Single-Nucleotide Polymorphisms, PITX2 and Abnormal Electrical Activity in Atrial Fibrillation
Abstract
1. Introduction
2. Single-Nucleotide Polymorphisms Affecting Atrial Electrical Activity
2.1. Genome-Wide Association Studies
2.2. Impact of Risk SNPs on PITX2 Expression or Function
2.3. Impact of Risk SNPs on Cardiomyocyte Function
2.4. Impact of Risk SNPs on Atrial Function
2.5. Impact of Risk SNPs on AF Therapy
3. Modulation of Electrical Activity by PITX2
3.1. Relationship Between PITX2 and AF
3.2. PITX2 Point Mutations and Non-Coding RNA
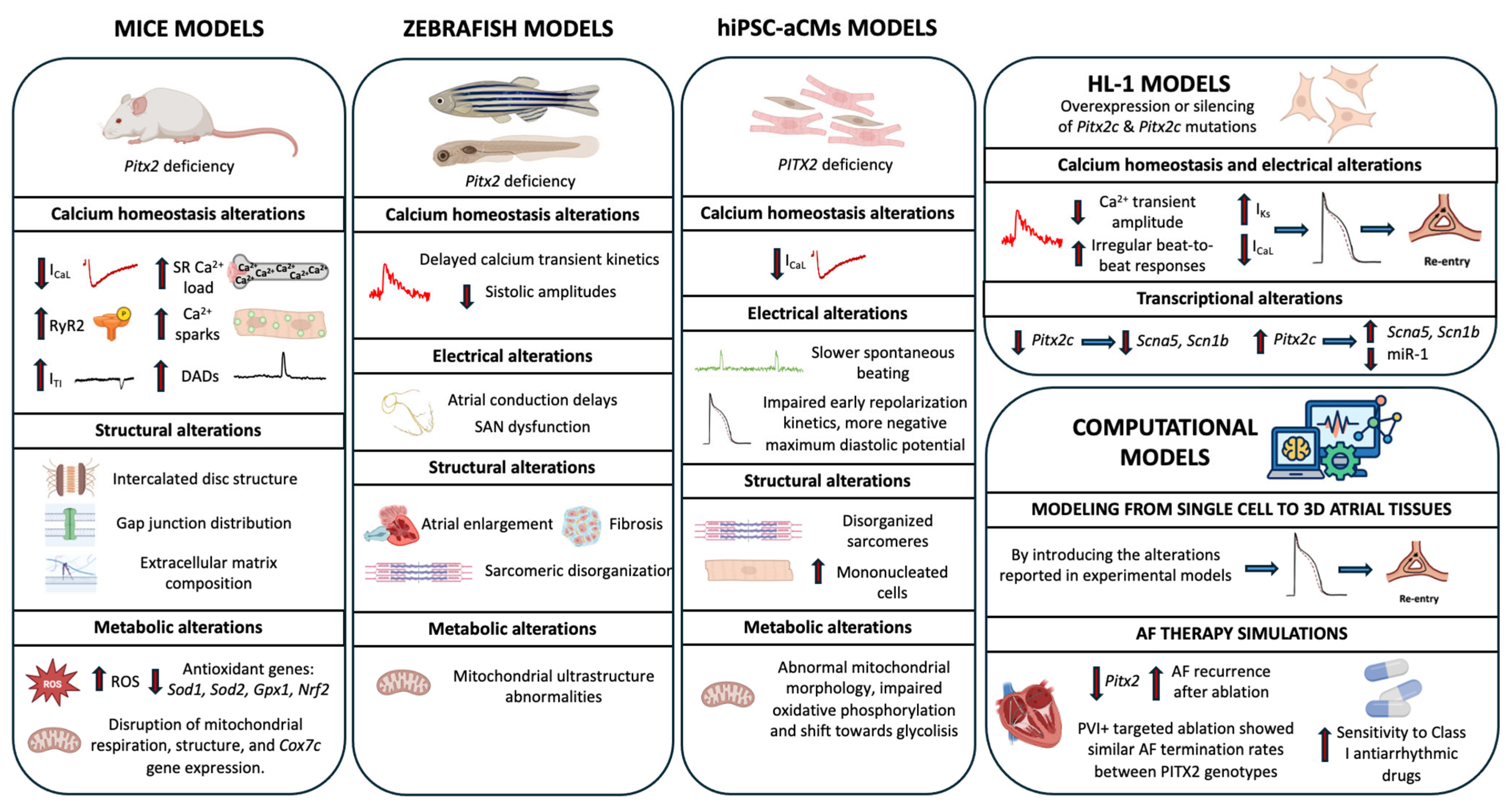
3.3. Genetic Manipulation of PITX2 and Experimental Models of Pitx2 Deficiency
3.3.1. Mouse Models
3.3.2. Zebrafish Model
3.3.3. Human Induced Pluripotent Stem Cell (hiPSC) Model
3.3.4. HL-1 Model
3.3.5. Computational Models
3.4. Relationship Between PITX2 and AF Therapy
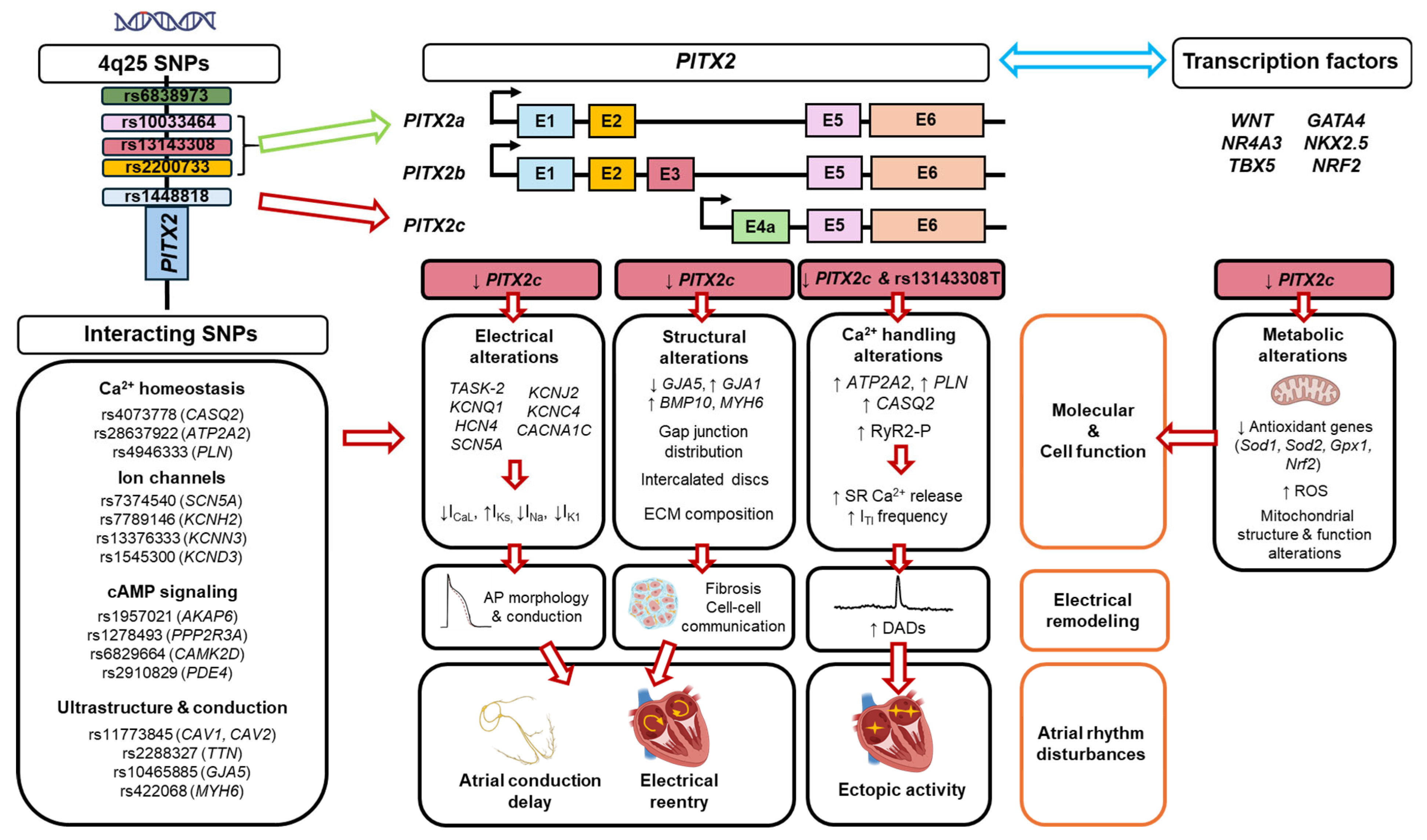
4. Cross-Talk Between PITX2 Signaling, Risk SNPs and Other Transcription Factors
4.1. PITX2 and 4q25
4.2. PITX2 and Other Transcription Factors
4.3. Pitx2 and Other SNPs
5. Future Directions and Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| APD | Action potential duration |
| GWAS | Genome Wide Association Studies |
| SR | Sarcoplasmic reticulum |
| RyR2 | Ryanodine receptor 2 |
| ICaL | L-type calcium current |
| SNPs | Single-Nucleotide Polymorphisms |
| IKs | Slow rectifier potassium current |
| ITI | Inward transient current |
References
- Freedman, B.; Hindricks, G.; Banerjee, A.; Baranchuk, A.; Ching, C.K.; Du, X.; Fitzsimons, D.; Healey, J.S.; Ikeda, T.; Lobban, T.C.A.; et al. World Heart Federation Roadmap on Atrial Fibrillation—A 2020 Update. Glob. Heart 2021, 16, 41. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of Atrial Fibrillation on the Risk of Death. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef]
- Wolf, P.A.; Abbott, R.D.; Kannel, W.B. Atrial Fibrillation as an Independent Risk Factor for Stroke: The Framingham Study. Stroke 1991, 22, 983–988. [Google Scholar] [CrossRef]
- Morseth, B.; Geelhoed, B.; Linneberg, A.; Johansson, L.; Kuulasmaa, K.; Salomaa, V.; Iacoviello, L.; Costanzo, S.; Söderberg, S.; Niiranen, T.J.; et al. Age-Specific Atrial Fibrillation Incidence, Attributable Risk Factors and Risk of Stroke and Mortality: Results from the MORGAM Consortium. Open Heart 2021, 8, e001624. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.A.; Giulianini, F.; Geelhoed, B.; Lunetta, K.L.; Misialek, J.R.; Niemeijer, M.N.; Rienstra, M.; Rose, L.M.; Smith, A.V.; Arking, D.E.; et al. Genetic Obesity and the Risk of Atrial Fibrillation: Causal Estimates from Mendelian Randomization. Circulation 2017, 135, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Mahamat-Saleh, Y.; Kobeissi, E.; Feng, T.; Heath, A.K.; Janszky, I. Blood Pressure, Hypertension and the Risk of Atrial Fibrillation: A Systematic Review and Meta-Analysis of Cohort Studies. Eur. J. Epidemiol. 2023, 38, 145–178. [Google Scholar] [CrossRef] [PubMed]
- Christophersen, I.E.; Ravn, L.S.; Budtz-Joergensen, E.; Skytthe, A.; Haunsoe, S.; Svendsen, J.H.; Christensen, K. Familial Aggregation of Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2009, 2, 378–383. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Arnar, D.O.; Helgadottir, A.; Gretarsdottir, S.; Holm, H.; Sigurdsson, A.; Jonasdottir, A.; Baker, A.; Thorleifsson, G.; Kristjansson, K.; et al. Variants Conferring Risk of Atrial Fibrillation on Chromosome 4q25. Nature 2007, 448, 353–357. [Google Scholar] [CrossRef]
- Ellinor, P.T.; Lunetta, K.L.; Albert, C.M.; Glazer, N.L.; Ritchie, M.D.; Smith, A.V.; Arking, D.E.; Müller-Nurasyid, M.; Krijthe, B.P.; Lubitz, S.A.; et al. Meta-Analysis Identifies Six New Susceptibility Loci for Atrial Fibrillation. Nat. Genet. 2012, 44, 670–675. [Google Scholar] [CrossRef]
- Lubitz, S.A.; Sinner, M.F.; Lunetta, K.L.; Makino, S.; Pfeufer, A.; Rahman, R.; Veltman, C.E.; Barnard, J.; Bis, J.C.; Danik, S.P.; et al. Independent Susceptibility Markers for Atrial Fibrillation on Chromosome 4q25. Circulation 2010, 122, 976–984. [Google Scholar] [CrossRef]
- Tessari, A.; Pietrobon, M.; Notte, A.; Cifelli, G.; Gage, P.J.; Schneider, M.D.; Lembo, G.; Campione, M. Myocardial Pitx2 Differentially Regulates the Left Atrial Identity and Ventricular Asymmetric Remodeling Programs. Circ. Res. 2008, 102, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, W.; Lu, M.-F.; Brown, N.A.; Martin, J.F. Regulation of Left-Right Asymmetry by Thresholds of Pitx2c Activity. Development 2001, 128, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Campione, M.; Steinbeisser, H.; Schweickert, A.; Deissler, K.; van Bebber, F.; Lowe, L.A.; Nowotschin, S.; Viebahn, C.; Haffter, P.; Kuehn, M.R.; et al. The Homeobox Gene Pitx2 Mediator of Asymmetric Left-Right Signaling in Vertebrate Heart and Gut Looping. Development 1999, 126, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Tarifa, C.; Serra, S.A.; Herraiz-Martínez, A.; Lozano-Velasco, E.; Benítez, R.; Aranega, A.; Franco, D.; Hove-Madsen, L. Pitx2c Deficiency Confers Cellular Electrophysiological Hallmarks of Atrial Fibrillation to Isolated Atrial Myocytes. Biomed. Pharmacother. 2023, 162, 114577. [Google Scholar] [CrossRef]
