4-Phenylbutyric Acid Improves Gait Ability of UBAP1-Related Spastic Paraplegia Mouse Model: Therapeutic Potential for SPG80
Abstract
1. Introduction
2. Results
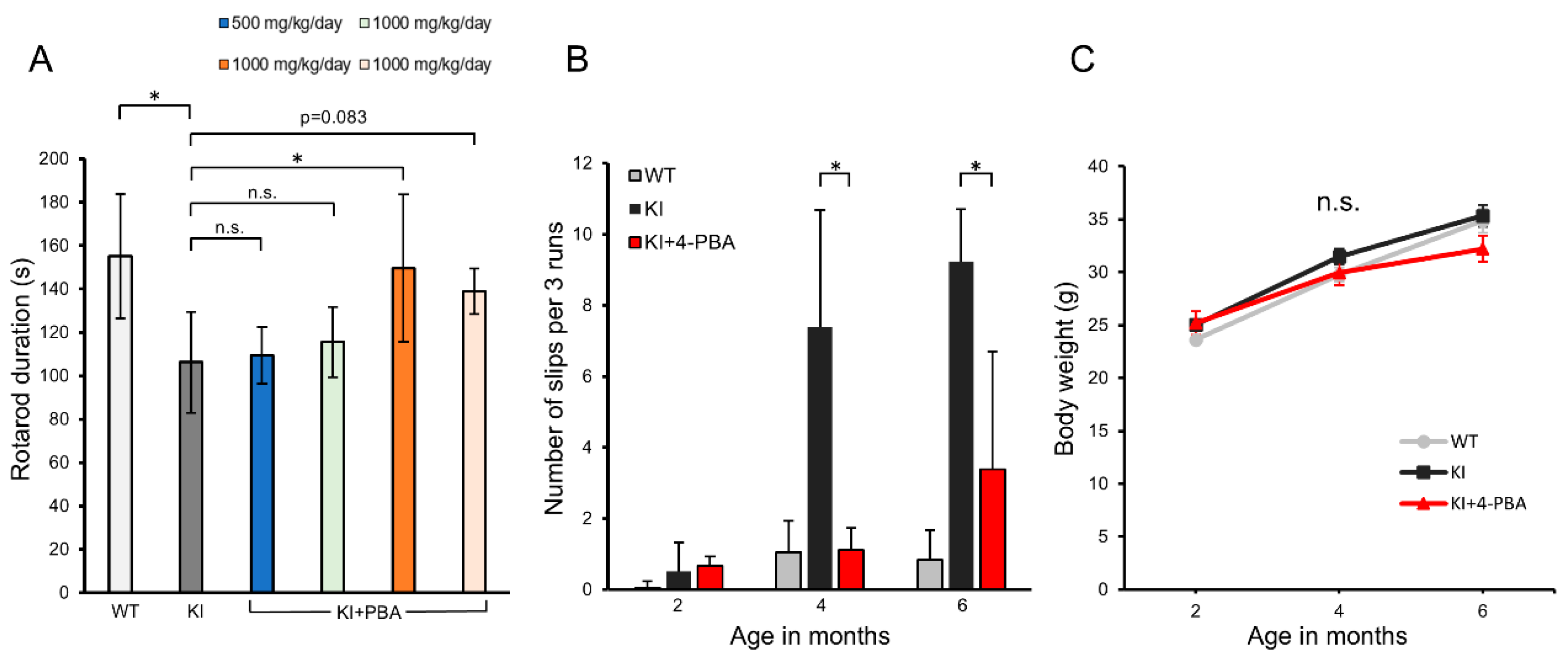
2.1. Therapeutic Effects of 4-PBA on Motor Function and Coordination in Ubap1 KI Mice
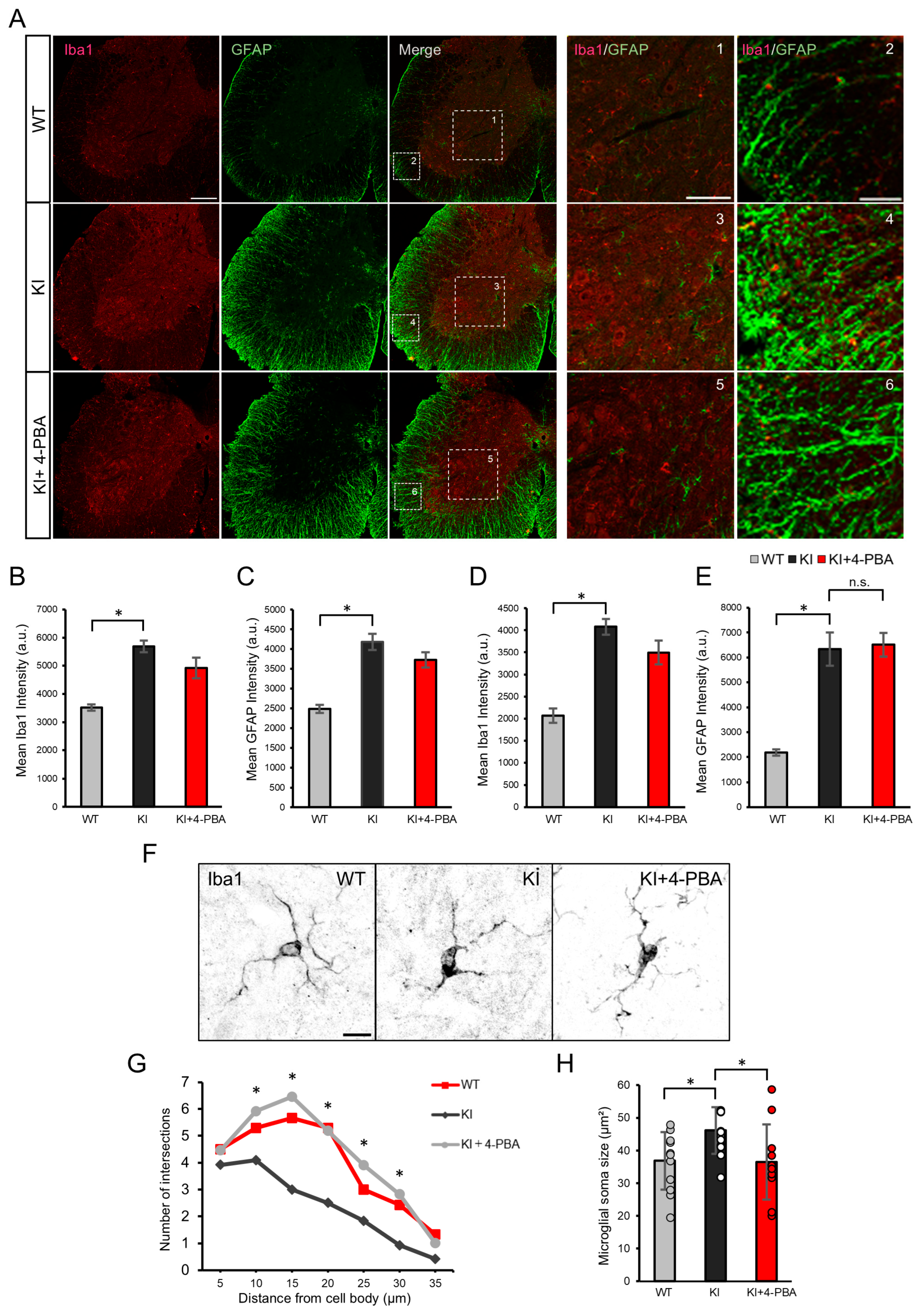
2.2. Glial Activation and Effects of 4-PBA on the Spinal Cord of Ubap1 KI Mice
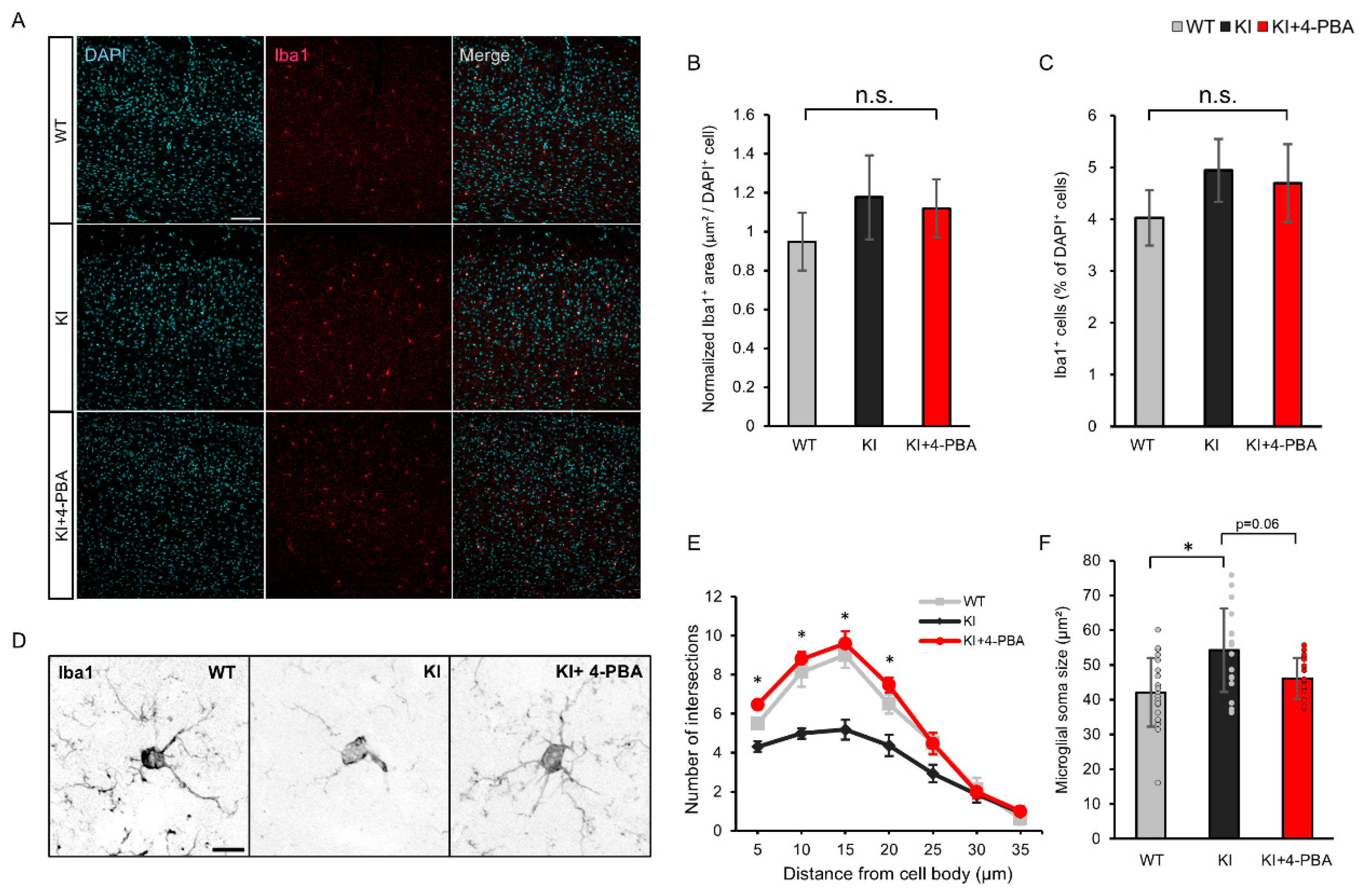
2.3. Microglial Morphology and Therapeutic Effects of 4-PBA on the Cerebral Cortex
