SPAchips: Microparticles Used for the Selective In Vitro Labelling of Microglia
Abstract
1. Introduction
2. Results
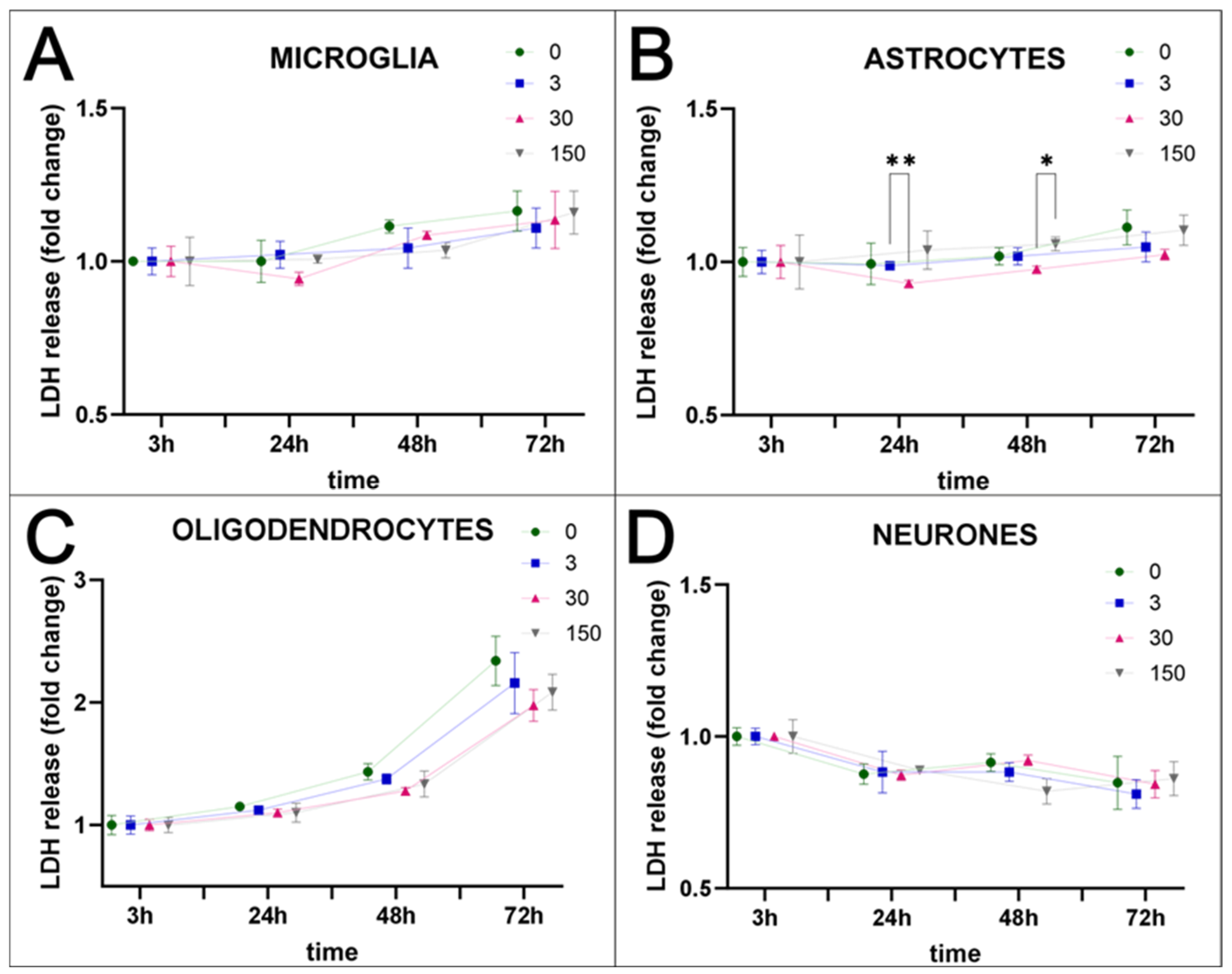
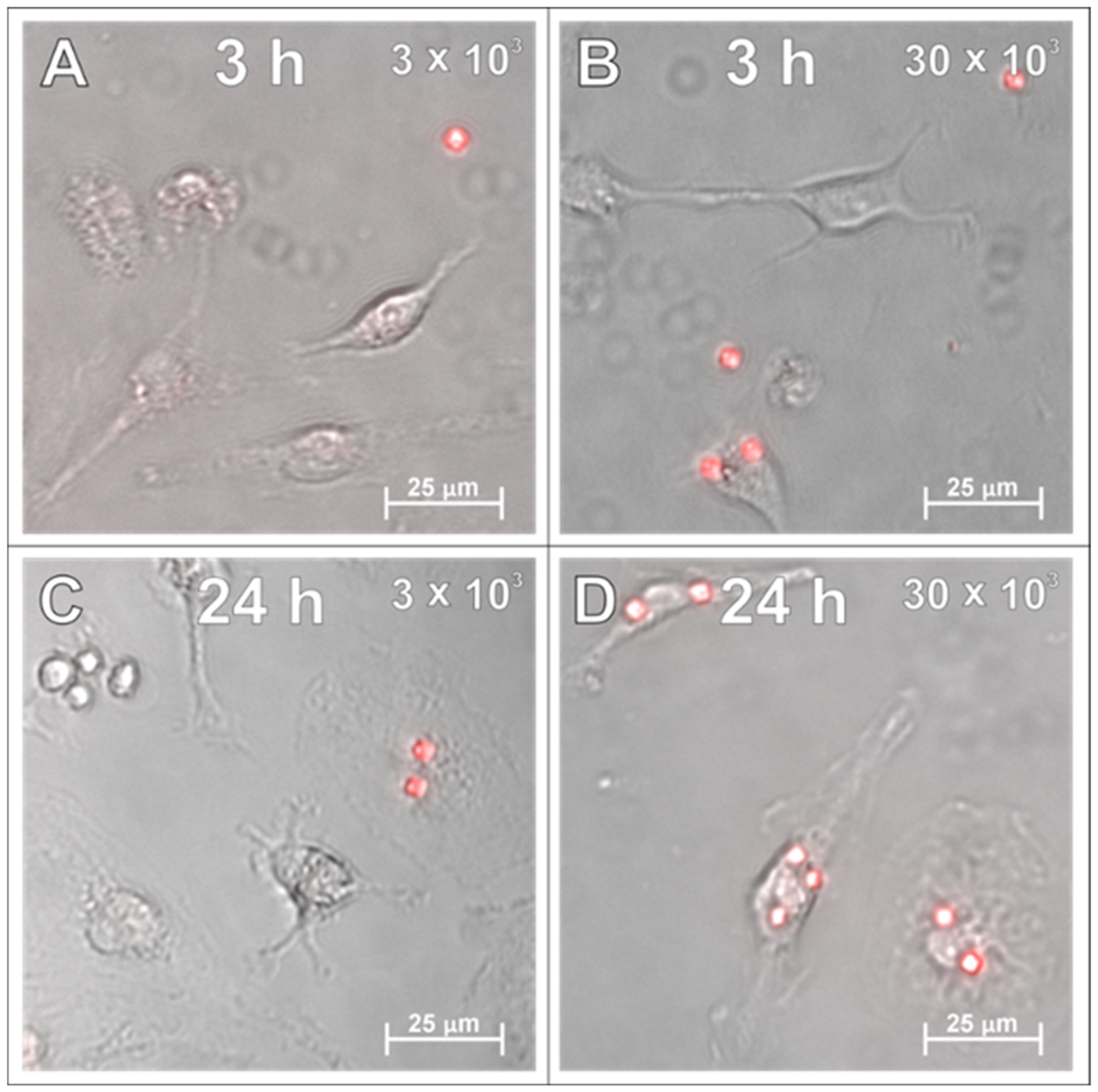
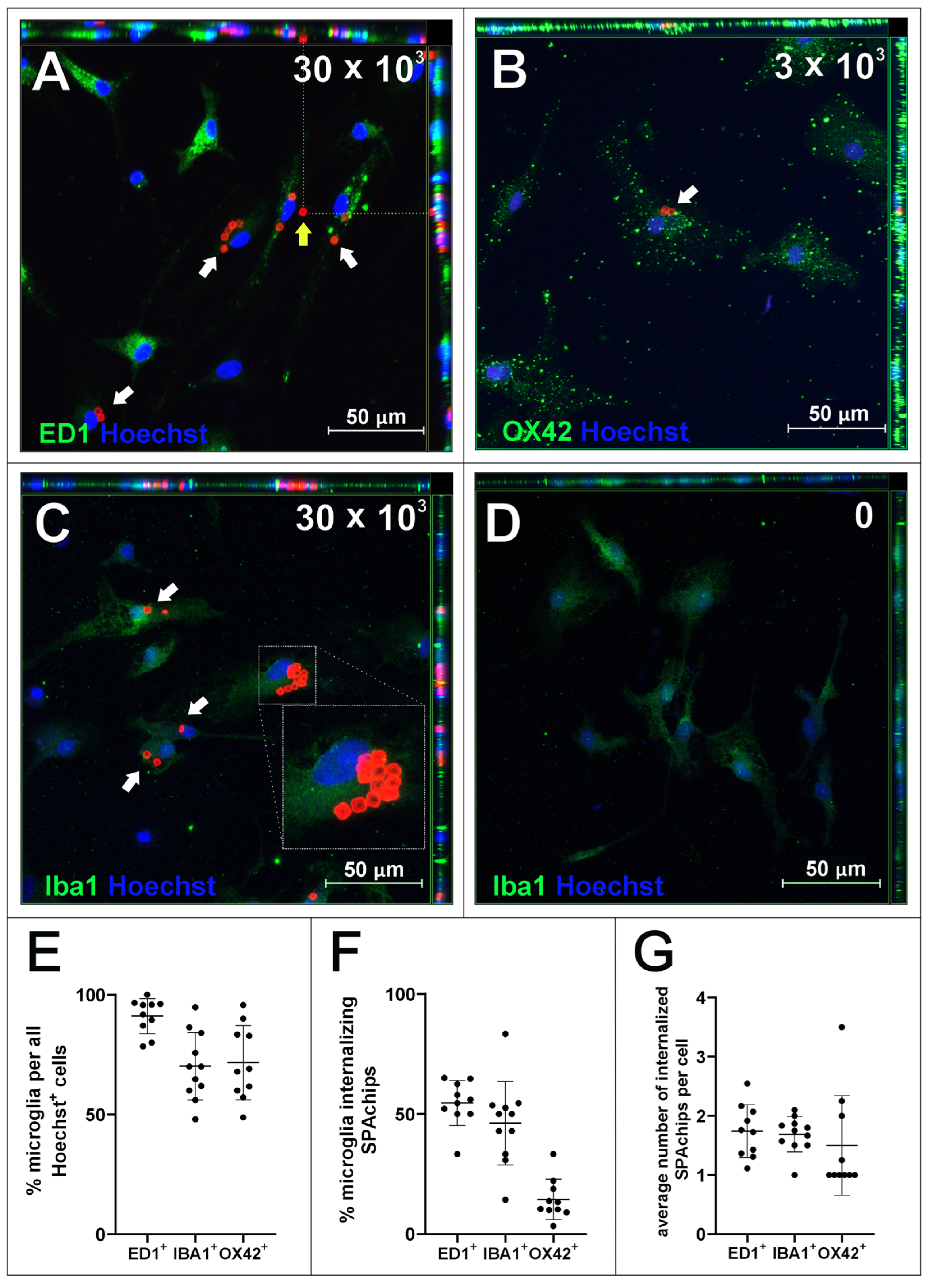
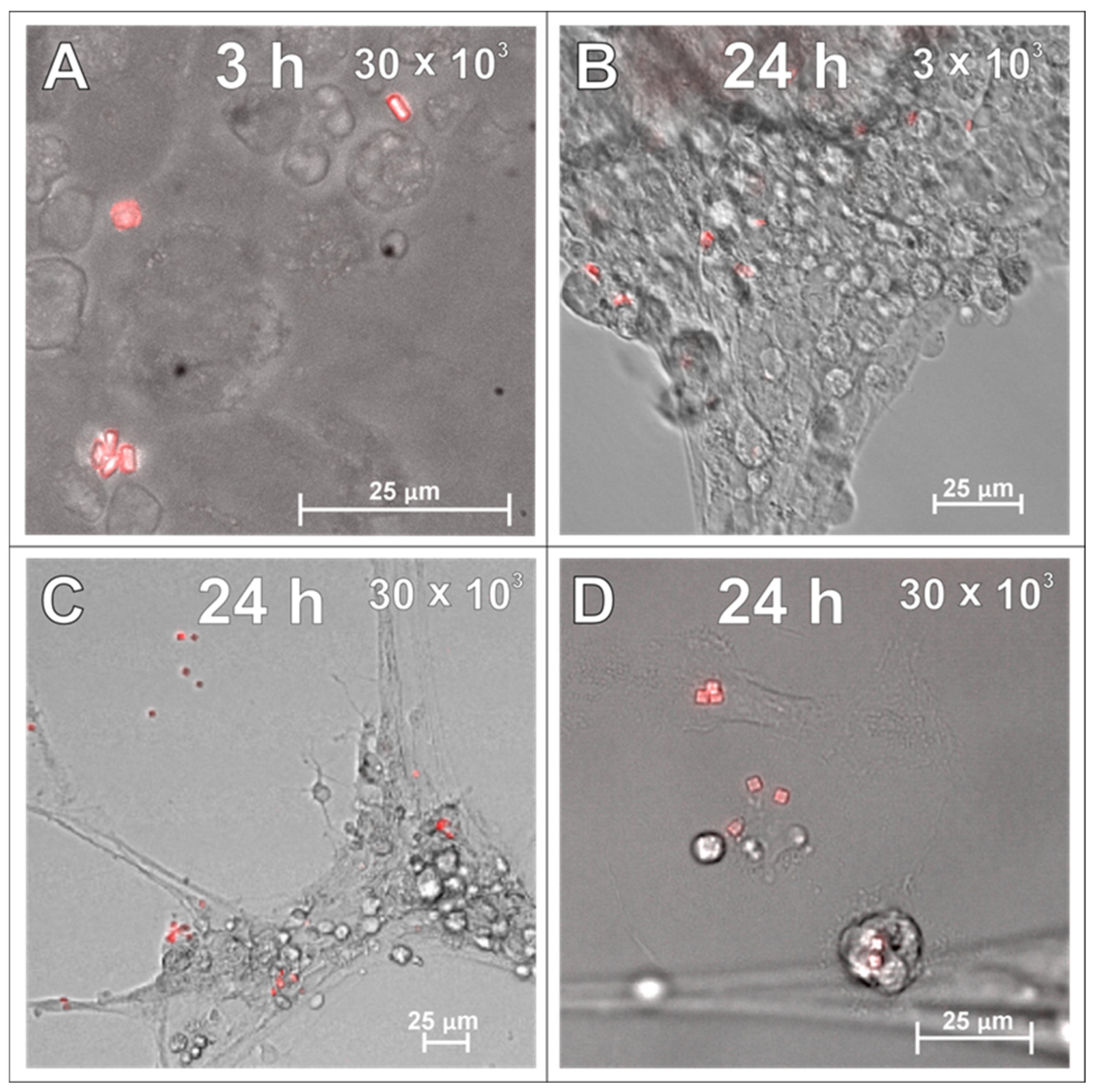
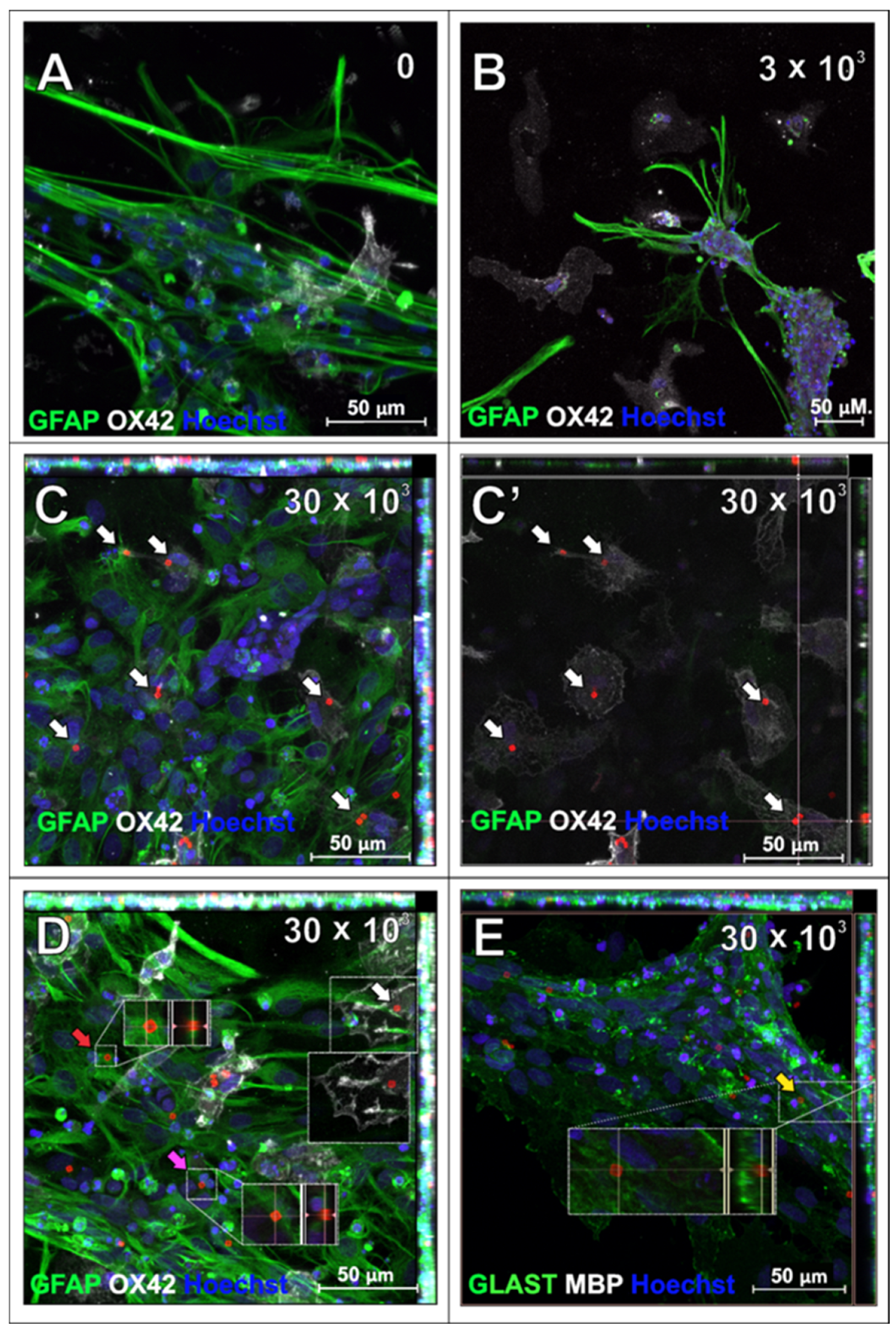
2.1. SPAchips® in Microglia Culture
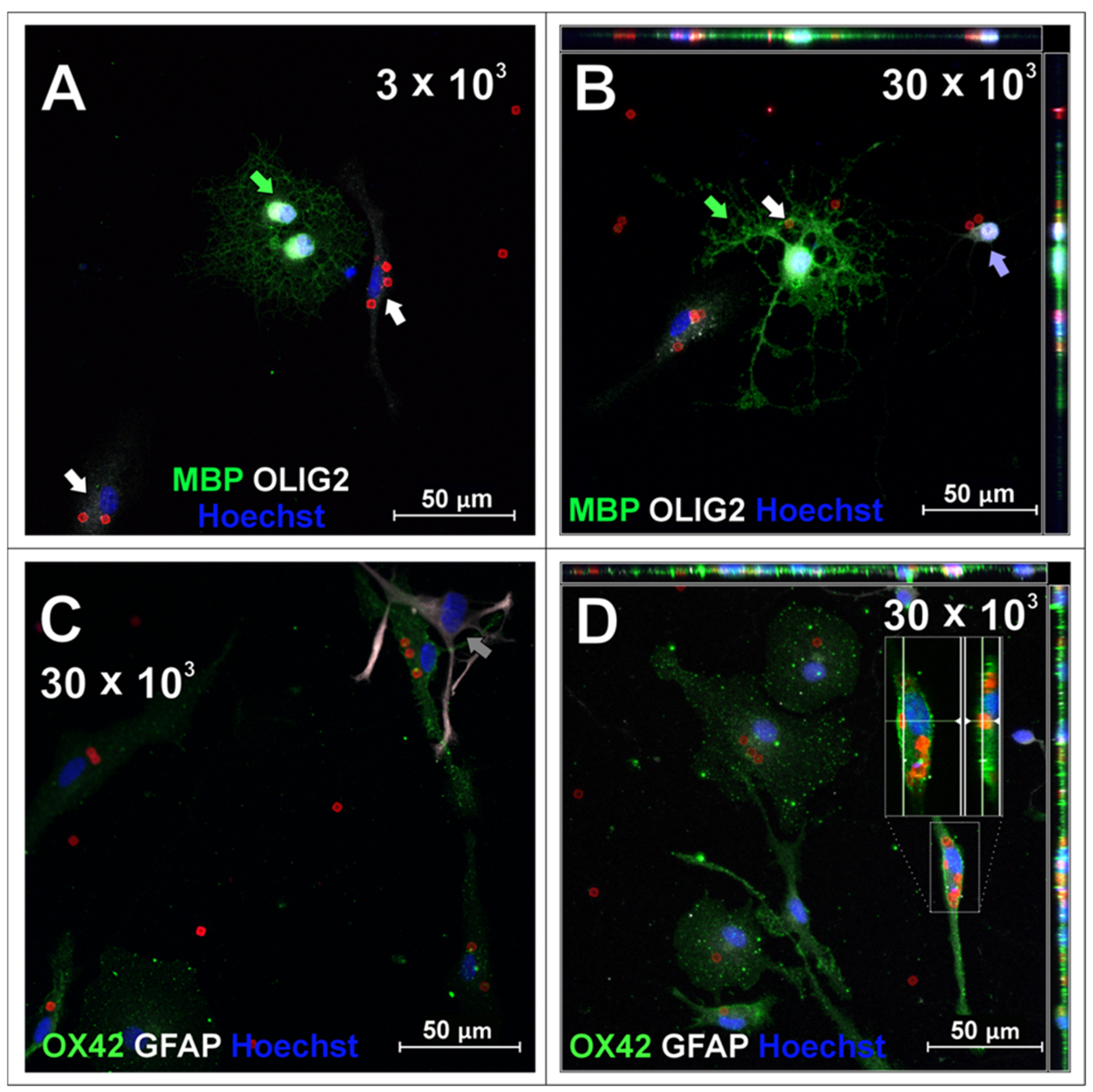
2.2. SPAchips® in Oligodendrocyte Culture
2.3. SPAchips® in Astrocytes Culture
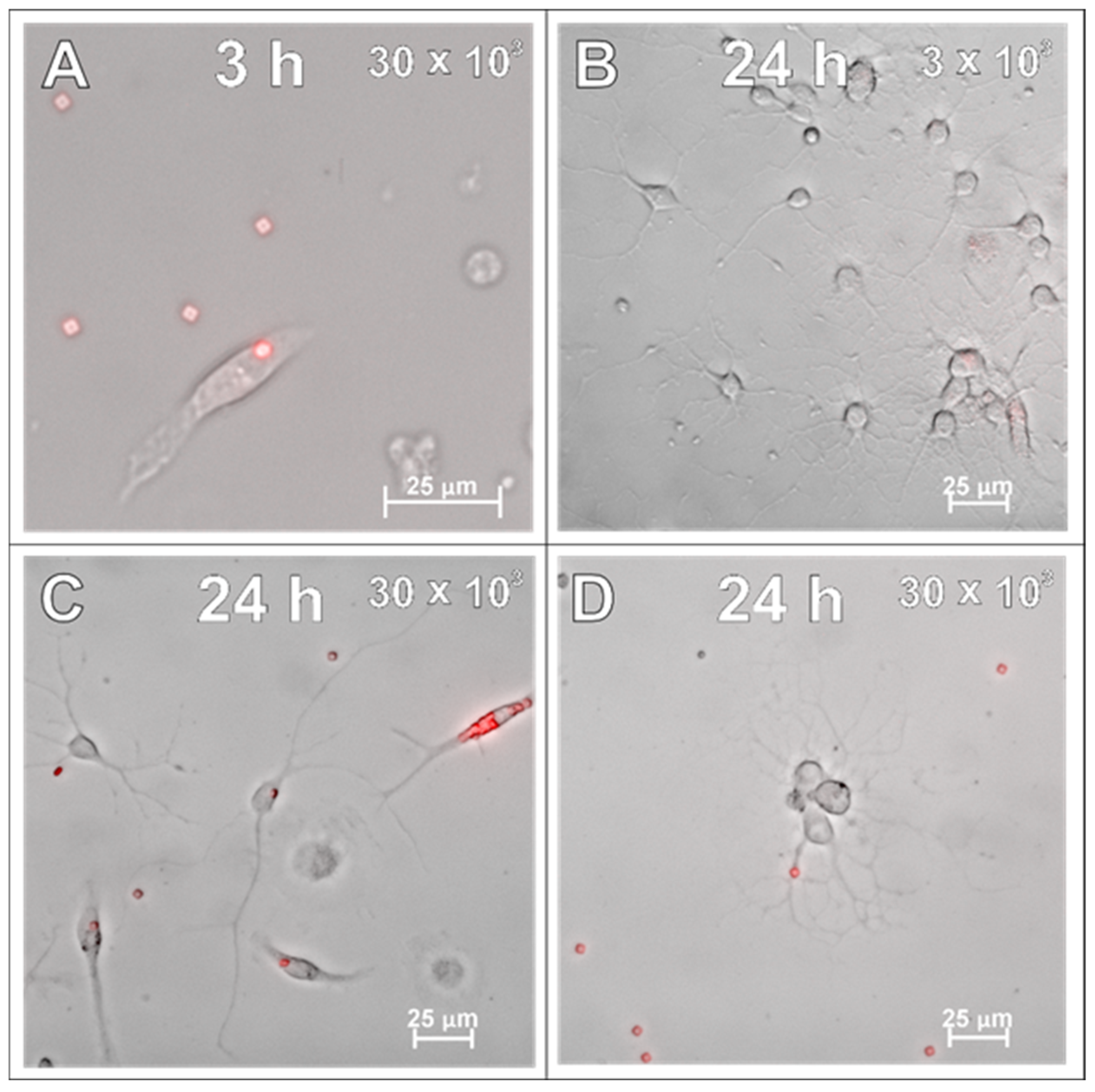
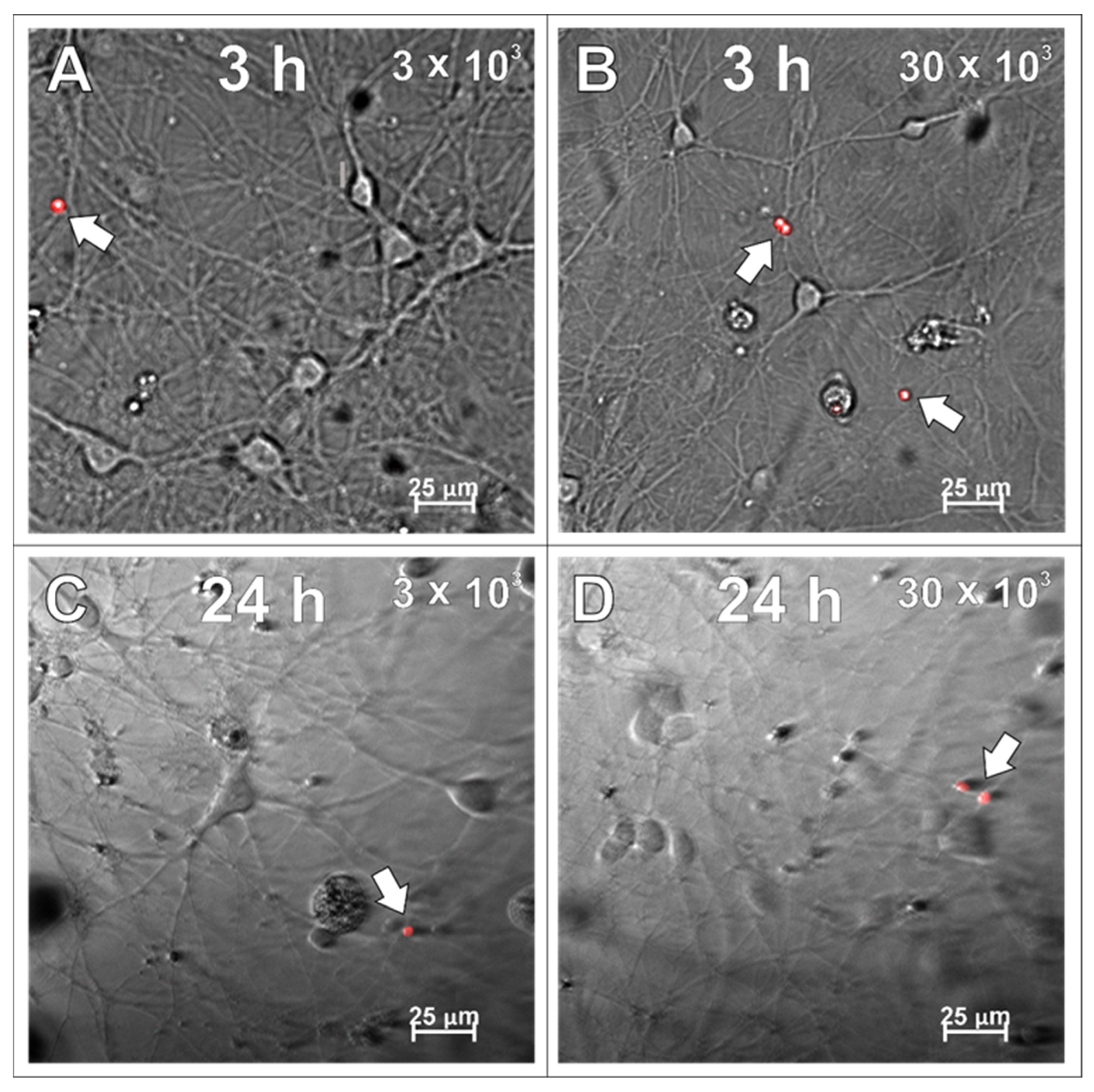
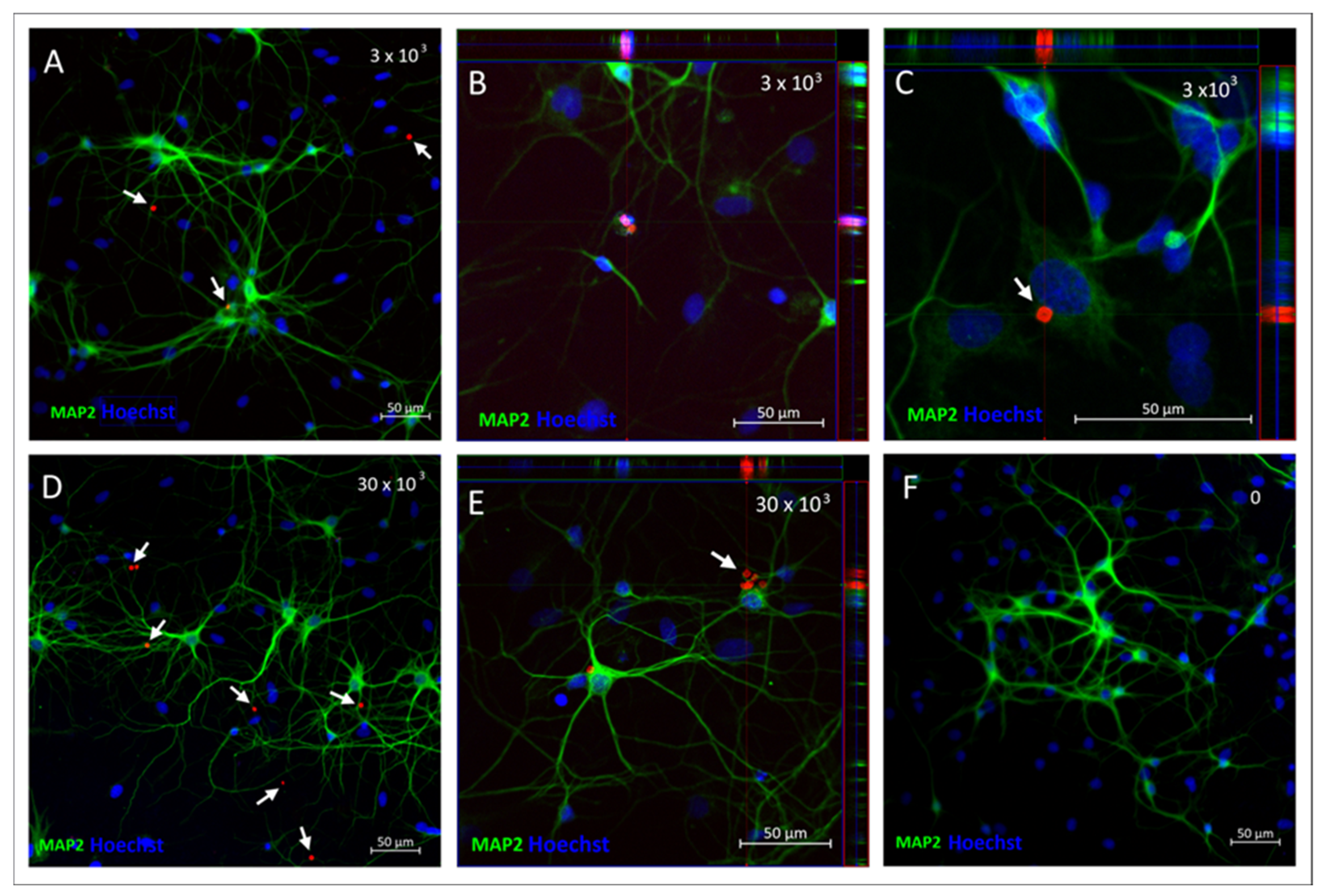
2.4. SPAchips® in Neuron Culture
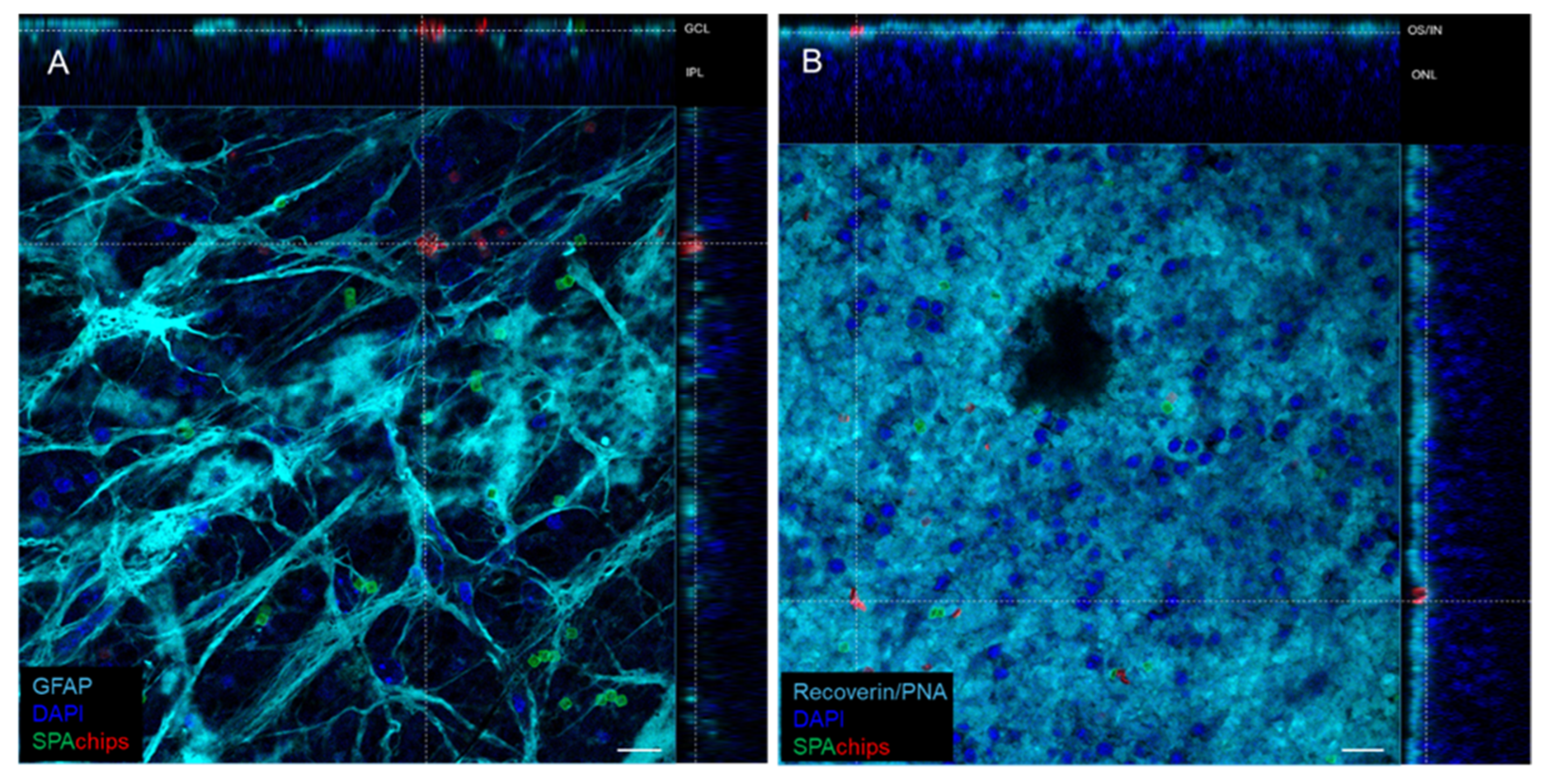
2.5. SPAchips® as Tool to Stain Cells of Retina Explants
3. Discussion
4. Materials and Methods
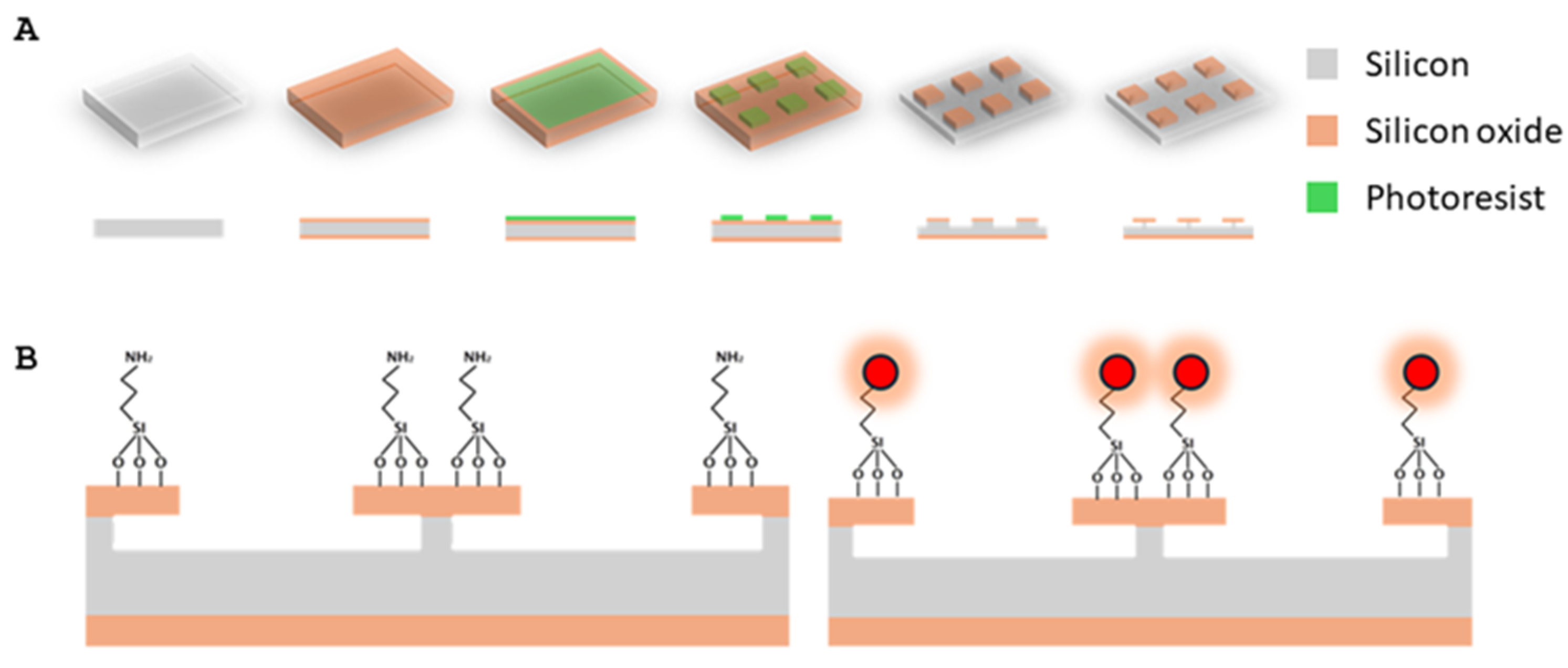
4.1. Manufacturing and Labelling of SPAchip®
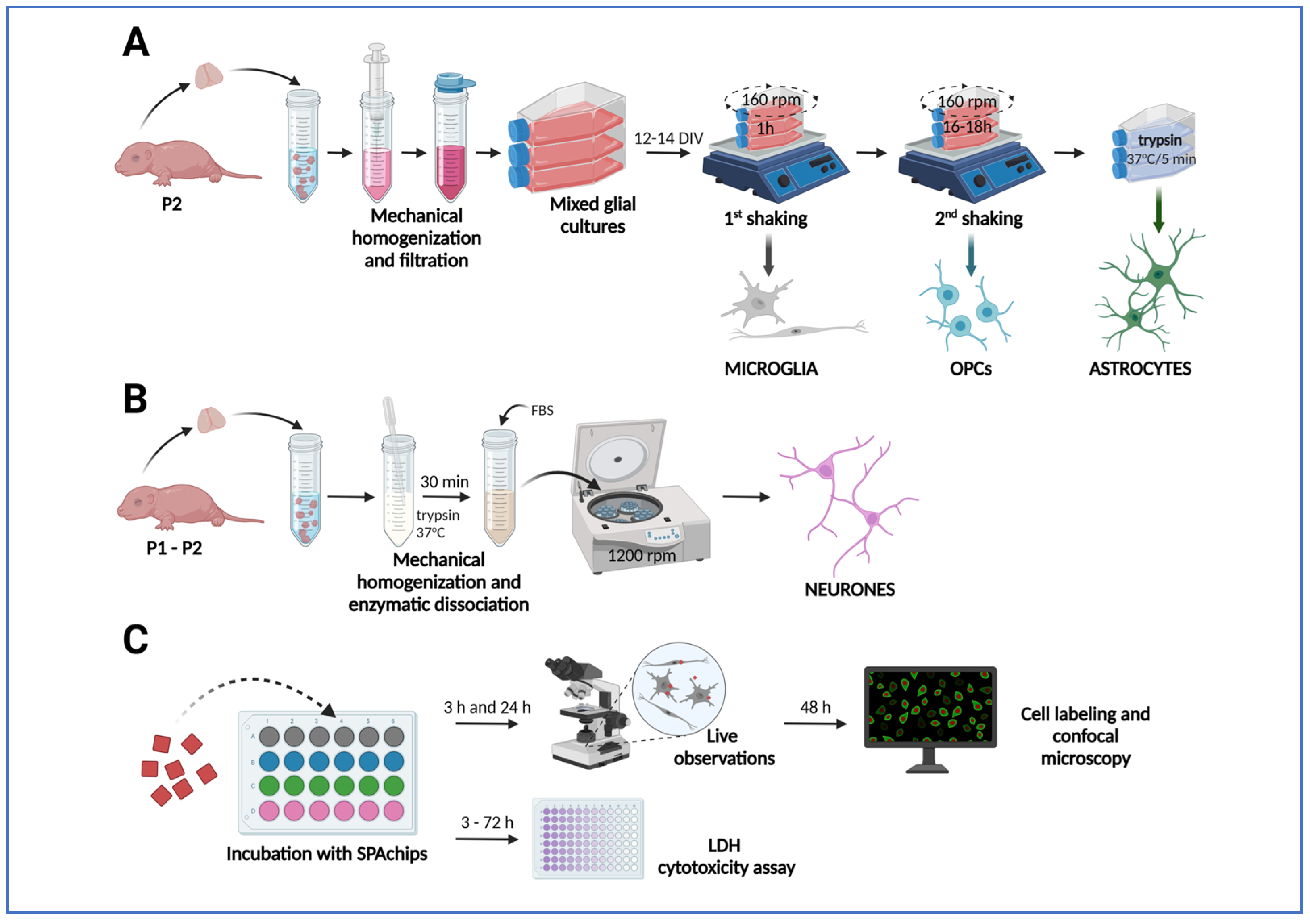
4.2. Isolation and Primary Cultures of Rat Macroglial and Microglial Cells
4.3. Establishing Primary Cultures of Rat Neurons
4.4. Incubation with SPAchip®
4.5. Culturing the Retina Explants
4.6. LDH Assay
4.7. Immunofluorescence Labelling
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACSA-1/GLAST | astrocyte cell surface antigen-1 |
| BBB | blood–brain barrier |
| CNS | central nervous system |
| GCL | ganglion cell layer |
| GDNF | glial-derived neurotrophic factor |
| GFAP | glial fibrillary acidic protein |
| iNOS | inducible nitric oxide synthase |
| IPL | inner plexiform layer |
| MAP2 | Microtubule-Associated Protein 2 |
| MBP | myelin basic protein |
| OLIG2 | transcriptional factor characteristic for oligodendroglial lineage |
| OS/IS | outer/inner segment |
| PDL | poly-D-lysine |
| ROS | reactive oxygen species |
| RGC | retinal ganglion cell layer |
| SPAchips® | suspended planar array chips |
| VACNF | vertically aligned carbon nanofiber |
References
- Abram, S.-L.; Tavernaro, I.; Johnston, L.J.; Zou, S.; Resch-Genger, U. Nanoscale Reference and Test Materials for the Validation of Characterization Methods for Engineered Nanomaterials—Current State, Limitations, and Needs. Anal. Bioanal. Chem. 2025, 417, 2405–2425. [Google Scholar] [CrossRef]
- Noreen, S.; Ishaq, I.; Saleem, M.H.; Ali, B.; Muhammad Ali, S.; Iqbal, J. Electrochemical Biosensing in Oncology: A Review Advancements and Prospects for Cancer Diagnosis. Cancer Biol. Ther. 2025, 26, 2475581. [Google Scholar] [CrossRef]