- Lozano-Velasco, E.; Hernández-Torres, F.; Daimi, H.; Serra, S.A.; Herraiz, A.; Hove-Madsen, L.; Aránega, A.; Franco, D. Pitx2 Impairs Calcium Handling in a Dose-Dependent Manner by Modulating Wnt Signalling. Cardiovasc. Res. 2016, 109, 55–66. [Google Scholar] [CrossRef]
- Chinchilla, A.; Daimi, H.; Lozano-Velasco, E.; Dominguez, J.N.; Caballero, R.; Delpo, E.; Tamargo, J.; Cinca, J.; Hove, L.M.; Aranega, A.E.; et al. PITX2 Insufficiency Leads to Atrial Electrical and Structural Remodeling Linked to Arrhythmogenesis. Circ. Cardiovasc. Genet. 2011, 4, 269–279. [Google Scholar] [CrossRef]
- Kirchhof, P.; Kahr, P.C.; Kaese, S.; Piccini, I.; Vokshi, I.; Scheld, H.H.; Rotering, H.; Fortmueller, L.; Laakmann, S.; Verheule, S.; et al. PITX2c Is Expressed in the Adult Left Atrium, and Reducing Pitx2c Expression Promotes Atrial Fibrillation Inducibility and Complex Changes in Gene Expression. Circ. Cardiovasc. Genet. 2011, 4, 123–133. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, M.; Li, L.; Bai, Y.; Zhou, Y.; Moon, A.M.; Kaminski, H.J.; Martin, J.F. Pitx2, an Atrial Fibrillation Predisposition Gene, Directly Regulates Ion Transport and Intercalated Disc Genes. Circ. Cardiovasc. Genet. 2014, 7, 23–32. [Google Scholar] [CrossRef]
- Syeda, F.; Holmes, A.P.; Yu, T.Y.; Tull, S.; Kuhlmann, S.M.; Pavlovic, D.; Betney, D.; Riley, G.; Kucera, J.P.; Jousset, F.; et al. PITX2 Modulates Atrial Membrane Potential and the Antiarrhythmic Effects of Sodium-Channel Blockers. J. Am. Coll. Cardiol. 2016, 68, 1881–1894. [Google Scholar] [CrossRef]
- Kaab, S.; Darbar, D.; van Noord, C.; Dupuis, J.; Pfeufer, A.; Newton-Cheh, C.; Schnabel, R.; Makino, S.; Sinner, M.F.; Kannankeril, P.J.; et al. Large Scale Replication and Meta-Analysis of Variants on Chromosome 4q25 Associated with Atrial Fibrillation. Eur. Heart J. 2008, 30, 813–819. [Google Scholar] [CrossRef]
- Christophersen, I.E.; Rienstra, M.; Roselli, C.; Yin, X.; Geelhoed, B.; Barnard, J.; Lin, H.; Arking, D.E.; Smith, A.V.; Albert, C.M.; et al. Large-Scale Analyses of Common and Rare Variants Identify 12 New Loci Associated with Atrial Fibrillation. Nat. Genet. 2017, 49, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, F.; Knight, A.C.; Zhao, J.; Xiao, J. Common Variants for Atrial Fibrillation: Results from Genome-Wide Association Studies. Hum. Genet. 2012, 131, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Roselli, C.; Chaffin, M.D.; Weng, L.C.; Aeschbacher, S.; Ahlberg, G.; Albert, C.M.; Almgren, P.; Alonso, A.; Anderson, C.D.; Aragam, K.G.; et al. Multi-Ethnic Genome-Wide Association Study for Atrial Fibrillation. Nat. Genet. 2018, 50, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Roselli, C.; Surakka, I.; Olesen, M.S.; Sveinbjornsson, G.; Marston, N.A.; Choi, S.H.; Holm, H.; Chaffin, M.; Gudbjartsson, D.; Hill, M.C.; et al. Meta-Analysis of Genome-Wide Associations and Polygenic Risk Prediction for Atrial Fibrillation in More than 180,000 Cases. Nat. Genet. 2025, 57, 539–547. [Google Scholar] [CrossRef]
- Gore-Panter, S.R.; Hsu, J.; Hanna, P.; Gillinov, A.M.; Pettersson, G.; Newton, D.W.; Moravec, C.S.; Van Wagoner, D.R.; Chung, M.K.; Barnard, J.; et al. Atrial Fibrillation Associated Chromosome 4q25 Variants Are Not Associated with PITX2c Expression in Human Adult Left Atrial Appendages. PLoS ONE 2014, 9, e86245. [Google Scholar] [CrossRef]
- Martin, R.I.R.; Babaei, M.S.; Choy, M.K.; Owens, W.A.; Chico, T.J.A.; Keenan, D.; Yonan, N.; Koref, M.S.; Keavney, B.D. Genetic Variants Associated with Risk of Atrial Fibrillation Regulate Expression of PITX2, CAV1, MYOZ1, C9orf3 and FANCC. J. Mol. Cell. Cardiol. 2015, 85, 207–214. [Google Scholar] [CrossRef]
- Reyat, J.S.; Chua, W.; Cardoso, V.R.; Witten, A.; Kastner, P.M.; Kabir, S.N.; Sinner, M.F.; Wesselink, R.; Holmes, A.P.; Pavlovic, D.; et al. Reduced Left Atrial Cardiomyocyte PITX2 and Elevated Circulating BMP10 Predict Atrial Fibrillation after Ablation. JCI Insight 2020, 5, e139179. [Google Scholar] [CrossRef]
- Aguirre, L.A.; Alonso, M.E.; Badía-Careaga, C.; Rollán, I.; Arias, C.; Fernández-Miñán, A.; López-Jiménez, E.; Aránega, A.; Gómez-Skarmeta, J.L.; Franco, D.; et al. Long-Range Regulatory Interactions at the 4q25 Atrial Fibrillation Risk Locus Involve PITX2c and ENPEP. BMC Biol. 2015, 13, 26. [Google Scholar] [CrossRef]
- Ye, J.; Tucker, N.R.; Weng, L.C.; Clauss, S.; Lubitz, S.A.; Ellinor, P.T. A Functional Variant Associated with Atrial Fibrillation Regulates PITX2c Expression through TFAP2a. Am. J. Hum. Genet. 2016, 99, 1281–1291. [Google Scholar] [CrossRef]
- Wang, J.; Klysik, E.; Sood, S.; Johnson, R.L.; Wehrens, X.H.T.; Martin, J.F. Pitx2 Prevents Susceptibility to Atrial Arrhythmias by Inhibiting Left-Sided Pacemaker Specification. Proc. Natl. Acad. Sci. USA 2010, 107, 9753–9758. [Google Scholar] [CrossRef]
- Herraiz-Martínez, A.; Llach, A.; Tarifa, C.; Gandía, J.; Jiménez-Sabado, V.; Lozano-Velasco, E.; Serra, S.A.; Vallmitjana, A.; Vázquez Ruiz De Castroviejo, E.; Benítez, R.; et al. The 4q25 Variant Rs13143308T Links Risk of Atrial Fibrillation to Defective Calcium Homoeostasis. Cardiovasc. Res. 2019, 115, 578–589. [Google Scholar] [CrossRef]