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
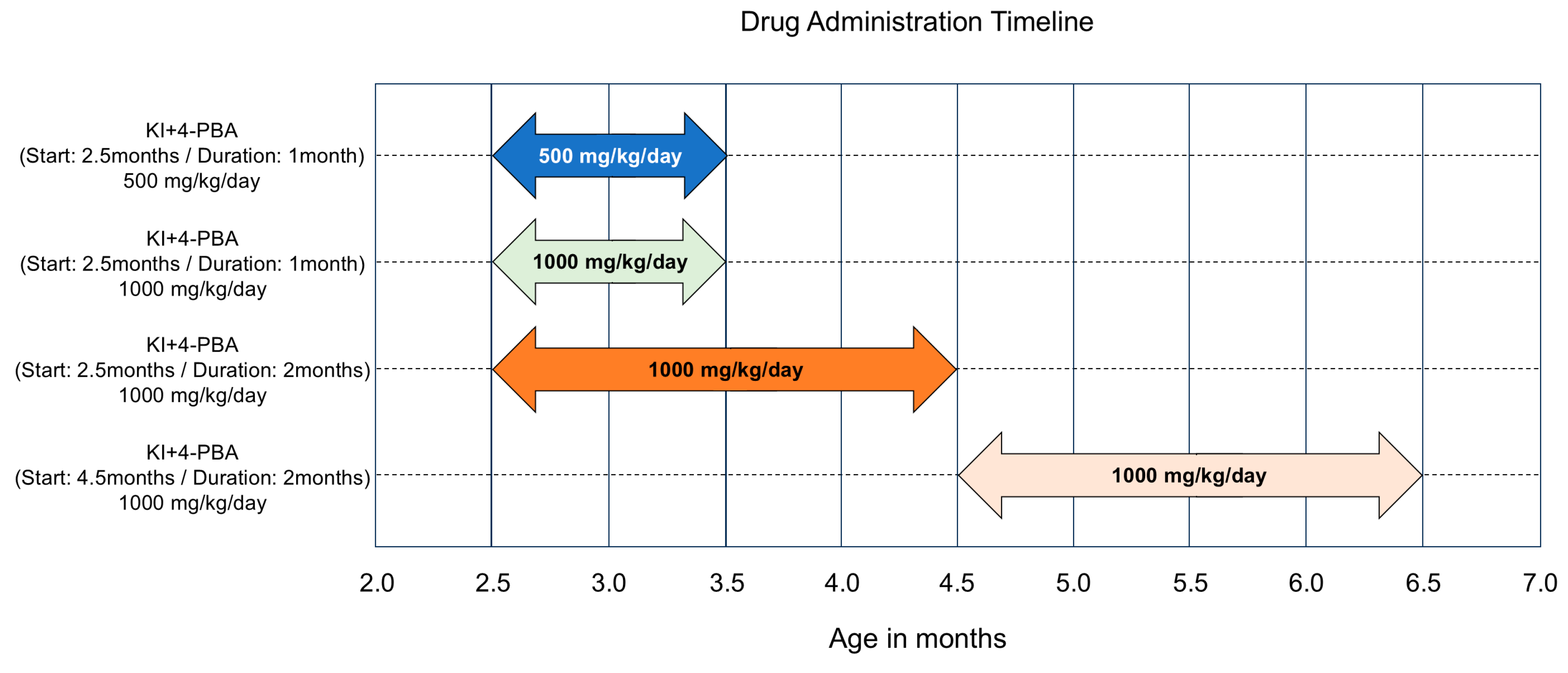
4.2. Drug Administration Timeline
4.3. Behavioral Tests
4.4. Tissue Preparation and Immunofluorescence Staining
4.5. Sholl Analysis
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HSP | hereditary spastic paraplegia |
| SPG80 | spastic paraplegia 80 |
| SPG15 | spastic paraplegia 15 |
| SPG11 | spastic paraplegia 11 |
| UBAP1 | ubiquitin-associated protein 1 |
| 4-PBA | 4-phenylbutyric acid |
| KI | knock-in |
| WT | wild type |
| MVB | multivesicular body |
| ESCRT-1 | endosomal sorting complex required for transport-1 |
| ER | endoplasmic reticulum |
| HDAC | histone deacetylase |
| Iba1 | ionized calcium binding adapter protein 1 |
| GFAP | glial fibrillary acidic protein |
| CRISPR/Cas 9 | clustered regularly interspaced short palindromic repeats/CRISPR associated proteins |
| a.u. | arbitrary units |
| DNA | deoxyribonucleic acid |
| PFA | paraformaldehyde |
| PBS | phosphate-buffered saline |
| NGS | normal goat serum |
| ANOVA | analysis of variance |
| HSD | honest significant difference |
| Tukey’s HSD test | Tukey’s honestly significant difference test |
References
- Fink, J.K. Hereditary spastic paraplegia. Curr. Neurol. Neurosci. Rep. 2006, 6, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Harding, A.E. Classification of the hereditary ataxias and paraplegia. Lancet 1983, 1, 1151–1155. [Google Scholar] [CrossRef]
- Available online: https://neuromuscular.wustl.edu/spinal/fsp.html (accessed on 3 October 2025).