- Gabizon, A.A. Cancer Nanomedicine from a Clinician-Scientist Perspective: Lessons and Prospects. J. Control. Release 2025, 382, 113731. [Google Scholar] [CrossRef] [PubMed]
- Almehdi, A.M.; Aboubaker, D.H.; Hamdy, R.; El-Keblawy, A. Nanotherapeutic Smart Approaches for Combating Alzheimer’s Disease and Overcoming Existing Obstacles: A Novel Eco-Friendly Green Approach. Toxicol. Rep. 2025, 14, 101906. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start Small, Think Big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Abdel-Megeed, R.M. Biogenic Nanoparticles as a Promising Drug Delivery System. Toxicol. Rep. 2025, 14, 101887. [Google Scholar] [CrossRef]
- Rust, R.; Sagare, A.P.; Zhang, M.; Zlokovic, B.V.; Kisler, K. The Blood–Brain Barrier as a Treatment Target for Neurodegenerative Disorders. Expert Opin. Drug Deliv. 2025, 22, 673–692. [Google Scholar] [CrossRef]
- Das, S.; Carnicer-Lombarte, A.; Fawcett, J.W.; Bora, U. Bioinspired Nanotools for Neuroscience. Prog. Neurobiol. 2016, 142, 1–22. [Google Scholar] [CrossRef]
- Yu, Z.; McKnight, T.E.; Ericson, M.N.; Melechko, A.V.; Simpson, M.L.; Morrison, B. Vertically Aligned Carbon Nanofiber Arrays Record Electrophysiological Signals from Hippocampal Slices. Nano Lett. 2007, 7, 2188–2195. [Google Scholar] [CrossRef]
- Bareket, L.; Waiskopf, N.; Rand, D.; Lubin, G.; David-Pur, M.; Ben-Dov, J.; Roy, S.; Eleftheriou, C.; Sernagor, E.; Cheshnovsky, O.; et al. Semiconductor Nanorod-Carbon Nanotube Biomimetic Films for Wire-Free Photostimulation of Blind Retinas. Nano Lett. 2014, 14, 6685–6692. [Google Scholar] [CrossRef]
- Torras, N.; Agusil, J.P.; Vázquez, P.; Duch, M.; Hernández-Pinto, A.M.; Samitier, J.; de la Rosa, E.J.; Esteve, J.; Suárez, T.; Pérez-García, L.; et al. Suspended Planar-Array Chips for Molecular Multiplexing at the Microscale. Adv. Mater. 2016, 28, 1449–1454. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as Architects of Central Nervous System Formation and Function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Bigbee, J.W. Cells of the Central Nervous System: An Overview of Their Structure and Function. Adv. Neurobiol. 2023, 29, 41–64. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef]
- Simons, M.; Gibson, E.M.; Nave, K.-A. Oligodendrocytes: Myelination, Plasticity, and Axonal Support. Cold Spring Harb. Perspect. Biol. 2024, 16, a041359. [Google Scholar] [CrossRef]