- Husser, D.; Adams, V.; Piorkowski, C.; Hindricks, G.; Bollmann, A. Chromosome 4q25 Variants and Atrial Fibrillation Recurrence After Catheter Ablation. J. Am. Coll. Cardiol. 2010, 55, 747–753. [Google Scholar] [CrossRef]
- Mints, Y.; Yarmohammadi, H.; Khurram, I.M.; Hoyt, H.; Hansford, R.; Zimmerman, S.L.; Steinberg, S.J.; Judge, D.P.; Tomaselli, G.F.; Calkins, H.; et al. Association of Common Variations on Chromosome 4q25 and Left Atrial Volume in Patients with Atrial Fibrillation. Clin. Med. Insights Cardiol. 2015, 9, CMC.S21712. [Google Scholar] [CrossRef]
- Kolek, M.J.; Parvez, B.; Muhammad, R.; Shoemaker, M.B.; Blair, M.A.; Stubblefield, T.; Kucera, G.A.; Denny, J.C.; Roden, D.M.; Darbar, D. A Common Variant on Chromosome 4q25 Is Associated with Prolonged PR Interval in Subjects with and Without Atrial Fibrillation. Am. J. Cardiol. 2014, 113, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, M.B.; Bollmann, A.; Lubitz, S.A.; Ueberham, L.; Saini, H.; Montgomery, J.; Edwards, T.; Yoneda, Z.; Sinner, M.F.; Arya, A.; et al. Common Genetic Variants and Response to Atrial Fibrillation Ablation. Circ. Arrhythmia Electrophysiol. 2015, 8, 296–302. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, W.; Yu, Y.; Hu, J.; Hong, K. Variant Rs2200733 and Rs10033464 on Chromosome 4q25 Are Associated with Increased Risk of Atrial Fibrillation after Catheter Ablation: Evidence from a Meta-Analysis. Cardiol. J. 2018, 25, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yang, Y.; Zhang, R.; Zhang, S.; Dong, Y.; Yin, X.; Chang, D.; Yang, Z.; Wang, K.; Gao, L.; et al. Polymorphism Rs2200733 at Chromosome 4q25 Is Associated with Atrial Fibrillation Recurrence after Radiofrequency Catheter Ablation in the Chinese Han Population. Am. J. Transl. Res. 2016, 8, 688–697. [Google Scholar]
- Zhao, L.; Zhang, G.; Wen, Z.; Huang, C.; Wu, H.; Xu, J.; Qi, B.; Wang, Z.; Shi, Y.; Liu, S. Common Variants Predict Recurrence after Nonfamilial Atrial Fibrillation Ablation in Chinese Han Population. Int. J. Cardiol. 2017, 227, 360–366. [Google Scholar] [CrossRef]
- Rattanawong, P. A Chromosome 4q25 Variant Is Associated with Atrial Fibrillation Recurrence After Catheter Ablation: A Systematic Review and Meta-Analysis. J. Atr. Fibrillation 2018, 10, 1666. [Google Scholar] [CrossRef]
- Miyazaki, S.; Ebana, Y.; Liu, L.; Nakamura, H.; Hachiya, H.; Taniguchi, H.; Takagi, T.; Kajiyama, T.; Watanabe, T.; Igarashi, M.; et al. Chromosome 4q25 Variants and Recurrence after Second-Generation Cryoballoon Ablation in Patients with Paroxysmal Atrial Fibrillation. Int. J. Cardiol. 2017, 244, 151–157. [Google Scholar] [CrossRef]
- Parvez, B.; Shoemaker, M.B.; Muhammad, R.; Richardson, R.; Jiang, L.; Blair, M.A.; Roden, D.M.; Darbar, D. Common Genetic Polymorphism at 4q25 Locus Predicts Atrial Fibrillation Recurrence after Successful Cardioversion. Heart Rhythm 2013, 10, 849–855. [Google Scholar] [CrossRef]
- Ulus, T. Genetic Polymorphism on Chromosome 4q25 (Rs17570669) May Predict Recurrence After Successful Electrical Cardioversion in Patients with Persistent Atrial Fibrillation. Turk Kardiyol. Dern. Ars.-Arch. Turk. Soc. Cardiol. 2023, 51, 550–556. [Google Scholar] [CrossRef]
- Weng, L.-C.; Hall, A.W.; Choi, S.H.; Jurgens, S.J.; Haessler, J.; Bihlmeyer, N.A.; Grarup, N.; Lin, H.; Teumer, A.; Li-Gao, R.; et al. Genetic Determinants of Electrocardiographic P-Wave Duration and Relation to Atrial Fibrillation. Circ. Genom. Precis. Med. 2020, 13, 387–395. [Google Scholar] [CrossRef]
- Parvez, B.; Vanglio, J.; Rowan, S.; Muhammad, R.; Kucera, G.; Stubblefield, T.; Carter, S.; Roden, D.; Darbar, D. Symptomatic Response to Antiarrhythmic Drug Therapy Is Modulated by a Common Single Nucleotide Polymorphism in Atrial Fibrillation. J. Am. Coll. Cardiol. 2012, 60, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, M.; Matamoros, M.; Barana, A.; Amorós, I.; Gómez, R.; Núñez, M.; Sacristán, S.; Pinto, Á.; Fernández-Avilés, F.; Tamargo, J.; et al. Pitx2c Increases in Atrial Myocytes from Chronic Atrial Fibrillation Patients Enhancing IKs and Decreasing ICa,L. Cardiovasc. Res. 2016, 109, 431–441. [Google Scholar] [CrossRef]
- Herraiz-Martínez, A.; Tarifa, C.; Lozano-Velasco, E.; Jiménez-Sábado, V.; Casabella, S.; Hernández-Torres, F.; Daimi, H.; Vázquez Ruiz de Castroviejo, E.; Delpón, E.; Caballero, R.; et al. Novel PITX2 Homeodomain-Contained Mutations from ATRIAL Fibrillation Patients Deteriorate Calcium Homeostasis. Hearts 2021, 2, 251–269. [Google Scholar] [CrossRef]
- Zhou, Y.-M.; Zheng, P.-X.; Yang, Y.-Q.; Ge, Z.-M.; Kang, W.-Q. A Novel PITX2c Loss-of-Function Mutation Underlies Lone Atrial Fibrillation. Int. J. Mol. Med. 2013, 32, 827–834. [Google Scholar] [CrossRef]
- Qiu, X.-B.; Xu, Y.-J.; Li, R.-G.; Xu, L.; Liu, X.; Fang, W.-Y.; Yang, Y.-Q.; Qu, X.-K. PITX2C Loss-of-Function Mutations Responsible for Idiopathic Atrial Fibrillation. Clinics 2014, 69, 15–22. [Google Scholar] [CrossRef]
- Yang, Y.-Q.; Xu, Y.-J.; Li, R.-G.; Qu, X.-K.; Fang, W.-Y.; Liu, X. Prevalence and Spectrum of PITX2c Mutations Associated with Familial Atrial Fibrillation. Int. J. Cardiol. 2013, 168, 2873–2876. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, D.-F.; Sun, Y.-M.; Yang, Y.-Q. A Novel PITX2c Loss-of-Function Mutation Associated with Familial Atrial Fibrillation. Eur. J. Med. Genet. 2014, 57, 25–31. [Google Scholar] [CrossRef]