- Blackstone, C. Cellular pathways of hereditary spastic paraplegia. Annu. Rev. Neurosci. 2012, 35, 25–47. [Google Scholar] [CrossRef] [PubMed]
- Nan, H.; Ichinose, Y.; Tanaka, M.; Koh, K.; Ishiura, H.; Mitsui, J.; Mizukami, H.; Morimoto, M.; Hamada, S.; Ohtsuka, T.; et al. UBAP1 mutations cause juvenile-onset hereditary spastic paraplegias (SPG80) and impair UBAP1 targeting to endosomes. J. Hum. Genet. 2019, 64, 1055–1065. [Google Scholar] [CrossRef]
- Fard, M.A.F.; Rebelo, A.P.; Buglo, E.; Nemati, H.; Dastsooz, H.; Gehweiler, I.; Reich, S.; Reichbauer, J.; Quintáns, B.; Ordóñez-Ugalde, A.; et al. Truncating mutations in UBAP1 cause hereditary spastic paraplegia. Am. J. Hum. Genet. 2019, 104, 767–773. [Google Scholar] [CrossRef]
- Lin, X.; Su, H.-Z.; Dong, E.-L.; Lin, X.-H.; Zhao, M.; Yang, C.; Wang, C.; Wang, J.; Chen, Y.-J.; Yu, H.; et al. Stop-gain mutations in UBAP1 cause pure autosomal-dominant spastic paraplegia. Brain 2019, 142, 2238–2252. [Google Scholar] [CrossRef]
- Katzmann, D.J.; Babst, M.; Emr, S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 2001, 106, 145–155. [Google Scholar] [CrossRef]
- Agromayor, M.; Soler, N.; Caballe, A.; Kueck, T.; Freund, S.M.; Allen, M.D.; Bycroft, M.; Perisic, O.; Ye, Y.; McDonald, B.; et al. The UBAP1 subunit of ESCRT-I interacts with ubiquitin via a SOUBA domain. Structure 2012, 20, 414–428. [Google Scholar] [CrossRef]
- Doyotte, A.; Russell, M.R.G.; Hopkins, C.R.; Woodman, P.G. Depletion of TSG101 forms a mammalian ‘Class E’ compartment: A multicisternal early endosome with multiple sorting defects. J. Cell. Sci. 2005, 118, 3003–3017. [Google Scholar] [CrossRef]
- Shirk, A.J.; Anderson, S.K.; Hashemi, S.H.; Chance, P.F.; Bennett, C.L. SIMPLE interacts with NEDD4 and TSG101: Evidence for a role in lysosomal sorting and implications for Charcot-Marie-Tooth disease. J. Neurosci. Res. 2005, 82, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Olzmann, J.A.; Barsh, G.S.; Chin, L.-S.; Li, L. Spongiform neurodegeneration-associated E3 ligase mahogunin ubiquitylates TSG101 and regulates endosomal trafficking. Mol. Biol. Cell 2007, 18, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Shimozono, K.; Nan, H.; Hata, T.; Saito, K.; Kim, Y.-J.; Nagatomo, H.; Ohtsuka, T.; Koizumi, S.; Takiyama, Y. Ubap1 knock-in mice reproduced the phenotype of SPG80. J. Hum. Genet. 2022, 67, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Frolov, A.; Huang, H.; Schutz, D.; Kohne, M.; Blank-Stein, N.; Osei-Sarpong, C.; Buttner, M.; Elmzzahi, T.; Khundadze, M.; Zahid, M.; et al. Microglia and CD8+ T cell activation precede neuronal loss in a murine model of spastic paraplegia 15. J. Exp. Med. 2025, 222, e20232357. [Google Scholar] [CrossRef]
- González-Sanmiguel, J.; Schuh, C.M.A.P.; Muñoz-Montesino, C.; Contreras-Kallens, P.; Aguayo, L.G.; Aguayo, S. Complex interaction between resident microbiota and misfolded proteins: Role in neuroinflammation and neurodegeneration. Cells 2020, 9, 2476. [Google Scholar] [CrossRef]