- Cui, Q.-L.; Mohammadnia, A.; Yaqubi, M.; Weng, C.; Dorion, M.-F.; Pernin, F.; Hall, J.A.; Dudley, R.; Stratton, J.A.; Kennedy, T.E.; et al. Myelination Potential and Injury Susceptibility of Grey versus White Matter Human Oligodendrocytes. Brain 2025, 148, 921–932. [Google Scholar] [CrossRef]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Lee, H.-G.; Wheeler, M.A.; Quintana, F.J. Function and Therapeutic Value of Astrocytes in Neurological Diseases. Nat. Rev. Drug Discov. 2022, 21, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Won, W.; Bhalla, M.; Lee, J.-H.; Lee, C.J. Astrocytes as Key Regulators of Neural Signaling in Health and Disease. Annu. Rev. Neurosci. 2025, 48, 251–276. [Google Scholar] [CrossRef]
- Vidal-Itriago, A.; Radford, R.A.W.; Aramideh, J.A.; Maurel, C.; Scherer, N.M.; Don, E.K.; Lee, A.; Chung, R.S.; Graeber, M.B.; Morsch, M. Microglia Morphophysiological Diversity and Its Implications for the CNS. Front. Immunol. 2022, 13, 997786. [Google Scholar] [CrossRef]
- Muzio, L.; Perego, J. CNS Resident Innate Immune Cells: Guardians of CNS Homeostasis. Int. J. Mol. Sci. 2024, 25, 4865. [Google Scholar] [CrossRef]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 1181–1194. [Google Scholar] [CrossRef]
- Jiang, X.; Yi, S.; Liu, Q.; Zhang, J. The Secretome of Microglia Induced by IL-4 of IFN-γ Differently Regulate Proliferation, Differentiation and Survival of Adult Neural Stem/Progenitor Cell by Targeting the PI3K-Akt Pathway. Cytotechnology 2022, 74, 407–420. [Google Scholar] [CrossRef]
- Subramani, M.; Lambrecht, B.; Ahmad, I. Human Microglia-Derived Proinflammatory Cytokines Facilitate Human Retinal Ganglion Cell Development and Regeneration. Stem Cell Rep. 2024, 19, 1092–1106. [Google Scholar] [CrossRef]
- Ferro, A.; Auguste, Y.S.S.; Cheadle, L. Microglia, Cytokines, and Neural Activity: Unexpected Interactions in Brain Development and Function. Front. Immunol. 2021, 12, 703527. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Olsen, M.L.; Sofroniew, M.V. Reactive Astrocytes and Emerging Roles in Central Nervous System (CNS) Disorders. Cold Spring Harb. Perspect. Biol. 2024, 16, a041356. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.E.; Lukens, J.R. Harnessing Microglia-Based Cell Therapies for the Treatment of Neurodegenerative Diseases. Curr. Opin. Immunol. 2025, 94, 102552. [Google Scholar] [CrossRef]
- Kim, M.E.; Lee, J.S. Mechanisms and Emerging Regulators of Neuroinflammation: Exploring New Therapeutic Strategies for Neurological Disorders. Curr. Issues Mol. Biol. 2024, 47, 8. [Google Scholar] [CrossRef]
