aquatic Plants, Duckweeds. International Journal of Molecular Sciences. 2025; 26(19):9824. https://doi.org/10.3390/ijms26199824
Chicago/Turabian StyleIwashita, Tomoki, Ayaka Makino, Ryosuke Nakai, Yasuko Yoneda, Yoichi Kamagata, Tadashi Toyama, Kazuhiro Mori, Yasuhiro Tanaka, and Hideyuki Tamaki. 2025. "Diverse Members of the Phylum Armatimonadota Promote the Growth of Aquatic Plants, Duckweeds" International Journal of Molecular Sciences 26, no. 19: 9824. https://doi.org/10.3390/ijms26199824
APA StyleIwashita, T., Makino, A., Nakai, R., Yoneda, Y., Kamagata, Y., Toyama, T., Mori, K., Tanaka, Y., & Tamaki, H. (2025). Diverse Members of the Phylum Armatimonadota Promote the Growth of Aquatic Plants, Duckweeds. International Journal of Molecular Sciences, 26(19), 9824. https://doi.org/10.3390/ijms26199824