- Vijayaraghavan, M.; Murali, S.P.; Thakur, G.; Li, X.-J. Role of glial cells in motor neuron degeneration in hereditary spastic paraplegias. Front. Cell Neurosci. 2025, 19, 1553658. [Google Scholar] [CrossRef]
- Gumeni, S.; Vantaggiato, C.; Montopoli, M.; Orso, G. Hereditary Spastic Paraplegia and Future Therapeutic Directions: Beneficial Effects of Small Compounds Acting on Cellular Stress. Front. Neurosci. 2021, 15, 660714. [Google Scholar] [CrossRef] [PubMed]
- Lichter-Konecki, U.; Diaz, G.A.; Merritt, J.L., 2nd; Feigenbaum, A.; Jomphe, C.; Marier, J.F.; Beliveau, M.; Mauney, J.; Dickinson, K.; Martinez, A.; et al. Ammonia control in children with urea cycle disorders (UCDs); phase 2 comparison of sodium phenylbutyrate and glycerol phenylbutyrate. Mol. Genet. Metab. 2011, 103, 323–329. [Google Scholar] [CrossRef]
- Özcan, U.; Yilmaz, E.; Özcan, L.; Furuhashi, M.; Vaillancourt, E.; Smith, R.O.; Görgün, C.Z.; Hotamisligil, G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006, 313, 1137–1140. [Google Scholar] [CrossRef]
- Qi, X.; Hosoi, T.; Okuma, Y.; Kaneko, M.; Nomura, Y. Sodium 4-phenylbutyrate protects against cerebral ischemic injury. Mol. Pharmacol. 2004, 66, 899–908. [Google Scholar] [CrossRef]
- Tochiya, M.; Hagiwara, D.; Azuma, Y.; Miyata, T.; Morishita, Y.; Suga, H.; Onoue, T.; Tsunekawa, T.; Takagi, H.; Ito, Y.; et al. Chemical chaperone 4-phenylbutylate reduces mutant protein accumulation in the endoplasmic reticulum of arginine vasopressin neurons in a mouse model for familial neurohypophysial diabetes insipidus. Neurosci. Lett. 2018, 682, 50–55. [Google Scholar] [CrossRef]
- Hommen, F.; Bilican, S.; Vilchez, D. Protein clearance strategies for disease intervention. J. Neural. Transm. 2022, 129, 141–172. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Jingzhen, Z.; Xinliang, Y.; Bishao, S.; Xin, L.; Ji, Z.; Zhenqiang, F. 4-PBA inhibits endoplasmic reticulum stress to improve autophagic flux in the treatment of protamine/lipopolysaccharide-induced interstitial cystitis in rats. Sci. Rep. 2023, 13, 14057. [Google Scholar] [CrossRef]
- Hetz, C.; Mollereau, B. Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat. Rev. Neurosci. 2014, 15, 233–249. [Google Scholar] [CrossRef]
- Huang, W.; Liu, Y.; Li, J.; Gao, Y.; Tang, J.; Yip, S.; Wang, X.; Zhang, H.; Ma, Y.; Su, S.; et al. Endoplasmic reticulum stress drives neuroinflammation through lipocalin 2 upregulation in retinal microglia after optic nerve injury. Investig. Ophthalmol. Vis. Sci. 2025, 66, 12. [Google Scholar] [CrossRef]
- Zhu, Y.; Yu, J.; Gong, J.; Shen, J.; Ye, D.; Cheng, D.; Xie, Z.; Zeng, J.; Xu, K.; Shen, J.; et al. PTP1B inhibitor alleviates deleterious microglial activation and neuronal injury after ischemic stroke by modulating the ER stress-autophagy axis via PERK signaling in microglia. Aging 2021, 13, 3405–3427. [Google Scholar] [CrossRef]
- Weaver, F.E.; White, E.; Peek, A.M.; A Nurse, C.; Austin, R.C.; A Igdoura, S. 4-phenylbutyric acid mitigates ER stress-induced neurodegeneration in the spinal cords of a GM2 gangliosidosis mouse model. Hum. Mol. Genet. 2025, 34, 32–46. [Google Scholar] [CrossRef]