- Solomou, G.; Young, A.M.H.; Bulstrode, H.J.C.J. Microglia and Macrophages in Glioblastoma: Landscapes and Treatment Directions. Mol. Oncol. 2024, 18, 2906–2926. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; So, K.-F.; Jiang, W.; Chiu, K. Targeting Microglia in Alzheimer’s Disease: Pathogenesis and Potential Therapeutic Strategies. Biomolecules 2024, 14, 833. [Google Scholar] [CrossRef]
- Shui, X.; Chen, J.; Fu, Z.; Zhu, H.; Tao, H.; Li, Z. Microglia in Ischemic Stroke: Pathogenesis Insights and Therapeutic Challenges. J. Inflamm. Res. 2024, 17, 3335–3352. [Google Scholar] [CrossRef] [PubMed]
- Quarta, A.; Berneman, Z.; Ponsaerts, P. Neuroprotective Modulation of Microglia Effector Functions Following Priming with Interleukin 4 and 13: Current Limitations in Understanding Their Mode-of-Action. Brain Behav. Immun. 2020, 88, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Zhang, R. Microglia Dynamic Response and Phenotype Heterogeneity in Neural Regeneration Following Hypoxic-Ischemic Brain Injury. Front. Immunol. 2023, 14, 1320271. [Google Scholar] [CrossRef]
- Saab, A.S.; Tzvetanova, I.D.; Nave, K.-A. The Role of Myelin and Oligodendrocytes in Axonal Energy Metabolism. Curr. Opin. Neurobiol. 2013, 23, 1065–1072. [Google Scholar] [CrossRef]
- Nave, K.-A.; Asadollahi, E.; Sasmita, A. Expanding the Function of Oligodendrocytes to Brain Energy Metabolism. Curr. Opin. Neurobiol. 2023, 83, 102782. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuan, Z.; Yang, S.; Zhu, Y.; Xue, M.; Zhang, J.; Leng, L. Brain Energy Metabolism: Astrocytes in Neurodegenerative Diseases. CNS Neurosci. Ther. 2023, 29, 24–36. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tiwari, S.P. Bioenergetics of Axon Integrity and Its Regulation by Oligodendrocytes and Schwann Cells. Mol. Neurobiol. 2024, 61, 5928–5934. [Google Scholar] [CrossRef]
- Kempuraj, D.; Dourvetakis, K.D.; Cohen, J.; Valladares, D.S.; Joshi, R.S.; Kothuru, S.P.; Anderson, T.; Chinnappan, B.; Cheema, A.K.; Klimas, N.G.; et al. Neurovascular Unit, Neuroinflammation and Neurodegeneration Markers in Brain Disorders. Front. Cell Neurosci. 2024, 18, 1491952. [Google Scholar] [CrossRef]
- Nam, J.; Won, N.; Bang, J.; Jin, H.; Park, J.; Jung, S.; Jung, S.; Park, Y.; Kim, S. Surface Engineering of Inorganic Nanoparticles for Imaging and Therapy. Adv. Drug Deliv. Rev. 2013, 65, 622–648. [Google Scholar] [CrossRef]