- Mechakra, A.; Footz, T.; Walter, M.; Aránega, A.; Hernández-Torres, F.; Morel, E.; Millat, G.; Yang, Y.-Q.; Chahine, M.; Chevalier, P.; et al. A Novel PITX2c Gain-of-Function Mutation, p.Met207Val, in Patients with Familial Atrial Fibrillation. Am. J. Cardiol. 2019, 123, 787–793. [Google Scholar] [CrossRef]
- Mora, C.; Serzanti, M.; Giacomelli, A.; Beltramone, S.; Marchina, E.; Bertini, V.; Piovani, G.; Refsgaard, L.; Olesen, M.S.; Cortellini, V.; et al. Generation of Induced Pluripotent Stem Cells (IPSC) from an Atrial Fibrillation Patient Carrying a PITX2 p.M200V Mutation. Stem Cell Res. 2017, 24, 8–11. [Google Scholar] [CrossRef]
- Tao, G.; Kahr, P.C.; Morikawa, Y.; Zhang, M.; Rahmani, M.; Heallen, T.R.; Li, L.; Sun, Z.; Olson, E.N.; Amendt, B.A.; et al. Pitx2 Promotes Heart Repair by Activating the Antioxidant Response after Cardiac Injury. Nature 2016, 534, 119–123. [Google Scholar] [CrossRef]
- Benzoni, P.; Da Dalt, L.; Elia, N.; Popolizio, V.; Cospito, A.; Giannetti, F.; Dell’Era, P.; Olesen, M.S.; Bucchi, A.; Baruscotti, M.; et al. PITX2 Gain-of-Function Mutation Associated with Atrial Fibrillation Alters Mitochondrial Activity in Human IPSC Atrial-like Cardiomyocytes. Front. Physiol. 2023, 14, 1250951. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Duron, H.E.; Chaudhuri, D. Beyond the TCA Cycle: New Insights into Mitochondrial Calcium Regulation of Oxidative Phosphorylation. Biochem. Soc. Trans. 2023, 51, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Mason, F.E.; Pronto, J.R.D.; Alhussini, K.; Maack, C.; Voigt, N. Cellular and Mitochondrial Mechanisms of Atrial Fibrillation. Basic Res. Cardiol. 2020, 115, 72. [Google Scholar] [CrossRef] [PubMed]
- Kohlhaas, M.; Nickel, A.G.; Maack, C. Mitochondrial Energetics and Calcium Coupling in the Heart. J. Physiol. 2017, 595, 3753–3763. [Google Scholar] [CrossRef]
- Cooley, N.; Cowley, M.J.; Lin, R.C.Y.; Marasco, S.; Wong, C.; Kaye, D.M.; Dart, A.M.; Woodcock, E.A. Influence of Atrial Fibrillation on MicroRNA Expression Profiles in Left and Right Atria from Patients with Valvular Heart Disease. Physiol. Genom. 2012, 44, 211–219. [Google Scholar] [CrossRef]
- García-Padilla, C.; Aránega, A.; Franco, D. The Role of Long Non-Coding RNAs in Cardiac Development and Disease. AIMS Genet. 2018, 5, 124–140. [Google Scholar] [CrossRef]
- Wang, J.; Bai, Y.; Li, N.; Ye, W.; Zhang, M.; Greene, S.B.; Tao, Y.; Chen, Y.; Wehrens, X.H.T.; Martin, J.F. Pitx2 -MicroRNA Pathway That Delimits Sinoatrial Node Development and Inhibits Predisposition to Atrial Fibrillation. Proc. Natl. Acad. Sci. USA 2014, 111, 9181–9186. [Google Scholar] [CrossRef]
- Torrado, M.; Franco, D.; Lozano-Velasco, E.; Hernández-Torres, F.; Calviño, R.; Aldama, G.; Centeno, A.; Castro-Beiras, A.; Mikhailov, A. A MicroRNA-Transcription Factor Blueprint for Early Atrial Arrhythmogenic Remodeling. Biomed Res. Int. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Gore-Panter, S.R.; Hsu, J.; Barnard, J.; Moravec, C.S.; Van Wagoner, D.R.; Chung, M.K.; Smith, J.D. PANCR, the PITX2 Adjacent Noncoding RNA, Is Expressed in Human Left Atria and Regulates PITX2c Expression. Circ. Arrhythmia Electrophysiol. 2016, 9, e003197. [Google Scholar] [CrossRef]
- Grote, P.; Wittler, L.; Hendrix, D.; Koch, F.; Währisch, S.; Beisaw, A.; Macura, K.; Bläss, G.; Kellis, M.; Werber, M.; et al. The Tissue-Specific LncRNA Fendrr Is an Essential Regulator of Heart and Body Wall Development in the Mouse. Dev. Cell 2013, 24, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Grote, P.; Herrmann, B.G. The Long Non-Coding RNA Fendrr Links Epigenetic Control Mechanisms to Gene Regulatory Networks in Mammalian Embryogenesis. RNA Biol. 2013, 10, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Welsh, I.C.; Kwak, H.; Chen, F.L.; Werner, M.; Shopland, L.S.; Danko, C.G.; Lis, J.T.; Zhang, M.; Martin, J.F.; Kurpios, N.A. Chromatin Architecture of the Pitx2 Locus Requires CTCF- and Pitx2-Dependent Asymmetry That Mirrors Embryonic Gut Laterality. Cell Rep. 2015, 13, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Subati, T.; Kim, K.; Yang, Z.; Murphy, M.B.; Van Amburg, J.C.; Christopher, I.L.; Dougherty, O.P.; Woodall, K.K.; Smart, C.D.; Johnson, J.E.; et al. Oxidative Stress Causes Mitochondrial and Electrophysiologic Dysfunction to Promote Atrial Fibrillation in Pitx2 +/− Mice. Circ. Arrhythmia Electrophysiol. 2025, 18, e013199. [Google Scholar] [CrossRef]
- Li, L.; Tao, G.; Hill, M.C.; Zhang, M.; Morikawa, Y.; Martin, J.F. Pitx2 Maintains Mitochondrial Function during Regeneration to Prevent Myocardial Fat Deposition. Development 2018, 145, dev168609. [Google Scholar] [CrossRef]
- Steimle, J.D.; Grisanti Canozo, F.J.; Park, M.; Kadow, Z.A.; Samee, A.H.; Martin, J.F. Decoding the PITX2-Controlled Genetic Network in Atrial Fibrillation. JCI Insight 2022, 7, e158895. [Google Scholar] [CrossRef]
- Llach, A.; Molina, C.E.; Fernandes, J.; Padró, J.; Cinca, J.; Hove-Madsen, L. Sarcoplasmic Reticulum and L-type Ca2+ Channel Activity Regulate the Beat-to-beat Stability of Calcium Handling in Human Atrial Myocytes. J. Physiol. 2011, 589, 3247–3262. [Google Scholar] [CrossRef]