- van Olst, L.; Simonton, B.; Edwards, A.J.; Forsyth, A.V.; Boles, J.; Jamshidi, P.; Watson, T.; Shepard, N.; Krainc, T.; Argue, B.M.; et al. Microglia mechanisms drive amyloid-βclearance in immunized patients with Alzheimer’s disease. Nat. Med. 2025, 31, 1604–1616, Correction in Nat. Med. 2025, 31, 1712. [Google Scholar] [CrossRef] [PubMed]
- Hamby, M.E.; Sofroniew, M.V. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics 2010, 7, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Mai, X.; Wang, Y.; Wang, X.; Liu, M.; Teng, F.; Liu, Z.; Su, M.-Y.; Stjepanovic, G. Structural basis for membrane remodeling by the AP5-SPG11-SPG15 complex. Nat. Struct. Mol. Biol. 2025, 32, 1334–1346. [Google Scholar] [CrossRef]
- Isik, S.; Kiyak, B.Y.; Akbayir, R.; Seyhali, R.; Arpaci, T. Microglia Mediated Neuroinflammation in Parkinson’s Disease. Cells 2023, 12, 1012. [Google Scholar] [CrossRef]
- Roberts, K.; Zeineddine, R.; Corcoran, L.; Li, W.; Campbell, I.L.; Yerbury, J.J. Extracellular aggregated Cu/Zn superoxide dismutase activates microglia to give a cytotoxic phenotype. Glia 2013, 61, 409–419. [Google Scholar] [CrossRef]
- Krumm, L.; Pozner, T.; Zagha, N.; Coras, R.; Arnold, P.; Tsaktanis, T.; Scherpelz, K.; Davis, M.Y.; Kaindl, J.; Stolzer, I.; et al. Neuroinflammatory disease signatures in SPG11-related hereditary spastic paraplegia patients. Acta Neuropathol. 2024, 147, 28. [Google Scholar] [CrossRef]
- Allison, R.; Edgar, J.R.; Reid, E. Spastin MIT domain disease-associated mutations disrupt lysosomal function. Front. Neurosci. 2019, 13, 1179. [Google Scholar] [CrossRef]
- Renvoisé, B.; Parker, R.L.; Yang, D.; Bakowska, J.C.; Hurley, J.H.; Blackstone, C. SPG20 protein spartin is recruited to midbodies by ESCRT-III protein Ist1 and participates in cytokinesis. Mol. Biol. Cell 2010, 21, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Klionsky, D.J. A delicate decision between repair and degradation of damaged lysosomes. Autophagy 2024, 20, 1471–1472. [Google Scholar] [CrossRef]
- Ziviny-Elboum, Y.; Westbroek, W.; Kfir, N.; Savitzki, D.; Shoval, Y.; Bloom, A.; Rod, R.; Khayat, M.; Gross, B.; Samri, W.; et al. A founder mutation in Vps37A causes autosomal recessive complex hereditary spastic paraparesis. J. Med. Genet. 2012, 49, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Glinton, K.E.; Minard, C.G.; Liu, N.; Sun, Q.; Elsea, S.H.; Burrage, L.C.; Nagamani, S.C. Monitoring the treatment of urea cycle disorders using phenylbutyrate metabolite analyses: Still many lessons to learn. Mol. Genet. Metab. 2023, 140, 107699. [Google Scholar] [CrossRef] [PubMed]
- Berthier, A.; Payá, M.; García-Cabrero, A.M.; Ballester, M.I.; Heredia, M.; Serratosa, J.M.; Sánchez, M.P.; Sanz, P. Pharmacological interventions to ameliorate neuropathological symptoms in a mouse model of Lafora disease. Mol. Neurobiol. 2016, 53, 1296–1309. [Google Scholar] [CrossRef]