- Zajdel, K.; Janowska, J.; Frontczak-Baniewicz, M.; Sypecka, J.; Sikora, B. Upconverting Nanoparticles as a New Bio-Imaging Strategy—Investigating Intracellular Trafficking of Endogenous Processes in Neural Tissue. Int. J. Mol. Sci. 2023, 24, 1122. [Google Scholar] [CrossRef]
- Yuan, D.; He, H.; Wu, Y.; Fan, J.; Cao, Y. Physiologically-Based Pharmacokinetic Modeling of Nanoparticles. J. Pharm. Sci. 2019, 108, 58–72. [Google Scholar] [CrossRef]
- De Araújo, R.F.; de Araújo, A.A.; Pessoa, J.B.; Freire Neto, F.P.; da Silva, G.R.; Leitão Oliveira, A.L.C.S.; de Carvalho, T.G.; Silva, H.F.O.; Eugênio, M.; Sant’Anna, C.; et al. Anti-Inflammatory, Analgesic and Anti-Tumor Properties of Gold Nanoparticles. Pharmacol. Rep. 2017, 69, 119–129. [Google Scholar] [CrossRef]
- Bhalala, U.S.; Koehler, R.C.; Kannan, S. Neuroinflammation and Neuroimmune Dysregulation after Acute Hypoxic-Ischemic Injury of Developing Brain. Front. Pediatr. 2015, 2, 144. [Google Scholar] [CrossRef]
- Sun, C.; Yin, N.; Wen, R.; Liu, W.; Jia, Y.; Hu, L.; Zhou, Q.; Jiang, G. Silver Nanoparticles Induced Neurotoxicity through Oxidative Stress in Rat Cerebral Astrocytes Is Distinct from the Effects of Silver Ions. Neurotoxicology 2016, 52, 210–221. [Google Scholar] [CrossRef]
- Mohamed, E.M.; Kattaia, A.A.A.; Abdul-Maksoud, R.S.; Abd El-Baset, S.A. Cellular, Molecular and Biochemical Impacts of Silver Nanoparticles on Rat Cerebellar Cortex. Cells 2020, 10, 7. [Google Scholar] [CrossRef]
- Chang, X.; Niu, S.; Shang, M.; Li, J.; Guo, M.; Zhang, W.; Sun, Z.; Li, Y.; Zhang, R.; Shen, X.; et al. ROS-Drp1-Mediated Mitochondria Fission Contributes to Hippocampal HT22 Cell Apoptosis Induced by Silver Nanoparticles. Redox Biol. 2023, 63, 102739. [Google Scholar] [CrossRef]
- He, J.; Ma, Y.; Niu, X.; Pei, J.; Yan, R.; Xu, F.; Ma, J.; Ma, X.; Jia, S.; Ma, W. Silver Nanoparticles Induce Endothelial Cytotoxicity through ROS-Mediated Mitochondria-Lysosome Damage and Autophagy Perturbation: The Protective Role of N-Acetylcysteine. Toxicology 2024, 502, 153734. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver Nanoparticles Induce Oxidative Cell Damage in Human Liver Cells through Inhibition of Reduced Glutathione and Induction of Mitochondria-Involved Apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhang, H.; Guo, Q.; Shan, S.; Chen, S.; Li, C.; Yang, X.; Li, Z.; Mu, Y.; Shao, H.; et al. Oxidative Stress-Mediated Mitochondrial Pathway-Dependent Apoptosis Is Induced by Silica Nanoparticles in H9c2 Cardiomyocytes. Toxicol. Mech. Methods 2020, 30, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaran, S.; Athira, S.S.; Mohanan, P.V. Zinc Oxide Nanoparticle Induced Neurotoxic Potential upon Interaction with Primary Astrocytes. NeuroToxicology 2019, 73, 213–227. [Google Scholar] [CrossRef]