- Hove-Madsen, L.; Llach, A.; Bayes-Genís, A.; Roura, S.; Font, E.R.; Arís, A.; Cinca, J. Atrial Fibrillation Is Associated with Increased Spontaneous Calcium Release From the Sarcoplasmic Reticulum in Human Atrial Myocytes. Circulation 2004, 110, 1358–1363. [Google Scholar] [CrossRef]
- Llach, A.; Molina, C.E.; Prat-Vidal, C.; Fernandes, J.; Casado, V.; Ciruela, F.; Lluis, C.; Franco, R.; Cinca, J.; Hove-Madsen, L. Abnormal Calcium Handling in Atrial Fibrillation Is Linked to Up-Regulation of Adenosine A2A Receptors. Eur. Heart J. 2011, 32, 721–729. [Google Scholar] [CrossRef]
- Vest, J.A.; Wehrens, X.H.T.; Reiken, S.R.; Lehnart, S.E.; Dobrev, D.; Chandra, P.; Danilo, P.; Ravens, U.; Rosen, M.R.; Marks, A.R. Defective Cardiac Ryanodine Receptor Regulation during Atrial Fibrillation. Circulation 2005, 111, 2025–2032. [Google Scholar] [CrossRef]
- Neef, S.; Dybkova, N.; Sossalla, S.; Ort, K.R.; Fluschnik, N.; Neumann, K.; Seipelt, R.; Schöndube, F.A.; Hasenfuss, G.; Maier, L.S. CaMKII-Dependent Diastolic SR Ca2+ Leak and Elevated Diastolic Ca2+ Levels in Right Atrial Myocardium of Patients with Atrial Fibrillation. Circ. Res. 2010, 106, 1134–1144. [Google Scholar] [CrossRef]
- Van Wagoner, D.R.; Pond, A.L.; Lamorgese, M.; Rossie, S.S.; McCarthy, P.M.; Nerbonne, J.M. Atrial L-Type Ca2+ Currents and Human Atrial Fibrillation. Circ. Res. 1999, 85, 428–436. [Google Scholar] [CrossRef]
- Greiser, M.; Schotten, U. Dynamic Remodeling of Intracellular Ca2+ Signaling during Atrial Fibrillation. J. Mol. Cell. Cardiol. 2013, 58, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Voigt, N.; Heijman, J.; Wang, Q.; Chiang, D.Y.; Li, N.; Karck, M.; Wehrens, X.H.T.; Nattel, S.; Dobrev, D. Cellular and Molecular Mechanisms of Atrial Arrhythmogenesis in Patients with Paroxysmal Atrial Fibrillation. Circulation 2014, 129, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Kaese, S.; Verheule, S. Cardiac Electrophysiology in Mice: A Matter of Size. Front. Physiol. 2012, 3, 345. [Google Scholar] [CrossRef] [PubMed]
- Kantharia, B.K.; Zhao, S.; Linz, D.; Heijman, J.; Wehrens, X.H.T. Hypertension and Atrial Fibrillation: Insight from Basic to Translational Science Into the Mechanisms and Management. J. Cardiovasc. Electrophysiol. 2025. [Google Scholar] [CrossRef]
- Balan, A.I.; Halațiu, V.B.; Scridon, A. Oxidative Stress, Inflammation, and Mitochondrial Dysfunction: A Link between Obesity and Atrial Fibrillation. Antioxidants 2024, 13, 117. [Google Scholar] [CrossRef]
- Fein, A.S.; Shvilkin, A.; Shah, D.; Haffajee, C.I.; Das, S.; Kumar, K.; Kramer, D.B.; Zimetbaum, P.J.; Buxton, A.E.; Josephson, M.E.; et al. Treatment of Obstructive Sleep Apnea Reduces the Risk of Atrial Fibrillation Recurrence After Catheter Ablation. J. Am. Coll. Cardiol. 2013, 62, 300–305. [Google Scholar] [CrossRef]
- Linz, D.; Nattel, S.; Kalman, J.M.; Sanders, P. Sleep Apnea and Atrial Fibrillation. Card. Electrophysiol. Clin. 2021, 13, 87–94. [Google Scholar] [CrossRef]
- Lebek, S.; Pichler, K.; Reuthner, K.; Trum, M.; Tafelmeier, M.; Mustroph, J.; Camboni, D.; Rupprecht, L.; Schmid, C.; Maier, L.S.; et al. Enhanced CaMKII-Dependent Late INa Induces Atrial Proarrhythmic Activity in Patients with Sleep-Disordered Breathing. Circ. Res. 2020, 126, 603–615, Correction in Circ. Res. 2020, 126, e60. [Google Scholar] [CrossRef]
- Vicente, M.; Cevallos-Salvador, M.P.; Martinez-Sielva, A.; Collins, M.M.; Salgado-Almario, J.; Domingo, B.; Llopis, J. Loss of Pitx2c Causes Early Alterations in Atrial Calcium Handling in Zebrafish. Cardiovasc. Res. 2024, 120, cvae088.105. [Google Scholar] [CrossRef]
- Collins, M.M.; Ahlberg, G.; Hansen, C.V.; Guenther, S.; Marín-Juez, R.; Sokol, A.M.; El-Sammak, H.; Piesker, J.; Hellsten, Y.; Olesen, M.S.; et al. Early Sarcomere and Metabolic Defects in a Zebrafish Pitx2c Cardiac Arrhythmia Model. Proc. Natl. Acad. Sci. USA 2019, 116, 24115–24121. [Google Scholar] [CrossRef] [PubMed]
- González-Rosa, J.M. Zebrafish Models of Cardiac Disease: From Fortuitous Mutants to Precision Medicine. Circ. Res. 2022, 130, 1803–1826. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Lemoine, M.D.; Mearini, G.; Koivumäki, J.; Sani, J.; Schwedhelm, E.; Kirchhof, P.; Ghalawinji, A.; Stoll, M.; Hansen, A.; et al. PITX2 Knockout Induces Key Findings of Electrical Remodeling as Seen in Persistent Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2023, 16, e011602. [Google Scholar] [CrossRef]
- Reyat, J.S.; Sommerfeld, L.C.; O’Reilly, M.; Roth Cardoso, V.; Thiemann, E.; Khan, A.O.; O’Shea, C.; Harder, S.; Müller, C.; Barlow, J.; et al. PITX2 Deficiency Leads to Atrial Mitochondrial Dysfunction. Cardiovasc. Res. 2024, 120, 1907–1923. [Google Scholar] [CrossRef]
- Babini, H.; Jiménez-Sábado, V.; Stogova, E.; Arslanova, A.; Butt, M.; Dababneh, S.; Asghari, P.; Moore, E.D.W.; Claydon, T.W.; Chiamvimonvat, N.; et al. HiPSC-Derived Cardiomyocytes as a Model to Study the Role of Small-Conductance Ca2+-Activated K+ (SK) Ion Channel Variants Associated with Atrial Fibrillation. Front. Cell Dev. Biol. 2024, 12, 1298007. [Google Scholar] [CrossRef]