- Sugiyama, T.; Nishitoh, H. Neurodegenerative diseases associated with the disruption of proteostasis and their therapeutic strategies using chemical chaperones. J. Biochem. 2024, 176, 179–186. [Google Scholar] [CrossRef]
- Paganoni, S.; Macklin, E.A.; Hendrix, S.; Berry, J.D.; Elliott, M.A.; Maiser, S.; Karam, C.; Caress, J.B.; Owegi, M.A.; Quick, A.; et al. Trial of sodium phenylbutyrate-taurusodiol for amyotrophic lateral sclerosis. N. Engl. J. Med. 2020, 383, 919–930. [Google Scholar] [CrossRef]
- Ono, K.; Ikemoto, M.; Kawarabayashi, T.; Ikeda, M.; Nishinakagawa, T.; Hosokawa, M.; Shoji, M.; Takahashi, M.; Nakashima, M. A chemical chaperone, sodium 4-phenylbutyric acid, attenuates the pathogenic potency in human alpha-synuclein A30P + A53T transgenic mice. Park. Relat. Disord. 2009, 15, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Varga, R.-E.; Khundadze, M.; Damme, M.; Nietzsche, S.; Hoffmann, B.; Stauber, T.; Koch, N.; Hennings, J.C.; Franzka, P.; Huebner, A.K.; et al. In vivo evidence for lysosomal depletion and impaired autophagic clearance in hereditary spastic paraplegia type SPG11. PLoS Genet. 2015, 11, e1005454. [Google Scholar] [CrossRef] [PubMed]
- Ferreirinha, F.; Quattrini, A.; Pirozzi, M.; Valsecchi, V.; Dina, G.; Broccoli, V.; Auricchio, A.; Piemonte, F.; Tozzi, G.; Gaeta, L.; et al. Axonal degeneration in paraplegin-deficient mice is associated with abnormal mitochondria and impairment of axonal transport. J. Clin. Investig. 2004, 113, 231–242. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimozono, K.; Kim, Y.-J.; Hata, T.; Nan, H.; Saito, K.; Mori, Y.; Ueno, Y.; Isono, F.; Iwasaki, M.; Koizumi, S.; et al. 4-Phenylbutyric Acid Improves Gait Ability of UBAP1-Related Spastic Paraplegia Mouse Model: Therapeutic Potential for SPG80. Int. J. Mol. Sci. 2025, 26, 9779. https://doi.org/10.3390/ijms26199779
Shimozono K, Kim Y-J, Hata T, Nan H, Saito K, Mori Y, Ueno Y, Isono F, Iwasaki M, Koizumi S, et al. 4-Phenylbutyric Acid Improves Gait Ability of UBAP1-Related Spastic Paraplegia Mouse Model: Therapeutic Potential for SPG80. International Journal of Molecular Sciences. 2025; 26(19):9779. https://doi.org/10.3390/ijms26199779
Chicago/Turabian StyleShimozono, Keisuke, Yeon-Jeong Kim, Takanori Hata, Haitian Nan, Kozo Saito, Yasunori Mori, Yuji Ueno, Fujio Isono, Masaru Iwasaki, Schuichi Koizumi, and et al. 2025. "4-Phenylbutyric Acid Improves Gait Ability of UBAP1-Related Spastic Paraplegia Mouse Model: Therapeutic Potential for SPG80" International Journal of Molecular Sciences 26, no. 19: 9779. https://doi.org/10.3390/ijms26199779
APA StyleShimozono, K., Kim, Y.-J., Hata, T., Nan, H., Saito, K., Mori, Y., Ueno, Y., Isono, F., Iwasaki, M., Koizumi, S., Ohtsuka, T., & Takiyama, Y. (2025). 4-Phenylbutyric Acid Improves Gait Ability of UBAP1-Related Spastic Paraplegia Mouse Model: Therapeutic Potential for SPG80. International Journal of Molecular Sciences, 26(19), 9779. https://doi.org/10.3390/ijms26199779