- Chang, X.; Li, J.; Niu, S.; Xue, Y.; Tang, M. Neurotoxicity of Metal-Containing Nanoparticles and Implications in Glial Cells. J. Appl. Toxicol. 2021, 41, 65–81. [Google Scholar] [CrossRef]
- Alaei, M.; Koushki, K.; Taebi, K.; Yousefi Taba, M.; Keshavarz Hedayati, S.; Keshavarz Shahbaz, S. Metal Nanoparticles in Neuroinflammation: Impact on Microglial Dynamics and CNS Function. RSC Adv. 2025, 15, 5426–5451. [Google Scholar] [CrossRef] [PubMed]
- Paisley, C.E.; Kay, J.N. Seeing Stars: Development and Function of Retinal Astrocytes. Dev. Biol. 2021, 478, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Koizumi, S.; Kiyama, H. Phagocytic Astrocytes: Emerging from the Shadows of Microglia. Glia 2022, 70, 1009–1026. [Google Scholar] [CrossRef]
- Wakida, N.M.; Cruz, G.M.S.; Ro, C.C.; Moncada, E.G.; Khatibzadeh, N.; Flanagan, L.A.; Berns, M.W. Phagocytic Response of Astrocytes to Damaged Neighboring Cells. PLoS ONE 2018, 13, e0196153. [Google Scholar] [CrossRef]
- Wan, T.; Zhu, W.; Zhao, Y.; Zhang, X.; Ye, R.; Zuo, M.; Xu, P.; Huang, Z.; Zhang, C.; Xie, Y.; et al. Astrocytic Phagocytosis Contributes to Demyelination after Focal Cortical Ischemia in Mice. Nat. Commun. 2022, 13, 1134. [Google Scholar] [CrossRef]
- Xu, T.; Liu, C.; Deng, S.; Gan, L.; Zhang, Z.; Yang, G.-Y.; Tian, H.; Tang, Y. The Roles of Microglia and Astrocytes in Myelin Phagocytosis in the Central Nervous System. J. Cereb. Blood Flow. Metab. 2023, 43, 325–340. [Google Scholar] [CrossRef]
- Minami, S.S.; Sun, B.; Popat, K.; Kauppinen, T.; Pleiss, M.; Zhou, Y.; Ward, M.E.; Floreancig, P.; Mucke, L.; Desai, T.; et al. Selective Targeting of Microglia by Quantum Dots. J. Neuroinflamm. 2012, 9, 22. [Google Scholar] [CrossRef]
- Portnoy, E.; Polyak, B.; Inbar, D.; Kenan, G.; Rai, A.; Wehrli, S.L.; Roberts, T.P.L.; Bishara, A.; Mann, A.; Shmuel, M.; et al. Tracking Inflammation in the Epileptic Rat Brain by Bi-Functional Fluorescent and Magnetic Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Peviani, M.; Capasso Palmiero, U.; Cecere, F.; Milazzo, R.; Moscatelli, D.; Biffi, A. Biodegradable Polymeric Nanoparticles Administered in the Cerebrospinal Fluid: Brain Biodistribution, Preferential Internalization in Microglia and Implications for Cell-Selective Drug Release. Biomaterials 2019, 209, 25–40. [Google Scholar] [CrossRef]