- Bai, J.; Lu, Y.; Lo, A.; Zhao, J.; Zhang, H. PITX2 Upregulation Increases the Risk of Chronic Atrial Fibrillation in a Dose-Dependent Manner by Modulating IKs and ICaL—Insights from Human Atrial Modelling. Ann. Transl. Med. 2020, 8, 191. [Google Scholar] [CrossRef]
- Bai, J.; Lo, A.; Gladding, P.A.; Stiles, M.K.; Fedorov, V.V.; Zhao, J. In Silico Investigation of the Mechanisms Underlying Atrial Fibrillation Due to Impaired Pitx2. PLoS Comput. Biol. 2020, 16, e1007678. [Google Scholar] [CrossRef]
- Jin, Z.; Hwang, I.; Lim, B.; Kwon, O.-S.; Park, J.-W.; Yu, H.-T.; Kim, T.-H.; Joung, B.; Lee, M.-H.; Pak, H.-N. Ablation and Antiarrhythmic Drug Effects on PITX2+/− Deficient Atrial Fibrillation: A Computational Modeling Study. Front. Cardiovasc. Med. 2022, 9, 942998. [Google Scholar] [CrossRef]
- Bai, J.; Zhu, Y.; Lo, A.; Gao, M.; Lu, Y.; Zhao, J.; Zhang, H. In Silico Assessment of Class I Antiarrhythmic Drug Effects on Pitx2-Induced Atrial Fibrillation: Insights from Populations of Electrophysiological Models of Human Atrial Cells and Tissues. Int. J. Mol. Sci. 2021, 22, 1265. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Jin, Z.; Park, J.-W.; Kwon, O.-S.; Lim, B.; Hong, M.; Kim, M.; Yu, H.-T.; Kim, T.-H.; Uhm, J.-S.; et al. Computational Modeling for Antiarrhythmic Drugs for Atrial Fibrillation According to Genotype. Front. Physiol. 2022, 12, 650449, Correction in Front. Physiol. 2022, 13, 9919917. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.P.; Saxena, P.; Kabir, S.N.; O’Shea, C.; Kuhlmann, S.M.; Gupta, S.; Fobian, D.; Apicella, C.; O’Reilly, M.; Syeda, F.; et al. Atrial Resting Membrane Potential Confers Sodium Current Sensitivity to Propafenone, Flecainide and Dronedarone. Heart Rhythm 2021, 18, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Sutanto, H.; Crijns, H.J.G.M.; Nattel, S.; Trayanova, N.A. Computational Models of Atrial Fibrillation: Achievements, Challenges, and Perspectives for Improving Clinical Care. Cardiovasc. Res. 2021, 117, 1682–1699. [Google Scholar] [CrossRef]
- Tarifa, C.; Vallmitjana, A.; Jiménez-Sábado, V.; Marchena, M.; Llach, A.; Herraiz-Martínez, A.; Godoy-Marín, H.; Nolla-Colomer, C.; Ginel, A.; Viñolas, X.; et al. Spatial Distribution of Calcium Sparks Determines Their Ability to Induce Afterdepolarizations in Human Atrial Myocytes. JACC Basic Transl. Sci. 2023, 8, 1–15. [Google Scholar] [CrossRef]
- Herraiz-Martínez, A.; Tarifa, C.; Jiménez-Sábado, V.; Llach, A.; Godoy-Marín, H.; Colino, H.; Nolla-Colomer, C.; Casabella, S.; Izquierdo-Castro, P.; Benítez, I.; et al. Influence of Sex on Intracellular Calcium Homeostasis in Patients with Atrial Fibrillation. Cardiovasc. Res. 2021, 118, 1033–1045. [Google Scholar] [CrossRef]
- Zhang, M.; Hill, M.C.; Kadow, Z.A.; Suh, J.H.; Tucker, N.R.; Hall, A.W.; Tran, T.T.; Swinton, P.S.; Leach, J.P.; Margulies, K.B.; et al. Long-Range Pitx2c Enhancer–Promoter Interactions Prevent Predisposition to Atrial Fibrillation. Proc. Natl. Acad. Sci. USA 2019, 116, 22692–22698. [Google Scholar] [CrossRef]
- Berry, F.B.; Lines, M.A.; Oas, J.M.; Footz, T.; Underhill, D.A.; Gage, P.J.; Walter, M.A. Functional Interactions between FOXC1 and PITX2 Underlie the Sensitivity to FOXC1 Gene Dose in Axenfeld–Rieger Syndrome and Anterior Segment Dysgenesis. Hum. Mol. Genet. 2006, 15, 905–919. [Google Scholar] [CrossRef]
- Suszko, M.I.; Antenos, M.; Balkin, D.M.; Woodruff, T.K. Smad3 and Pitx2 Cooperate in Stimulation of FSHβ Gene Transcription. Mol. Cell. Endocrinol. 2008, 281, 27–36. [Google Scholar] [CrossRef]
- Nadadur, R.D.; Broman, M.T.; Boukens, B.; Mazurek, S.R.; Yang, X.; van den Boogaard, M.; Bekeny, J.; Gadek, M.; Ward, T.; Zhang, M.; et al. Pitx2 Modulates a Tbx5-Dependent Gene Regulatory Network to Maintain Atrial Rhythm. Sci. Transl. Med. 2016, 8, 354ra115. [Google Scholar] [CrossRef]
- Kioussi, C.; Briata, P.; Baek, S.H.; Rose, D.W.; Hamblet, N.S.; Herman, T.; Ohgi, K.A.; Lin, C.; Gleiberman, A.; Wang, J.; et al. Identification of a Wnt/Dvl/β-Catenin → Pitx2 Pathway Mediating Cell-Type-Specific Proliferation during Development. Cell 2002, 111, 673–685. [Google Scholar] [CrossRef]
- Mommersteeg, M.T.M.; Brown, N.A.; Prall, O.W.J.; de Gier-de Vries, C.; Harvey, R.P.; Moorman, A.F.M.; Christoffels, V.M. Pitx2c and Nkx2-5 Are Required for the Formation and Identity of the Pulmonary Myocardium. Circ. Res. 2007, 101, 902–909. [Google Scholar] [CrossRef]
- Zhao, C.-M.; Peng, L.-Y.; Li, L.; Liu, X.-Y.; Wang, J.; Zhang, X.-L.; Yuan, F.; Li, R.-G.; Qiu, X.-B.; Yang, Y.-Q. PITX2 Loss-of-Function Mutation Contributes to Congenital Endocardial Cushion Defect and Axenfeld-Rieger Syndrome. PLoS ONE 2015, 10, e0124409. [Google Scholar] [CrossRef]
- Kharlap, M.S.; Timofeeva, A.V.; Goryunova, L.E.; Khaspekov, G.L.; Dzemeshkevich, S.L.; Ruskin, V.V.; Akchurin, R.S.; Golitsyn, S.P.; Beabealashvilli, R.S. Atrial Appendage Transcriptional Profile in Patients with Atrial Fibrillation with Structural Heart Diseases. Ann. N. Y. Acad. Sci. 2006, 1091, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Chilukoti, R.K.; Giese, A.; Malenke, W.; Homuth, G.; Bukowska, A.; Goette, A.; Felix, S.B.; Kanaan, J.; Wollert, H.-G.; Evert, K.; et al. Atrial Fibrillation and Rapid Acute Pacing Regulate Adipocyte/Adipositas-Related Gene Expression in the Atria. Int. J. Cardiol. 2015, 187, 604–613. [Google Scholar] [CrossRef] [PubMed]