- Ma, W.; Kong, Y.; Sheng, X.; Liu, T.; Zhang, K.; Wen, R.; Liu, D.; Long, G.; Li, X. A Hydrogel-Based Nanoparticles Sustained-Release System Selectively Targeting pro-Inflammatory Microglia for Spinal Cord Injury Repair. J. Control. Release 2025, 387, 114180. [Google Scholar] [CrossRef]
- Ziemka-Nalecz, M.; Jaworska, J.; Zalewska, T. Insights Into the Neuroinflammatory Responses After Neonatal Hypoxia-Ischemia. J. Neuropathol. Exp. Neurol. 2017, 76, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Schulz, L.N.; Varghese, A.; Michenkova, M.; Wedemeyer, M.; Pindrik, J.A.; Leonard, J.R.; Garcia-Bonilla, M.; McAllister, J.P.; Cassady, K.; Wilson, R.K.; et al. Neuroinflammatory Pathways and Potential Therapeutic Targets in Neonatal Post-Hemorrhagic Hydrocephalus. Pediatr. Res. 2025, 97, 1345–1357. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Md, S.; Alhakamy, N.A.; Alfaleh, M.A.; Afzal, O.; Altamimi, A.S.A.; Iqubal, A.; Shaik, R.A. Mechanisms Involved in Microglial-Interceded Alzheimer’s Disease and Nanocarrier-Based Treatment Approaches. J. Pers. Med. 2021, 11, 1116. [Google Scholar] [CrossRef]
- Li, H.; Guan, M.; Zhang, N.-N.; Wang, Y.; Liang, T.; Wu, H.; Wang, C.; Sun, T.; Liu, S. Harnessing Nanomedicine for Modulating Microglial States in the Central Nervous System Disorders: Challenges and Opportunities. Biomed. Pharmacother. 2024, 177, 117011. [Google Scholar] [CrossRef]
- Wu, J.; Cui, X.; Bao, L.; Liu, G.; Wang, X.; Chen, C. A Nanoparticle-Based Wireless Deep Brain Stimulation System That Reverses Parkinson’s Disease. Sci. Adv. 2025, 11, eado4927. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Xu, X.; Wei, C.; Li, Y.; Wang, M.; Lv, C.; Wu, J.; Dai, Y.; Han, Y.; Lesniak, M.S.; et al. The Dynamic Interactions between Nanoparticles and Macrophages Impact Their Fate in Brain Tumors. Small 2021, 17, 2103600. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Tripathi, T.; Venkidasamy, B.; Monziani, A.; Rajakumar, G.; Alomary, M.N.; Alyahya, S.A.; Onimus, O.; D’souza, N.; Barkat, M.A.; et al. Multifunctional Nanocarriers for Alzheimer’s Disease: Befriending the Barriers. Mol. Neurobiol. 2024, 61, 3042–3089. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tan, H.; Wen, H.; Xin, P.; Liu, Y.; Deng, Z.; Xu, Y.; Gao, F.; Zhang, L.; Ye, Z.; et al. Recent Progress in Nanomedicine for the Diagnosis and Treatment of Alzheimer’s Diseases. ACS Nano 2024, 18, 33792–33826. [Google Scholar] [CrossRef] [PubMed]
- Gargas, J.; Janowska, J.; Ziabska, K.; Ziemka-Nalecz, M.; Sypecka, J. Neonatal Rat Glia Cultured in Physiological Normoxia for Modeling Neuropathological Conditions In Vitro. Int. J. Mol. Sci. 2022, 23, 6000. [Google Scholar] [CrossRef]
- Janowska, J.; Gargas, J.; Sypecka, J. Pearls and Pitfalls of Isolating Rat OPCs for In Vitro Culture with Different Methods. Cell. Mol. Neurobiol. 2023, 43, 3705–3722. [Google Scholar] [CrossRef]
- Arroba, A.I.; Alvarez-Lindo, N.; van Rooijen, N.; de la Rosa, E.J. Microglia-Mediated IGF-I Neuroprotection in the Rd10 Mouse Model of Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9124–9130. [Google Scholar] [CrossRef]
- Caffé, A.R.; Ahuja, P.; Holmqvist, B.; Azadi, S.; Forsell, J.; Holmqvist, I.; Söderpalm, A.K.; van Veen, T. Mouse Retina Explants after Long-Term Culture in Serum Free Medium. J. Chem. Neuroanat. 2001, 22, 263–273. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargas, J.; Janowska, J.; Dabrowska-Bouta, B.; Sidoryk-Wegrzynowicz, M.; Hernández-Pinto, A.M.; Miguez, R.; Suárez, T.; Struzynska, L.; Sypecka, J. SPAchips: Microparticles Used for the Selective In Vitro Labelling of Microglia. Int. J. Mol. Sci. 2025, 26, 9773. https://doi.org/10.3390/ijms26199773
Gargas J, Janowska J, Dabrowska-Bouta B, Sidoryk-Wegrzynowicz M, Hernández-Pinto AM, Miguez R, Suárez T, Struzynska L, Sypecka J. SPAchips: Microparticles Used for the Selective In Vitro Labelling of Microglia. International Journal of Molecular Sciences. 2025; 26(19):9773. https://doi.org/10.3390/ijms26199773
Chicago/Turabian StyleGargas, Justyna, Justyna Janowska, Beata Dabrowska-Bouta, Marta Sidoryk-Wegrzynowicz, Alberto M. Hernández-Pinto, Rubén Miguez, Teresa Suárez, Lidia Struzynska, and Joanna Sypecka. 2025. "SPAchips: Microparticles Used for the Selective In Vitro Labelling of Microglia" International Journal of Molecular Sciences 26, no. 19: 9773. https://doi.org/10.3390/ijms26199773
APA StyleGargas, J., Janowska, J., Dabrowska-Bouta, B., Sidoryk-Wegrzynowicz, M., Hernández-Pinto, A. M., Miguez, R., Suárez, T., Struzynska, L., & Sypecka, J. (2025). SPAchips: Microparticles Used for the Selective In Vitro Labelling of Microglia. International Journal of Molecular Sciences, 26(19), 9773. https://doi.org/10.3390/ijms26199773