- van Ouwerkerk, A.F.; Hall, A.W.; Kadow, Z.A.; Lazarevic, S.; Reyat, J.S.; Tucker, N.R.; Nadadur, R.D.; Bosada, F.M.; Bianchi, V.; Ellinor, P.T.; et al. Epigenetic and Transcriptional Networks Underlying Atrial Fibrillation. Circ. Res. 2020, 127, 34–50, Correction in Circ. Res. 2020, 127, e143–e146. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Rice, K.M.; Arking, D.E.; Pfeufer, A.; van Noord, C.; Smith, A.V.; Schnabel, R.B.; Bis, J.C.; Boerwinkle, E.; Sinner, M.F.; et al. Variants in ZFHX3 Are Associated with Atrial Fibrillation in Individuals of European Ancestry. Nat. Genet. 2009, 41, 879–881. [Google Scholar] [CrossRef]
- Gudbjartsson, D.F.; Holm, H.; Gretarsdottir, S.; Thorleifsson, G.; Walters, G.B.; Thorgeirsson, G.; Gulcher, J.; Mathiesen, E.B.; Njølstad, I.; Nyrnes, A.; et al. A Sequence Variant in ZFHX3 on 16q22 Associates with Atrial Fibrillation and Ischemic Stroke. Nat. Genet. 2009, 41, 876–878. [Google Scholar] [CrossRef]
- Luo, Z.; Yan, C.; Zhang, W.; Shen, X.; Zheng, W.; Chen, F.; Cao, X.; Yang, Y.; Lin, X.; Wang, Z.; et al. Association between SNP Rs13376333 and Rs1131820 in the KCNN3 Gene and Atrial Fibrillation in the Chinese Han Population. Clin. Chem. Lab. Med. (CCLM) 2014, 52, 1867–1873. [Google Scholar] [CrossRef]
- Chang, S.-H.; Chang, S.-N.; Hwang, J.-J.; Chiang, F.-T.; Tseng, C.-D.; Lee, J.-K.; Lai, L.-P.; Lin, J.-L.; Wu, C.-K.; Tsai, C.-T. Significant Association of Rs13376333 in KCNN3 on Chromosome 1q21 with Atrial Fibrillation in a Taiwanese Population. Circ. J. 2012, 76, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.-L.; Zhou, Y.-F.; Yang, X.-J.; Qian, X.-D.; Jiang, W.-P. KCNN3 SNP Rs13376333 on Chromosome 1q21 Confers Increased Risk of Atrial Fibrillation. Int. Heart J. 2015, 56, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Bosada, F.M.; Rivaud, M.R.; Uhm, J.-S.; Verheule, S.; van Duijvenboden, K.; Verkerk, A.O.; Christoffels, V.M.; Boukens, B.J. A Variant Noncoding Region Regulates Prrx1 and Predisposes to Atrial Arrhythmias. Circ. Res. 2021, 129, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, C.; Wang, X.; Xu, C.; Wu, M.; Wang, P.; Tu, X.; Wang, Q.K. Significant Association Between CAV1 Variant Rs3807989 on 7p31 and Atrial Fibrillation in a Chinese Han Population. J. Am. Heart Assoc. 2015, 4, e001980. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, B.; Lin, Y.; Chen, X.-G.; Chen, M.; Hu, Z.; Zhang, F. The Rs3807989 G/A Polymorphism in CAV1 Is Associated with the Risk of Atrial Fibrillation in Chinese Han Populations. Pacing Clin. Electrophysiol. 2015, 38, 164–170. [Google Scholar] [CrossRef]
- Wass, S.Y.; Offerman, E.J.; Sun, H.; Hsu, J.; Rennison, J.H.; Cantlay, C.C.; McHale, M.L.; Gillinov, A.M.; Moravec, C.; Smith, J.D.; et al. Novel Functional Atrial Fibrillation Risk Genes and Pathways Identified from Coexpression Analyses in Human Left Atria. Heart Rhythm 2023, 20, 1219–1226. [Google Scholar] [CrossRef]
- Schunkert, H.; Di Angelantonio, E.; Inouye, M.; Patel, R.S.; Ripatti, S.; Widen, E.; Sanderson, S.C.; Kaski, J.P.; McEvoy, J.W.; Vardas, P.; et al. Clinical Utility and Implementation of Polygenic Risk Scores for Predicting Cardiovascular Disease. Eur. Heart J. 2025, 46, 1372–1383. [Google Scholar] [CrossRef]
- Zhou, H.; Liang, X.; Zhang, X.; Wu, J.; Jiang, Y.; Guo, B.; Wang, J.; Meng, Q.; Ding, X.; Baima, Y.; et al. Associations of Long-Term Exposure to Fine Particulate Constituents with Cardiovascular Diseases and Underlying Metabolic Mediations: A Prospective Population-Based Cohort in Southwest China. J. Am. Heart Assoc. 2024, 13, e033455. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Q.; Gao, Q.; Xu, M.; Fang, N.; Mu, L.; Han, X.; Yu, H.; Zhang, S.; Li, Y.; et al. Microplastics and Nanoplastics Increase Major Adverse Cardiac Events in Patients with Myocardial Infarction. J. Hazard. Mater. 2025, 489, 137624. [Google Scholar] [CrossRef]
- Herraiz-Martínez, A.; Álvarez-García, J.; Llach, A.; Molina, C.E.; Fernandes, J.; Ferrero-Gregori, A.; Rodríguez, C.; Vallmitjana, A.; Benítez, R.; Padró, J.M.; et al. Ageing Is Associated with Deterioration of Calcium Homeostasis in Isolated Human Right Atrial Myocytes. Cardiovasc Res 2015, 106, 76–86. [Google Scholar] [CrossRef]
- Vad, O.B.; Monfort, L.M.; Paludan-Müller, C.; Kahnert, K.; Diederichsen, S.Z.; Andreasen, L.; Lotta, L.A.; Nielsen, J.B.; Lundby, A.; Svendsen, J.H.; et al. Rare and Common Genetic Variation Underlying Atrial Fibrillation Risk. JAMA Cardiol. 2024, 9, 732. [Google Scholar] [CrossRef]
- Carnes, C.A.; Janssen, P.M.L.; Ruehr, M.L.; Nakayama, H.; Nakayama, T.; Haase, H.; Bauer, J.A.; Chung, M.K.; Fearon, I.M.; Gillinov, A.M.; et al. Atrial Glutathione Content, Calcium Current, and Contractility. J. Biol. Chem. 2007, 282, 28063–28073. [Google Scholar] [CrossRef]
- Xie, W.; Santulli, G.; Reiken, S.R.; Yuan, Q.; Osborne, B.W.; Chen, B.X.; Marks, A.R. Mitochondrial Oxidative Stress Promotes Atrial Fibrillation. Sci. Rep. 2015, 5, 11427. [Google Scholar] [CrossRef]
- Hu, Y.-F.; Chen, Y.-J.; Lin, Y.-J.; Chen, S.-A. Inflammation and the Pathogenesis of Atrial Fibrillation. Nat. Rev. Cardiol. 2015, 12, 230–243. [Google Scholar] [CrossRef]
- Stojadinovic, P.; Wichterle, D.; Fukunaga, M.; Peichl, P.; Melenovsky, V.; Franekova, J.; Kautzner, J.; Sramko, M. Acute Effect of Atrial Fibrillation on Circulating Natriuretic Peptides: The Influence of Heart Rate, Rhythm Irregularity, and Left Atrial Pressure Overload. Am. J. Cardiol. 2023, 208, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Pan, Y.; Kong, B.; Meng, H.; Shuai, W.; Huang, H. Ubiquitin-Specific Protease 38 Promotes Inflammatory Atrial Fibrillation Induced by Pressure Overload. Europace 2023, 26, euad366. [Google Scholar] [CrossRef] [PubMed]
- Bode, D.; Pronto, J.R.D.; Schiattarella, G.G.; Voigt, N. Metabolic Remodelling in Atrial Fibrillation: Manifestations, Mechanisms and Clinical Implications. Nat. Rev. Cardiol. 2024, 21, 682–700. [Google Scholar] [CrossRef] [PubMed]
- Nolla-Colomer, C.; Casabella-Ramon, S.; Jimenez-Sabado, V.; Vallmitjana, A.; Tarifa, C.; Herraiz-Martínez, A.; Llach, A.; Tauron, M.; Montiel, J.; Cinca, J.; et al. Β2-Adrenergic Stimulation Potentiates Spontaneous Calcium Release By Increasing Signal Mass and Co-Activation of Ryanodine Receptor Clusters. Acta Physiol. 2022, 234, e13736. [Google Scholar] [CrossRef]
- Tarifa, C.; Jiménez-Sábado, V.; Franco, R.; Montiel, J.; Guerra, J.; Ciruela, F.; Hove-Madsen, L. Expression and Impact of Adenosine A3 Receptors on Calcium Homeostasis in Human Right Atrium. Int. J. Mol. Sci. 2023, 24, 4404. [Google Scholar] [CrossRef]
- Jiménez-Sábado, V.; Casabella-Ramón, S.; Llach, A.; Gich, I.; Casellas, S.; Ciruela, F.; Chen, S.R.W.; Guerra, J.M.; Ginel, A.; Benítez, R.; et al. Beta-Blocker Treatment of Patients with Atrial Fibrillation Attenuates Spontaneous Calcium Release-Induced Electrical Activity. Biomed. Pharmacother. 2023, 158, 114169. [Google Scholar] [CrossRef]
- Godoy-Marín, H.; Duroux, R.; Jacobson, K.A.; Soler, C.; Colino-Lage, H.; Jiménez-Sábado, V.; Montiel, J.; Hove-Madsen, L.; Ciruela, F. Adenosine A2A Receptors Are Upregulated in Peripheral Blood Mononuclear Cells from Atrial Fibrillation Patients. Int. J. Mol. Sci. 2021, 22, 3467. [Google Scholar] [CrossRef]
- Casabella-Ramón, S.; Jiménez-Sábado, V.; Tarifa, C.; Casellas, S.; Lu, T.T.; Izquierdo-Castro, P.; Gich, I.; Jiménez, M.; Ginel, A.; Guerra, J.M.; et al. Impact of R-Carvedilol on Β2-Adrenergic Receptor-Mediated Spontaneous Calcium Release in Human Atrial Myocytes. Biomedicines 2022, 10, 1759. [Google Scholar] [CrossRef]

| Risk SNP | Effect on PITX2 Activity | Impact on Atrial Function | Risk Stratification | Therapeutic Relevance |
|---|---|---|---|---|
| rs2200733 | Inconsistent effects Enhancer-related | ↓ refractory period ↑ PR interval | Predictor of AF recurrence after ablation/cardioversion | ↑ recurrence after ablation |
| rs10033464 | Unclear PITX2c effect Enhancer-related | ↑ LA volume Impaired compliance | ↑ AF risk | AAD response: Class I > Class III in carriers |
| rs17042171 (linked with rs2200733)/rs6843082 (linked with rs10033464) | ↑ PITX2a, =PITX2c | Isoform-specific effects To be tested | Isoform-dependent risk To be tested | rs17042171 (↑ recurrence after CV) rs6843082 (Exploratory) |
| rs2595104 (linked with rs1448818) | ↓ PITX2c Enhancer: TFAP2a | Remains to be tested | ↑ AF susceptibility | Exploratory |
| rs13143308 | Not reported | ↑ Ca2+ release ↑ afterdepolarizations | ↑ AF risk | Therapy targeting SR Ca2+ release |
| Alteration | Mechanism | Pitx2c Deficient Models | AF Patients |
|---|---|---|---|
| Calcium handling | ICaL density | ↓ | ↓ χ |
| CACNA1C expression | ↓/↑ | ↓ | |
| SR Ca2+ load | ↑ | ↑/= # | |
| SERCA2a | ↑ | ↓ | |
| PLN | ↑ | = | |
| CASQ2 | ↑ | ↓ | |
| RyR2-P | ↑ | ↑ | |
| Ca2+ sparks/waves | ↑ | ↑ | |
| ITI | ↑ | ↑ | |
| Electrical remodeling | APD | ↓ | ↓/↑ |
| RMP | Slightly depolarized | Slightly depolarized | |
| Conduction | Slowed | Slowed | |
| DADs | ↑ | ↑ | |
| SA Node Function | Altered | Altered | |
| Structural remodeling | Gap junctions | ↓ Cx40, ↑Cx43 | ↓ Cx40, ↓ Cx43 |
| ECM | ↑ Fibrosis | ↑ Fibrosis & collagen | |
| Atrial size | Enlarged; ↑ BMP10 | Enlarged | |
| Sarcomeric structure | Altered | Altered | |
| Metabolic & Mitochondrial Alterations | ROS | ↑ | ↑ |
| Mitochondrial Function & Structure | Altered | Altered | |
| Adipose-like tissue | ↑ | ↑ | |
| Glycolysis | ↑ | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Sábado, V.; Hove-Madsen, L. Single-Nucleotide Polymorphisms, PITX2 and Abnormal Electrical Activity in Atrial Fibrillation. Int. J. Mol. Sci. 2025, 26, 9780. https://doi.org/10.3390/ijms26199780
Jiménez-Sábado V, Hove-Madsen L. Single-Nucleotide Polymorphisms, PITX2 and Abnormal Electrical Activity in Atrial Fibrillation. International Journal of Molecular Sciences. 2025; 26(19):9780. https://doi.org/10.3390/ijms26199780
Chicago/Turabian StyleJiménez-Sábado, Verónica, and Leif Hove-Madsen. 2025. "Single-Nucleotide Polymorphisms, PITX2 and Abnormal Electrical Activity in Atrial Fibrillation" International Journal of Molecular Sciences 26, no. 19: 9780. https://doi.org/10.3390/ijms26199780
APA StyleJiménez-Sábado, V., & Hove-Madsen, L. (2025). Single-Nucleotide Polymorphisms, PITX2 and Abnormal Electrical Activity in Atrial Fibrillation. International Journal of Molecular Sciences, 26(19), 9780. https://doi.org/10.3390/ijms26199780





